Abstract
Patient: Female, 18
Final Diagnosis: Simultanous presentation of Kaposi Sarcoma and Lymphoma
Symptoms: —
Medication: —
Clinical Procedure: —
Specialty: Oncology
Objective
Rare disease
Background:
KSHV/HHV-8 is associated with Kaposi’s sarcoma (KS) as well as with a few categories of lymphoproliferative diseases, mostly occurring in patients with HIV infection/AIDS. Although the association between lymphomas and Kaposi’s sarcoma has been described, the simultaneous presence of the 2 entities within the same organ is rare and mainly associated with HIV/ AIDS.
Case Report:
We report a case of simultaneous occurrence of Kaposi‘s sarcoma and large B-cell lymphoma in the same lymph node in a 18-year-old African woman who was HIV-negative. We found concurrent infection with Kaposi’s sarcoma herpes virus (KSHV) and Epstein-Barr virus (EBV), confirmed by PCR amplification of DNA obtained from distinct tumor areas selected in the paraffin block.
Conclusions:
The possibility of occurrence of 2 lesions with distinct features in the same organ may be unexpected for pathologists performing fine-needle aspiration cytology (FNAC) evaluation but must be considered, even in HIV-negative individuals, despite its rare occurrence, as was demonstrated by this case.
Keywords: FNAC, Kaposi’s sarcoma, large B-cell lymphoma, HHV8, EBV, lymph node
Background
Kaposi’s sarcoma (KS) is a low-grade mesenchymal angioproliferative disorder occurring most commonly in human immunodeficiency virus (HIV)-infected patients. Lymph node involvement is a rare event in classical KS but occurs frequently in endemic (African) and epidemic (AIDS-related) KS. KS-associated herpes virus (KSHV), also known as human herpes virus type 8 (HHV8) was first identified in AIDS-related KS [1]. KSHV/HHV-8 is also associated with a few categories of lymphoproliferative diseases, mostly occurring in patients with HIV infection/AIDS [2–6].
We report a case of simultaneous presentation of KS and large B-cell lymphoma (LBCL) in the same lymph node in an HIV-negative patient with a concurrent infection with KSHV/HHV-8 and EBV.
Case Reports
The patient was an 18-year-old black woman with a history of weakness, weight loss, productive cough, and night fever for 3 months who was admitted to the Hospital Central de Maputo. She also described abdominal distension and left flank pain. On admission, the patient presented with these symptoms and was found to have moderate anemia, thrombocytopenia, hypoalbuminemia, peripheral generalized lymphadenopathy, and splenomegaly. Testing for Mycobacterium tuberculosis in sputum was negative. She was treated with broad-spectrum antibiotics. Ultrasonography confirmed grade IV homogenous splenomegaly and showed para-aortic lymphadenopathy and liver features consistent with hepatic steatosis.
The HIV test by was consistently negative for HIV-1 and -2 (serologic test with Determine HIV1/2, Uni-Gold HIV-1/2 and SD BioLine HIV 1/2; molecular test by PCR for HIV-1 was also performed and was negative, and viral load for HIV-1 was undetectable). CD4+ count performed during admission was 579/ ml blood. A clinical diagnosis of lymphoma was suspected and the case was subjected to FNAC.
During admission, the patient progressed to have hallucinations, headache, and nuchal rigidity. Her disease progressed to multiorgan failure and she died. No autopsy was performed.
Morphological Findings
Two FNAC were performed in the cervical lymph node. Aspirations were obtained using a 0.6-mm-diameter (23-gauge) needle attached to a 10-cc syringe held in a standard metal syringe holder. The material obtained was placed on standard glass slides, thinly smeared, and air-dried for May Grünwald Giemsa staining.
The cytological examination showed bloody smear disclosing a mixed population of isolated large and small lymphocytes. The large lymphocytes presented non-cleaved nuclei with prominent nucleoli and a rim of basophilic cytoplasm. Isolated and groups of overlapping spindle cells with spindle nuclei were also found scattered throughout the smears, sometimes in a cohesive cluster with radial arrangements and nuclear crush artifacts. Because of the presence of 2 distinct patterns, FNAC was repeated. This showed the same results, confirming the cytological picture. On the basis of these cytological features, a diagnosis of lymphoproliferative process compatible with lymphoma with possible simultaneous fusocellular tumor was made and excisional biopsy was done.
A tissue sample obtained by excisional biopsy from the same cervical lymph node as previously aspirated. It was fixed in 10% formalin, embedded in paraffin, cut into 4 μm sections and hematoxylin and eosin stained.
