Abstract
Modifications of α-synuclein resulting in changes in its conformation are considered to be key pathological events for Lewy body diseases (LBD), which include Parkinson’s disease (PD) and dementia with Lewy bodies (DLB). We have previously described a histopathological Unified Staging System for LBD that classifies the spread of α-synuclein phosphorylated at serine 129 (pS129-α-synuclein) from olfactory bulb to brainstem or limbic regions, and finally neocortex. Lewy bodies and Lewy neurites are highly enriched in pS129-α-synuclein. Increased formation of pS129-α-synuclein changes its solubility properties enhancing its tendency to aggregate and disrupt normal function. As in vitro and animal studies have shown that inhibiting formation of pS129-α-synuclein can prevent toxic consequences, this has become one of the therapeutic targets for LBD. However, detailed biochemical descriptions of the changes in pS129-α-synuclein properties in diseased human brains are needed to further our understanding of how these might contribute to molecular pathogenesis. In this study, we used 130 separate brain samples from cingulate cortex (limbic cortex) and 131 from temporal cortex (neocortex) that had been staged according to our Unified Staging System to examine progressive changes in properties of pS129-α-synuclein with the formation of progressively more severe histological Lewy-type pathology. The brain samples from these staged cases had been separated into cytosol-enriched, membrane-enriched (detergent soluble) and insoluble (ureas/SDS soluble) fractions. We also characterized the nature and appearance of higher molecular weight forms of pS129-α-synuclein. The major species was the 16 kD monomeric form; this accumulated with increasing stage with a large increase in Stage IV samples. By comparing two brain regions, we showed higher accumulation of insoluble pS129-α-synuclein in cingulate cortex, where histological deposits occur first, than in temporal cortex in samples with advanced (Stage IV) LB pathology.
Keywords: Western blots, Parkinson’s disease, antibodies, fractionation, post-translational modification, postmortem brain tissue, dementia with Lewy bodies, incidental Lewy body disease, pathogenesis, aggregation
Introduction
The purpose of this study was to define changes in the formation of pS129-α-synuclein as well as changes in its solubility properties in a group of brain samples from Lewy Body disorder (LBD) cases staged histologically for increasing severity according to our Unified Staging System (Beach et al, 2009).
There are a number of schemes for describing and classifying the severity of LB pathology in human neurodegenerative diseases (Alafuzoff et al, 2009; Braak et al, 2002; Braak et al, 2003; Deramecourt et al, 2006; Halliday et al, 2006; Marui et al, 2002; McKeith et al., 1996; McKeith et al, 2005;); we described a Unified Staging System that aimed to classify not only PD and DLB, but also incidental Lewy body disease (ILBD) and Alzheimer’s disease with LBs (ADLB) (Beach et al, 2009). This scheme as well as prior ones initially used the neuroanatomical distribution of abnormal α-synuclein to classify and stage individual subjects with LBDs but there are also implications for theories of pathogenesis, as many investigators have suggested that Lewy pathology structures may spread within the brain by trans-synaptic passage. Aggregated α-synuclein is a major component of LBs and Lewy neurites (Baba et al, 1998). In human LBDs, all Lewy pathological structures are highly enriched in pS129-α-synuclein (Anderson et al, 2006; Fujiwara et al, 2002; Saito et al, 2003). It is possible that pS129-α-synuclein may be critical to this spreading process, either by seeding the initial α-synuclein aggregates, which are then passed neuron to neuron, or, alternatively, pS129-α-synuclein itself may be the transmissible factor, initiating α-synuclein aggregation anew with each successive trans-synaptic passage. There is currently much research being carried out to investigate whether pS129-α-synuclein levels in cerebrospinal fluid will be a good biomarker for PD and related LBD (Wang et al., 2012).
pS129-α-synuclein may have some function in normal brain, as it is biochemically detectable in the cerebral cortex, substantia nigra and nucleus basalis of Meynert of normal control subjects at low levels, but its levels became significantly enhanced with the development of pathology (Lue et al., 2012; Muntane et al, 2012). Other α-synuclein post-translational modifications have been described, including truncation, nitration (Duda et al, 2000; Gao et al, 2008; Gomez-Tortosa et al, 2002; Paxinou et al, 2001), oxidative modifications and ubiquitination (Hasegawa et al, 2002; Tofaris et al, 2003), as well as phosphorylation at serine 87 and tyrosine 125 (Chen et al, 2009; Paleologou et al, 2010), but the pathological significance of pS129-α-synuclein modification has been the most widely investigated (Anderson et al, 2006; Beach et al, 2010; Cavallarin et al, 2010; Sato et al, 2011; Schulz-Schaeffer 2010; Sugeno et al, 2008). Understanding the role of α-synuclein in the pathology of diseases that involve Lewy body (LB) formation (synucleinopathies), in particular Parkinson’s disease (PD) has become a central research focus (Jellinger 2009; Lees et al, 2009).
Two key questions arise from studying pS129-α-synuclein in relation to LBD pathology; firstly, is there evidence for increased biochemical phosphorylation of α-synuclein before overt appearance of histopathology, and secondly, how does solubility of pS129-α-synuclein change with increased formation of LBD pathology? In the first part of this investigation, we recently demonstrated, using the same series of cases as described in this report, that in unfractionated brain samples, there were increased levels of total monomeric pS129-α-synuclein in temporal cortex samples from LBD cases but yet without temporal cortex histological deposits (Lue et al, 2012). This provided evidence that increased phosphorylation of α-synuclein can occur early in the disease process and therefore could be contributing to the formation of LB pathological structures. To extend these studies and define changes in solubility properties of pS129-α-synuclein with progression of LBD, we measured levels of all forms pS129-α-synuclein in samples in the cytosol-enriched (soluble fraction), membrane-enriched (detergent soluble) and aggregated (detergent insoluble/urea-SDS soluble) fractions.
Materials and Methods
Human brain tissue samples
Human brain tissue samples of cingulate and temporal cortex used in this study were obtained from the Banner Sun Health Research Institute Brain and Body Donation Program (Beach et al, 2008). We used 131 separate samples of temporal cortex (Table 1A) and 130 samples of cingulate cortex (Table 1B). These tables show key demographic details, including mean age, gender distribution, postmortem intervals, apolipoprotein E status and histological LB, plaque and tangle scores. For a subset of these cases, scores were available for Mini-Mental Status Examination (MMSE) and Unified Parkinson Disease Rating Scale (UPDRS)(motor score). Samples from both brain regions were available for many of the cases; there were 116 cases where samples from both brain regions were available (Table 1C). There were no significant differences in demographic features between these separate groups. The cases used in this study were a subset of those examined histologically for LB pathology to establish the Unified Staging System (Beach et al., 2009).
Table 1A.
Case demographics: Temporal Cortex
| Group | Stage 0 | Stage I | Stage IIa | Stage IIb | Stage III | Stage IV |
|---|---|---|---|---|---|---|
| N | 19 | 17 | 4 | 15 | 34 | 42 |
| Male:Female | 12:7 | 8:9 | 2:2 | 8:7 | 20:14 | 24:18 |
| Age at death (years) | 84.1±6.4 | 86.1±4.5 | 87.2±4.0 | 79.8±7.7 | 80.9±6.5 | 79.5±5.2* |
| PMD (hours) | 3.2±2.9 | 3.1±0.9 | 2.4±0.8 | 4.0±2.6 | 3.4±1.4 | 3.1±0.8 |
| ApoE (ε2:ε3:ε4 (%)) | 10.5:76.3:13.2 | 3.1:68.8:28.1 | 12.5:75:12.5 | 3.3:63.3:33.3 | 5.9:73.5:20.6 | 5.9:63.1:30.5 |
| LTS (Temp) | 0 | 0 | 0 | 0.27+0.46 | 0.91+0.29 | 2.54+0.74 |
| Plaques | 4.3±4.2 | 12.5±2.1* | 9.2±4.9 | 13.2±2.4* | 6.5±5.1 | 10.9±3.9*@ |
| Tangles | 3.9±2.3 | 11.5±4.7* | 6.5±4.2 | 12.0±3.7* | 4.5±2.9* | 8.0±4.4*@ |
| MMSE (N) | 28.7±1.6 (17) | 7.8±8** (9) | 25.7±4.2 (3) | 8.0±7.9** (15) | 20.1±7.2 (24) | 11.5±10.1**@ (28) |
| UPDRS (N) | 7.6±5.7 (16) | 36.8±27.2 (6) | 20.5±16.1 (4) | 30±31 (4) | 38.2±19.4** (28) | 45.3±25.7** (21) |
Numbers represent mean + standard deviation
PMD: Postmortem delay. LTS (Temp): Lewy-Type Density Score (temporal cortex)
MMSE: Mini-Mental Score Examination. UPDRS: Unified Parkinson Disease Rating Score (Motor Section)
significant difference (P< 0.05) between group and stage 0 samples (ANOVA with post hoc test)
significant difference (P<0.05) between stage 4 and stage 3 samples (ANOVA with post hoc test)
Table 1B.
