Abstract
Patients with systemic lupus erythematosus (SLE) produce excessive amounts of autoantibodies. It has also been demonstrated in several systems that such patients have a relative loss of suppressor thymus-derived (T) cells that inhibit the immune response. This loss of suppressor cells has been suggested as one of the causes of the excessive production of antibodies in patients with SLE.
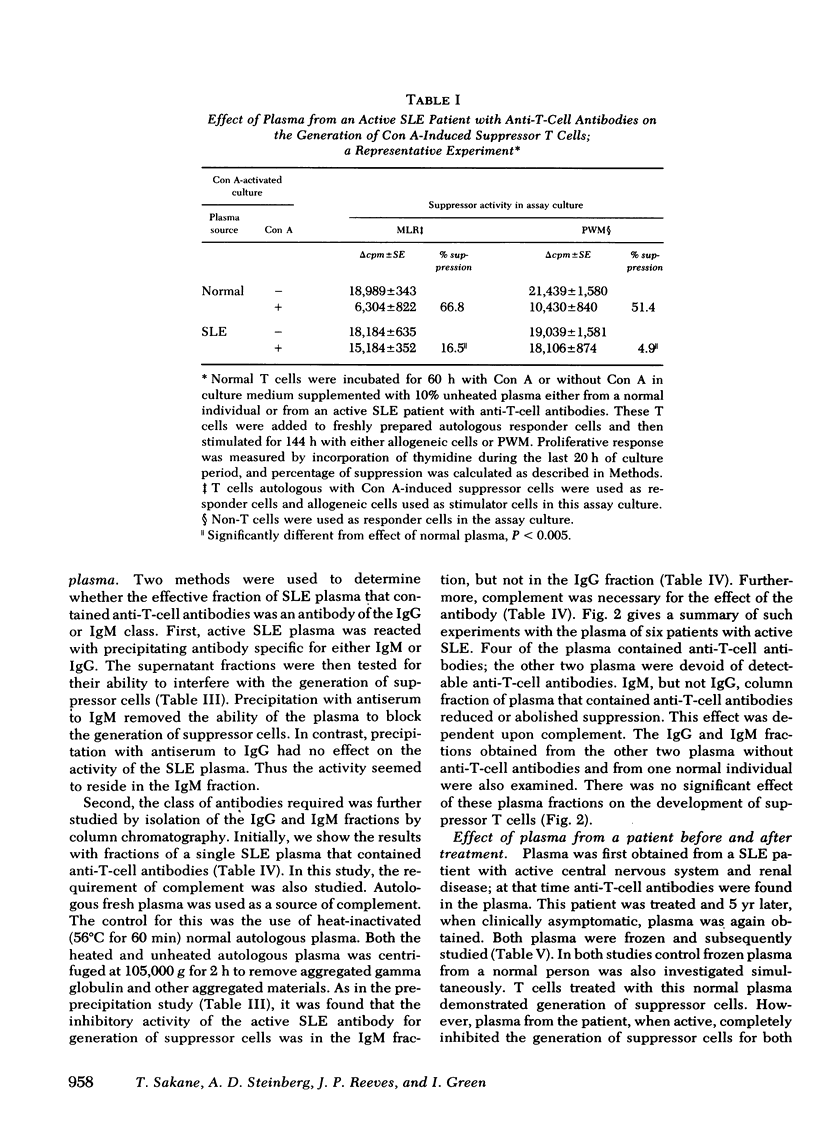
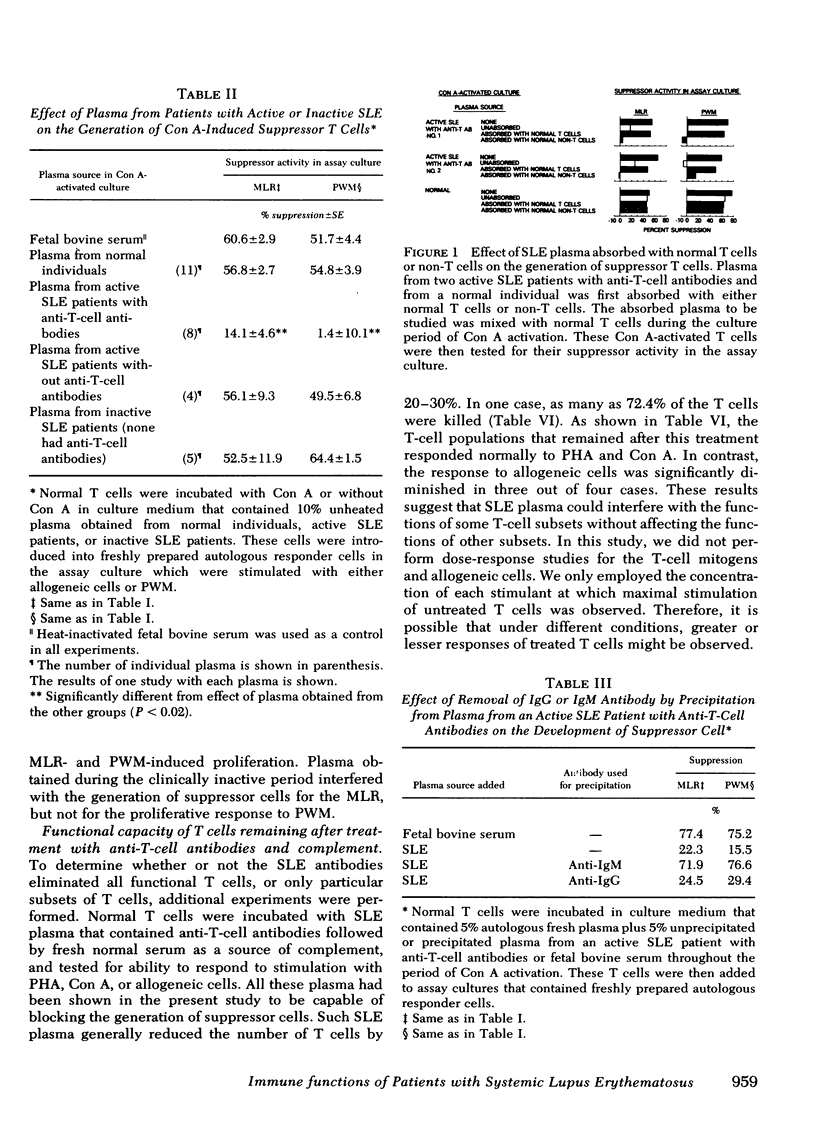
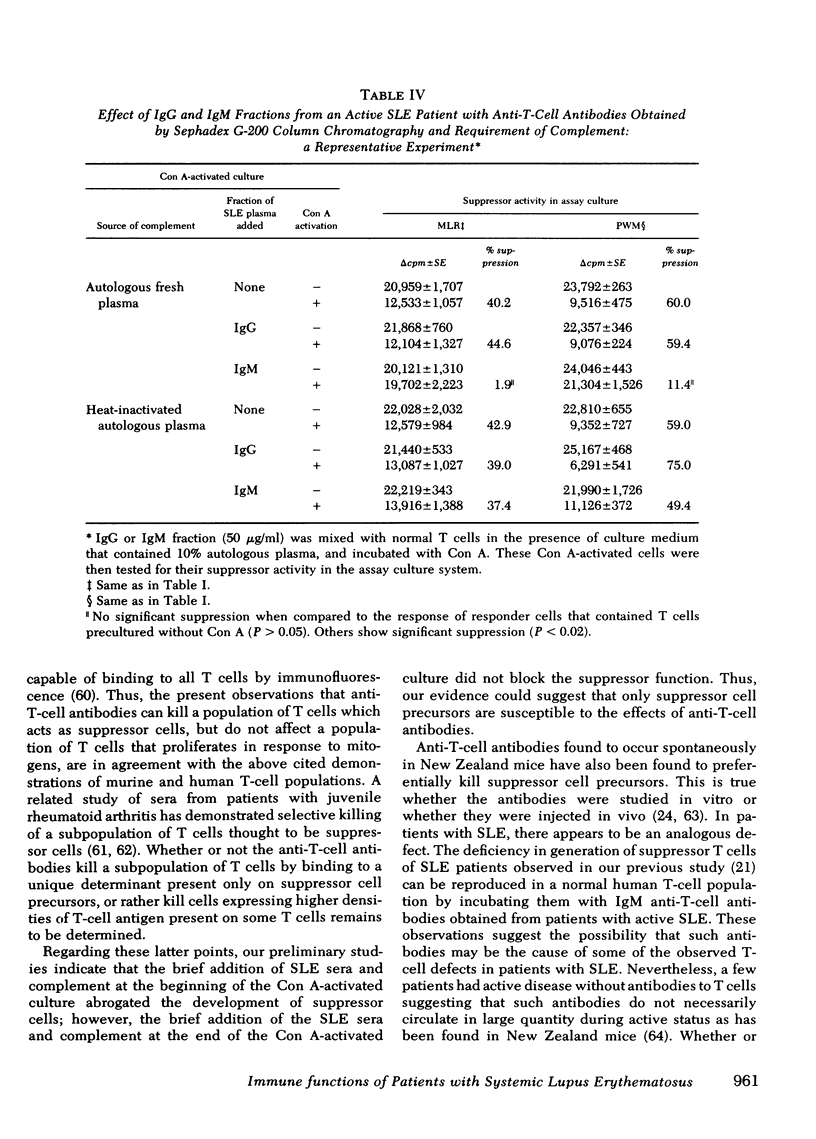
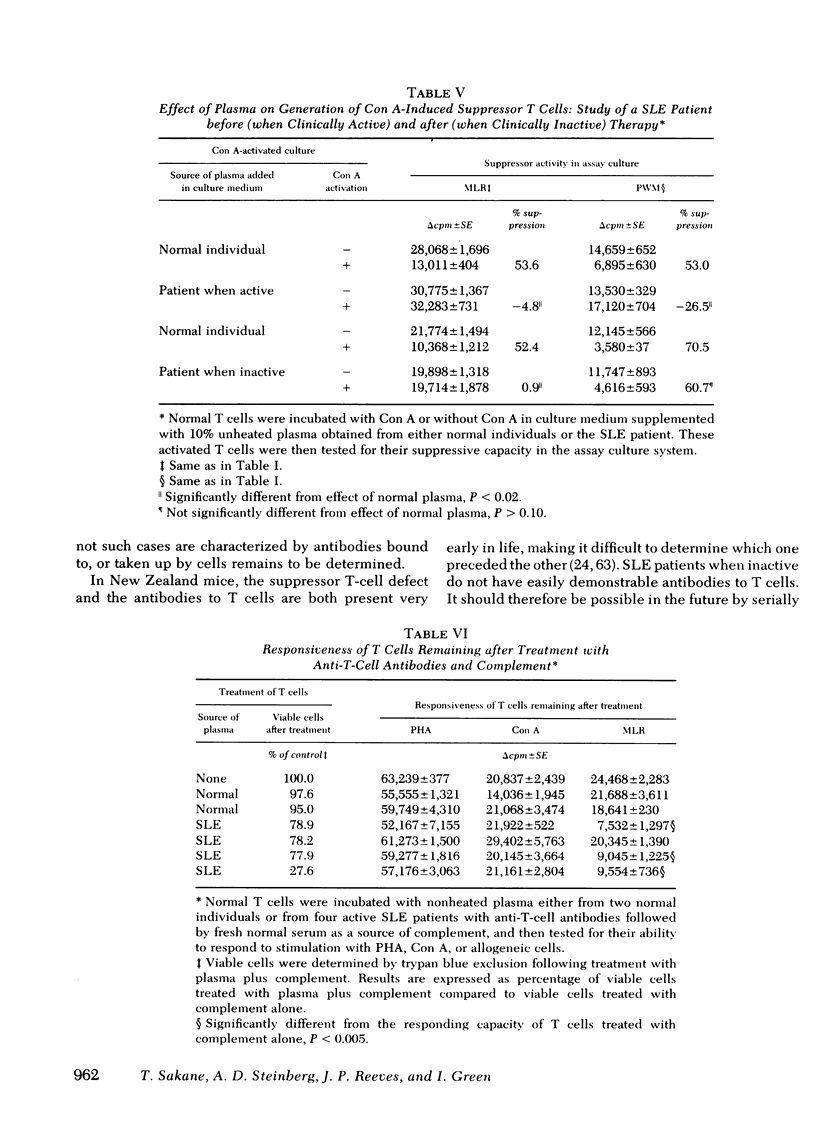
In the present report we have tested the hypothesis that anti-T-cell antibodies found in the plasma of some patients with SLE preferentially kill suppressor cells. T cells from normal individuals can be activated by concanavalin A to develop suppressor cell activity. We therefore cultured normal T cells together with concanavalin A in the presence of plasma or plasma fractions from patients with SLE. We found that plasma from patients with active SLE, in which anti-T-cell antibodies were present, inhibited the development of suppressor activity in such cultures. In contrast, plasma from other active patients and patients with inactive SLE, in which no anti-T-cell antibodies could be detected, failed to block the development of such suppressor activity. Absorption of the plasma that contained anti-T-cell antibodies with T cell, but not non-T cells, could eliminate the suppressor-inhibiting activity of the SLE plasma that contained anti-T-cell antibodies. The immunoglobulin (Ig)M, but not the IgG, fraction of the plasma was shown to possess the inhibiting property and complement was found to be necessary for the effect of such anti-T-cell antibodies. We also demonstrated that exposure of normal T cells to such anti-T-cell antibodies and complement did not affect another population of T cells that could proliferate in response to mitogens.
