Abstract
The effects of continuous infusions of insulin in physiologic doses on glucose kinetics and circulating counterregulatory hormones (epinephrine, norepinephrine, glucagon, cortisol, and growth hormone) were determined in normal subjects and diabetics. The normals received insulin at two dose levels (0.4 and 0.25 mU/kg per min) and the diabetics received the higher dose (0.4 mU/kg per min) only.
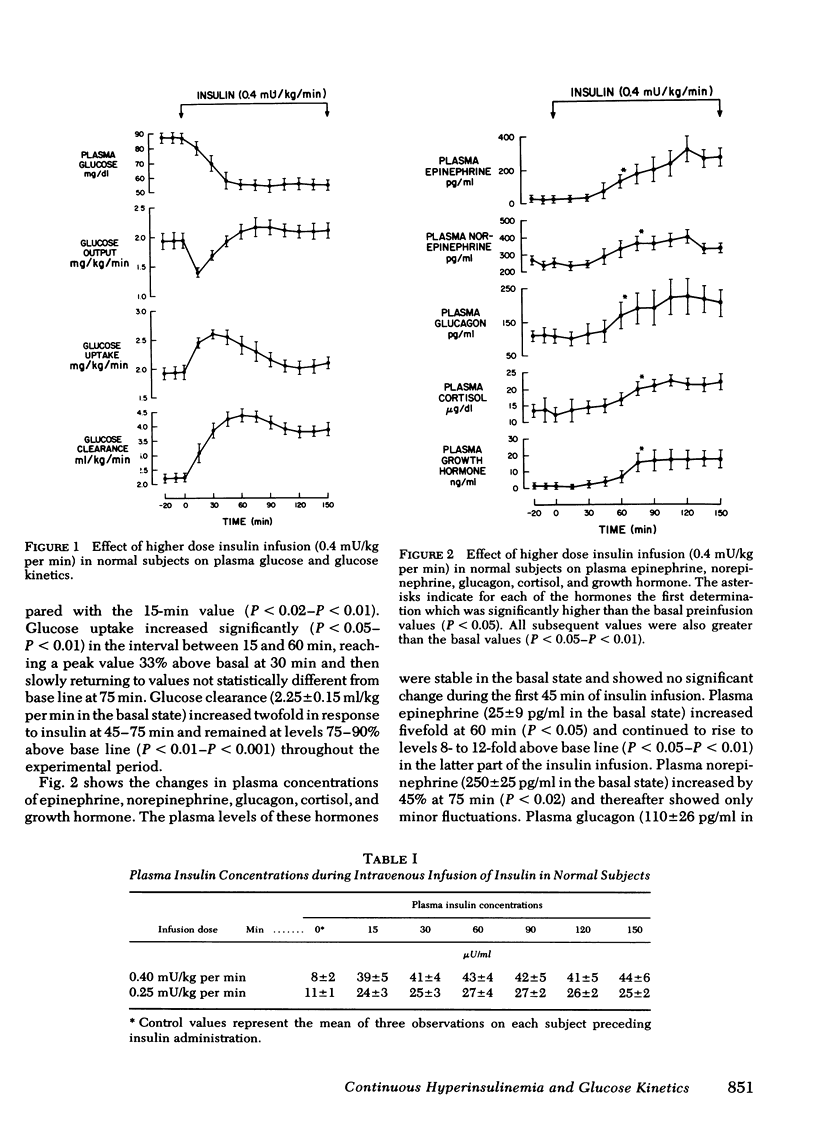
In all three groups of studies, continuous infusion of insulin resulted in an initial decline in plasma glucose followed by stabilization after 60-180 min. In the normal subjects, with the higher insulin dose there was a fivefold rise in plasma insulin. Plasma glucose fell at a rate of 0.73±0.12 mg/min for 45 min and then stabilized at 55±3 mg/dl after 60 min. The initial decline in plasma glucose was a result of a rapid, 27% fall in glucose output and a 33% rise in glucose uptake. Subsequent stabilization was a result of a return of glucose output and uptake to basal levels. The rebound increment in glucose output was significant (P < 0.05) by 30 min after initiation of the insulin infusion and preceded, by 30-45 min, a significant rise in circulating counterregulatory hormones.
With the lower insulin infusion dose, plasma insulin rose two- to threefold, plasma glucose initially fell at a rate of 0.37±0.04 mg/min for 75 min and stabilized at 67±3 mg/dl after 75 min. The changes in plasma glucose were entirely a result of a fall in glucose output and subsequent return to base line, whereas glucose uptake remained unchanged. Plasma levels of counterregulatory hormones showed no change from basal throughout the insulin infusion.