Case demographics: Cingulate Cortex
| Group | Stage 0 | Stage I | Stage IIa | Stage IIb | Stage III | Stage IV |
|---|---|---|---|---|---|---|
| N | 16 | 16 | 5 | 17 | 31 | 45 |
| Male:Female | 11:5 | 8:8 | 3:2 | 9:8 | 19:12 | 26:19 |
| Age at death (years) | 83.4±6.6 | 85.2±4.8 | 87.6±3.5 | 80.7±7.6 | 81.1±6.6 | 79.2±5.3* |
| PMD (hours) | 3.3±3.2 | 3.0±0.9 | 2.5±0.8 | 3.9±2.5 | 3.3±1.3 | 3.0±0.8 |
| ApoE (ε2:ε3:ε4 (%)) | 12.5:75.0:12.5 | 3.1:65.7:31.2 | 10:70:20 | 5.9:55.9:38.2 | 6.4:71.0:22.6 | 5.5:63.4:31.1 |
| LTS (Cing) | 0 | 0 | 0.2+0.45 | 0.53+0.51 | 1.86+0.83 | 3.18+0.72 |
| Plaques | 4.6±4.2 | 12.5±2.2* | 9.3±4.2 | 13.2±2.3* | 6.6±5.1 | 10.6±4.3*@ |
| Tangles | 3.9±2.1 | 12.0±4.4* | 5.8±3.9 | 12.3±3.6* | 4.9±2.8* | 7.9±4.4*@ |
| MMSE (N) | 28.6±1.7 (16) | 7.4±7.6** (7) | 25.7±4.2 (3) | 8.2±7.5** (17) | 19.7±7.2 (24) | 11.2±10.3**@ (31) |
| UPDRS (N) | 7.2±5.8 (16) | 46.5±26.5 (4) | 20.5±16.1 (4) | 30±31 (4) | 38.0±19.8** (27) | 46.9±26.1** (22) |
Numbers represent mean + standard deviation
PMD: Postmortem delay. LTS (Cing): Lewy-Type Density Score (cingulate cortex)
MMSE: Mini-Mental Score Examination: UPDRS: Unified Parkinson Disease Rating Scale (Motor section)
significant difference (P< 0.05) between group and stage 0 samples (ANOVA with post hoc test)
significant difference (P<0.05) between stage 4 and stage 3 samples (ANOVA with post hoc test)
Table 1C.
Case demographics: Common Cases
| Group | Stage 0 | Stage I | Stage IIa | Stage IIb | Stage III | Stage IV |
|---|---|---|---|---|---|---|
| N | 15 | 15 | 4 | 14 | 30 | 38 |
| Male:Female | 10:5 | 7:8 | 2:2 | 7:7 | 18:12 | 23:15 |
| Age at death (years) | 83.5±6.8 | 85.8±4.6 | 87.2±3.9 | 79.2±7.6 | 81.3±6.6 | 79.5±5.4* |
| PMD (hours) | 3.3±3.3 | 3.0±1.0 | 2.4±0.8 | 4.1±2.7 | 3.2±1.2 | 3.0±0.8 |
| ApoE (ε2:ε3:ε4 (%)) | 13.3:73.4:13.3 | 3.3:70.0:26.7 | 12.5:75:12.5 | 3.6:64.3:32.1 | 6.7:71.7:21.6 | 6.6:64.5:28.9 |
| LDS (Cing) | 0 | 0 | 0.2+0.5 | 0.43+0.51 | 1.80+0.85 | 3.20+0.73 |
| LDS (Temp) | 0 | 0 | 0 | 0.21±0.43 | 0.9±0.3 | 2.5±0.76 |
| Plaques | 4.6±4.4 | 12.4±2.2* | 9.2±4.9 | 13.1±2.5* | 6.4±5.2 | 10.8±4.0*@ |
| Tangles | 3.9±2.2 | 11.8±4.5* | 6.5±4.2 | 11.9±3.8* | 5.0±2.8* | 8.2±4.6*@ |
Numbers represent mean + standard deviation
PMD: Postmortem delay. LTS (Cing): Lewy Type Density Score (cingulate cortex); LTS (Temp): Lewy Type Density Score (temporal cortex)
significant difference (P< 0.05) between group and stage 0 samples (ANOVA with post hoc test)
significant difference (P<0.05) between stage 4 and stage 3 samples (ANOVA with post hoc test)
Samples were dissected on dry ice from cingulate cortex and temporal cortex from each case, and were selected to include neurologically normal controls without Lewy body disease, classified as stage 0, and a range of cases from stage I (Lewy pathology only in olfactory bulb), stage IIa (Lewy pathology predominantly in olfactory bulb and brainstem), stage IIb (Lewy pathology predominantly in olfactory bulb and limbic regions), stage III (Lewy pathology in olfactory bulb, brain stem and limbic regions) and stage IV (significant Lewy pathology extended into neocortex). To categorize these cases according to our Unified Stage system (Beach et al, 2009), the mean Lewy-type histopathology density scores (LTS), derived from DLB Consortium templates (McKeith et al 1996, 2005) for the aggregate density of both Lewy bodies and neurites, were used as a measure of Lewy pathology in 10 brain regions. Mean LTS scores for samples used in this study are shown in Table 1A, 1B and 1C.
These data were considered in conjunction with detailed neuropathology in order to obtain final clinicopathological diagnoses. Consensus criteria were used to diagnose AD, DLB and PD in these cases (Hyman et al, 2012; McKeith et al, 2005; Mirra et al., 1991).
Tissue fractionation procedure
To identify alterations in solubility of α-synuclein in brain samples, the following three-step procedure was adopted from published protocols to allow for the processing of large numbers of samples (Figure 1A) (Anderson et al, 2006; Fujiwara et al, 2002; Kahle et al, 2001; Klucken et al, 2006). Each dissected brain sample was sonicated in 5 volumes (weight/volume-w/v) of Tris-buffered saline (25 mM Tris-HCl, pH 7.5, 150 mM NaCl) supplemented with proteinase/phosphatase inhibitors (Pierce - Thermo-Fisher, Rockland, IL) and 5 mM EDTA. The resulting extract was centrifuged (18,000 g for 30 min) in a refrigerated microcentrifuge. The supernatant (designated the soluble or cytosol-enriched fraction) was recovered and aliquoted. The pellet, which contains membrane and nuclear fractions, was resuspended by sonication in 5 volumes (original w/v) of radioimmunoprecipitation assay (RIPA) buffer (Pierce-Thermo-Fisher: 25mM Tris-HCl (pH 7.6), 150mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) supplemented with proteinase/phosphatase inhibitors and EDTA. After 30 min incubation at 4°C, the extract was centrifuged (18,000 g for 30 min, 4°C). The supernatant (designated the membrane-enriched fraction or detergent soluble fraction) was recovered and aliquoted. To ensure that the insoluble fraction was not contaminated, the resulting pellet was re-extracted in the same manner with RIPA buffer, centrifuged but the supernatant was discarded. The resulting pellet was extracted in 5 volumes of 6 M urea/2% SDS for 18 h at 70°C. The supernatant fraction (designated the insoluble fraction) was recovered and aliquoted. The pellet was not further extracted. Protein concentrations in each fraction were determined using a Micro BCA method and samples analyzed by the western blot procedure described below. The degree of contamination of the soluble-enriched fraction with membrane proteins was assessed by western blot analyses of selected Stage 0 and Stage IV samples using CD200 as the marker for membrane contamination (Walker et al., 2009).
Figure 1.
Figure 1A: Fractionation scheme for brain tissue to identify changes in solubility of pS129- α-synuclein. A three stage extraction scheme was used for processing cingulate cortex (127 staged cases) and temporal cortex (134 staged cases). Fractions were considered as soluble (cytosol-enriched), detergent soluble (membrane enriched fraction) and insoluble (dissolved in 6M Urea/2%SDS with heat).
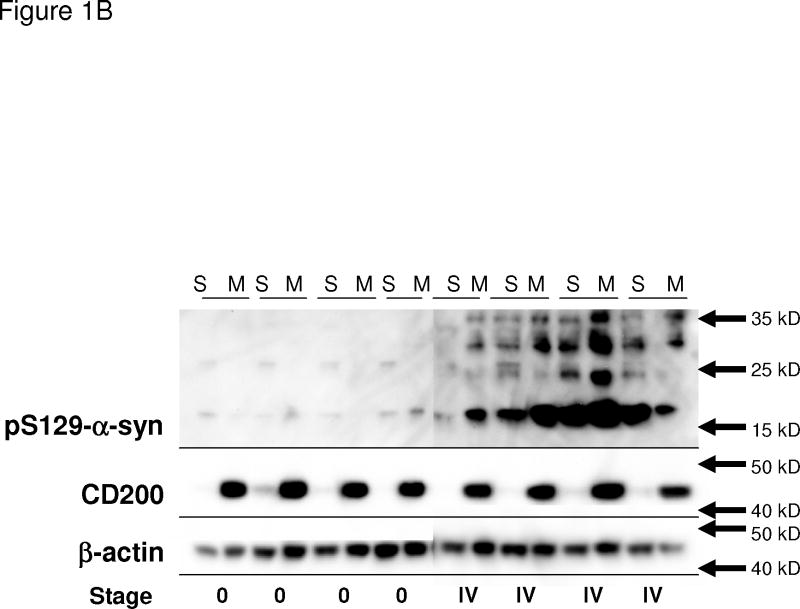
Figure 1B: Assessment of purity of soluble and membrane fractions. To determine the level of contamination of the soluble (cytosol-enriched) (S) fractions with membrane (M) components, paired samples from selected fractionated temporal cortex Stage 0 and Stage IV cases were analyzed for pS129-α–synuclein (top panel), CD200 as a marker for membrane proteins (middle panel), and β-actin (lower panel). It can be seen that CD200 levels in soluble fractions were slight.