Thus, certain patients with SLE have in their plasma an antibody of the IgM class that can selectively eliminate a population of T cells capable of developing suppressor function. The loss of suppressor T cells in patients with SLE may be the result of the effects of such antibody activity in vivo.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdou N. I., Sagawa A., Pascual E., Hebert J., Sadeghee S. Suppressor T-cell abnormality in idiopathic systemic lupus erythematosus. Clin Immunol Immunopathol. 1976 Sep;6(2):192–199. doi: 10.1016/0090-1229(76)90110-0. [DOI] [PubMed] [Google Scholar]
- Bell D. A., Clark C., Blomgren S. E., Vaughan J. H. Anti DNA antibody production by lymphoid cells of NZB-W mice and human systemic lupus erythematosus (SLE). Clin Immunol Immunopathol. 1973 Apr;1(3):293–303. doi: 10.1016/0090-1229(73)90046-9. [DOI] [PubMed] [Google Scholar]
- Bluestein H. G., Zvaifler N. J. Brain-reactive lymphocytotoxic antibodies in the serum of patients with systemic lupus erythematosus. J Clin Invest. 1976 Feb;57(2):509–516. doi: 10.1172/JCI108303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresnihan B., Jasin H. E. Suppressor function of peripheral blood mononuclear cells in normal individuals and in patients with systemic lupus erythematosus. J Clin Invest. 1977 Jan;59(1):106–116. doi: 10.1172/JCI108607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budman D. R., Merchant E. B., Steinberg A. D., Doft B., Gershwin M. E., Lizzio E., Reeves J. P. Increased spontaneous activity of antibody-forming cells in the peripheral blood of patients with active SLE. Arthritis Rheum. 1977 Apr;20(3):829–833. doi: 10.1002/art.1780200312. [DOI] [PubMed] [Google Scholar]
- Cantor H., Shen F. W., Boyse E. A. Separation of helper T cells from suppressor T cells expressing different Ly components. II. Activation by antigen: after immunization, antigen-specific suppressor and helper activities are mediated by distinct T-cell subclasses. J Exp Med. 1976 Jun 1;143(6):1391–1340. doi: 10.1084/jem.143.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbarre F., Le Gô A., Kahan A. Hyperbasophilic immunoblasts in circulating blood in chronic inflammatory rheumatic and collagen diseases. Ann Rheum Dis. 1975 Oct;34(5):422–430. doi: 10.1136/ard.34.5.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbarre F., Pompidou A., Kahan A., Brouilhet H., Le Gô A., Amor B. Etude des lymphocytes du sang au cours de la maladie lupique. Pathol Biol (Paris) 1971 Apr;19(7):379–387. [PubMed] [Google Scholar]
