Abstract
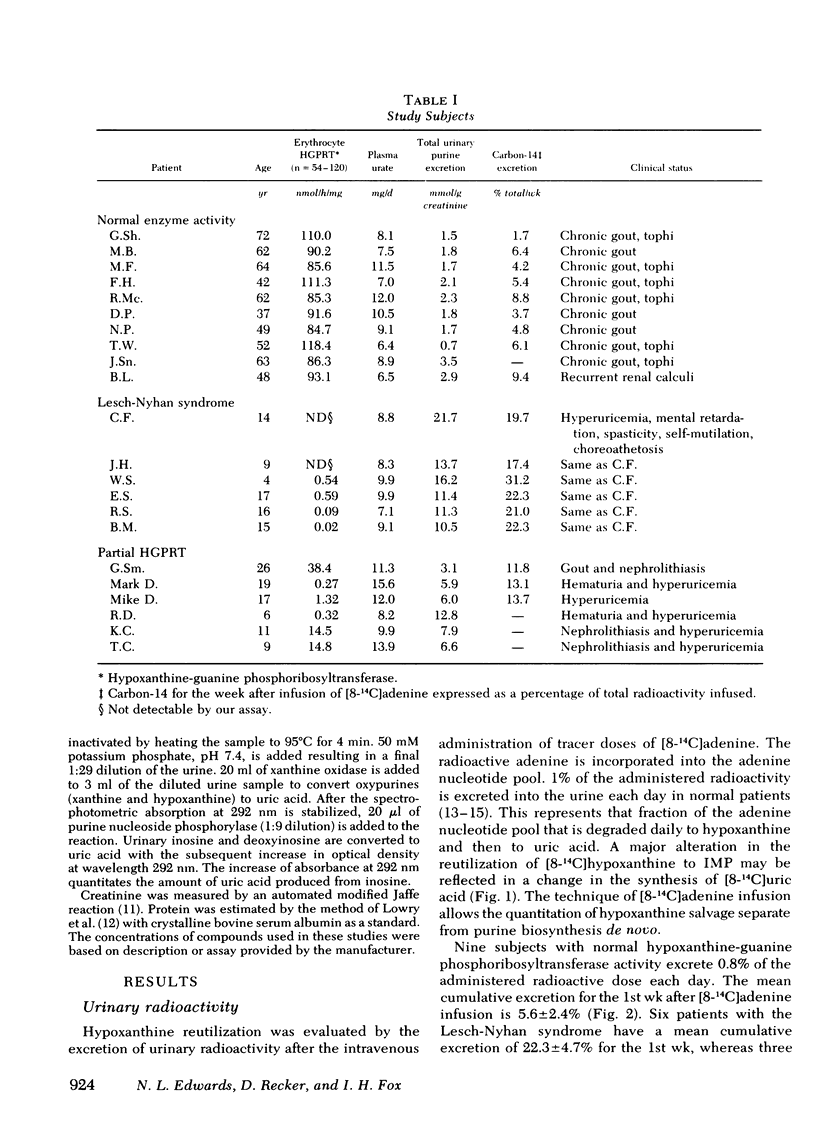
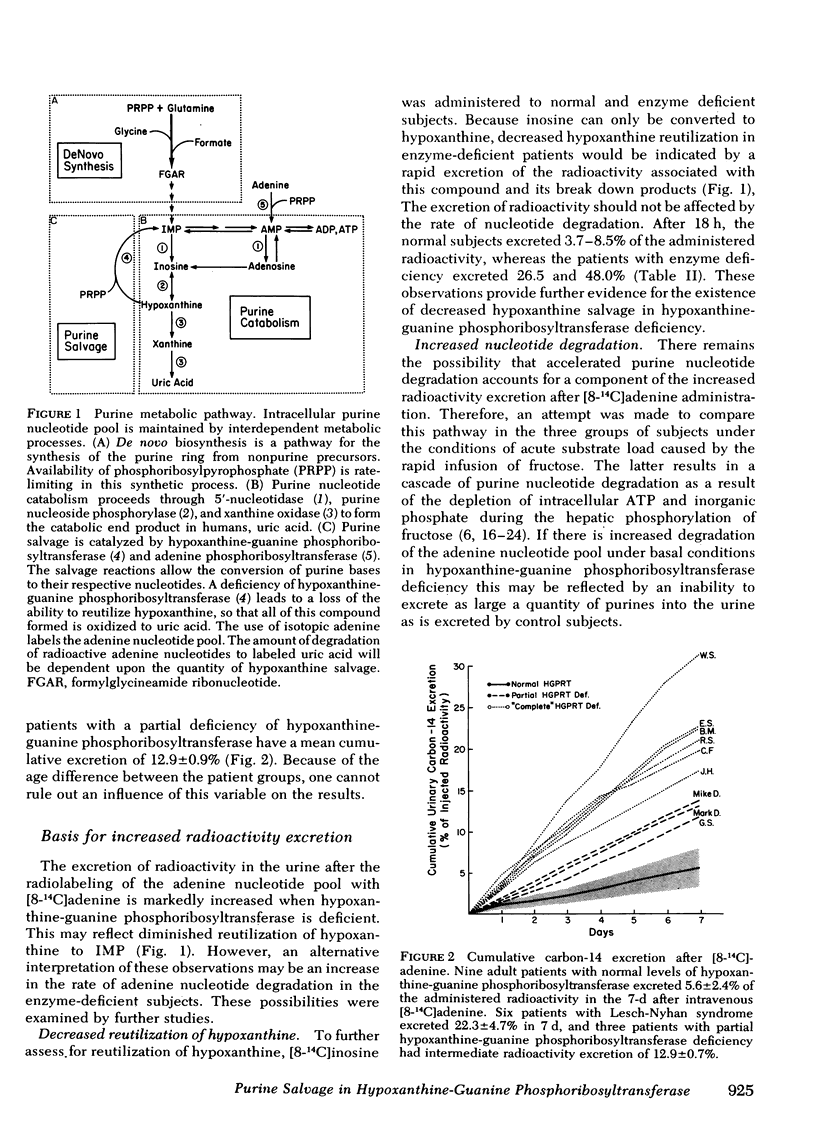
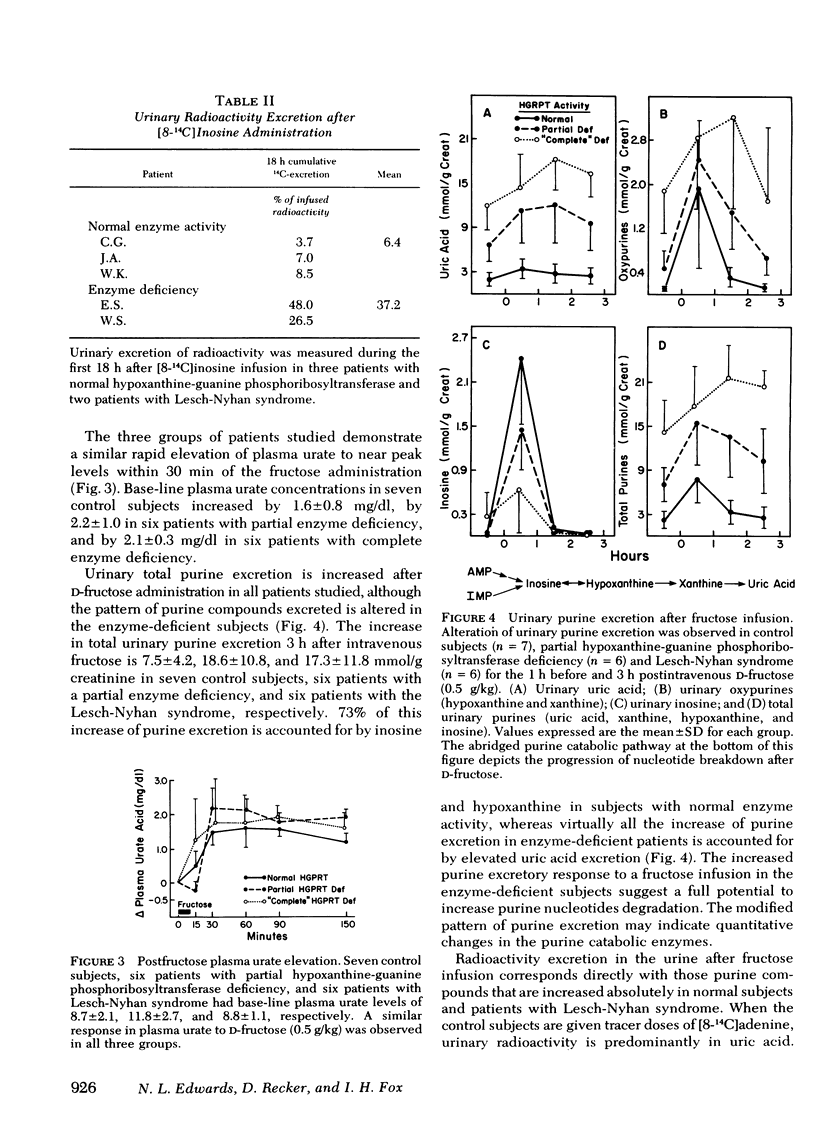
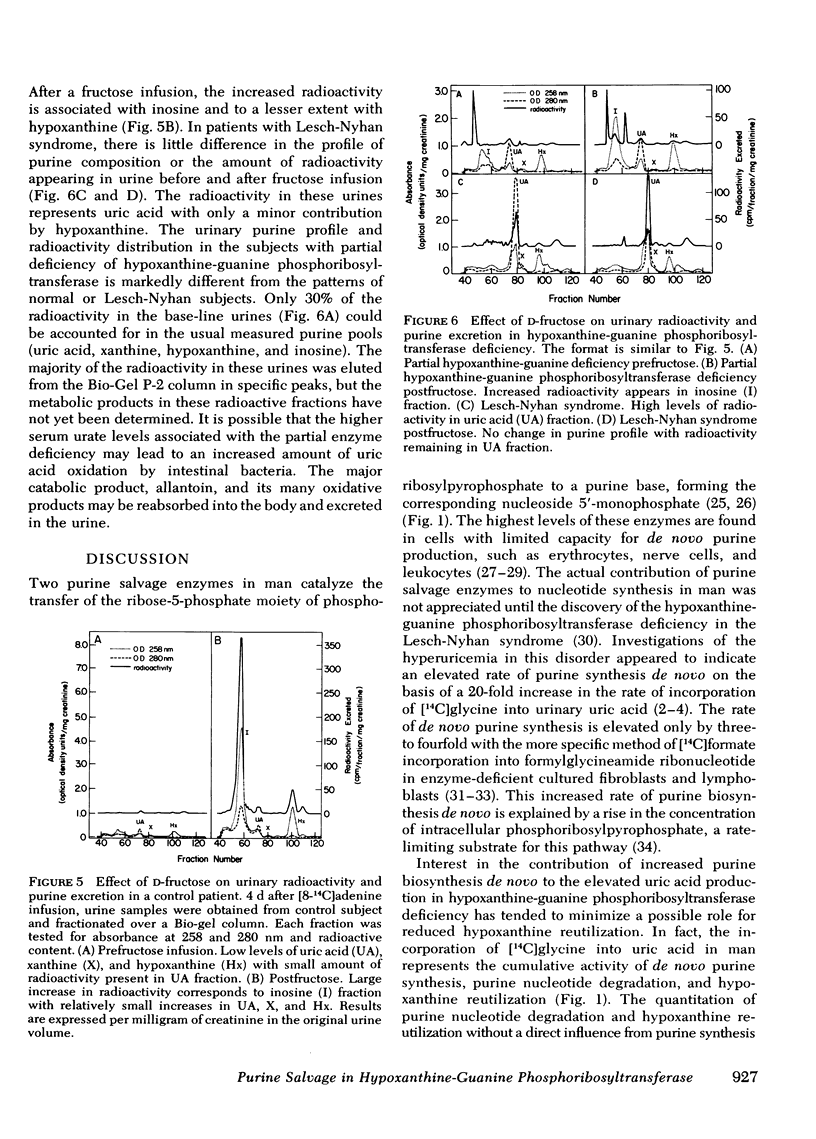
The contribution of reduced purine salvage to the hyperuricemia associated with hypoxanthine-guanine phosphoribosyltransferase deficiency was measured by the intravenous administration of tracer doses of [8-14C]adenine to nine patients with normal enzyme activity, three patients with a partial deficiency of hypoxanthine-guanine phosphoribosyltransferase, and six patients with the Lesch-Nyhan syndrome. The mean cumulative excretion of radioactivity 7 d after the adenine administration is 5.6±2.4, 12.9±0.9, and 