In vitro phosphorylation and alkaline phosphatase treatment
To confirm phosphorylation specificity of pS129-α–syn (Tokyo), the following control experiments were carried out. Recombinant α-synuclein (RPeptide, GA) (20 μg) was treated with casein kinase 2 (500 units) (New England Biolabs) for 16 h at 37°C (Machiya et al, 2010); as a control, another sample was treated in the same manner but without addition of ATP as the phosphate donor. Dilutions of these samples were separated by gel electrophoresis and transferred to PVDF membranes for western blot detection with phosphorylation-specific antibodies, and with a total α-synuclein antibody. To show that the signal detected with pS129-α–syn (Tokyo) and pS129-α–syn (Epitomics) in tissue extracts was phosphorylation-specific, western blot membranes were prepared with a range of samples showing high levels of signal. Membranes were prepared in duplicate; one was treated with calf intestinal alkaline phosphatase (CIAP-50 units/ml) (New England Biolabs) in reaction buffer at 37°C for 30 min, while the parallel membrane was treated in reaction buffer alone. The membranes were then rinsed, blocked and incubated in primary antibodies (pS129-α–syn (Tokyo) or pS129-α–syn (Epitomics)). Western blot detections were carried out as described below.
Western Immunoblot Procedure
Samples were dissolved at a concentration of 1 μg/μl protein in western blot sample buffer (NUPAGE LDS – Invitrogen Corp, Carlsbad, CA) containing 0.1 M DTT and heated at 70°C for 10 min. Samples grouped into soluble, membrane or insoluble fractions from cingulate cortex or temporal cortex were analyzed separately. Samples were separated on 4–12% NuPAGE Bis–Tris MIDI gels using MES-SDS running buffer (Invitrogen). Proteins were transferred to polyvinylidene fluoride (PVDF) membranes according to the manufacturer’s suggestion using a semi-dry blotting apparatus (20V for 45 min). Following drying, membranes were blocked in 5% skim milk solution dissolved in Tris-buffered saline (TBST—25mM Tris–HCl (pH 7.3), 150mM NaCl, 0.1% (w/v) Tween 20), and then reacted for 18 h in appropriate dilutions of antibodies in TBST containing 5% milk. Following washes with TBST, bound antibody was detected by reaction for 2 h with the appropriate horseradish peroxidase (HRP) labeled anti-immunoglobulin (Thermo-Fisher, Rockland, IL. at 1:10,000 dilution). Bound antibodies were detected by reaction of membranes with HRP chemiluminescent substrate (Supersignal Dura, Thermo Fisher) with direct imaging using a Fluorochem Q imaging system (Cell Biosciences, San Leandro, CA). Protein loading was measured by reaction with an antibody to β-actin (mouse monoclonal: Sigma at 1:5000 dilution) or by staining membranes with 1% Ponceau S solution. Intensities of chemiluminescence bands were quantified using Fluorochem Q SA software (Cell Biosciences-Alpha Innotech). To ensure reproducible measurements across multiple blots, the following scheme was carried out. Each set of gels included a repeated series of 3–4 samples that were used for correction between blots. Using the direct imaging system, 4 membranes were imaged directly at the same time. Each set of membranes was imaged multiple times to ensure absence of signal saturation. Using this protocol, the reproducibility between blots imaged under these conditions was high. Mean values of the repeated samples were used to normalize values across different series of samples. In these experiments, all samples (cingulate and temporal cortex) from either soluble, membrane or insoluble fractions were processed and imaged together. Due to the presence of multiple higher molecular species of pS129-α-synuclein in many brain samples, the use of synthetic standards of pS129-α-synuclein for blot calibration purposes did not prove feasible for these studies.
Antibodies used for western blots in this study were pS129-α–syn (Tokyo), a custom rabbit polyclonal (Obi et al, 2008) (1:10,000), pS129-α-syn (Epitomics)(Burlingame, CA), a rabbit monoclonal (1:1000): both of these antibodies are specific for serine 129 phosphorylated α-synuclein and do not recognize non-phosphorylated α-synuclein (Figure 3). To detect unmodified forms of α-synuclein, mouse monoclonal antibodies syn-1 (BD Biosciences, MA -1:2000) and LB509 (Covance, Inc, CA, 1:2500) were used.
Figure 3. Further characterization α-synuclein species in Staged temporal cortex samples using immunoprecipitation/western blot procedures.
A and B) Identification of α-synuclein reactive bands immunoprecipitated with phospho-specific antibody pS129-α-syn (Tokyo) and detected using LB509 antibody to α-synuclein. Selected samples (n=4) from each stage were analyzed. In Stage IV samples (DLB cases), there were 5 distinct α-synuclein species and also higher molecular weight (mw > 50 –250 kD) aggregated α-synuclein. Panel B represents a longer exposure of panel A and also shows trace amounts of pS129-α-synuclein precipitated from certain Stage 0 –Stage III samples.
C) Identification of ubiquitin-reactive bands immunoprecipitated with phosphorylation specific antibody pS129-α-syn (Tokyo) and detected using an antibody to ubiquitin. Three of the high molecular weight p129-α-synuclein bands (approximate molecular weight 36 kD, 42 kD and 48–50 kD) were immunoreactive for ubiquitin. Monomeric pS129-α-synuclein (18 kD) and 26 kD bands (panel A) were not reactive for ubiquitin.
D) Identification of α-synuclein reactive bands immunoprecipitated with LB509, a non-phosphorylation dependent antibody to α-synuclein and detected using phosphorylation specific antibody pS129-α-syn (Tokyo). The pattern of Stage IV bands was essentially the same as the reverse analyses (panel A).
E–H) Defining soluble oligomer forms of α-synuclein. Samples were analyzed for pS129-α-synuclein. E) non-phosphorylation dependent α-synuclein using LB509 (F); and non-phosphorylation dependent α-synuclein using syn-1 (G). Equivalent loading was demonstrated with β-actin (H). A major soluble oligomeric species (O) was detected using LB509 all of these staged samples.
Immunoprecipitation Procedure
Brain samples of temporal cortex from selected Stage 0 to Stage IV cases were extracted in 6 volumes of RIPA buffer containing protease/phosphatase inhibitors. Samples were centrifuged (18,000g/30 min) and the clarified supernatants used as a source of α-synuclein. For each sample, 500 μg of total protein was reacted with antibody. Protein was diluted to 500 μl with Immunoprecipitation (IP) lysis buffer containing protease/phosphatase inhibitors and incubated with either 2.5 μl of pS129-α–syn (Tokyo) antibody or 10 μg of LB509 antibody for 2 h. Immune complexes were isolated by reaction with protein A/G coated magnetic beads (Protein A/G magnetic IP/Co-IP kit, Pierce-Thermo Scientific – Pierce) for 1 hour according to the manufacturer’s instructions. Proteins released from magnetic beads in sample buffer were separated by gel electrophoresis and transferred to PVDF membranes using the above described western blot procedures. As the samples contain large amounts of immunoglobulin from the precipitating antibody, western blot detection was carried out with a primary antibody from a different species. To identify ubiquitinated species of pS129-α-synuclein, mouse monoclonal antibody to ubiquitin (1:2000 dilution; clone P4D1, Covance) was employed, which reacts with free ubiquitin, polyubiquitin and ubiquitin-conjugated proteins.
Data Analysis and Statistical Analysis
Data were obtained for the intensities of pS129-α–synuclein reactive bands (monomeric and higher molecular weight species) for each fraction from each brain region. The ratios of pS129-α–synuclein to β actin were calculated for soluble and membrane samples; for insoluble (urea/SDS soluble) samples, β actin could not be used for normalization as it is not present in this fraction. Instead, the membranes with insoluble fraction-separated proteins were assessed with a total protein dye for equal loading. The mean intensities for each measure were calculated for each group of staged samples and presented in each figure with Standard Error of the Mean (SEM). For statistical analysis, non-parametric Kruskall-Wallis analysis of variance was carried out with the Dunn’s post-hoc test for significance testing. Correlation analysis of histopathological LTS with levels of pS129-α-synuclein in each fraction employed Spearman non-parametric analysis. Pearson correlations were performed to determine if the levels of p129-α-synuclein in fractions from any given brain region showed any degree of correlation with the levels in other fractions and regions. For this last test, data were analyzed to show whether it was normal distributed using the D’Agostino-Pearson test and Kolmogorov-Smirnov test for normalcy using MedCalc 12 software. All other statistical analyses were carried out using Graphpad Prism 4 Software. For each measure, significant differences were assumed if P values were less than 0.05.
Results
pS129-α-synuclein immunoreactive proteins in staged human brains
The aim of this study was to determine how the relative levels of the different forms of pS129-α-synuclein present in a large group of samples from human brain cases changed with LBD Stage. These samples had been staged from Stage 0 to Stage IV according to the Unified Staging System (Beach et al, 2009). A goal was to determine how the different properties of pS129-α-synuclein changed with progressively increasing severity of LBD; these properties included changes in solubility, aggregation and post-translational modifications. Samples from cingulate cortex and temporal cortex were used to determine whether there was a progressive change between the regions as severity of LBD increased. These two brain regions were cingulate cortex and temporal cortex. Cingulate cortex becomes affected by Lewy Body pathology earlier than temporal cortex (Beach et al, 2009).