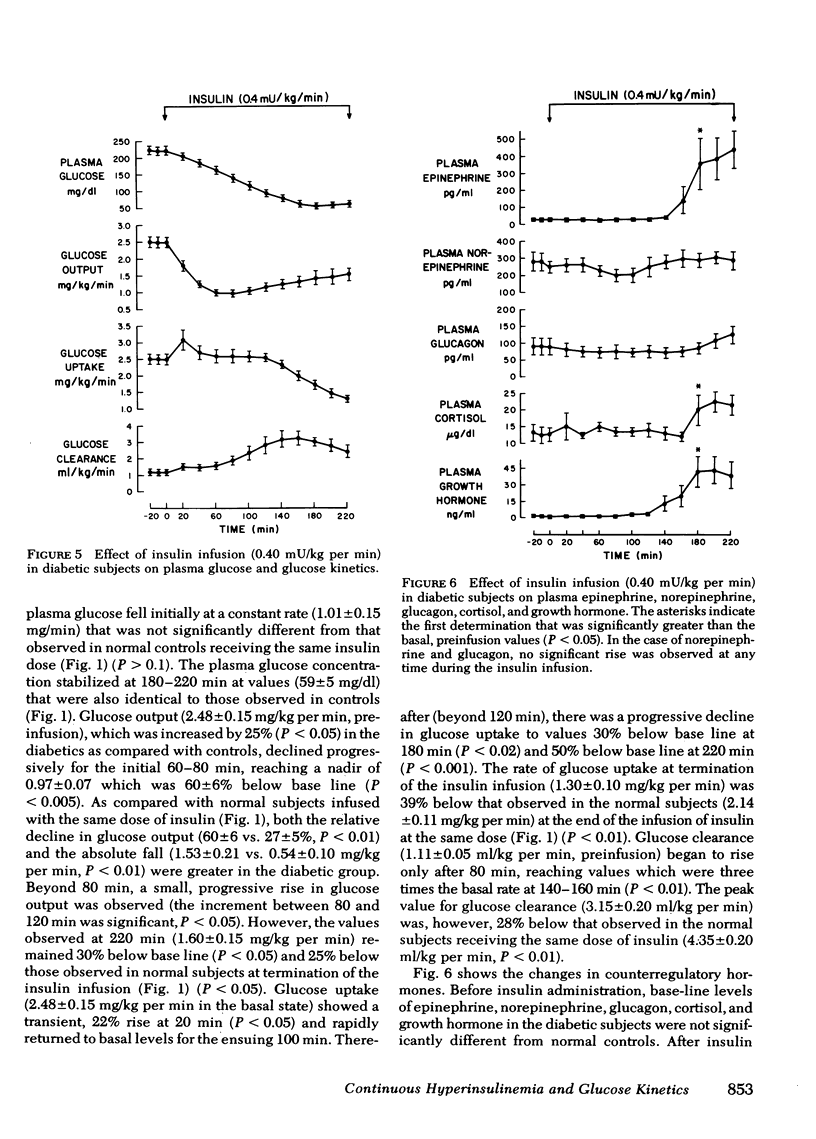
In the diabetic group (plasma glucose levels 227±7 mg/dl in the basal state), the initial rate of decline in plasma glucose (1.01±0.15 mg/dl) and the plateau concentration of plasma glucose (59±5 mg/dl) were comparable to controls receiving the same insulin dose. However, the initial fall in plasma glucose was almost entirely a result of suppression of glucose output, which showed a twofold greater decline (60±6%) than in controls (27±5%, P <0.01) and remained suppressed throughout the insulin infusion. In contrast, the late stabilization in plasma glucose was a result of a fall in glucose uptake to values 50% below basal (P < 0.001) and 39% below that observed in controls at termination of the insulin infusion (P < 0.01). Plasma norepinephrine and glucagon failed to rise during the insulin infusion, whereas plasma epinephrine, cortisol, and growth hormone rose to values comparable to controls receiving the same insulin dose.