22.3±4.7% of infused radioactivity for control subjects, partial hypoxanthine-guanine phosphoribosyltransferase-deficient subjects, and Lesch-Nyhan patients, respectively. To assess relative rates of nucleotide degradation in control and hypoxanthine-guanine phosphoribosyltransferase-deficient patients two separate studies were employed. With [8-14C]inosine administration, three control subjects excreted 3.7-8.5% and two enzyme-deficient patients excreted 26.5-48.0% of the injected radioactivity in 18 h. The capacity of the nucleotide catabolic pathway to accelerate in response to d-fructose was evaluated in control and enzyme-deficient patients. The normal metabolic response to intravenous fructose is a 7.5±4.2-mmol/g creatinine increase in total urinary purines during the 3-h after the infusion. The partial hypoxanthine-guanine phosphoribosyltransferase-deficient subjects and Lesch-Nyhan patients show increases of 18.6±10.8 and 17.3±11.8 mmol/g creatinine, respectively. Of the observed rise in purine exretion in control subjects, 40% occurs from inosine excretion and 32% occurs from oxypurine excretion. The rise in total purine excretion with Lesch-Nyhan syndrome is almost entirely accounted for by an elevated uric acid excretion. Increases in urine radioactivity after fructose infusion are distributed in those purines that are excreted in elevated quantities.
