Abstract
Background
Fibromyalgia (FM) is a chronic condition characterized by diffused musculoskeletal pain and overwhelming fatigue.
Purpose
To compare the gene expression profiles of fatigued FM women with different levels of pain and catastrophizing.
Methods
Nine FM women enrolled in an active Medstar Research Institute protocol were included in the gene expression analyses of peripheral blood RNA using Affymetrix GeneChip® human genome U133 Plus 2.0 array. Scores from Brief Pain Inventory, Pain Catastrophizing Scale, and Multidimensional Fatigue Inventory categorized the 9 participants into pain (high, n = 3; low, n = 6) and catastrophizing groups (high, n = 5; low, n = 4).
Discussion
Differential expression of 107 genes between the high and low pain groups and 139 genes between the high and low catastrophizing groups (over 2.0-fold change, p < 0.05) were observed. Network analyses showed interferon signaling and interferon regulatory activation factor pathways distinguished between the pain groups while dendritic cell maturation delineated between the catastrophizing groups.
Conclusion
Findings provide preliminary evidence that specific physiological pathways may possibly delineate pain and catastrophizing mechanisms. Further investigation using larger and more homogenous sample is warranted.
Keywords: fibromyalgia, gene expression, microarray, pain, fatigue, catastrophizing
Fibromyalgia (FM) is a syndrome that is manifested by chronic, widespread bodily pain (Low & Schweinhardt, 2012). Based on the American College of Rheumatology (ACR) diagnostic criteria in 1990, fibromyalgia was diagnosed by the self-report history of widespread pain with at least 11 out of 18 tender sites on exam (Wolfe et al., 1990). While defined by widespread pain, FM is also characterized by overwhelming fatigue, sleep disturbance, cognitive dysfunction, and other somatic symptoms such as headache, gastrointestinal discomfort and stiffness, bladder symptoms, diarrhea, constipation, and paresthesia (Wolfe et al., 2010; Wolfe & Hawley, 1999). This realization led to the proposal of revising the 1990 ACR diagnostic criteria broadening the manifestations of FM not only with widespread pain, but also to include fatigue, non-refreshing sleep, cognitive difficulties, and other somatic complaints (Wolfe at al., 2010). While three medications have been approved by the United States Food and Drug Administration (FDA) to manage FM, no treatment has been shown to be particularly effective to date.
Fatigue is a common symptom in FM, seen in close to 70% of patients (White, Speechley, Harth, & Ostbye, 2000). This fatigue is not alleviated by rest and sleep (Humphrey et al., 2010) and is often reported as the most bothersome FM symptom, significantly contributing to the decline in the patients’ health-related quality of life (Arnold et al., 2008). Gene expression profiles have been used to explore biologic underpinnings of disease (Finnan, et al., 2011; Gursoy, et al., 2003; Macedo, et al., 2008) and also in the development of predictive algorithms of treatment/disease outcomes (Andersen & Skorpen, 2009; Neckley, et al., 2007). Considering the burden of symptoms in the lives of individuals with FM (Bellato, et al., 2012; Mease, 2005), this study attempted to explore the distinct biologic underpinnings of specific symptoms that define FM. This study compared the gene expression profiles of fatigued FM subjects with high and low pain, as well as the gene expression profiles of the same FM subjects with high and low catastrophizing, or their exaggerated, negative attention to their symptoms (Edwards, Chalan, Mensing, Smith, & Haythornthwaite, 2011).
The cause of FM is unknown; however, its etiology is assumed to be related to the combination of inherited susceptibility and traumatic or stressful environmental exposure (Ablin & Buskila, 2006; Ablin, Cohen, & Buskila, 2006a; Ablin, Shoenfeld, & Buskila, 2006b). The symptom experience of individuals with FM is thought to be influenced by both physiological and psychological factors. Suspected physiological factors include dysfunction in the stress systems, such as the sympathetic (adrenergic) and hypothalamic-pituitary-adrenal (HPA) pathways (Sabban, 2007). Altered central mechanisms that are related to pain transmission, sleep regulation, and depression may also be involved in the pathogenesis of FM fatigue (Finan et al., 2010).
Previous genomic studies in FM were limited in focus and investigated the expression of genes specific to the proposed pathways of interest (e.g., sympathetic nervous system and inflammation) using reverse transcriptase polymerase chain reaction (RT-PCR) and real time, quantitative PCR (qPCR) (Salemi et al., 2003; Light, White, Hughen & Light, 2009). For example, down regulation of Catechol-O-Methyl-Transferase (COMT) gene by qPCR was associated with severe psychological distress (Desmeules et al., 2012). Low COMT in high catastrophizing individuals was also reported to increase risk for the development of chronic pain syndromes (George et al., 2008). Genes related to immune modulation, oxidative stress, and apoptosis were also shown to be differentially expressed in fatigued patients post infection (Gow et al., 2009). An unbiased approach of investigating genes that are associated with FM symptoms has not been conducted previously.
Psychological factors, such as negative personality traits, are also suspected to play a role in FM pathogenesis (Clauw, 2009). Catastrophizing has been well-established as a predictor of the severity of a pain experience (Aaron, 1999; Buenaver, Edwards, & Haythornthwaite, 2007; Buitenhuis, De Jong, Jaspers, & Groothoff, 2008; Burckhardt, Clark, & Bennett, 2001; Burckhardt, Clark, O'Reilly, & Bennett, 1997). In addition, pain catastrophizing is thought to influence the experience of FM symptoms (Burgmer et al., 2011). Being able to distinguish the biological mechanisms responsible for these known physiologic and psychological contributors to FM symptoms would be a major advance in the field, because identification of physiologic pathways related to specific symptoms defining FM will provide a foundation for new interventions. This study report is on a preliminary finding from a subset analysis of an ongoing study that seeks to differentiate the gene expression profiles between women who are healthy and those with fibromyalgia.