To firstly identify which pS129-α-synuclein bands could be detected in these samples, representative samples from Stage 0 to Stage IV cases were analyzed by western blot using two antibodies to pS129-α-synuclein and with a widely-used antibody (syn-1) to α-synuclein that recognizes a non-phosphorylation dependent epitope (Figure 2A). For this study, we primarily utilized a rabbit polyclonal raised against a peptide sequence of α-synuclein (amino acids 124 – 134) with phosphorylated serine at epitope 129. This antibody, designated —pS129-α-syn (Tokyo), has been described previously in histological studies (Obi et al, 2008). This antibody has been used in our recent pathological studies of LBDs and appears to selectively identify abnormal accumulations of α-synuclein in LBs, LNs and intracellular aggregates, but not normal presynaptic α-synuclein. As biochemical characterization of this antibody has not been extensive, confirmatory studies utilized a rabbit monoclonal to pS129-α-synuclein (Epitomics, Burlingame, CA). In these samples, which were derived from membrane-enriched fractions, we could detect strongly reactive bands for pS129-α-synuclein in the Stage IV cingulate cortex samples with only trace amounts in samples from stages IIb and III. Results showed a band that corresponded to the molecular weight of monomeric pS129-α-synuclein (16–17 kD) along with 6 different higher molecular bands. The patterns of bands were almost identical using these two pS129-α-synuclein antibodies (Figure 2A). A completely different pattern was seen in these same samples using the antibody syn-1. As the higher molecular weight bands were not prominently detected with syn-1 antibody, this lead to the question whether these bands were species of α-synuclein.
Figure 2. Characterization of α-synuclein species in Staged temporal cortex samples showing phosphorylation dependent specificity of pS129-α–synuclein antibodies.
A) Progression of increased pS129-α–synuclein immunoreactivity with increased LB staging. Randomly selected samples (temporal cortex RIPA extracted fraction) from each LB stage were analyzed using the pS129 α-synuclein antibodies (pS129-α–syn (Tokyo) and pS129-α–syn (Epitomics) and an antibody to total α-synuclein (syn-1). Panels show a large increase in intensity in the Stage IV sample with the pS129-α–syn specific antibodies with total α-synuclein being detected in all samples. Blots were reprobed with antibody to β-actin to show equivalent loadings.
B) Loss of immunoreactivity with pS129-α– syn (Tokyo) following treatment with alkaline phosphatase. Samples were temporal cortex membrane-enriched fraction from four different Stage IV cases. One membrane (+ Alk phosph) was treated with alkaline phosphatase while the other was incubated with buffer (− Alk phosph). Dephosphorylation of separated proteins resulted in loss of immunoreactive bands. There was equivalent reactivity on alkaline phosphatase-treated and untreated membranes with antibody to β-actin.
C) In vitro phosphorylation of recombinant α-synuclein with casein kinase 2 (CK2). Dilutions of CK2 treated α-synuclein (P α-syn), or control treated α-synuclein (NonP α-syn) probed with pS129-α–synuclein antibodies pS129-α-syn (Tokyo) and pS129 α-syn (Epitomics) and with antibody syn-1 which recognizes unmodified forms of α-synuclein.
Immunoreactivity of pS129-α-synuclein bands is phosphorylation-specific
The phosphorylation specific nature of immunoreactivity detected with pS129-α–syn (Tokyo) in tissue samples was proven by treating western blot membranes containing different Stage IV cases with alkaline phosphatase. There was almost complete loss of immunoreactivity for all bands with pS129-α–syn (Tokyo) antibody on the membranes treated with alkaline phosphatase (Figure 2B), while the buffer-treated membranes showed the expected pattern of monomeric and higher molecular weight bands. This same result was also seen with the pS129-α–syn (Epitomics) antibody (data not shown). Finally, to further confirm that pS129 α-synuclein immunoreactive bands on blots were solely dependent on α-synuclein phosphorylation, recombinant α-synuclein was phosphorylated in vitro with recombinant casein kinase 2 (CK2). Membranes were probed with pS129-α-syn (Tokyo), pS129-α-syn (Epitomics), and syn-1 (Figure 2C). The phosphorylation-specific antibodies only recognized α-synuclein treated with CK2 and ATP, and were unreactive with non-phosphorylated α-synuclein (Figure 2C).
Immunoprecipitation studies of selected LBD samples
Immunoprecipitation was used to show that the higher molecular weight pS129 α–synuclein-immunoreactive bands were modified forms of α-synuclein (Figure 3A–3D). Selected temporal cortex samples from Stage 0 to Stage IV cases (4 samples from each group) were directly extracted in RIPA buffer; these samples would contain soluble and membrane-associated (detergent soluble) forms of α-synuclein. After precipitation with pS129-α-SYN-Tokyo antibody, western blot analyses and detection using a non-phosphorylation dependent α-synuclein antibody (LB509) was carried out. In the selected Stage IV samples (diagnosed as PD or DLB), at least 6 different bands were identified (Figure 3A). This confirmed the identity of all bands (except those indicated as IgG) as forms of α-synuclein. In Figure 3B, a more intense image of the same blot showed that these Stage IV samples also contained significant amounts of very high molecular weight (> 50 kD to 250 kD) smeared aggregates of pS129-α-synuclein. The complementary experiment (precipitation with LB509 and western blot detection with p129-α-SYN Tokyo) identified the same 6 bands, though in this experiment there was stronger cross reaction with the precipitating IgG, which comigrated with the highest band at around 50 kD (Fig 3C). To determine which bands represented pS129-α-synuclein modified with ubiquitin, these same samples were immunoprecipitated with pS129-α-SYN-Tokyo and detected using an antibody to ubiquitin (Figure 3D). This showed 4 higher molecular weight bands, along with smeared aggregated α-synuclein (Figure 3D). By comparison, the band at 26 kD and monomeric α-synuclein showed no reactivity for ubiquitin. The 26 kD band had been identified as being mono-ubiquitinated by others (Anderson et al, 2006). There was no evidence of ubiquitinated species of p129S-α-synuclein in earlier Stage 0-III samples suggesting this modification occurred very late in the formation of LB pathological structures.
Defining soluble α-synuclein oligomer species
One additional set of experiments were carried out to address the issue of which of the higher molecular-weight forms of pS129-α-synuclein species might be defined as soluble oligomers, the potential toxic form. Using a small series of cingulate cortex cases (5 stage IV cases, I stage III case, and 2 stage 0 cases), we utilized methodology involving direct detergent extract of brain tissue followed by an ultracentrifugation step (Tsika et al, 2010) to remove all forms of insoluble α-synuclein, and thus identify putative soluble oligomeric species. In Figure 3E, there was an increase in intensity of monomeric pS129-α–synuclein in the Stage IV compared to Stage 0 samples, while antibodies LB509 (Figure 3F) and syn-1 (Figure 3G) detected total monomeric α synuclein in all samples. In panel E, the strongly reactive Stage IV sample contained higher molecular weight bands ranging from 25 kD to 60 kD, while the other stage IV samples had the same but less intense bands. With the inclusion of an ultracentrifugation step, these results show the higher molecular weight species detected were not due to contamination of microcentrifuged fractions with insoluble material. Most of these samples also had prominent bands of approximately 35 kD, detected with LB509 antibody (Figure 3 F, indicated on Figure with D). This band meets the criteria of soluble oligomer (a presumptive stable dimer of α-synuclein), but its level does not appear to be disease-related being present in Stage 0 and most Stage IV samples at approximately the same intensities. Interestingly, it was present in the majority of samples detected with LB509, but could not be detected with syn-1 antibody, suggesting a change in conformation.
Characterization of pS129-α-synuclein in fractionated staged LBD cases
The above results showed that we could detect 6–7 different bands with the antibodies to pS129-α-synuclein in these selected LBD brain samples, and that this immunoreactivity was phosphorylation-specific. We next quantified the progressive changes in intensities of these pS129-α-synuclein immunoreactive bands in a large series of brain samples fractionated as described in Figure 1A. Using the membrane protein CD200 as a marker for membrane contamination, we showed in selected temporal cortex samples that the presence of CD200 in soluble fractions was slight or undetectable (Figure 1B). We sought to address the question whether increased phosphorylation in the cytosol enriched pool of α-synuclein (soluble fraction) could make the α-synuclein more prone to associate with detergent-soluble membranes, and then further aggregate into a more insoluble fraction, defined by its resistance to detergents but solubility in urea-SDS.
Representative western blot images of soluble and membrane fractions from some of the staged temporal cortex samples are shown in Figure 4A. Similar patterns were seen for the corresponding cingulate cortex samples (data not shown). The two sets of images are aligned to show the relative levels of pS129-α–synuclein, α-synuclein forms detected with LB509 and β-actin levels in these respective fractions. The different species of α-synuclein that were measured are annotated on these images (molecular weights are approximate and based on migration of dyed marker proteins). M refers to 16–17 kD monomeric pS129-α-synuclein or unmodified α-synuclein, which is the most abundant form of α-synuclein. HMW refers to a group of at least 5–6 species of higher molecular weight pS129-α–synuclein (this group was measured collectively) ranging from 26 kD to 50 kD. As can be seen, these are present in Stage IV soluble fractions but enriched in the membrane fractions, and represent aggregated and/or ubiquitinated forms of pS129-α– synuclein (as seen in Figure 3D). There is a species (designated D – dimer) with molecular weight of 36 kD detected with a non-phosphorylation dependent antibody (LB509) whose levels did not change across the LB staging spectrum. A truncated form of pS129-α–synuclein (T) of approximately 12 kD was detected only in soluble fractions. Since all samples were analyzed under standardized conditions, comparison of intensities between cingulate cortex and temporal cortex samples for each fraction were possible.