The observations suggest that purine salvage is a major contributor to increased purine excretion and that the purine catabolic pathway responds differently to an increased substrate load in hypoxanthine-guanine phosphoribosyltransferase deficiency. The purine salvage pathway is normally an important mechanism for the reutilization of hypoxanthine in man.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AYVAZIAN J. H., SKUPP S. THE STUDY OF PURINE UTILIZATION AND EXCRETION IN A XANTHINURIC MAN. J Clin Invest. 1965 Jul;44:1248–1260. doi: 10.1172/JCI105231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENNETT E. L., KRUECKEL B. J. Renewal of nucleotides and nucleic acids in C57 mice studied with adenine-4, 6-14C1. Biochim Biophys Acta. 1955 Aug;17(4):503–514. doi: 10.1016/0006-3002(55)90413-3. [DOI] [PubMed] [Google Scholar]
- BUZARD J., BISHOP C., TALBOTT J. H. The fate of uric acid in the normal and gouty human being. J Chronic Dis. 1955 Jul;2(1):42–49. doi: 10.1016/0021-9681(55)90106-5. [DOI] [PubMed] [Google Scholar]
- Bartlett G. R. Metabolism by man of intravenously administered adenine. Transfusion. 1977 Jul-Aug;17(4):367–373. doi: 10.1046/j.1537-2995.1977.17477216865.x. [DOI] [PubMed] [Google Scholar]
- Bartlett G. R. Metabolism by the rabbit of intravenously administered adenine. Transfusion. 1977 Jul-Aug;17(4):351–357. doi: 10.1046/j.1537-2995.1977.17477216863.x. [DOI] [PubMed] [Google Scholar]
- Bode J. C., Zelder O., Rumpelt H. J., Wittkamp U. Depletion of liver adenosine phosphates and metabolic effects of intravenous infusion of fructose or sorbitol in man and in the rat. Eur J Clin Invest. 1973 Sep;3(5):436–441. doi: 10.1111/j.1365-2362.1973.tb02211.x. [DOI] [PubMed] [Google Scholar]
- Brenton D. P., Astrin K. H., Cruikshank M. K., Seegmiller J. E. Measurement of free nucleotides in cultured human lymphoid cells using high pressure liquid chromatography. Biochem Med. 1977 Jun;17(3):231–247. doi: 10.1016/0006-2944(77)90029-1. [DOI] [PubMed] [Google Scholar]
- Burch H. B., Lowry O. H., Meinhardt L., Max P., Jr, Chyu K. Effect of fructose, dihydroxyacetone, glycerol, and glucose on metabolites and related compounds in liver and kidney. J Biol Chem. 1970 Apr 25;245(8):2092–2102. [PubMed] [Google Scholar]
- Carcassi A. Xanthine oxidase activity in a gouty patient with partial deficiency of HGPRT. Adv Exp Med Biol. 1977;76A:346–350. doi: 10.1007/978-1-4613-4223-6_43. [DOI] [PubMed] [Google Scholar]
- Edwards N. L., Gelfand E. W., Biggar D., Fox I. H. Partial deficiency of purine nucleoside phosphorylase: studies of purine and pyrimidine metabolism. J Lab Clin Med. 1978 May;91(5):736–749. [PubMed] [Google Scholar]
- Fontenelle L. J., Henderson J. F. An enzymatic basis for the inability of erythrocytes to synthesize purine ribonucleotides de novo. Biochim Biophys Acta. 1969 Feb 18;177(1):175–176. doi: 10.1016/0304-4165(69)90085-3. [DOI] [PubMed] [Google Scholar]
- Fox I. H., Kelley W. N. Phosphoribosylpyrophosphate in man: biochemical and clinical significance. Ann Intern Med. 1971 Mar;74(3):424–433. doi: 10.7326/0003-4819-74-3-424. [DOI] [PubMed] [Google Scholar]