Methods
Participants
The data were derived from a prospective, longitudinal, observational study from an Institutional Review Board-approved Medstar Health Research Institute protocol. Participants diagnosed with FM using the 1990 (self-report history of widespread pain with at least 11 out of 18 tender sites on exam) or the 2010 American College of Rheumatology criteria (Widespread Pain Index (WPI) ≥ 7 and Symptom Severity (SS) ≥ 5, or WPI = 3 – 6 and the SS ≥ 9) were included in the analyses. Data analyzed in this study were obtained during one outpatient visit.
Measures
All participants were evaluated for the following:
Fatigue was measured by the Multidimensional Fatigue Inventory (MFI), a 20-item, self-report questionnaire composed of five subscales: general fatigue, physical fatigue, reduced activity, reduced motivation, and mental fatigue (Smets, Garssen, Bonke, & De Haes, 1995). Each of the five subscales was measured with 4 items using a rating scale of 1 (completely true) to 5 (no, not true), which have been found to have acceptable internal consistency reliability (Cronbach’s alpha > 0.80) (Smets, Garssen, Bonke, De Haes, 1995). Scores that are greater than 13 on the general fatigue subscale or higher than 10 on the reduced activity subscale were suggested as cutoff scores for clinically significant fatigue (Reeves et al., 2005).
Pain was measured by the Brief Pain Inventory-Short Form (BPI-SF) (Cleeland, Ladinsky, Serlin, & Nugyen, 1988). This self-report instrument measures two concepts: pain intensity (4 items) and pain interference (7 items) using a numeric rating scale of 0 (no pain / interference) to 10 (pain as bad as you can imagine / complete interference). The BPI-SF was found to have very good internal consistency, both for pain intensity (Cronbach’s alpha = 0.88) and pain interference (Cronbach’s alpha = 0.87) (Kapstad, Rokne, & Stavem, 2010). Findings from a longitudinal study in the prediction of worsening health outcomes provided empirical support for setting the cut point score for clinically significant pain intensity at five for the BPI-SF (Castel et al., 2007).
Catastrophizing was measured using the Pain Catastrophizing Scale (PCS), a 13-item, self-report questionnaire consisting of three subscales: rumination, magnification, and helplessness. Participants were asked to rate their thoughts and feelings on a 0 (not at all) to 4 (all the time) numeric scale. The PCS total score had acceptable internal consistency in past research (Cronbach’s alpha = 0.87) (Osman et al., 2000; Osman et al., 1997). A PCS score of 16 or higher has been considered to be the cutoff for high catastrophizing (Riddle, Wade, Jiranek, & Kong, 2010).
Biological Sample Collection
Peripheral blood (2.5 mL) was collected from each patient using PAXgene™ Blood RNA tubes (Qiagen, Frederick, Maryland) containing red blood cell lysis buffer and a RNA-stabilizing solution. The collected blood was stored at −80°C until ready for RNA extraction.
RNA extraction and microarray experiments
Total RNA was extracted using the PAXgene™ Blood RNA system (Qiagen, Frederick, Maryland) according to manufacturer’s instructions. The quantity of total RNA was measured by a spectrophotometer at optical density of 260 nanometers. RNA quality was assessed using the RNA 6000 Nano LabChip® on a Bioanalyzer Agilent 2100 (Agilent Technologies, Palo Alto, CA). RNA purification, cDNA and cRNA synthesis, amplication, hybridization, scanning and data analyses were conducted by one laboratory technician following standard protocols as previously described (Wang et al., 2007). Affymetrix microarray chips (HG-U133 Plus 2.0, Santa Clara, California) were used for gene expression analysis. The Affymetrix HG-U133 Plus 2.0 microarray chip is comprised of more than 54,000 probe sets and 1,3000,000 distinct oligonucleotide features, which analyzed the expression level of over 47,000 transcripts, including 38,500 well characterized human genes. Affymetrix GeneChip Command Console (AGCC, 3.0 V) was used to scan the images for data acquisition.
Gene expression analysis
Affrymetrix CEL files (the file format that store the results of the intensity calculations of the pixel values of the scanned image from a microarray chip) were imported into Partek® Genomic Suite™ (Partek Inc., St. Louis, MO) using the default Partek® normalization parameters. Probe-level data or transcripts were pre-processed, background corrected, normalized, and summarized, using the robust multi-array average (RMA) method (Wu & Irizarry, 2007). Differential gene expression analysis using two-way analysis of variance (ANOVA) was performed between the high and low pain and the high and low catastrophizing groups. Batch effects were controlled in the analysis by including the scanned dates. Pairwise comparison was used to determine the differential gene expression between the high and low pain and high and low catastrophizing groups. The differentially expressed gene lists for the pain and catastrophizing groups were developed based on the filtering criteria of a > 2.0 or < −2.0 fold change and an unadjusted p-value of < 0.05. Quality assurance and quality control of the microarray data was confirmed by examining the histograms of the microarray data from the samples, which showed no outlier. Ingenuity Pathway analysis (IPA) (Ingenuity® Systems, www.ingenuity.com, Redwood City, California) identified functional networks of the differentially expressed probesets from the Ingenuity’s Knowledge Base. Right-tailed Fisher’s exact test was used to calculate p-values determining the probability that each biological function and/or disease assigned to these networks was not due to chance alone. A Monte Carlo simulation method was used to confirm the results obtained from the Ingenuity pathway analyses.
Results
Sample Characteristics
Nine Caucasian women (26 to 50 years old, mean = 41.22) diagnosed with FM (based on the 1990 or 2010 diagnostic criteria) who scored greater than 13 on the MFI general fatigue subscale or more than 10 on the MFI reduced activity subscale were included in the analyses. Their pain (BPI-SF) scores ranged from 0.5 – 6.3 (mean = 4.11) and catastrophizing (PCS) scores ranged from 4 – 36 (mean = 17) (table 1).