- Engleman E. G., McDevitt H. O. A suppressor T cell of the mixed lymphocyte reaction specific for the HLA-D region in man. J Clin Invest. 1978 Mar;61(3):828–838. doi: 10.1172/JCI108997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. L., Breard J. M., Lazarus H., Schlossman S. F., Chess L. Detection, isolation, and functional characterization of two human T-cell subclasses bearing unique differentiation antigens. J Exp Med. 1977 Jan 1;145(1):221–233. doi: 10.1084/jem.145.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershwin M. E., Chused T. M., Steinberg A. D. Cytotoxic activity of anti-theta antibody found in the gamma m fraction. Transplantation. 1974 Oct;18(4):377–380. doi: 10.1097/00007890-197410000-00015. [DOI] [PubMed] [Google Scholar]
- Glinski W., Gershwin M. E., Budman D. R., Steinberg A. D. Study of lymphocyte subpopulations in normal humans and patients with systemic lupus erythematosus by fractionation of peripheral blood lymphocytes on a discontinuous Ficoll gradient. Clin Exp Immunol. 1976 Nov;26(2):228–238. [PMC free article] [PubMed] [Google Scholar]
- Glinski W., Gershwin M. E., Steinberg A. D. Fractionation of cells on a discontinuous Ficoll gradient. Study of subpopulations of human T cells using anti-T-cell antibodies from patients with systemic lupus erythematosus. J Clin Invest. 1976 Mar;57(3):604–614. doi: 10.1172/JCI108316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldblum R., Pillarisetty R., Talal N. Independent appearance of anti-thymocyte and anti-RNA antibodies in NZB/NZW F1 mice. Immunology. 1975 Apr;28(4):621–628. [PMC free article] [PubMed] [Google Scholar]
- HARVEY A. M., SHULMAN L. E., TUMULTY P. A., CONLEY C. L., SCHOENRICH E. H. Systemic lupus erythematosus: review of the literature and clinical analysis of 138 cases. Medicine (Baltimore) 1954 Dec;33(4):291–437. [PubMed] [Google Scholar]
