Abstract
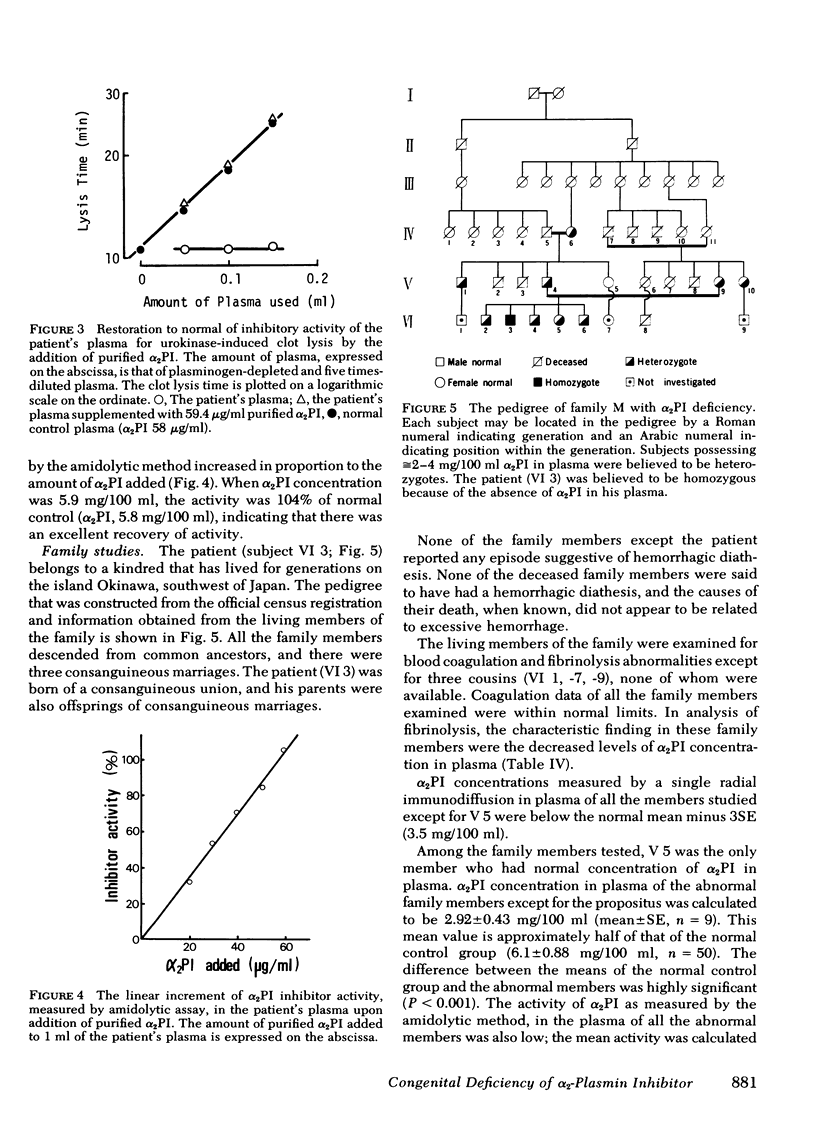
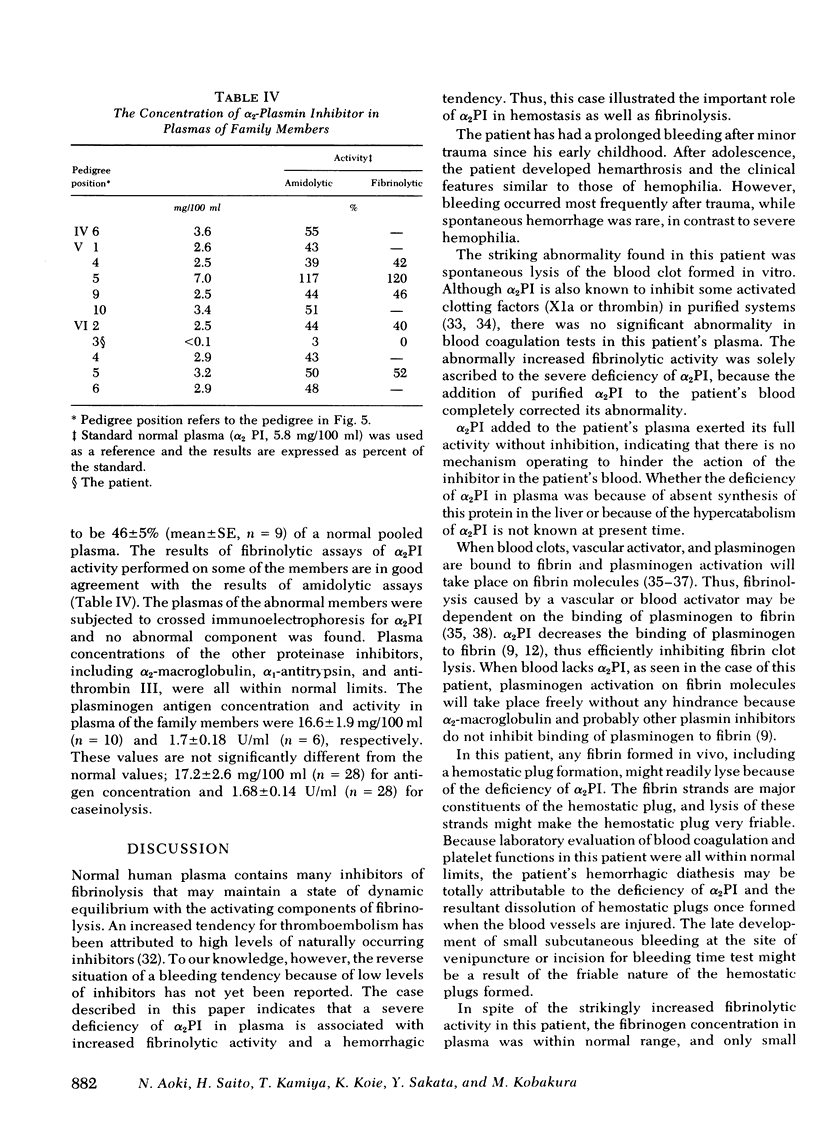
α2-Plasmin inhibitor (α2PI) is a recently characterized, fast-reacting plasmin inhibitor in human plasma that appears to play an important role in regulation of in vivo fibrinolysis. We report here a case of complete deficiency of α2PI in man. The patient, a 25-yr-old Japanese man, had a life-long severe bleeding tendency (hemarthrosis and excessive bleeding after trauma). The following tests were within normal limits: platelet count, bleeding time, thrombin time, prothrombin time, partial thromboplastin time, titers of known clotting factors, platelet glass bead retention, Factor VIII-related antigen, platelet aggregation by ADP, collagen and ristocetin, and clot retraction. Routine liver function tests were also normal. The only abnormal finding was that whole blood clot lysis was extemely rapid and was complete in 4-8 h. The concentration of plasma protease inhibitors, including α2-macro-globulin, antithrombin III, α1-antitrypsin, and C1̄INH, were all normal. The concentration of α2-PI in the patient's plasma, assayed by immunological methods, was <0.1 mg/100 ml (normal concentration, 6.1±0.88 mg/100 ml [mean±SE]) and functional assays showed a complete deficiency of α2PI. Addition of purified α2PI to the patient's whole blood completely corrected the accelerated fibrinolysis. The patient's parents, four siblings, and four other members of this family were asymptomatic, but the titers of α2PI in their plasmas were ≅50% of normal pooled plasma. There were three consanguineous marriages in this family, and the α2PI deficiency appears to have been inherited as an autosomal recessive trait. We speculate that α2PI deficiency in this patient has led to uninhibited in vivo fibrinolysis that probably causes the severe hemorrhagic tendency. Thus, this study indicates the important role of α2PI in hemostasis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allain J. P., Cooper H. A., Wagner R. H., Brinkhous K. M. Platelets fixed with paraformaldehyde: a new reagent for assay of von Willebrand factor and platelet aggregating factor. J Lab Clin Med. 1975 Feb;85(2):318–328. [PubMed] [Google Scholar]