Table 1.
Characteristics of Total Sample
| Min | Max | Mean | SD | Clinical cutoff | |
|---|---|---|---|---|---|
| Age | 26 | 50 | 41.22 | 7.31 | |
| Pain severity | 0.5 | 6.3 | 4.11 | 1.91 | ≥ 5 |
| Pain interference | 0.0 | 8.43 | 5.16 | 2.63 | |
| Pain sensitivity (Dolorimeter) | |||||
| Tender point (tolerated less than 4 kg of pressure) | 11 | 18 | 15.75 | 3.15 | |
| Average pain threshold | 0.5 | 3.1 | 1.86 | 0.94 | |
| Symptoms severity | 3 | 10 | 7.11 | 1.97 | |
| Wide spread pain index | 5 | 18 | 11.11 | 4.08 | |
| Fatigue (Multidimensional Fatigue Inventory) | |||||
| General fatigue | 12 | 20 | 17.11 | 2.71 | ≥ 13 |
| Reduced activity | 9 | 19 | 12.89 | 3.89 | ≥ 10 |
| Catastrophizing | 4 | 36 | 17.00 | 9.79 | ≥ 16 |
Using the pain score of 5 as a cut point for clinical significance, six of the nine FM women were categorized into the low pain group (mean= 3.12 ± 1.5), and three subjects into the high pain group (mean= 6.08 ± 0.1). Table 2 shows symptom characteristics of the two pain groups. The high pain group had significantly higher pain severity than the low pain group t (7) = −3.245, p = 0.015. However, no significant differences in pain interference, pain threshold, symptoms severity, widespread pain index, general fatigue, tender points, physical activity, motivation, or mental fatigue were found between the high and low pain groups.
Table 2.
Symptom differences between high pain vs. low pain and high catastrophizing vs. low catastrophizing groups
| Variables | Pain severity Mean (SD)
|
t | df | Catastrophizing Mean (SD)
|
t | df | ||
|---|---|---|---|---|---|---|---|---|
| Low (n = 6) | High (n = 3) | Low (n = 4) | High (n = 5) | |||||
| Age | 42.33 (8.7) | 39.00 (3.6) | 0.619 | 7 | 43.50 (5.3) | 39.40 (8.8) | 0.819 | 7 |
| Pain severity | 3.12 (1.5) | 6.08 (0.1) | −3.245* | 7 | 3.44 (2.4) | 4.65 (1.5) | −0.940 | 7 |
| Pain interference | 4.55 (2.9) | 6.38 (1.8) | −0.981 | 7 | 3.93 (3.0) | 6.14 (2.0) | −1.309 | 7 |
| Pain sensitivity | ||||||||
| Tender points | 16.60 (2.0) | 14.33 (4.7) | 0.983 | 6 | 15.00 (4.2) | 16.50 (1.9) | −0.645 | 6 |
| Pain threshold | 1.75 (1.0) | 2.04 (1.0) | −0.396 | 6 | 1.41 (1.1) | 2.3 (0.5) | −1.444 | 6 |
| Symptoms severity | 6.50 (2.0) | 8.33 (1.5) | −1.395 | 7 | 6.25 (2.2) | 7.8 (1.6) | −1.209 | 7 |
| Wide spread pain index | 10.50 (3.9) | 12.33 (4.9) | −0.611 | 7 | 10.50 (5.8) | 11.6 (2.7) | −0.380 | 7 |
| Fatigue | ||||||||
| General fatigue | 16.67 (3.1) | 18.00 (2.0) | −0.671 | 7 | 17.50 (2.5) | 16.80 (3.1) | 0.363 | 7 |
| Physical fatigue | 16.00 (1.7) | 16.33 (1.2) | −0.306 | 7 | 15.75 (1.5) | 16.40 (1.5) | −0.642 | 7 |
| Reduced activity | 13.33 (3.6) | 12.00 (5.2) | −0.461 | 7 | 12.25 (4.7) | 13.40 (3.6) | −0.418 | 7 |
| Reduced motivation | 12.00 (3.2) | 10.67 (2.1) | −0.641 | 7 | 11.00 (1.8) | 12.00 (3.6) | −0.501 | 7 |
| Mental fatigue | 14.00 (5.8) | 13.00 (4.6) | 0.259 | 7 | 10.00 (5.9) | 16.60 (1.5) | −2.447* | 7 |
| Catastrophizing | ||||||||
| Rumination | 7.67 (3.0) | 6.67 (7.6) | 0.294 | 7 | 4.25 (3.1) | 9.80 (4.1) | −2.239 | 7 |
| Magnification | 4.00 (2.8) | 1.67 (1.5) | 1.306 | 7 | 1.50 (1.3) | 4.60 (2.7) | −2.091 | 7 |
| Helpless | 6.50 (5.0) | 6.33 (3.2) | 0.052 | 7 | 3.00 (1.4) | 9.20 (3.7) | −3.136* | 7 |
| Total catastrophizing | 18.17 (10.5) | 14.74 (9.7) | 0.480 | 7 | 8.75 (4.4) | 23.60 (7.4) | −3.513* | 7 |
Note. = p value ≤ 0.05
Using the catastrophizing score of 16 as a cut point for clinical significance, based on a previous study (Riddle, Wade, Jiranek & Kong, 2010), four of the same nine FM women were categorized into the low catastrophizing group (mean = 8.75 ± 4.4), and five subjects into the high catastrophizing group (mean = 23.60 ± 7.4). Subjects in the high catastrophizing group (n = 5) reported higher scores in almost all of the symptoms reported and had significantly higher mental fatigue than the low catastrophizing group (n = 4) t (7) = −2.447, p = 0.044 (table 2).