- Hahn B. H., Bagby K. K., Osterland C. K. Abnormalities of delayed hypersensitivity in systemic lupus erythematosus. Am J Med. 1973 Jul;55(1):25–31. doi: 10.1016/0002-9343(73)90146-0. [DOI] [PubMed] [Google Scholar]
- Harbeck R. J., Bardana E. J., Kohler P. F., Carr R. I. DNA:anti-DNA complexes: their detection in systemic lupus erythematosus sera. J Clin Invest. 1973 Apr;52(4):789–795. doi: 10.1172/JCI107242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F., Fauci A. S. Activation of human B lymphocytes. III. Concanavalin A-induced generation of suppressor cells of the plaque-forming cell response of normal human B lymphocytes. J Immunol. 1977 Jun;118(6):2281–2287. [PubMed] [Google Scholar]
- Horowitz S., Borcherding W., Moorthy A. V., Chesney R., Schulte-Wisserman H., Hong R. Induction of suppressor T cells in systemic lupus erythematosus by thymosin and cultured thymic epithelium. Science. 1977 Sep 2;197(4307):999–1001. doi: 10.1126/science.302032. [DOI] [PubMed] [Google Scholar]
- Horwitz D. A., Cousar J. B. A relationship between impaired cellular immunity humoral suppression of lymphocyte function and severity of systemic lupus erythematosus. Am J Med. 1975 Jun;58(6):829–835. doi: 10.1016/0002-9343(75)90639-7. [DOI] [PubMed] [Google Scholar]
- Horwitz D. A. Impaired delayed hypersensitivity in systemic lupus erythematosus. Arthritis Rheum. 1972 Jul-Aug;15(4):353–359. doi: 10.1002/art.1780150406. [DOI] [PubMed] [Google Scholar]
- Jandinski J., Cantor H., Tadakuma T., Peavy D. L., Pierce C. W. Separation of helper T cells from suppressor T cells expressing different Ly components. I. Polyclonal activation: suppressor and helper activities are inherent properties of distinct T-cell subclasses. J Exp Med. 1976 Jun 1;143(6):1382–1390. doi: 10.1084/jem.143.6.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen L. W., Krakauer R. S., Steinberg A. D. Selective loss of suppressor cell function in New Zealand mice induced by NTA. J Immunol. 1977 Sep;119(3):830–830. [PubMed] [Google Scholar]