Figure 4. Comparison of relative levels of pS129-α– synuclein with increased LB staging in soluble and membrane fractions of cingulate and temporal cortex.
A) Representative western blots of staged temporal cortex samples separated into SOLUBLE and MEMBRANE samples showing different species of α-synuclein. Figure represents a composite from individual western blots to show a representation of bands across all blots analyzed. Membranes probed with pS129-α-syn (Tokyo) showed monomeric (M) α-synuclein in both fraction, a truncated (T) species in the soluble fraction and an oligomer form enriched in the soluble fraction (O). Higher molecular weight (HMW) pS129-α-synuclein bands (6 separate bands with molecular weights 24–50 kD) were found primarily in Stage IV samples. An abundant dimeric form of unphosphorylated α-synuclein (D) was present in all fractions. The band identified as (O) and that identified with (D) were not the same species and differed slightly in molecular weight. Figure shows LB staging for the selected samples. Similar patterns of bands in soluble and membrane fractions of cingulate samples were observed (not shown).
B) and C) Changes in pS129-α-synuclein levels with increased staging; comparison between cingulate and temporal cortex samples. Large increases in levels of pS129-α–synuclein were apparent in both soluble (A) and membrane (B) fractions of Stage IV samples compared to Stage 0, but with progressive increase between Stage 0 and Stage III. Results represent normalized mean values ± standard error of mean (S.E.M.).
D and E) Changes in higher molecular weight pS129-α-synuclein in soluble and membrane fractions from cingulate and temporal cortex samples with increased staging. Significant increases in all stage IV samples compared to Stage 0-III. Results represent normalized mean values ± standard error of mean (S.E.M.).
The relative levels of pS129-α–synuclein in staged soluble fractions (Figure 4B), and staged membrane fractions (Figure 4C) are shown. Most noticeable from these figures is the large increase in levels of pS129-α–synuclein in the Stage IV samples, both membrane and soluble, compared to earlier staged samples. The differences between stage 0 and IV for both fractions and both brain regions were significant (P<0.01 – one way ANOVA with Newman Keuls post test). It can be seen in Figure 4B that there was an increase in soluble temporal cortex pS129-α-synuclein levels in Stage I, Stage IIb, and Stage III compared to Stage 0, but these increases did not reach statistical significance.
There were no significant differences between levels of soluble pS129-α–synuclein in Stage IV samples of cingulate and temporal cortex. In membrane fractions of Stage IV cases, temporal cortex samples had significantly higher levels compared to cingulate cortex (P< 0.01). There was an average 6.2-fold increase of pS129-α–synuclein levels in soluble fraction stage IV cingulate cortex samples compared to stage 0 samples, and a 6.45 fold increase between stage 0 and Stage IV in temporal cortex samples. By comparison, in the membrane fractions the increase in levels was 57.7-fold for cingulate and 136.2-fold for temporal samples. This is suggestive of a progressive partitioning of pS129-α-synuclein into the membrane fraction from the cytosolic soluble fraction, perhaps because higher concentrations promote conformation change. A salient feature of these studies is that in all fractions, there were very large increases in pS129-α–synuclein levels between stage III and stage IV. Another significant finding, as shown in Figure 4B, was the increase in pS129-α–synuclein in membrane fractions of Stage III temporal cortex samples compared to Stage 0 (P<0.01).
We also measured the relative levels of higher molecular weight forms of pS129 α-synuclein in soluble (Figure 4 D) and membrane fractions of cingulate and temporal cortex (Figure 4 E). The molecular weights of these bands (approximately 24–26 kD, 34–36 kD, 40 kD and 50 kD) (examples of patterns shown in Figure 2A and 4A) correspond to the ubiquitinated pS129-α–synuclein species with different ubiquitin residues (Figure 3D) and/or oligomeric aggregates (Anderson et al, 2006; Hasegawa et al, 2002). To obtain these measurements, we calculated the combined intensities of these higher molecular weight bands for each sample and fraction. The mean levels of higher molecular weight bands were significantly increased in all Stage IV samples; samples that would have significant densities of Lewy bodies and neurites.
Insoluble pS129-α–synuclein
Defining the biochemical solubility state of α-synuclein in brain tissues has varied between different laboratories based on the tissue fractionation schemes being employed. We characterized in a quantitative manner the relative levels of pS129-α-synuclein defined as insoluble after two rounds of extraction with RIPA buffer, which efficiently dissolve cellular membranes but not highly aggregated α-synuclein. The resulting insoluble materials were dissolved in 6M urea/2%SDS with heating. We have defined this as insoluble pS129-a-synuclein; it is possible that there was an additional fraction of aggregated pS129-α-synuclein that would require formic acid extraction, but this feature was not investigated.
We measured relative levels of monomeric (Figure 5A) and higher molecular weight pS129-α-synuclein (Figure 5B) in the insoluble fractions of staged samples derived from cingulate cortex and temporal cortex. A representative western blot of a series of cingulate cortex insoluble samples shows the distribution of immunoreactive bands for pS129-α–synuclein (Figure 5C). As seen, some Stage I samples had detectable levels of insoluble pS129-α–synuclein, despite the absence of cingulate or temporal cortex Lewy histopathology at this stage. In the Stage III and IV samples, there were significantly higher levels of monomeric pS129-α–synuclein and higher molecular weight pS129-α-synuclein species in cingulate compared to temporal cortex samples (P< 0.01 (Stage III) and P< 0.001 (Stage IV)). Analyses indicated that Stage III cases from both regions had higher levels of monomeric pS129-α-synuclein compared to Stage 0 cases, but these differences did not reach statistical significance. Separating Stage IV samples into disease categories of PD or DLB showed that there was no difference between these two groups for cingulate cortex (Figure 5D), but a significant increase (6.4 fold) in DLB cases compared to PD for the temporal cortex samples (P< 0.001) (Figure 5E). In Figures 5D and 5E, the individual range of pS129-α-synuclein values in stage IV PD and DLB cases can be compared. As these are matched samples for cingulate and temporal cortex, these results again suggest that formation of insoluble α-synuclein has progressed earlier and further in cingulate compared to temporal Stage IV cases. The higher levels in temporal cortex DLB cases compared to PD cases suggest DLB to be a more aggressive LB disease, although these cases were not specifically selected to be representative of their disease class. The increased severity of Lewy body disease in DLB relative to PD was also shown in the original Unified Staging System publication (Beach et al, 2009), where 50% of PD cases were assessed as Stage III and 40% were Stage IV, while 30% of DLB cases were assessed as Stage III and 60% were Stage IV. Analyzing the levels of insoluble pS129-α-synuclein in both brain regions according to disease pathology provided some additional insight into disease progression (Figure 5F and Figure 5G). PD cases with dementia and sufficient pathology to meet the diagnosis of AD (PDAD) had significantly higher levels of insoluble pS129-α-synuclein than PD or PDD cases.
Figure 5. Comparison of relative levels of insoluble pS129-α-synuclein species in staged LB cingulate and temporal cortex samples.
A and B) Significantly higher levels of insoluble monomeric (A) and higher molecular weight (B) pS129-α-synuclein in cingulate cortex samples compared to temporal cortex samples. Significantly higher levels of pS129-α-synuclein in cingulate stage IV samples (***P<0.001) and stage III samples compared to temporal cortex samples (** P<0.01) for monomeric (B) and higher molecular weight samples (C). Results represent normalized mean values ± standard error of mean (S.E.M.).
C) Representative western blots showing patterns of pS129-α-synuclein immunoreactive bands for a selection of staged cingulate cortex insoluble fraction samples. Major bands being detected were monomeric pS129 α-synuclein (16–17 kD) and higher molecular weight (hmw) species ranging for 25–50 kD. Insoluble pS129-α-synuclein can be detected in some Stage I samples.
D) and E) Significantly higher levels of insoluble monomeric pS129-α–synuclein in temporal cortex insoluble DLB compared to PD samples but not in cingulate cortex. Stage IV samples were separated into two groups based on neuropathological diagnosis of PD or DLB.
Comparison of pS129-α-synuclein intensities with densities of Lewy histopathological structures
The Unified Staging System (Beach et al, 2009), which is the basis of classifying these samples from different LBDs, used a ranking system to determine density of Lewy-type histopathological structures in each brain region. As samples with, for example, Stage IV classification may have a Lewy Density Score (LTS) ranging between 2 and 4, and as this range likely represents several orders of magnitude, this may explain in part the biochemical variability seen even within a single stage. We assessed using Spearman non-parametric analyses whether levels of pS129-α-synuclein in different fractions correlated with their individual histopathological Lewy density scores (LTS). There were strong and highly significant correlations between temporal cortex or cingulate cortex LTS scores and membrane and insoluble fraction pS129-α-synuclein levels in these respective brain regions (Figure 6). By comparison, there were weaker but still significant correlations between LTS scores and intensities of pS129-α–synuclein in soluble fractions.
Figure 6. Correlation of pS129-α-synuclein levels with Lewy Type Density Scores in each fraction and brain region.