Differential Gene Expression
The gene list was obtained from the microarray analysis comparing the gene expression profiles from peripheral blood of subjects with high and low pain, and those with high and low catastrophizing groups. Using the filtering criteria, 107 probesets were differentially expressed between high and low pain groups. Table 3 lists the top 10 differentially expressed genes between subjects in the high and low pain severity group. Based on the p-values (the statistical significance of the occurrence of genes), the most up regulated genes in the high pain group are the basic leucine zipper protein (BATF2) and two immune response genes (CASP5 and CCR1).
Table 3.
Top 10 differentially expressed genes between the high and low pain groups based on p-values
| Gene Symbol | Genes title | Function | p-value | Fold-Change | Expression in high pain group |
|---|---|---|---|---|---|
| BATF2 | basic leucine zipper transcription factor, ATF-like 2 | Protein binding/protein dimerization activity | 0.0002 | 2.064 | up |
| CASP5 | caspase 5, apoptosis-related cysteine peptidase | Cysteine-type endopeptidase activity | 0.0003 | 2.732 | up |
| CCR1 | chemokine (C-C motif) receptor 1 | C-C chemokine binding/ C-C chemokine receptor activity | 0.0004 | 2.220 | up |
| CD69 | CD69 molecule | transmembraine signaling receptor activity | 0.0008 | 4.217 | up |
| CEACAM1 | carcinoembryonic antigen-related cell adhesion molecule 1 (biliary glycoprotein) | molecular function/protein binding | 0.0010 | 2.493 | up |
| LY6E | lymphocyte antigen 6 complex, locus E | adrenal gland development/ epinephrine secrertion/norepinephrine metabolic process | 0.0100 | −2.102 | down |
| PARP14 | poly (ADP-ribose) polymerase family, member 14 | NAD+ADP-ribosyltransferase activity | 0.0112 | −2.048 | down |
| RPL23 | ribosomal protein L23 | Structoral constituent of ribosome | 0.0191 | −2.166 | down |
| RPL7 | ribosomal protein L7 | DNA, RNA, mRNA binding/ protein homodimerization activity | 0.0237 | −2.869 | down |
| SCO2 | SCO2 cytochrome c oxidase assembly protein | Copper ion binding | 0.0312 | −2.243 | down |
| SERPING1 | serpin peptidase inhibitor, clade G (C1 inhibitor), member 1 | protein binding/ serine-type endopeptidase inhibitor activity | 0.0316 | −2.074 | down |
| SH2D1B | SH2 domain containing 1B | protien binding, bridging | 0.0360 | −2.767 | down |
There were 139 probesets that were differentially expressed between subjects in the high and low catastrophizing groups, after the filtering criteria were applied. In the high catastrophizing group, the genes involved in chemokine activity and heparin binding (PF4V1) and the GABA regulation gene (USP46) were found to be down regulated and the genes involved in actin binding (TNS1), ferric iron binding (LTF), and cytokinesis (SPP1) were found to be up regulated, based on the p values (table 4).
Table 4.
Top 10 differentially expressed genes between the high and low catastrophizing groups based on p-values
| Gene Symbol | Genes title | Function | p-value | Fold-Change | Expression in high catastrophizing |
|---|---|---|---|---|---|
| TNS1 | Tensin 1 | Actin binding/protein binding | 0.026 | 4.005 | up |
| LTF | Lactotransferrin | Ferric iron binding/serune-type endopeptidase activity/DNA binding | 0.029 | 3.345 | up |
| SLC6A8 | solute carrier family 6 (neurotransmitter transporter, creatine), member 8 | Creatine transmembrane transporter activity/creatine:sodium symporter activity | 0.014 | 3.064 | up |
| TMTC1 | transmembrane and tetratricopeptide repeat containing 1 | function is yet unknown | 0.023 | 2.647 | up |
| SPP1 | secreted phosphoprotein 1 | Cytokine activity/extracellular metrix binding | 0.046 | 2.363 | up |
| PF4V1 | Platelet factor 4 variant 1 | Chemokine activity/ heparin binding | 0.008 | −5.639 | down |
| CLEC4D | C-type lectin domain family 4, member D | Carbohydrate binding | 0.001 | −3.151 | down |
| 10SEP | septin 10 | GTP binding | 0.003 | −2.353 | down |
| FAS | Fas (TNF receptor superfamily, member 6) | Identical protein binding/ signal transducer activity | 0.005 | −2.214 | down |
| USP46 | ubiquitin specific peptidase 46 | Ubiquitin-specific protease activity | 0.006 | −2.211 | down |
Network and Pathway Analyses
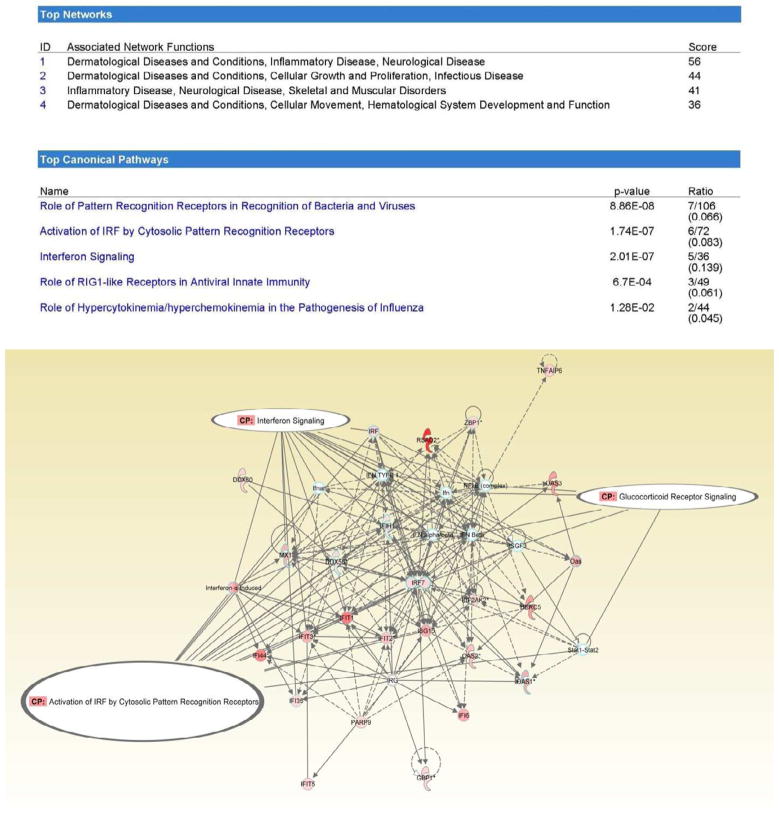
The 107 differentially expressed probesets between the high and low pain severity groups were analyzed using Ingenuity Pathway analysis and revealed several common physiological networks: dermatological diseases and conditions; inflammatory disease; neurological disease; cellular growth, movement, and proliferation; infectious disease; skeletal and muscular disorders; and hematological system development and function (figure 1A). Further Ingenuity Pathway analysis of the differentially expressed probesets, revealed distinct canonical pathways between the high and low pain groups to include: role of pattern recognition of bacteria and viruses, activation of interferon regulatory factor (IRF) cytosolic pattern recognition receptors, interferon signaling, role of retinoic acid-inducible 1 (RIG1)-like receptors in antiviral innate immunity, and the role of hypercytokinemia / hyperchemokinemia in the pathogenesis of influenza (figure 1B). Overlay of physiological networks and canonical pathways revealed the activation of IRF by cytosolic pattern recognition receptor, interferon signaling, and glucocorticoid receptor signaling as the networks that were associated with the differentially expressed probesets between the high and low pain groups (figure 1C).