- Koffler D., Schur P. H., Kunkel H. G. Immunological studies concerning the nephritis of systemic lupus erythematosus. J Exp Med. 1967 Oct 1;126(4):607–624. doi: 10.1084/jem.126.4.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lies R. B., Messner R. P., Williams R. C., Jr Relative T-cell specificity of lymphocytotoxins from patients with systemic lupus erythematosus. Arthritis Rheum. 1973 May-Jun;16(3):369–375. doi: 10.1002/art.1780160312. [DOI] [PubMed] [Google Scholar]
- Lohrmann H. P., Novikovs L., Graw R. G., Jr Cellular interactions in the proliferative response of human T and B lymphocytes to phytomitogens and allogeneic lymphocytes. J Exp Med. 1974 Jun 1;139(6):1553–1567. doi: 10.1084/jem.139.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malavé I., Layrisse Z., Layrisse M. Dose-dependent hyporreactivity to phytohemagglutinin in systemic lupus erythematosus. Cell Immunol. 1975 Feb;15(2):231–236. doi: 10.1016/0008-8749(75)90001-5. [DOI] [PubMed] [Google Scholar]
- Messner R. P., Kennedy M. S., Jelinek J. G. Antilymphocyte antibodies in systemic lupus erythematosus. Effect on lymphocyte surface characteristics. Arthritis Rheum. 1975 May-Jun;18(3):201–206. doi: 10.1002/art.1780180302. [DOI] [PubMed] [Google Scholar]
- Messner R. P., Lindström F. D., Williams R. C., Jr Peripheral blood lymphocyte cell surface markers during the course of systemic lupus erythematosus. J Clin Invest. 1973 Dec;52(12):3046–3056. doi: 10.1172/JCI107503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal K. K., Rossen R. D., Sharp J. T., Lidsky M. D., Butler W. T. Lymphocyte cytotoxic antibodies in systemic lupus erythematosus. Nature. 1970 Mar 28;225(5239):1255–1256. doi: 10.1038/2251255a0. [DOI] [PubMed] [Google Scholar]
- Moretta L., Webb S. R., Grossi C. E., Lydyard P. M., Cooper M. D. Functional analysis of two human T-cell subpopulations: help and suppression of B-cell responses by T cells bearing receptors for IgM or IgG. J Exp Med. 1977 Jul 1;146(1):184–200. doi: 10.1084/jem.146.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto C., Abe T., Hara M., Homma M. In vitro TNP-specific antibody formation by peripheral lymphocytes from patients with systemic lupus erythematosus. Scand J Immunol. 1977;6(6-7):575–579. doi: 10.1111/j.1365-3083.1977.tb02135.x. [DOI] [PubMed] [Google Scholar]