- Aoki N., Moroi M., Matsuda M., Tachiya K. The behavior of alpha2-plasmin inhibitor in fibrinolytic states. J Clin Invest. 1977 Aug;60(2):361–369. doi: 10.1172/JCI108784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki N., Moroi M., Sakata Y., Yoshida N., Matsuda M. Abnormal plasminogen. A hereditary molecular abnormality found in a patient with recurrent thrombosis. J Clin Invest. 1978 May;61(5):1186–1195. doi: 10.1172/JCI109034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki N., Moroi M., Tachiya K. Effects of alpha2-plasmin inhibitor on fibrin clot lysis. Its comparison with alpha2-macroglobulin. Thromb Haemost. 1978 Feb 28;39(1):22–31. [PubMed] [Google Scholar]
- Aoki N., Yamanaka T. The alpha2-plasmin inhibitor levels in liver diseases. Clin Chim Acta. 1978 Mar 1;84(1-2):99–105. doi: 10.1016/0009-8981(78)90481-3. [DOI] [PubMed] [Google Scholar]
- BORN G. V. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1962 Jun 9;194:927–929. doi: 10.1038/194927b0. [DOI] [PubMed] [Google Scholar]
- Bohn H., Haupt H. Eine quantitative Bestimmung von Faktor 13 mit Anti-Faktor-13-Serum. Thromb Diath Haemorrh. 1968 Jul 31;19(3):309–315. [PubMed] [Google Scholar]
- Collen D. Identification and some properties of a new fast-reacting plasmin inhibitor in human plasma. Eur J Biochem. 1976 Oct 1;69(1):209–216. doi: 10.1111/j.1432-1033.1976.tb10875.x. [DOI] [PubMed] [Google Scholar]
- Gurewich V., Hyde E., Lipinski B. The resistance of fibrinogen and soluble fibrin monomer in blood to degradation by a potent plasminogen activator derived from cadaver limbs. Blood. 1975 Oct;46(4):555–565. [PubMed] [Google Scholar]
- Harpel P. C. Plasmin inhibitor interactions. The effectiveness of alpha2-plasmin inhibitor in the presence of alpha2-macroglobulin. J Exp Med. 1977 Oct 1;146(4):1033–1040. doi: 10.1084/jem.146.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highsmith R. F., Weirich C. J., Burnett C. J. Protease inhibitors and human plasmin: interaction in a whole plasma system. Biochem Biophys Res Commun. 1977 Dec 7;79(3):648–656. doi: 10.1016/0006-291x(77)91161-5. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Matsuda M., Yoshida N., Aoki N., Wakabayashi K. Distribution of cold-insoluble globulin in plasma and tissues. Ann N Y Acad Sci. 1978 Jun 20;312:74–92. doi: 10.1111/j.1749-6632.1978.tb16794.x. [DOI] [PubMed] [Google Scholar]