The histological examination showed complete distortion of the lymph node architecture (Figure 1A), mostly by a lymphoproliferative lesion composed of a diffuse population of large lymphoid cells displaying vesicular nuclei with prominent nucleoli and high mitotic index, intermingled with abundant small lymphocytes and histiocytes (Figure 1B and 1D); the remaining lymph node was occupied by a vascular lesion composed of a discrete proliferation of slit-like vessels, filled with red blood cells, surrounded by slightly atypical spindle cells with 12 mitoses/10 high-power fields (Figure 1C and 1D), focal hyaline globules, and hemosiderin deposits.
Figure 1.
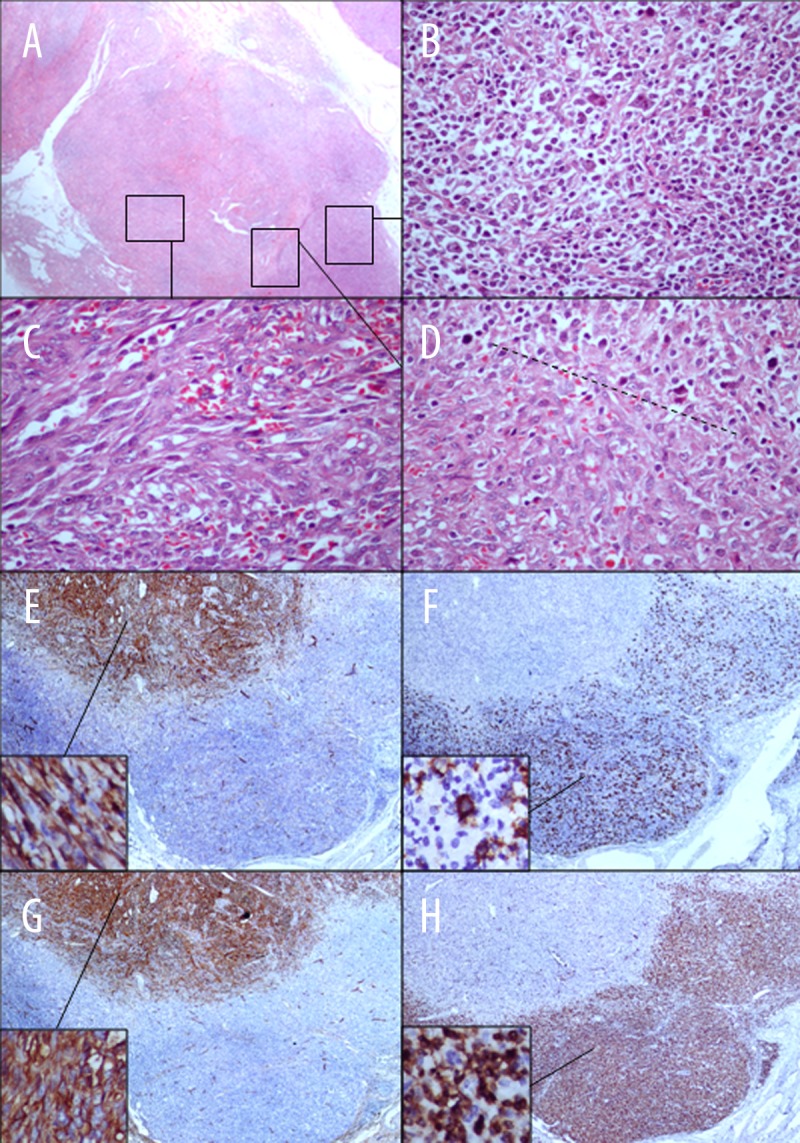
Histological features of a single lymph node consistent with synchronous T cell rich B cell lymphoma and Kaposi sarcoma. The lymph node architecture (A – HE, 40×) is distorted by a diffuse proliferation of large lymphoid cells intermingled with small lymphocytes and histiocytes (B and D – HE, 40×) co-existent with a proliferation of slit-like vessels, filled with red blood cells, surrounded by slightly atypical spindle cells with 12 mitoses/10 high-power fields (C and D – HE, 40×). The immunohistochemical study of the vascular lesion showed expression of CD31 and CD34 in the spindle cells (E and F – HE, 4× and 40×). In the lymphoproliferative lesion, the large lymphoid cells disclosed distinctive membrane CD20 positivity (G – HE, 4× and 40×) whereas the small lymphocytes expressed membrane CD3 positivity (H – HE, 4× and 40×).
Immunohistochemical analyses were performed by the avidin-biotin-peroxidase method with the Ventana Benchmark autostaining system on formalin-fixed, paraffin-embedded sections from the same sample. Stains were performed with the following antibodies: CD31, CD34, CD20, CD3, CD15, and CD30.