- Nies K. M., Brown J. C., Dubois E. L., Quismorio F. P., Friou G. J., Terasaki P. I. Histocompatibility (HL-A) antigens and lymphocytotoxic antibodies in systemic lupus erythematosus (SLE). Arthritis Rheum. 1974 Jul-Aug;17(4):397–402. doi: 10.1002/art.1780170409. [DOI] [PubMed] [Google Scholar]
- Ooi B. S., Orlina A. R., Pesce A. J., Mendoza N., Masaitis L., Pollak V. E. Lymphocytotoxic antibodies in patients with systemic lupus erythematosus. Clin Exp Immunol. 1974 Jun;17(2):237–243. [PMC free article] [PubMed] [Google Scholar]
- Parker L. M., Chused T. M., Steinberg A. D. Immunofluorescence studies on thymocytotoxic antibody from New Zealand Black mice. J Immunol. 1974 Jan;112(1):285–292. [PubMed] [Google Scholar]
- Reinertsen J. L., Klippel J. H., Johnson A. H., Steinberg A. D., Decker J. L., Mann D. L. B-lymphocyte alloantigens associated with systemic lupus erythematosus. N Engl J Med. 1978 Sep 7;299(10):515–518. doi: 10.1056/NEJM197809072991004. [DOI] [PubMed] [Google Scholar]
- Rosenthal C. J., Franklin E. C. Depression of cellular-mediated immunity in systemic lupus erythematosus. relation to disease activity. Arthritis Rheum. 1975 May-Jun;18(3):207–217. doi: 10.1002/art.1780180303. [DOI] [PubMed] [Google Scholar]
- Sakane T., Green I. Human suppressor T cells induced by concanavalin A: suppressor T cells belong to distinctive T cell subclasses. J Immunol. 1977 Sep;119(3):1169–1178. [PubMed] [Google Scholar]
- Sakane T., Green I. Protein A from Staphylococcus aureus-a mitogen for human T lymphocytes and B lymphocytes but not L lymphocytes. J Immunol. 1978 Jan;120(1):302–311. [PubMed] [Google Scholar]
- Sakane T., Steinberg A. D., Green I. Failure of autologous mixed lymphocyte reactions between T and non-T cells in patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3464–3468. doi: 10.1073/pnas.75.7.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakane T., Steinberg A. D., Green I. Studies of immune functions of patients with systemic lupus erythematosus. I. Dysfunction of suppressor T-cell activity related to impaired generation of, rather than response to, suppressor cells. Arthritis Rheum. 1978 Jul-Aug;21(6):657–664. doi: 10.1002/art.1780210608. [DOI] [PubMed] [Google Scholar]