- Mattler L. E., Bang N. U. Serine protease specificity for peptide chromogenic substrates. Thromb Haemost. 1977 Dec 15;38(4):776–792. [PubMed] [Google Scholar]
- Merskey C., Kleiner G. J., Johnson A. J. Quantitative estimation of split products of fibrinogen in human serum, relation to diagnosis and treatment. Blood. 1966 Jul;28(1):1–18. [PubMed] [Google Scholar]
- Moroi M., Aoki N. Inhibition of plasminogen binding to fibrin by alpha2-plasmin inhibitor. Thromb Res. 1977 Jun;10(6):851–856. doi: 10.1016/0049-3848(77)90142-6. [DOI] [PubMed] [Google Scholar]
- Moroi M., Aoki N. Inhibition of proteases in coagulation, kinin-forming and complement systems by alpha2-plasmin inhibitor. J Biochem. 1977 Oct;82(4):969–972. doi: 10.1093/oxfordjournals.jbchem.a131801. [DOI] [PubMed] [Google Scholar]
- Moroi M., Aoki N. Isolation and characterization of alpha2-plasmin inhibitor from human plasma. A novel proteinase inhibitor which inhibits activator-induced clot lysis. J Biol Chem. 1976 Oct 10;251(19):5956–5965. [PubMed] [Google Scholar]
- Müllertz S., Clemmensen I. The primary inhibitor of plasmin in human plasma. Biochem J. 1976 Dec 1;159(3):545–553. doi: 10.1042/bj1590545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NILSSON I. M., KROOK H., STERNBY N. H., SODERBERG E., SODERSTROM N. Severe thrombotic disease in a young man with bone marrow and skeletal changes and with a high content of an inhibitor in the fibrinolytic system. Acta Med Scand. 1961 Mar;169:323–337. doi: 10.1111/j.0954-6820.1961.tb07838.x. [DOI] [PubMed] [Google Scholar]
- Naito K., Aoki N. Assay of alpha2-plasmin inhibitor activity by means of a plasmin specific tripeptide substrate. Thromb Res. 1978 Jun;12(6):1147–1156. doi: 10.1016/0049-3848(78)90069-5. [DOI] [PubMed] [Google Scholar]
- Ogston D., Bennett B., Mackie M. Properties of a partially purified preparation of a circulating plasminogen activator. Thromb Res. 1976 Mar;8(3):275–284. doi: 10.1016/0049-3848(76)90022-0. [DOI] [PubMed] [Google Scholar]
- PROCTOR R. R., RAPAPORT S. I. The partial thromboplastin time with kaolin. A simple screening test for first stage plasma clotting factor deficiencies. Am J Clin Pathol. 1961 Sep;36:212–219. doi: 10.1093/ajcp/36.3.212. [DOI] [PubMed] [Google Scholar]