It is concluded that (a) in normal and diabetic subjects, physiologic hyperinsulinemia results in an initial decline followed by stabilization of plasma glucose despite ongoing infusion of insulin; (b) in the normal subjects, a rebound increase in glucose output is the initial or principal mechanism counteracting the fall in plasma glucose and occurs (with an insulin dose of 0.25 mU/kg per min) in the absence of a rise in circulating counterregulatory hormones; (c) in diabetics, although the changes in plasma glucose are comparable to controls, the initial decline is a result of an exaggerated suppression of glucose output, whereas the stabilization of plasma glucose occurs primarily as a consequence of an exaggerated fall in glucose uptake; and (d) failure of plasma norepinephrine as well as glucagon to rise in the diabetics may contribute to the exaggerated suppression of glucose output.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahrén B., Ericsson M., Hedner P., Ingemansson S., Westgren U. Somatostatin inhibits thyroid hormone secretion induced by exogenous TSH in man. J Clin Endocrinol Metab. 1978 Nov;47(5):1156–1159. doi: 10.1210/jcem-47-5-1156. [DOI] [PubMed] [Google Scholar]
- Alberti K. G. Low-dose insulin in the treatment of diabetic ketoacidosis. Arch Intern Med. 1977 Oct;137(10):1367–1376. [PubMed] [Google Scholar]
- Altszuler N., Steele R., Rathgeb I., De Bodo R. C. Glucose metabolism and plasma insulin level during epinephrine infusion in the dog. Am J Physiol. 1967 Mar;212(3):677–682. doi: 10.1152/ajplegacy.1967.212.3.677. [DOI] [PubMed] [Google Scholar]
- Axelrod J., Weinshilboum R. Catecholamines. N Engl J Med. 1972 Aug 3;287(5):237–242. doi: 10.1056/NEJM197208032870508. [DOI] [PubMed] [Google Scholar]
- Brodows R. G., Pi-Sunyer F. X., Campbell R. G. Neural control of counter-regulatory events during glucopenia in man. J Clin Invest. 1973 Aug;52(8):1841–1844. doi: 10.1172/JCI107366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen N. J. Plasma norepinephrine and epinephrine in untreated diabetics, during fasting and after insulin administration. Diabetes. 1974 Jan;23(1):1–8. doi: 10.2337/diab.23.1.1. [DOI] [PubMed] [Google Scholar]
- Cowan J. S., Hetenyi G., Jr Glucoregulatory responses in normal and diabetic dogs recorded by a new tracer method. Metabolism. 1971 Apr;20(4):360–372. doi: 10.1016/0026-0495(71)90098-9. [DOI] [PubMed] [Google Scholar]
- DE BODO R. C., STEELE R., ALTSZULER N., DUNN A., ARMSTRONG D. T., BISHOP J. S. Further studies on the mechanism of action of insulin. Metabolism. 1959 Jul 2;8(4 Pt 2):520–530. [PubMed] [Google Scholar]
- DE MOOR P., STEENO O., RASKIN M., HENDRIKX A. Fluorimetric determination of free plasma 11-hydroxycorticosteroids in man. Acta Endocrinol (Copenh) 1960 Feb;33:297–307. doi: 10.1530/acta.0.xxxiii0297. [DOI] [PubMed] [Google Scholar]
- Eigler N., Saccà L., Sherwin R. S. Synergistic interactions of physiologic increments of glucagon, epinephrine, and cortisol in the dog: a model for stress-induced hyperglycemia. J Clin Invest. 1979 Jan;63(1):114–123. doi: 10.1172/JCI109264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensinck J. W., Walter R. M., Palmer J. P., Brodows R. G., Campbell R. G. Glucagon responses to hypoglycemia in adrenalectomized man. Metabolism. 1976 Feb;25(2):227–232. doi: 10.1016/0026-0495(76)90053-6. [DOI] [PubMed] [Google Scholar]
- Felig P., Wahren J. Influence of endogenous insulin secretion on splanchnic glucose and amino acid metabolism in man. J Clin Invest. 1971 Aug;50(8):1702–1711. doi: 10.1172/JCI106659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber A. J., Cryer P. E., Santiago J. V., Haymond M. W., Pagliara A. S., Kipnis D. M. The role of adrenergic mechanisms in the substrate and hormonal response to insulin-induced hypoglycemia in man. J Clin Invest. 1976 Jul;58(1):7–15. doi: 10.1172/JCI108460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genuth S. M. Metabolic clearance of insulin in man. Diabetes. 1972 Oct;21(10):1003–1012. doi: 10.2337/diab.21.10.1003. [DOI] [PubMed] [Google Scholar]
- Gerich J. E., Langlois M., Noacco C., Karam J. H., Forsham P. H. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science. 1973 Oct 12;182(4108):171–173. doi: 10.1126/science.182.4108.171. [DOI] [PubMed] [Google Scholar]
- Glinsmann W. H., Hern E. P., Lynch A. Intrinsic regulation of glucose output by rat liver. Am J Physiol. 1969 Apr;216(4):698–703. doi: 10.1152/ajplegacy.1969.216.4.698. [DOI] [PubMed] [Google Scholar]