Figure 1.
Top networks and top canonical pathways for the differentially expressed probesets between high and low pain groups. The interferon signaling, glucocorticoid receptor signaling and activation of IRF by cytosolic pattern recognition receptors pathways were associated with differentially expressed probesets between high and low pain groups
The 139 differentially expressed probesets between the high and low castratrophizing groups were analyzed by IPA and revealed four common physiologic networks to include: cellular movement / hematological system development and function / immune cell trafficking; amino acid metabolism / small molecular biochemistry / cellular movement; cell death / cancer / hereditary disorder; and cellular development/ gene expression (figure 2A). Top canonical pathways associated with the differentially expressed probesets between the two catastrophizing groups included type 2 diabetes signaling; valine, leucine, and isoleucine biosynthesis; pantothenate and coenzyme A (CoA) biosynthesis; role of Janus kinases (JAK2) in hormone-like cytokine signaling; and inhibition of angiogenesis by thrombospondin-1 (TSP1) (figure 2B). Overlay of the physiologic networks and canonical pathways revealed pathways related to dendritic cell maturation and glucocorticoid receptor signaling as common networks that were associated with the differentially expressed genes between the catastrophizing groups (figure 2C).
Figure 2.
Top networks and top canonical pathways for the differentially expressed probesets between high and low catastrophizing groups. The dendritic cell maturation and glucocorticoid receptor signaling pathways were associated with differentially expressed probesets between high and low catastrophizing groups.
To confirm that physiologic pathways were not influenced by outlying conditions (e.g., acute viral infection from one of the study subjects), especially between the pain groups, a Monte Carlo stimulation method was performed by randomly excluding one subject at a time and re-running the differential gene expression analysis and determining if physiologic pathways remain consistently the same during the analyses. The Monte Carlo simulation method confirmed that the top 5 canonical pathways (role of pattern recognition of bacteria and viruses, activation of interferon regulatory factor [IRF] cytosolic pattern recognition receptors, interferon signaling, role of retinoic acid-inducible 1 [RIG1]-like receptors in antiviral innate immunity, and the role of hypercytokinemia / hyperchemokinemia in the pathogenesis of influenza) remain consistently observed in the high pain group.
Making causative associations between these genes and the occurrence of FM symptoms or their etiologies is premature because of the small sample and the preliminary nature of this study. Findings from this preliminary study do not assert accurate detection of specific genes that are directly associated with the symptom categories mentioned.
Discussion
This is the first study to our knowledge that investigated the whole genome differential gene expression of FM subjects according to self-reported clinical pain and catastrophization. This gap in knowledge may be related to the use of limited methodological genomic approaches used in previous studies and the new changes in the diagnostic criteria for FM. Results from this preliminary study suggest potential physiologic pathways that might delineate biologic mechanisms between pain and catastrophization. Physiologic pathways involving interferon signaling and activation of interferon regulatory factor can potentially distinguish between high and low pain experiences while dendritic cell maturation may possibly delineate between the high and low catastrophizing subjects. Enhanced glucocorticoid receptor signaling, which has been previously implicated in FM (Maletic & Raison, 2009), was found in both the high pain and catastrophizing categories and appears to be a potentially shared pathway.
The immunoreactivity of interferon-gamma receptor has been linked to the spinal nociceptive pathways in animal studies. One study observed that the stimulation of the interferon-gamma receptor signaling activates the spinal microglia producing long-lasting pain hypersensitivity (Tsuda et al., 2009). An earlier study demonstrated a functional interferon-gamma receptor in spinal nociceptive pathways that were associated with neuropathic pain (Brita et al., 1997). Further investigation of the role of interferon signaling in defining mechanisms behind pain symptoms in FM is worthwhile to pursue.