- RATNOFF O. D., MENZIE C. A new method for the determination of fibrinogen in small samples of plasma. J Lab Clin Med. 1951 Feb;37(2):316–320. [PubMed] [Google Scholar]
- SALZMAN E. W. MEASUREMENT OF PLATELET ADHESIVENESS. A SIMPLE IN VITRO TECHNIQUE DEMONSTRATING AN ABNORMALITY IN VON WILLEBRAND'S DISEASE. J Lab Clin Med. 1963 Nov;62:724–735. [PubMed] [Google Scholar]
- Saito H., Goldsmith G. H., Jr Plasma thromboplastin antecedent (PTA, factor XI): a specific and sensitive radioimmunoassay. Blood. 1977 Sep;50(3):377–385. [PubMed] [Google Scholar]
- Saito H., Ratnoff O. D., Waldmann R., Abraham J. P. Fitzgerald Trait: Deficiency of a Hitherto Unrecognized Agent, Fitzgerald Factor, Participating in Surface-Mediated Reactions of Clotting, Fibrinolysis, Generation of Kinins, and the Property of Diluted Plasma Enhancing Vascular Permeability (PF/Dil). J Clin Invest. 1975 May;55(5):1082–1089. doi: 10.1172/JCI108009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teger-Nilsson A. C., Friberger P., Gyzander E. Determination of a new rapid plasmin inhibitor in human blood by means of a plasmin specific tripeptide substrate. Scand J Clin Lab Invest. 1977 Sep;37(5):403–409. [PubMed] [Google Scholar]
- Thorsen S. Differences in the binding to fibrin of native plasminogen and plasminogen modified by proteolytic degradation. Influence of omega-aminocarboxylic acids. Biochim Biophys Acta. 1975 May 30;393(1):55–65. doi: 10.1016/0005-2795(75)90216-0. [DOI] [PubMed] [Google Scholar]
- Thorsen S., Glas-Greenwalt P., Astrup T. Differences in the binding to fibrin of urokinase and tissue plasminogen activator. Thromb Diath Haemorrh. 1972 Aug 31;28(1):65–74. [PubMed] [Google Scholar]
- VON KAULLA K. N., SCHULTZ R. L. Methods for the evaluation of human fibrinolysis; studies with two combined technics. Am J Clin Pathol. 1958 Feb;29(2):104–112. doi: 10.1093/ajcp/29.2.104. [DOI] [PubMed] [Google Scholar]
- Zimmerman T. S., Ratnoff O. D., Powell A. E. Immunologic differentiation of classic hemophilia (factor 8 deficiency) and von Willebrand's dissase, with observations on combined deficiencies of antihemophilic factor and proaccelerin (factor V) and on an acquired circulating anticoagulant against antihemophilic factor. J Clin Invest. 1971 Jan;50(1):244–254. doi: 10.1172/JCI106480. [DOI] [PMC free article] [PubMed] [Google Scholar]