- Hers H. G. The control of glycogen metabolism in the liver. Annu Rev Biochem. 1976;45:167–189. doi: 10.1146/annurev.bi.45.070176.001123. [DOI] [PubMed] [Google Scholar]
- Issekutz B., Jr, Issekutz T. B., Elahi D., Borkow I. Effect of insulin infusions on the glucose kinetics in alloxan-steptozotocin diabetic dogs. Diabetologia. 1974 Aug;10(4):323–328. doi: 10.1007/BF02627734. [DOI] [PubMed] [Google Scholar]
- McCraw E. F., Peterson M. J., Ashmore J. Autoregulation of glucose metabolism in the isolated perfused rat liver. Proc Soc Exp Biol Med. 1967 Oct;126(1):232–236. doi: 10.3181/00379727-126-32410. [DOI] [PubMed] [Google Scholar]
- Navalesi R., Pilo A., Ferrannini E. Kinetic analysis of plasma insulin disappearance in nonketotic diabetic patients and in normal subjects. A tracer study with 125I-insulin. J Clin Invest. 1978 Jan;61(1):197–208. doi: 10.1172/JCI108918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M. M., Alberti K. G., Greenwood R., Gumaa K. A., Hockaday T. D., Lowy C., Nabarro J. D., Pyke D. A., Sönksen P. H., Watkins P. J. Treatment of diabetic coma with continuous low-dose infusion of insulin. Br Med J. 1974 Jun 29;2(5921):687–690. doi: 10.1136/bmj.2.5921.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. P., Henry D. P., Benson J. W., Johnson D. G., Ensinck J. W. Glucagon response to hypoglycemia in sympathectomized man. J Clin Invest. 1976 Feb;57(2):522–525. doi: 10.1172/JCI108305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passon P. G., Peuler J. D. A simplified radiometric assay for plasma norepinephrine and epinephrine. Anal Biochem. 1973 Feb;51(2):618–631. doi: 10.1016/0003-2697(73)90517-4. [DOI] [PubMed] [Google Scholar]
- Pozefsky T., Felig P., Tobin J. D., Soeldner J. S., Cahill G. F., Jr Amino acid balance across tissues of the forearm in postabsorptive man. Effects of insulin at two dose levels. J Clin Invest. 1969 Dec;48(12):2273–2282. doi: 10.1172/JCI106193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosselin G., Assan R., Yalow R. S., Berson S. A. Separation of antibody-bound and unbound peptide hormones labelled with iodine-131 by talcum powder and precipitated silica. Nature. 1966 Oct 22;212(5060):355–357. doi: 10.1038/212355a0. [DOI] [PubMed] [Google Scholar]
- STEELE R., BISHOP J. S., DUNN A., ALTSZULER N., RATHBEB I., DEBODO R. C. INHIBITION BY INSULIN OF HEPATIC GLUCOSE PRODUCTION IN THE NORMAL DOG. Am J Physiol. 1965 Feb;208:301–306. doi: 10.1152/ajplegacy.1965.208.2.301. [DOI] [PubMed] [Google Scholar]
- STEELE R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959 Sep 25;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- Sacca L., Perez G., Carteni G., Rengo F. Evaluation of the role of the sympathetic nervous system in the glucoregulatory response to insulin-induced hypoglycemia in the rat. Endocrinology. 1977 Oct;101(4):1016–1022. doi: 10.1210/endo-101-4-1016. [DOI] [PubMed] [Google Scholar]
- Saccà L., Sherwin R., Felig P. Effect of sequential infusions of glucagon and epinephrine on glucose turnover in the dog. Am J Physiol. 1978 Sep;235(3):E287–E290. doi: 10.1152/ajpendo.1978.235.3.E287. [DOI] [PubMed] [Google Scholar]
- Saccá L., Perez G., Carteni G., Trimarco B., Rengo F. Role of glucagon in the glucoregulatory response to insulin-induced hypoglycemia in the rat. Horm Metab Res. 1977 May;9(3):209–212. doi: 10.1055/s-0028-1093538. [DOI] [PubMed] [Google Scholar]
- Semple P. F., White C., Manderson W. G. Continuous intravenous infusion of small doses of insulin in treatment of diabetic ketoacidosis. Br Med J. 1974 Jun 29;2(5921):694–698. doi: 10.1136/bmj.2.5921.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Service F. J., Nelson R. L., Rubenstein A. H., Go V. L. Direct effect of insulin on secretion of insulin, glucagon, gastric inhibitory polypeptide, and gastrin during maintenance of normoglycemia. J Clin Endocrinol Metab. 1978 Sep;47(3):488–493. doi: 10.1210/jcem-47-3-488. [DOI] [PubMed] [Google Scholar]
- Shulman G. I., Liljenquist J. E., Williams P. E., Lacy W. W., Cherrington A. D. Glucose disposal during insulinopenia in somatostatin-treated dogs. The roles of glucose and glucagon. J Clin Invest. 1978 Aug;62(2):487–491. doi: 10.1172/JCI109150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALAAS O., WALAAS E. Effect of epinephrine on rat diaphragm. J Biol Chem. 1950 Dec;187(2):769–776. [PubMed] [Google Scholar]