- Fox I. H., Kelley W. N. Studies on the mechanism of fructose-induced hyperuricemia in man. Metabolism. 1972 Aug;21(8):713–721. doi: 10.1016/0026-0495(72)90120-5. [DOI] [PubMed] [Google Scholar]
- GOLDWASSER E. The enzymic conversion of adenine to adenosine phosphates. Biochim Biophys Acta. 1954 Mar;13(3):341–346. doi: 10.1016/0006-3002(54)90339-x. [DOI] [PubMed] [Google Scholar]
- Hershfield M. S., Spector E. B., Seegmiller J. E. Purine synthesis and excretion in mutants of the WI-L2 human lymphoblastoid line deficient in adenosine kinase (AK) and adenine phosphoribosyltransferase (APRT). Adv Exp Med Biol. 1977;76A:303–313. doi: 10.1007/978-1-4613-4223-6_38. [DOI] [PubMed] [Google Scholar]
- Kelley W. N., Greene M. L., Rosenbloom F. M., Henderson J. F., Seegmiller J. E. Hypoxanthine-guanine phosphoribosyltransferase deficiency in gout. Ann Intern Med. 1969 Jan;70(1):155–206. doi: 10.7326/0003-4819-70-1-155. [DOI] [PubMed] [Google Scholar]
- Kelley W. N., Rosenbloom F. M., Henderson J. F., Seegmiller J. E. A specific enzyme defect in gout associated with overproduction of uric acid. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1735–1739. doi: 10.1073/pnas.57.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley W. N., Rosenbloom F. M., Seegmiller J. E. The effects of azathioprine (imuran) on purine synthesis in clinical disorders of purine metabolism. J Clin Invest. 1967 Sep;46(9):1518–1529. doi: 10.1172/JCI105643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinenberg J. R., Goldfinger S., Bradley K. H., Seegmiller J. E. An enzymatic spectrophotometric method for the determination of xanthine and hypoxanthine. Clin Chem. 1967 Oct;13(10):834–846. [PubMed] [Google Scholar]
- LAJTHA L. G., VANE J. R. Dependence of bone marrow cells on the liver for purine supply. Nature. 1958 Jul 19;182(4629):191–192. doi: 10.1038/182191a0. [DOI] [PubMed] [Google Scholar]
- LESCH M., NYHAN W. L. A FAMILIAL DISORDER OF URIC ACID METABOLISM AND CENTRAL NERVOUS SYSTEM FUNCTION. Am J Med. 1964 Apr;36:561–570. doi: 10.1016/0002-9343(64)90104-4. [DOI] [PubMed] [Google Scholar]
- LIDDLE L., SEEGMILLER J. E., LASTER L. The enzymatic spectrophotometric method for determination of uric acid. J Lab Clin Med. 1959 Dec;54:903–913. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lever J. E., Nuki G., Seegmiller J. E. Expression of purine overproduction in a series of 8-azaguanine-resistant diploid human lymphoblast lines. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2679–2683. doi: 10.1073/pnas.71.7.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäenpä P. H., Raivio K. O., Kekomäki M. P. Liver adenine nucleotides: fructose-induced depletion and its effect on protein synthesis. Science. 1968 Sep 20;161(3847):1253–1254. doi: 10.1126/science.161.3847.1253. [DOI] [PubMed] [Google Scholar]
- Narins R. G., Weisberg J. S., Myers A. R. Effects of carbohydrates on uric acid metabolism. Metabolism. 1974 May;23(5):455–465. doi: 10.1016/0026-0495(74)90093-6. [DOI] [PubMed] [Google Scholar]
- Nuki G., Astrin K., Brenton D., Cruikshank M., Lever J., Seegmiller J. E. Purine and pyrimidine nucleotide concentrations in cells with decreased hypoxanthine-guanine-phosphoribosyltransferase (HGPRT) activity. Adv Exp Med Biol. 1977;76A:326–340. doi: 10.1007/978-1-4613-4223-6_41. [DOI] [PubMed] [Google Scholar]