Panels A–E show correlation plots of pS129-α-synuclein levels in cingulate cortex soluble enriched fraction (A), cingulate membrane enriched fraction (B) and cingulate insoluble fraction C) against cingulate Lewy Type Density scores, and temporal cortex soluble enriched fraction (D), temporal membrane enriched fraction (E) and temporal insoluble fraction (F) against corresponding temporal cortex Lewy Type Density scores. Blots show linear regression line. Statistical analysis using Spearman non-parametric analysis (r) with corresponding P values.
Correlation analyses of pS129-α-synuclein levels between brain regions and different fractions
Pairwise analyses of pS129-α-synuclein levels between all cingulate and temporal cortex brain fractions were carried out to determine if there was a relationship between these measures in different brain regions. Table 3 shows the degree of correlation between these different measures. Selected analyses from this table are shown in graphical form as linear regression plots (Figure 7A–G). Of note from these analyses was the significant correlation between pS129-α-synuclein levels in the cingulate cortex insoluble fraction and all other measures. These analyses used Pearson correlation even though these sets of pathological data from cases that ranged from control to severely diseased (Stage 0 to Stage IV) were shown to not be normally distributed. This test provided greater discrimination between groups compared to other non-parametric tests. Although these statistical correlations need to be interpreted cautiously as outliers can affect the results, comparing the fractions where correlations were positive with those where there was no correlation, provides some indication of possible pathological interactions and point to further studies. There may be factors that led to increased formation of insoluble pS129-α-synuclein in cingulate cortex that might be affecting progression of increased pS129-α-synuclein aggregation in temporal cortex.
Table 3.
Correlation analyses for pS129-α-synuclein levels in different brain fractions of staged brain cases
| Factor | Pearson R | P value |
|---|---|---|
| Cingulate-Soluble | ||
| Cingulate-membrane | 0.045 | 0.689 NS |
| Cingulate-insoluble | 0.351 | <0.01** |
| Temporal-soluble | 0.051 | 0.617 NS |
| Temporal-membrane | 0.121 | 0.364 NS |
| Temporal-insoluble | 0.122 | 0.361 NS |
| Cingulate-Membrane | ||
| Cingulate-insoluble | 0.576 | <0.0001*** |
| Temporal-soluble | 0.092 | 0.413 NS |
| Temporal-membrane | 0.655 | < 0.0001*** |
| Temporal-insoluble | 0.042 | 0.717 NS |
| Cingulate-Insoluble | ||
| Temporal-soluble | 0.424 | < 0.001** |
| Temporal-membrane | 0.679 | < 0.0001*** |
| Temporal-insoluble | 0.391 | < 0.0001*** |
| Temporal-Soluble | ||
| Temporal-membrane | 0.443 | < 0.0001*** |
| Temporal-insoluble | 0.106 | 0.428 NS |
| Temporal-Membrane | ||
| Temporal-insoluble | 0.628 | < 0.0001*** |
Significant correlations are in bold, and R (Pearson correlation coefficients) and significances.
NSnot statistically significant
Figure 7. Selected regression plots of pS129-α-synuclein levels with different brain regions and fractions.
Correlation analysis between selected brain regions and fractions (complete list of all analyses shown in Table 3). Panel A and B shows negative correlations between cingulate-membrane and cingulate soluble (A) and between cingulate membrane and temporal soluble fractions (B). Panels C, D, E and F show positive correlations between cingulate membrane and cingulate insoluble (C); cingulate membrane and temporal membrane (D); cingulate and temporal insoluble (E) and temporal membrane and temporal insoluble (F). Graphs show Pearson R correlation coefficients with corresponding P values to indicate degree of significance.
Discussion
In this study, we measured the changes in the levels and solubility of α-synuclein phosphorylated at serine 129, the most abundant form of phosphorylated α-synuclein, in concert with stagewise changes in Lewy pathology in samples of cingulate cortex and temporal cortex. This study concentrated on what is now believed to be the pathological modification of α-synuclein (pS129-α-synuclein), whose levels are increased as the Lewy body disease progresses. Histological studies done by our group and others have shown that immunohistochemical detection of pS129-α-synuclein is a sensitive method of describing these types of diseases (Obi et al., 2008; Beach et al., 2009; Beach et al., 2010). By confirming these observations at the biochemical level, we attempted to dissect the progression of changes in pS129-α-synuclein.
This work is an extension of our recently published work on pS129-α-synuclein levels in unfractionated brain samples using the same group of cases as described in this publication (Lue et al., 2012). The major finding from this publication was demonstration that there were higher levels of pS129-α-synuclein in cingulate and temporal cortex in samples showing low level (Stage I) of LB pathology compared to Stage 0 samples. This was assessed by comparing cortical pS129-α-synuclein levels with LTS scores in olfactory bulb, substantia nigra and amygdala. This study showed that there were biochemical changes in pS129-α-synuclein in brain regions before histological pS129-α-synuclein deposition.
It is known that small amounts of pS129-α–synuclein are present in fractions of normal brains and thus may have normal cellular function (Muntane et al, 2012), but the large increases in levels of this modified form in the presence of Lewy pathology implies changes in the regulation of phosphorylation and/or dephosphorylation of α-synuclein leads to the formation of more pathogenic forms of α-synuclein (Cavallarin et al, 2010; Lee et al, 2011), and thus could initiate or accelerate Lewy body diseases. Defining the stage of the disease that this becomes significant is therefore an important issue. Low levels of pS129-α-synuclein are readily detectable in Stage O (normal cases) cytosol fraction (Figure 4) and in unfractionated Stage 0 samples (Lue et al., 2012), but not in membrane fractions. By measuring changes in solubility in different cellular fractions as well as relative levels of pS129-α–synuclein, one can see an apparent changing distribution. Increased pS129-α–synuclein appears to occur first in the soluble fraction. It is known that this changes its conformation and solubility and promotes its association with membrane structures (Auluck et al, 2010; Bartels et al, 2010; Uversky and Eliezer, 2009). Further changes in phosphorylation and ubiquitination presumably increase aggregation and formation of an insoluble fraction. As the solubility of α-synuclein decreases, phosphorylation appears to increase along with other modifications, with a fraction of pS129-α–synuclein becoming higher molecular weight modified forms. As cingulate cortex shows Lewy pathology earlier than neocortex, this result suggests that the earlier formation of modified α-synuclein in cingulate cortex results in earlier progression into the insoluble fraction compared to what is happening in temporal cortex. In cingulate cortex, the progression into the insoluble fraction would appear to be more advanced in both PD and DLB groups as there was no difference between them. The same analyses for temporal cortex suggest formation of aggregated/insoluble pS129-α–synuclein in DLB cases had progressed at a different and faster rate than for the PD cases.
A publication focusing on PD alone and using samples of putamen and frontal cortex addressed similar issues of progression of pS129-α synuclein formation and changes in solubility (Zhou et al, 2011) using PD cases classified as Stage IV (brainstem), stage V (limbic) or stage VI (neocortical) according to a different staging scheme (Harding and Halliday 1998). However, despite the different patient groups and brain regions, similar patterns of changes in solubility and increased phosphorylation were observed. There were significantly increased levels of pS129-α-synuclein in both soluble and membrane fractions of frontal cortex compared to putamen in the PD samples, and also increased levels of total insoluble α-synuclein in the insoluble fraction (Zhou et al, 2011). Levels of pS129-α-synuclein in putamen were significantly associated with levels in frontal cortex (Zhou et al, 2011). Both of our studies indicate, even though using different patient groups and different staging schemes, evidence for progression of pS129-α-synuclein.
We also showed that there were increased levels of pS129-α–synuclein in PD (Stage III and IV) cases with significant AD pathology. This agrees with studies suggesting an interaction of α-synuclein and amyloid beta accelerates their deposition (Mandal et al, 2006; Obi et al, 2008; Ono et al, 2012).
Analyses between values for all fractions showed that the cingulate pS129-α-synuclein insoluble levels correlated highly with all other measures, not only within that brain region, but for all temporal fractions as well (Table 3). This provides some evidence for factors affecting formation of insoluble pS129-α-synuclein in cingulate cortex might be affecting the progression of pS129-α-synuclein formation in temporal cortex. Since pS129-α-synuclein becomes highly enriched in Lewy pathologies in different LBDs, there is still the question of whether increased phosphorylation of pS129-α-synuclein occurs after aggregation into Lewy structures has started (Paleologou et al, 2008), or whether phosphorylation precedes and therefore could contribute to α-synuclein misfolding, aggregation and toxicity that contributes to initiation of LB formation. Our earlier study (Lue et al, 2012) suggests that phosphorylation at least precedes the formation of immunohistochemically-detectable LTS.