Although no studies have linked neuronal dendritic cell maturation with catastrophizing, an animal study reported suppression of dendritic maturation of immature neurons by light deprivation is observed in individuals with seasonal affective disorder (Lau, Jongstra-Bilen, & Cybulsky, 2011). Another animal study detected the association of the inhibition of hippocampal neurogenesis and neuronal dendritic spine formation with an increase in depression-like behaviors (Zhang, Tonelli, Regenold, & McCarthy, 2010). Catastrophizing is highly associated with depression (Edwards et al., 2011). Further investigation of a potential role of dendritic cell maturation in the development of behavior may provide insight into the physiologic mechanisms behind catastrophizing.
Previous studies have attempted to identify FM subgroups, based on pain and catastrophizing symptoms (de Souza et al., 2009; Giesecke et al., 2003; Rehm et al., 2010; Turk, Okifuji, Sinclair, & Starz, 1996, 1998). Some studies found significant roles of specific genes on the relationship of catastrophizing and pain intensity (Finan et al., 2011; George et al., 2008; Demeules et al., 2012). One chronic fatigue syndrome study found differential genes related to immune response, oxidative stress, and apoptosis expressed when compared with healthy controls (Gow et al., 2009). However, no studies have included fatigue, catastrophizing, and pain and explored the genomic associations distinct or shared by these FM symptoms. These studies suggest that psychological distress can influence the symptom responses of individuals with FM (de Souza et al., 2009; Giesecke et al., 2003). Individuals with high catastrophizing were found to also report high pain (Burckhardt et al., 2001; Burckhardt et al., 1997; Gracely et al., 2004) and fatigue (Jacobsen, Andrykowski, & Thors, 2004; Jacobsen, Azzarello, & Hann, 1999; Sohl & Friedberg, 2008).
Results of this preliminary study are limited because of the small sample size. The study findings should be interpreted with caution because of the major limitations mentioned. Careful selection of a similar homogenous sample will be necessary to confirm the findings reported in this study. However, our results demonstrate the potential application of a genomic approach to an illness defined only by a subjective complaint. Moreover, our findings highlighted potential physiologic pathways of interest, stimulating questions and suggesting a specific direction for continued investigation. It seems possible to use our techniques to understand the distinct mechanisms that delineate particular FM symptoms as well as common mechanisms that are shared by multiple symptoms.
Conclusion and Implications
This study provides preliminary evidence that a genomic approach can assist in understanding the etiology of an illness that is defined by its symptoms. Possible physiologic pathways such as interferon signaling and activation of interferon regulatory factor may potentially distinguish between individuals with FM experiencing high and low pain symptoms. Genes related to dendritic cell maturation may possibly delineate between the high and low catastrophizing FM subjects, while high pain and high catastrophizing subjects showed potentially differential expression of genes related to glucocorticoid receptor signaling. Further research is necessary to confirm these preliminary findings, taking particular attention to addressing the major limitations of this analysis and the inherent challenges phenotyping individuals with FM.
Acknowledgments
This study is part of a collaborative activity of NINR and the MedStar Research Institute as approved by the Office of Human Subjects Research (OHSR) of the National Institutes of Health (OHSR IRB-Exempt #4966), and is funded in part by the Intramural Research Program of the NINR and from a Medstar grant. We would like to extend our gratitude to Joan K. Austin, PhD, RN, FAAN for her guidance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aaron LA. Unpublished doctoral disseration. University of Alabama at Birmingham; Birmingham, Alabama: 1999. The moderating effects of catastrophizing as a response to daily pain in patients with fibromyalgia. [Google Scholar]
- Ablin JN, Buskila D. The genetics of fibromyalgia--closing Osler's backdoor. Journal of the Israel Medical Association. 2006;8:428–429. [PubMed] [Google Scholar]
- Ablin JN, Cohen H, Buskila D. Mechanisms of Disease: genetics of fibromyalgia. Nature Clinical Practice Rheumatology. 2006a;2:671–678. doi: 10.1038/ncprheum0349. [DOI] [PubMed] [Google Scholar]
- Ablin JN, Shoenfeld Y, Buskila D. Fibromyalgia, infection and vaccination: two more parts in the etiological puzzle. Journal of Autoimmunity. 2006b;27:145–152. doi: 10.1016/j.jaut.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Anderson S, Skorpen F. Variation in the COMT gene: implications for pain perception and pain treatment. Phamacogenomics. 2009;10(4):669–684. doi: 10.2217/pgs.09.13. [DOI] [PubMed] [Google Scholar]
- Arnold LM, Crofford LJ, Mease PJ, Burgess SM, Palmer SC, Abetz L, Martin SA. Patient perspectives on the impact of fibromyalgia. Patient Education and Counseling. 2008;73:114–120. doi: 10.1016/j.pec.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellato E, Marini E, Castoldi F, Barbasetti N, Mattei L, Bonasia DE, Blonna D. Fibromyalgia syndrome: Etiology, pathogenesis, diagnosis and treatment. Pain Research and Treatment. 2012 doi: 10.1155/2012/426130. Epub 2012 Nov 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buenaver LF, Edwards RR, Haythornthwaite JA. Pain-related catastrophizing and perceived social responses: Inter-relationships in the context of chronic pain. Pain. 2007;127:234–242. doi: 10.1016/j.pain.2006.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buitenhuis J, De Jong PJ, Jaspers JPC, Groothoff JW. Catastrophizing and causal beliefs in whiplash. Spine. 2008;33:2427–2433. doi: 10.1097/BRS.0b013e318183c6ca. [DOI] [PubMed] [Google Scholar]