The immunohistochemical study of the vascular lesion showed expression of CD31 and CD34 in the atypical spindle cells (Figure 1E and 1F). In the lymphoproliferative lesion, the large lymphoid cells had clear-cut membrane CD20 positivity (Figure 1G), whereas the small lymphocytes expressed membrane CD3 positivity (Figure 1H). CD15 and CD30 were not expressed by any of the lymphoid cell types.
The morphological and immunophenotypical features of the aforementioned lesions are consistent with the co-existence of a T cell-rich LBCL and a KS lesion in the same lymph node.
We tested for DNA from KSHV/HHV-8 and EBV by PCR amplification of DNA obtained from areas of distinct types selected in the paraffin block. These yielded KSHV/HHV-8 and EBV DNA in the sampled lymph node in the areas of KS and LBCL, respectively.
Discussion
We report a case of simultaneous presentation of KS and LBCL in the same lymph node in an HIV-negative patient, with a concurrent infection of KSHV/HHV-8 and EBV. KS is a low-grade angioproliferative disorder and occurs mainly in HIV-infected patients. Lymph node involvement is a rare event in classical KS but occurs frequently in endemic (African) and epidemic (AIDS-related) KS. The lymphadenopathic form of KS is more frequently observed in children and adolescents, independent of HIV status [7].
The association between lymphomas (Hodgkin and non-Hodgkin) and KS has been described [1,8,9]. However, the simultaneous presence of the 2 pathologic entities within the same organ is very rare. Few cases have been described in the literature. Carbone and Volpe [10] described a case of Hodgkin lymphoma (HL) occurring simultaneously with KS in a lymph node based on histology, and later confirmed by immunohystochemistry [11]. Another 4 cases of HL associated with KS occurring in the same lymph node were described, most of them in HIV-positive patients [8]. Licci et al. [9] described 2 cases of concurrent non-Hodgkin lymphoma (NHL) and KS in the same lymph node, one of which was an LBCL in an AIDS patient. The second one was, as in our case, in an HIV-negative patient and displayed the same type of lymphoma that we also described in the present case, namely a T cell-rich LBCL. Concurrent KS and MALT lymphoma has also been described [12]. More recently, a case report of simultaneous occurrence of KS and non-Hodgkin extracavitary lymphoma in the same lymph node was described in a HIV-positive patient [13].
We demonstrated in our case the presence of KSHV/HHV-8 and EBV infection, as reported in other cases [1,4,5,9,13]. Apart from the association of KSHV/HHV-8 with KS, this virus also associates with 3 distinct lymphoproliferative diseases, mainly in HIV infection/AIDS patients: primary effusion lymphoma [2], multi-centric Castleman’s disease [14], and multicentric Castleman’s disease – associated plasmablastic lymphoma [6]. KSHV/HHV-8 was also identified in HIV-negative patients with a rare entity known as germinotropic lymphoproliferative disorder [3], and in extracavitary solid disuse large cell lymphomas with PEL-like morphology [5,15]. Subsequently, B-lineage lymphomas with a post-germinal-center immunophenotype were also described in association with KSHV/HHV-8 [4].
The occurrence of 2 lesions with distinct features in the same organ may be unexpected for pathologists performing FNAC evaluation, but must be considered despite its rare occurrence. In the present case, the diagnosis of KS and NHL was confirmed by histology and by immunohistochemistry, which assisted in the classification of (EBV-infection related) T cell-rich LBCL and (KSHV/HHV-8 infection-related) KS in the same lymph node of an HIV-negative patient.
Conclusions
We described a case of an HIV-negative patient presenting with concurrent KS and a T cell-rich LBCL in the same lymph node, associated with the presence of KSHV/HHV-8 and EBV infection.
Acknowledgments
We are indebted to Fernando Schmitt MD, MSc, PhD, Institute of Molecular Pathology and Immunology of the University of Porto (IPATIMUP).
Grant sponsor
Fundação Calouste Gulbenkian – Projecto de Cooperação Moçambique – IPATIMUP: “Projecto de Reforço das Capacidades Institucionais do Serviço de Anatomia Patológica do Hospital Central de Maputo”.
The work of CC was supported by Grant Number R24TW008908 from the Fogarty International Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Fogarty International Center or the National Institutes of Health.” This award is supported by funds provided to the NIH and HRSA under the “Tom Lantos and Henry Hyde United States Leadership Against HIV/ AIDS, Tuberculosis, and Malaria Reauthorization Act of 2008,” Public Law 110-293, which is more commonly known as the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR). Co-funding is also provided by the NIH Office of Research on Women’s Health and the Office of AIDS Research.