The potential significance of increased formation of pS129-α-synuclein has been suggested by animal models and in vitro approaches. One study showed that in vitro formation of pS129-α-synuclein promoted fibril formation (Fujiwara et al, 2002). By contrast, another concluded that phosphorylation inhibited the tendency of α-synuclein to form fibrils, while a mutant peptide with an alanine substitution at serine 129, so that phosphorylation cannot occur at that residue, formed larger numbers of fibrils (Paleologou et al, 2008). A recent transgenic α-synuclein mouse model has provided the strongest evidence that formation of pS129-α-synuclein is associated with enhanced toxicity (Lee et al, 2011). Stimulating the activity of phosphoprotein phosphatase 2A (PP2A) using the coffee-extracted chemical eicosanoyl-5-hydroxytryptamide in a dose-dependent manner resulted in reduced levels of pS129-α–synuclein, and reduced levels of aggregated α-synuclein. The consequence of this was enhanced neuronal activity and improved motor performance, along with reduced astrocytic and microglial reactions. This latest study strongly supports the notion of pS129-α-synuclein as a pathogenic, not a protective, modification, since reducing the levels of pS129-α–synuclein had direct therapeutic benefit (Cavallarin et al, 2010). As we are observing some early changes in pS129-α–synuclein, prior to the development of histopathology, inhibiting this process could be an approach to treating LBDs, particularly if such an approach would also reduce the dramatic increases in pS129-α-synuclein occurring in advanced LBDs (Stage IV). The significance of this large increase in phosphorylation late in disease is unclear and has been used as evidence that pS129-α-synuclein might be sequestered into LB structures and is thus in a non-toxic form (Fujiwara et al, 2002). Increased formation of aggregated-α-synuclein into Lewy structures appears to inhibit the phosphatases that will dephosphorylate pS129-α-synuclein (Wu et al., 2012). Both events could be occurring simultaneously in different disease stages or different affected brain regions.
Defining solubility properties of α-synuclein based on biochemical extraction procedures has varied between publications and is likely responsible for differences found in the distribution of species of α-synuclein (Gao et al, 2008; Neumann et al, 2002; Pawlyk et al, 2003; Popescu et al, 2004). In this study, we measured, in different solubility-determined fractions, a number of different species of pS129-α–synuclein including monomeric pS129-α–synuclein (16–17 kD) as well as 5 different higher molecular weight pS129-α–synuclein forms. We also characterized 3 of the individual higher molecular weight species of pS129-α-synuclein as being ubiquitinated, as was the high molecular weight aggregated smear of pS129-α-synuclein. An additional non-phosphorylated soluble dimeric form of α-synuclein was identified in a subset of samples; this form was abundant in both control and most LB staged samples.
In summary, we have shown that increased levels of pS129-α–synuclein were a significant feature in soluble, detergent soluble (membrane-associated) and detergent insoluble brain fractions late in development of LB pathology. Additionally, there was some evidence of increased phosphorylation of α-synuclein at stages prior to LB pathology. Based on differences between staged groups, it can be suggested that increased phosphorylation was initially occurring in soluble fractions, leading to an increased association with membranes, and then an increased accumulation into insoluble LB aggregates. The very large increase in pS129-α-synuclein in stage IV samples in membrane and insoluble fractions does suggest that aggregated α-synuclein might be becoming further phosphorylated. As there is still controversy over which enzymes are the most significant for producing pS129-α-synuclein in vivo (Mbefo et al, 2010; Waxman and Giasson 2011; Waxman and Giasson 2008), the recent study that showed a therapeutic benefit for stimulating dephosphorylation could be the best approach for determining whether reducing pS129-α-synuclein is a valid therapeutic target in humans (Lee et al, 2011).
Table 2A.
Case demographics (Disease Duration)
| Group | Stage 0 | Stage I | Stage IIa | Stage IIb | Stage III | Stage IV |
|---|---|---|---|---|---|---|
| Disease Duration (yrs) – PD | 6.25±11.21 (n=5) | 6 (n=1) | 14.5±7.9 (n=27) | 13.4±8.8NS (n=16) | ||
| Disease Duration (yrs) – DLB | 6.2±2.9 (n=9) | 8±4.3NS (n=32) | ||||
| Disease Duration (yrs) – ADLB | 7.9±4.3 (n=17) | 9.7±5.5NS (n=17) |
Disease Duration: Time (yrs) + standard deviation from initial diagnosis of movement disorder (PD) or cognitive decline (DLB or ADLB) and death.
Table 2B.
Case demographics (Parkinson’s Disease)
| Group | Stage 0 | Stage I | Stage IIa | Stage IIb | Stage III | Stage IV | TOTAL |
|---|---|---|---|---|---|---|---|
| PD (no dementia)/(n) Disease duration (yrs) | 3 (4.7±4.2) | 4 (16,5±5.8) | 7 | ||||
| PD (AD dementia)/(n) Disease duration (yrs) | 1 (2) | 9 (16.3±9.3) | 10 (10.7±5.7) | 19 | |||
| PD (non-AD dementia)/(n) Disease duration (yrs) | 1 (23) | 17 (13.1±7.7) | 7 (17.3±11.3) | 25 |
Group: PD (AD dementia): PD with dementia due to Alzheimer’s disease. PD (non-AD dementia); PD with dementia but with insufficient pathology for AD diagnosis.
The highlights of this paper are as follows.
Changes in solubility of phosphorylated α-synuclein in Lewy Body disease (LBD) brains
Increase in phosphorylated α-synuclein enhanced in most severe LBD affected brains
Increase in phosphorylated α-synuclein shift from cingulate to temporal fractions
Increase in high molecular weight/ubiquitinated forms of phosphorylated α-synuclein
Acknowledgments
This work was supported by a grant from the Michael J. Fox Foundation for Parkinson’s research (TGB, DGW, L-FL). The operation of the Banner Sun Health Research Institute Brain and Body Donation Program is supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson’s Disease Consortium) and the Michael J. Fox Foundation for Parkinson’s Research (The Prescott Family Initiative). The study sponsors played no role in study design, study execution or data interpretation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alafuzoff I, Ince PG, Arzberger T, et al. Staging/typing of Lewy body related alpha-synuclein pathology: a study of the BrainNet Europe Consortium. Acta Neuropathol. 2009;117:635–652. doi: 10.1007/s00401-009-0523-2. [DOI] [PubMed] [Google Scholar]
- Anderson JP, Walker DE, Goldstein JM, et al. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J Biol Chem. 2006;281:29739–29752. doi: 10.1074/jbc.M600933200. [DOI] [PubMed] [Google Scholar]
- Auluck PK, Caraveo G, Lindquist S. alpha-Synuclein: membrane interactions and toxicity in Parkinson’s disease. Annu Rev Cell Dev Biol. 2010;26:211–233. doi: 10.1146/annurev.cellbio.042308.113313. [DOI] [PubMed] [Google Scholar]
- Baba M, Nakajo S, Tu PH, et al. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am J Pathol. 1998;152:879–884. [PMC free article] [PubMed] [Google Scholar]
- Bartels T, Ahlstrom LS, Leftin A, et al. The N-terminus of the intrinsically disordered protein alpha-synuclein triggers membrane binding and helix folding. Biophys J. 2010;99:2116–2124. doi: 10.1016/j.bpj.2010.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, Adler CH, Lue L, et al. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 2009;117:613–634. doi: 10.1007/s00401-009-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, Adler CH, Sue LI, et al. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119:689–702. doi: 10.1007/s00401-010-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, Sue LI, Walker DG, et al. The Sun Health Research Institute Brain Donation Program: description and experience, 1987–2007. Cell Tissue Bank. 2008;9:229–245. doi: 10.1007/s10561-008-9067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del TK, Bratzke H, et al. Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson’s disease (preclinical and clinical stages) J Neurol. 2002;249(Suppl 3):III/1–III/5. doi: 10.1007/s00415-002-1301-4. [DOI] [PubMed] [Google Scholar]
- Braak H, Del TK, Rub U, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Brown DR. Oligomeric alpha-synuclein and its role in neuronal death. IUBMB Life. 2010;62:334–339. doi: 10.1002/iub.316. [DOI] [PubMed] [Google Scholar]
- Cavallarin N, Vicario M, Negro A. The role of phosphorylation in synucleinopathies: focus on Parkinson’s disease. CNS Neurol Disord Drug Targets. 2010;9:471–481. doi: 10.2174/187152710791556140. [DOI] [PubMed] [Google Scholar]
- Chen L, Periquet M, Wang X, et al. Tyrosine and serine phosphorylation of alpha-synuclein have opposing effects on neurotoxicity and soluble oligomer formation. J Clin Invest. 2009;119:3257–3265. doi: 10.1172/JCI39088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deramecourt V, Bombois S, Maurage CA, et al. Biochemical staging of synucleinopathy and amyloid deposition in dementia with Lewy bodies. J Neuropathol Exp Neurol. 2006;65:278–288. doi: 10.1097/01.jnen.0000205145.54457.ea. [DOI] [PubMed] [Google Scholar]