- Burckhardt CS, Clark SR, Bennett RM. Pain coping strategies and quality of life in women with fibromyalgia: Does age make a difference? Journal of Musculoskeletal Pain. 2001;9:5–18. [Google Scholar]
- Burckhardt CS, Clark SR, O'Reilly CA, Bennett RM. Pain-Coping Strategies of women with fibromyalgia: Relationship to pain, fatigue, and quality of life. Journal of Musculoskeletal Pain. 1997;5:5–21. [Google Scholar]
- Burgmer M, Petzke F, Giesecke T, Gaubitz M, Heuft G, Pfleiderer B. Cerebral activation and catastrophizing during pain anticipation in patients with fibromyalgia. Psychosomatic Medicine. 2011;73:751–759. doi: 10.1097/PSY.0b013e318236588a. [DOI] [PubMed] [Google Scholar]
- Castel LD, Abernethy AP, Li Y, Depuy V, Saville BR, Hartmann KE. Hazards for pain severity and pain interference with daily living, with exploration of brief pain inventory cutpoints, among women with metastatic breast cancer. Journal of Pain and Symptom Management. 2007;34:380–392. doi: 10.1016/j.jpainsymman.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Clauw DJ. Fibromyalgia: an overview. American Journal of Medicine. 2009;122:S3–S13. doi: 10.1016/j.amjmed.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Cleeland CS, Ladinsky JL, Serlin RC, Nugyen CT. Multidimensional measurement of cancer pain: comparisons of US and Vietnamese patients. Journal of Pain and Symptom Management. 1988;3:23–27. doi: 10.1016/0885-3924(88)90134-0. [DOI] [PubMed] [Google Scholar]
- Desmeules J, Piguet V, Besson M, Chabert J, Rapiti E, Rabsamen M, Cedraschi C. Psychological distress in fibromyalgia patients: A role of Catechol-O-Methyle-Transferase Val158Met Polymorphism. Health Psychology. 2012;31(2):242–249. doi: 10.1037/a0025223. [DOI] [PubMed] [Google Scholar]
- de Souza JB, Goffaux P, Julien N, Potvin S, Charest J, Marchand S. Fibromyalgia subgroups: profiling distinct subgroups using the Fibromyalgia Impact Questionnaire. A preliminary study. Rheumatology International. 2009;29:509–515. doi: 10.1007/s00296-008-0722-5. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Cahalan C, Mensing G, Smith M, Haythornthwaite JA. Pain, catastrophizing, and depression in the rheumatic diseases. Nature Reviews Rheumatology. 2011;7:216–224. doi: 10.1038/nrrheum.2011.2. [DOI] [PubMed] [Google Scholar]
- Finan PH, Zautra AJ, Davis MC, Lemery-Chalfant K, Covault J, Tennen H. COMT moderates the relation of daily maladaptive coping and pain in fibromyalgia. Pain. 2011;152:300–307. doi: 10.1016/j.pain.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan PH, Zautra AJ, Davis MC, Lemery-Chalfant K, Covault J, Tennen H. Genetic influences on the dynamics of pain and affect in fibromyalgia. Health Psychology. 2010;29:134–142. doi: 10.1037/a0018647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SZ, Wallace MR, Wright TW, Moser MW, Greenfield WH, III, Sack BK, Fillingim RB. Evidence for a biopsychosocial influence on shoulder pain: Pain catastrophizing and catechol-O-methyltransferase (COMT) diplotype predict clinical pain rating. Pain. 2008;136:53–61. doi: 10.1016/j.pain.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesecke T, Williams DA, Harris RE, Cupps TR, Tian X, Tian TX, Clauw DJ. Subgrouping of fibromyalgia patients on the basis of pressure-pain thresholds and psychological factors. Arthritis and Rheumatism. 2003;48:2916–2922. doi: 10.1002/art.11272. [DOI] [PubMed] [Google Scholar]
- Gracely RH, Geisser ME, Giesecke T, Grant MA, Petzke F, Williams DA, Clauw DJ. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain. 2004;127:835–843. doi: 10.1093/brain/awh098. [DOI] [PubMed] [Google Scholar]
- Gow JW, Hagan S, Herzyk P, Cannon C, Behan PO, Chaudhuri A. A gene signature for post-infectious chronic fatigue syndrome. BMC medical Genomics. 2009;2:38. doi: 10.1186/1755-8794-2-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gursoy S, Erdal E, Herken H, Madenci E, Alasehirli B, Erdal N. Significance of catechol-O-methyltransferase gene polymorphism in fibromyalgia syndrome. Rheumatology International. 2003;23 (3):104–107. doi: 10.1007/s00296-002-0260-5. [DOI] [PubMed] [Google Scholar]
- Humphrey L, Arbuckle R, Mease P, Williams DA, Samsoe BD, Gilbert C. Fatigue in fibromyalgia: a conceptual model informed by patient interviews. BMC Musculoskeletal Disorders. 2010;11:216. doi: 10.1186/1471-2474-11-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen PB, Andrykowski MA, Thors CL. Relationship of Catastrophizing to Fatigue among Women Receiving Treatment for Breast Cancer. Journal of Consulting and Clinical Psychology. 2004;72:355–361. doi: 10.1037/0022-006X.72.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen PB, Azzarello LM, Hann DM. Relation of catastrophizing to fatigue severity in women with breast cancer. Cancer research Therapy and Control. 1999;8:155–164. [Google Scholar]
- Kapstad H, Rokne B, Stavem K. Psychometric properties of the Brief Pain Inventory among patients with osteoarthritis undergoing total hip replacement surgery. Health and Quality of Life Outcomes. 2010;8:148. doi: 10.1186/1477-7525-8-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau AC, Jongstra-Bilen J, Cybulsky MI. Eicosapentaenoic acid and regression of atherosclerotic lesions: a role for dendritic cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31:1943–1945. doi: 10.1161/ATVBAHA.111.231910. [DOI] [PubMed] [Google Scholar]