- Perheentupa J., Raivio K. Fructose-induced hyperuricaemia. Lancet. 1967 Sep 9;2(7515):528–531. doi: 10.1016/s0140-6736(67)90494-1. [DOI] [PubMed] [Google Scholar]
- Raivio K. O., Becker 7. A., Meyer L. J., Greene M. L., Nuki G., Seegmiller J. E. Stimulation of human purine synthesis de novo by fructose infusion. Metabolism. 1975 Jul;24(7):861–869. doi: 10.1016/0026-0495(75)90133-x. [DOI] [PubMed] [Google Scholar]
- Rosenbloom F. M., Henderson J. F., Caldwell I. C., Kelley W. N., Seegmiller J. E. Biochemical bases of accelerated purine biosynthesis de novo in human fibroblasts lacking hypoxanthine-guanine phosphoribosyltransferase. J Biol Chem. 1968 Mar 25;243(6):1166–1173. [PubMed] [Google Scholar]
- SARTORELLI A. C., LEPAGE G. A. Metabolic effects of 6-thioguanine. II. Biosynthesis of nucleic acid purines in vivo and in vitro. Cancer Res. 1958 Dec;18(11):1329–1335. [PubMed] [Google Scholar]
- SORENSEN L. B. Degradation of uric acid in man. Metabolism. 1959 Sep;8:687–703. [PubMed] [Google Scholar]
- SORENSEN L. B. The pathogenesis of gout. Arch Intern Med. 1962 Apr;109:379–390. doi: 10.1001/archinte.1962.03620160005002. [DOI] [PubMed] [Google Scholar]
- Schwarzmeier J. D., Müller M. M., Marktl W. Studies on the effect of fructose and xylitol in the rat liver: 5'-nucleotidase, adenosine deaminase, de novo purine synthesis. Adv Exp Med Biol. 1977;76A:542–552. doi: 10.1007/978-1-4613-4223-6_68. [DOI] [PubMed] [Google Scholar]
- Seegmiller J. E., Klinenberg J. R., Miller J., Watts R. W. Suppression of glycine-15N incorporation into urinary uric acid by adenine-8-13C in normal and gouty subjects. J Clin Invest. 1968 May;47(5):1193–1203. doi: 10.1172/JCI105808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegmiller J. E., Rosenbloom F. M., Kelley W. N. Enzyme defect associated with a sex-linked human neurological disorder and excessive purine synthesis. Science. 1967 Mar 31;155(3770):1682–1684. doi: 10.1126/science.155.3770.1682. [DOI] [PubMed] [Google Scholar]
- Simkin P. A. Hexose infusions in Cebus monkeys: effects on uric acid metabolism. Metabolism. 1972 Nov;21(11):1029–1036. doi: 10.1016/0026-0495(72)90033-9. [DOI] [PubMed] [Google Scholar]
- WILLIAMS W. J., BUCHANAN J. M. Biosynthesis of the purines. V. Conversion of hypoxanthine to inosinic acid by liver enzymes. J Biol Chem. 1953 Aug;203(2):583–593. [PubMed] [Google Scholar]
- WYNGAARDEN J. B., SEEGMILLER J. E., LASTER L., BLAIR A. E. Utilization of hypoxanthine, adenine and 4-amino-5-imidazole-carboxamide for uric acid synthesis in man. Metabolism. 1959 Jul 1;8(4 Pt 1):455–464. [PubMed] [Google Scholar]
- Wood A. W., Becker M. A., Seegmiller J. E. Purine nucleotide synthesis in lymphoblasts cultured from normal subjects and a patient with Lesch-Nyhan syndrome. Biochem Genet. 1973 Jul;9(3):261–274. doi: 10.1007/BF00485739. [DOI] [PubMed] [Google Scholar]
- Woods H. F., Eggleston L. V., Krebs H. A. The cause of hepatic accumulation of fructose 1-phosphate on fructose loading. Biochem J. 1970 Sep;119(3):501–510. doi: 10.1042/bj1190501. [DOI] [PMC free article] [PubMed] [Google Scholar]