References:
- 1.Carbone A. KSHV/HHV-8 associated Kaposi’s sarcoma in lymph nodes concurrent with Epstein-Barr virus associated Hodgkin lymphoma. J Clin Pathol. 2005;58:626–28. doi: 10.1136/jcp.2004.023465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cesarman E, Chang Y, Moore PS, et al. Kaposi’s sarcoma-associated herpes-virus-like DNA sequences in AIDS-related body cavity-based lymphomas. N Engl J Med. 1995;332:1186–91. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 3.Du M-Q, Diss TC, Liu H, et al. KSHV-and EBV-associated germinotropic lymphoproliferative disorder. Blood. 2002;100:3415–18. doi: 10.1182/blood-2002-02-0487. [DOI] [PubMed] [Google Scholar]
- 4.Ferry J, Sohani A, Longtine J, et al. HHV8-positive Hodgkin lymphoma-like large B-cell lymphoma and HHV8-positive intravascular large B-cell lymphoma. Mod Pathol. 2009;22:618–26. doi: 10.1038/modpathol.2009.36. [DOI] [PubMed] [Google Scholar]
- 5.Katano H, Suda T, Morishita Y, et al. Human herpesvirus 8-associated solid lymphomas that occur in AIDS patients take anaplastic large cell morphology. Mod Pathol. 2000;13:77–85. doi: 10.1038/modpathol.3880012. [DOI] [PubMed] [Google Scholar]
- 6.Oksenhendler E, Boulanger E, Galicier L, et al. High incidence of Kaposi sarcoma-associated herpesvirus-related non-Hodgkin lymphoma in patients with HIV infection and multicentric Castleman disease. Blood. 2002;99:2331–36. doi: 10.1182/blood.v99.7.2331. [DOI] [PubMed] [Google Scholar]
- 7.Parkin DM, Ferlay J, Hamdi-Cherif M, et al. Cancer in Africa: Epidemiology and Prevention. IARC Scientific Publications. 2003;153:286–91. [PubMed] [Google Scholar]
- 8.Ngan K-W, Kuo T-t. Simultaneous occurrence of Hodgkin’s Lymphoma and Kaposi’s sarcoma within the same lymph node of a non-AIDS patient. Int J Surg Pathol. 2006;14:85–88. doi: 10.1177/106689690601400117. [DOI] [PubMed] [Google Scholar]
- 9.Licci S, D’Antonio A, Boscaino A, et al. Non-Hodgkin lymphomas concurrent with HHV8-associatedd Kaposi’s sarcoma in the same lymph node in AIDS and non-AIDS patients. Acta Haematol. 2007;118:47–52. doi: 10.1159/000102587. [DOI] [PubMed] [Google Scholar]
- 10.Carbone A, Volpe R. Kaposi’s sarcoma in lymph nodes concurrent with Hodgkin’s disease. Am J Clin Pathol. 1983;80:228–30. doi: 10.1093/ajcp/80.2.228. [DOI] [PubMed] [Google Scholar]
- 11.Mitsuyasu RT, Colman MF, Sun NC. Simultaneous occurrence of Hodgkin’s disease and Kaposi’s sarcoma in a patient with the acquired immune deficiency syndrome. Am J Med. 1986;80:954–58. doi: 10.1016/0002-9343(86)90644-3. [DOI] [PubMed] [Google Scholar]
- 12.Mirabile A, Devizzi L, Gianni AM, et al. MALT lymphoma and Kaposi sarcoma in an HIV-negative patient. Am J Hemato. 2010;85:815–17. doi: 10.1002/ajh.21802. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, Yang KY, Hong T, et al. Kaposi sarcoma-associated herpesvirus (human herpesvirus type 8)-associated extracavitary lymphoma: Report of a case in an HIV-positive patient with simultaneous kaposi sarcoma and a review of the literature. Acta Haematol. 2010;123:237–41. doi: 10.1159/000314347. [DOI] [PubMed] [Google Scholar]
- 14.Soulier J, Grollet L, Oksenhendler E, et al. Kaposi’s sarcoma – associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood. 1995;86:1276–80. [PubMed] [Google Scholar]
- 15.Carbone A, Gloghini A, Vaccher E, et al. Kaposi’s sarcoma-associated herpesvirus/human herpesvirus type 8-positive solid lymphomas. A tissue-based variant of primary effusion lymphoma. J Mol Diagn. 2005;7:17–27. doi: 10.1016/S1525-1578(10)60004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