- Duda JE, Giasson BI, Chen Q, et al. Widespread nitration of pathological inclusions in neurodegenerative synucleinopathies. Am J Pathol. 2000;157:1439–1445. doi: 10.1016/S0002-9440(10)64781-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara H, Hasegawa M, Dohmae N, et al. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- Gao HM, Kotzbauer PT, Uryu K, et al. Neuroinflammation and oxidation/nitration of alpha-synuclein linked to dopaminergic neurodegeneration. J Neurosci. 2008;28:7687–7698. doi: 10.1523/JNEUROSCI.0143-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Tortosa E, Gonzalo I, Newell K, et al. Patterns of protein nitration in dementia with Lewy bodies and striatonigral degeneration. Acta Neuropathol. 2002;103:495–500. doi: 10.1007/s00401-001-0495-3. [DOI] [PubMed] [Google Scholar]
- Halliday GM, Del TK, Braak H. Critical appraisal of brain pathology staging related to presymptomatic and symptomatic cases of sporadic Parkinson’s disease. J Neural Transm. 2006;(Suppl):99–103. doi: 10.1007/978-3-211-45295-0_16. [DOI] [PubMed] [Google Scholar]
- Harding AJ, Halliday GM. Simplified neuropathological diagnosis of dementia with Lewy bodies. Neuropathol Appl Neurobiol. 1998;24:195–201. doi: 10.1046/j.1365-2990.1998.00115.x. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Fujiwara H, Nonaka T, et al. Phosphorylated alpha-synuclein is ubiquitinated in alpha-synucleinopathy lesions. J Biol Chem. 2002;277:49071–49076. doi: 10.1074/jbc.M208046200. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA. Formation and development of Lewy pathology: a critical update. J Neurol. 2009;256(Suppl 3):270–279. doi: 10.1007/s00415-009-5243-y. [DOI] [PubMed] [Google Scholar]
- Kahle PJ, Neumann M, Ozmen L, et al. Selective insolubility of alpha-synuclein in human Lewy body diseases is recapitulated in a transgenic mouse model. Am J Pathol. 2001;159:2215–2225. doi: 10.1016/s0002-9440(10)63072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucken J, Ingelsson M, Shin Y, et al. Clinical and biochemical correlates of insoluble alpha-synuclein in dementia with Lewy bodies. Acta Neuropathol. 2006;111:101–108. doi: 10.1007/s00401-005-0027-7. [DOI] [PubMed] [Google Scholar]
- Lee KW, Chen W, Junn E, et al. Enhanced Phosphatase Activity Attenuates {alpha}-Synucleinopathy in a Mouse Model. J Neurosci. 2011;31:6963–6971. doi: 10.1523/JNEUROSCI.6513-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees AJ, Hardy J, Revesz T. Parkinson’s disease. Lancet. 2009;373:2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- Lue LF, Walker DG, Adler CH, et al. Biochemical Increase in Phosphorylated Alpha-Synuclein Precedes Histopathology of Lewy-Type Synucleinopathies. Brain Pathol. 2012 doi: 10.1111/j.1750-3639.2012.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machiya Y, Hara S, Arawaka S, et al. Phosphorylated alpha-synuclein at Ser-129 is targeted to the proteasome pathway in a ubiquitin-independent manner. J Biol Chem. 2010;285:40732–40744. doi: 10.1074/jbc.M110.141952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal PK, Pettegrew JW, Masliah E, et al. Interaction between Abeta peptide and alpha synuclein: molecular mechanisms in overlapping pathology of Alzheimer’s and Parkinson’s in dementia with Lewy body disease. Neurochem Res. 2006;31:1153–1162. doi: 10.1007/s11064-006-9140-9. [DOI] [PubMed] [Google Scholar]
- Marui W, Iseki E, Nakai T, et al. Progression and staging of Lewy pathology in brains from patients with dementia with Lewy bodies. J Neurol Sci. 2002;195:153–159. doi: 10.1016/s0022-510x(02)00006-0. [DOI] [PubMed] [Google Scholar]
- Mbefo MK, Paleologou KE, Boucharaba A, et al. Phosphorylation of synucleins by members of the Polo-like kinase family. J Biol Chem. 2010;285:2807–2822. doi: 10.1074/jbc.M109.081950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–72. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- Mihajlovic M, Lazaridis T. Membrane-bound structure and energetics of alpha-synuclein. Proteins. 2008;70:761–778. doi: 10.1002/prot.21558. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Part II Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Muntane G, Ferrer I, Martinez-Vicente M. alpha-synuclein phosphorylation and truncation are normal events in the adult human brain. Neuroscience. 2012;200:106–119. doi: 10.1016/j.neuroscience.2011.10.042. [DOI] [PubMed] [Google Scholar]
- Neumann M, Kahle PJ, Giasson BI, et al. Misfolded proteinase K-resistant hyperphosphorylated alpha-synuclein in aged transgenic mice with locomotor deterioration and in human alpha-synucleinopathies. J Clin Invest. 2002;110:1429–1439. doi: 10.1172/JCI15777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obi K, Akiyama H, Kondo H, et al. Relationship of phosphorylated alpha-synuclein and tau accumulation to Abeta deposition in the cerebral cortex of dementia with Lewy bodies. Exp Neurol. 2008;210:409–420. doi: 10.1016/j.expneurol.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Ono K, Takahashi R, Ikeda T, et al. Cross-seeding effects of amyloid beta-protein and alpha-synuclein. J Neurochem. 2012;122:883–890. doi: 10.1111/j.1471-4159.2012.07847.x. [DOI] [PubMed] [Google Scholar]
- Paleologou KE, Oueslati A, Shakked G, et al. Phosphorylation at S87 is enhanced in synucleinopathies, inhibits alpha-synuclein oligomerization, and influences synuclein-membrane interactions. J Neurosci. 2010;30:3184–3198. doi: 10.1523/JNEUROSCI.5922-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paleologou KE, Schmid AW, Rospigliosi CC, et al. Phosphorylation at Ser-129 but not the phosphomimics S129E/D inhibits the fibrillation of alpha-synuclein. J Biol Chem. 2008;283:16895–16905. doi: 10.1074/jbc.M800747200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlyk AC, Giasson BI, Sampathu DM, et al. Novel monoclonal antibodies demonstrate biochemical variation of brain parkin with age. J Biol Chem. 2003;278:48120–48128. doi: 10.1074/jbc.M306889200. [DOI] [PubMed] [Google Scholar]
- Paxinou E, Chen Q, Weisse M, et al. Induction of alpha-synuclein aggregation by intracellular nitrative insult. J Neurosci. 2001;21:8053–8061. doi: 10.1523/JNEUROSCI.21-20-08053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu A, Lippa CF, Lee VM, et al. Lewy bodies in the amygdala: increase of alpha-synuclein aggregates in neurodegenerative diseases with tau-based inclusions. Arch Neurol. 2004;61:1915–1919. doi: 10.1001/archneur.61.12.1915. [DOI] [PubMed] [Google Scholar]
- Saito Y, Kawashima A, Ruberu NN, et al. Accumulation of phosphorylated alpha-synuclein in aging human brain. J Neuropathol Exp Neurol. 2003;62:644–654. doi: 10.1093/jnen/62.6.644. [DOI] [PubMed] [Google Scholar]
- Sato H, Arawaka S, Hara S, et al. Authentically phosphorylated alpha-synuclein at Ser129 accelerates neurodegeneration in a rat model of familial Parkinson’s disease. J Neurosci. 2011;31:16884–16894. doi: 10.1523/JNEUROSCI.3967-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz-Schaeffer WJ. The synaptic pathology of alpha-synuclein aggregation in dementia with Lewy bodies, Parkinson’s disease and Parkinson’s disease dementia. Acta Neuropathol. 2010;120:131–143. doi: 10.1007/s00401-010-0711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugeno N, Takeda A, Hasegawa T, et al. Serine 129 phosphorylation of alpha-synuclein induces unfolded protein response-mediated cell death. J Biol Chem. 2008;283:23179–23188. doi: 10.1074/jbc.M802223200. [DOI] [PubMed] [Google Scholar]
- Tofaris GK, Razzaq A, Ghetti B, et al. Ubiquitination of alpha-synuclein in Lewy bodies is a pathological event not associated with impairment of proteasome function. J Biol Chem. 2003;278:44405–44411. doi: 10.1074/jbc.M308041200. [DOI] [PubMed] [Google Scholar]
- Tsika E, Moysidou M, Guo J, et al. Distinct region-specific alpha-synuclein oligomers in A53T transgenic mice: implications for neurodegeneration. J Neurosci. 2010;30:3409–3418. doi: 10.1523/JNEUROSCI.4977-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uversky VN, Eliezer D. Biophysics of Parkinson’s disease: structure and aggregation of alpha-synuclein. Curr Protein Pept Sci. 2009;10:483–499. doi: 10.2174/138920309789351921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooijen BD, Claessens MM, Subramaniam V. Membrane interactions of oligomeric alpha-synuclein: potential role in Parkinson’s disease. Curr Protein Pept Sci. 2010a;11:334–342. doi: 10.2174/138920310791330659. [DOI] [PubMed] [Google Scholar]
- van Rooijen BD, Claessens MM, Subramaniam V. Membrane Permeabilization by Oligomeric alpha-Synuclein: In Search of the Mechanism. PLoS One. 2010b;5:e14292. doi: 10.1371/journal.pone.0014292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Shi M, Chung KA, et al. Phosphorylated α-synuclein in Parkinson’s disease. Sci Transl Med. 2012;4:121. doi: 10.1126/scitranslmed.3002566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman EA, Giasson BI. Specificity and regulation of casein kinase-mediated phosphorylation of alpha-synuclein. J Neuropathol Exp Neurol. 2008;67:402–416. doi: 10.1097/NEN.0b013e3186fc995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman EA, Giasson BI. Characterization of kinases involved in the phosphorylation of aggregated alpha-synuclein. J Neurosci Res. 2011;89:231–247. doi: 10.1002/jnr.22537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Lou H, Alerte TN, et al. Lewy-like aggregation of alpha-synuclein reduces protein phosphatase 2A activity in vitro and in vivo. Neuroscience. 2012;207:288–297. doi: 10.1016/j.neuroscience.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Broe M, Huang Y, et al. Changes in the solubility and phosphorylation of alpha-synuclein over the course of Parkinson’s disease. Acta Neuropathol. 2011;121:695–704. doi: 10.1007/s00401-011-0815-1. [DOI] [PubMed] [Google Scholar]