- Light AR, White AT, Hughen RW, Light KC. Moderate exercise increases expression for sensory, adrenergic, and immune genes in chronic fatigue syndrome patients but not in normal subjects. J Pain. 2009;10:1099–1112. doi: 10.1016/j.jpain.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low LA, Schweinhardt P. Early life adversity as a risk factor for fibromyalgia in ater life. Pain Research and Treatment. 2012;2012:140832. doi: 10.1155/2012/140832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo JA, Hesse J, Turner JD, Meyer J, Hellhammer DH, Muller CP. Glucocorticoid sensitivity in fibromyalgia patients: decreased expression of corticosteriod receptor and glucocorticoid-induced leucin zipper. Psychoneuroendocrinology. 2008;33 (6):799–809. doi: 10.1016/j.psyneuen.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Maletic V, Raison CL. Neurobiology of depression, fibromyalgia and neuropathic pain. Frontiers in Bioscience. 2009;14:5291–5338. doi: 10.2741/3598. [DOI] [PubMed] [Google Scholar]
- Mease P. Fibromyalgia syndrome: review of clinical presentation, pathogenesis, outcome measures, and treatment. Journal of Rheumatology. 2005;75(6):21. [PubMed] [Google Scholar]
- Osman A, Barrios FX, Gutierrez PM, Kopper BA, Merrifield T, Grittmann L. The Pain Catastrophizing Scale: further psychometric evaluation with adult samples. Journal of Behavioral Medicine. 2000;23:351–365. doi: 10.1023/a:1005548801037. [DOI] [PubMed] [Google Scholar]
- Osman A, Barrios FX, Kopper BA, Hauptmann W, Jones J, O'Neill E. Factor structure, reliability, and validity of the Pain Catastrophizing Scale. Journal of Behavioral Medicine. 1997;20:589–605. doi: 10.1023/a:1025570508954. [DOI] [PubMed] [Google Scholar]
- Reeves WC, Wagner D, Nisenbaum R, Jones JF, Gurbaxani B, Solomon L, Heim C. Chronic fatigue syndrome--a clinically empirical approach to its definition and study. BMC Medicine. 2005;3:19. doi: 10.1186/1741-7015-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm SE, Koroschetz J, Gockel U, Brosz M, Freynhagen R, Tolle TR, Baron R. A cross-sectional survey of 3035 patients with fibromyalgia: subgroups of patients with typical comorbidities and sensory symptom profiles. Rheumatology (Oxford) 2010;49:1146–1152. doi: 10.1093/rheumatology/keq066. [DOI] [PubMed] [Google Scholar]
- Riddle DL, Wade JB, Jiranek WA, Kong X. Preoperative pain catastrophizing predicts pain outcome after knee arthroplasty. Clinical Orthopaedics and Related Research. 2010;468:798–806. doi: 10.1007/s11999-009-0963-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabban EL. Catecholamines in stress: molecular mechanisms of gene expression. Endocrine Regulations. 2007;41:61–73. [PubMed] [Google Scholar]
- Salemi S, Rathage J, Wollina U, Michel BA, Gay RE, Gay S, Sprott H. Detecion of interleukin 1beta (IL-1beta), IL-6, and tumor necrosis factor-alpha in skin of patients with fibromyalgia. J Rheumatol. 2003;30(1):146–150. [PubMed] [Google Scholar]
- Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. Journal of Psychosomatic Research. 1995;39:315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- Sohl SJ, Friedberg F. Memory for fatigue in chronic fatigue syndrome: Relationships to fatigue variability, catastrophizing, and negative affect. Behavioral Medicine. 2008;34:29–35. doi: 10.3200/BMED.34.1.29-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Masuda T, Kitano J, Shimoyama H, Tozaki-Saitoh H, Inoue K. IFN-gamma receptor sognaling mediates spinal microglia activation driving neuropathic pain. Proceedings of the National Academy of Sciences USA. 2009;106:8032–8037. doi: 10.1073/pnas.0810420106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk DC, Okifuji A, Sinclair JD, Starz TW. Pain, disability, and physical functioning in subgroups of patients with fibromyalgia. The Journal of Rheumatology. 1996;23:1255–1262. [PubMed] [Google Scholar]
- Turk DC, Okifuji A, Sinclair JD, Starz TW. Differential responses by psychosocial subgroups of fibromyalgia syndrome patients to an interdisciplinary treatment. Arthritis Care & Research. 1998;11:397–404. doi: 10.1002/art.1790110511. [DOI] [PubMed] [Google Scholar]
- Wang XM, Wu TX, Hamza M, Ramsay ES, Wahl SM, Dionne RA. Rofecoxib modulates multiple gene expression pathways in a clinical model of acute inflammatory pain. Pain. 2007;128:136–147. doi: 10.1016/j.pain.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White KP, Speechley M, Harth M, Ostbye T. Co-existence of chronic fatigue syndrome with fibromyalgia syndrome in the general population. A controlled study. Scandinavian Journal of Rheumatology. 2000;29:44–51. doi: 10.1080/030097400750001798. [DOI] [PubMed] [Google Scholar]
- Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, Yunus MB. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care & Research. 2010;62:600–610. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- Wolfe F, Hawley DJ. Evidence of disordered symptom appraisal in fibromyalgia: increased rates of reported comorbidity and comorbidity severity. Clinical and Experimental Rheumatology. 1999;17:297–303. [PubMed] [Google Scholar]
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Sheon RP. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis & Rheumatism. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- Wu Z, Irizarry RA. A statistical framework for the analysis of microarray probe-level data. The Annals of Applied Statistics. 2007;1(2):333–357. doi: 10.1241/07-AOAS116. [DOI] [Google Scholar]
- Zhang JM, Tonelli L, Regenold WT, McCarthy MM. Effects of neonatal flutamide treatment on hippocampal neurogenesis and synaptogenesis correlate with depression-like behaviors in preadolescent male rats. Neuroscience. 2010;169:544–554. doi: 10.1016/j.neuroscience.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]