- Scheinberg M. A., Cathcart E. S. B cell and T cell lymphopenia in systemic lupus erythematosus. Cell Immunol. 1974 May;12(2):309–314. doi: 10.1016/0008-8749(74)90083-5. [DOI] [PubMed] [Google Scholar]
- Shirai T., Hayakawa K., Okumura K., Tada T. Differential cytotoxic effect of natural thymocytotoxic autoantibody of NZB mice on functional subsets of T cells. J Immunol. 1978 Jun;120(6):1924–1929. [PubMed] [Google Scholar]
- Shirai T., Mellors R. C. Natural thymocytotoxic autoantibody and reactive antigen in New Zealand black and other mice. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1412–1415. doi: 10.1073/pnas.68.7.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou L., Schwartz S. A., Good R. A. Suppressor cell activity after concanavalin A treatment of lymphocytes from normal donors. J Exp Med. 1976 May 1;143(5):1100–1110. doi: 10.1084/jem.143.5.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stastny P., Ziff M. Antibodies against cell membrane constituents in systemic lupus erythematosus and related diseases. I. Cytotoxic effect of serum from patients with systemic lupus erythematosus (SLE) for allogeneic and for autologous lymphocytes. Clin Exp Immunol. 1971 Apr;8(4):543–550. [PMC free article] [PubMed] [Google Scholar]
- Stastny P., Ziff M. Direct lysis of lymphocytes by complement in patients with systemic lupus erythematosus. Arthritis Rheum. 1971 Nov-Dec;14(6):733–736. doi: 10.1002/art.1780140608. [DOI] [PubMed] [Google Scholar]
- Stastny P., Ziff M. Lymphocyte and platelet autoantibodies in S.L.E. Lancet. 1971 Jun 12;1(7711):1239–1240. doi: 10.1016/s0140-6736(71)91751-x. [DOI] [PubMed] [Google Scholar]
- Steinberg A. D., Klassen L. W. Role of suppressor T cells in lymphopoietic disorders. Clin Haematol. 1977 Jun;6(2):439–478. [PubMed] [Google Scholar]
- Strelkauskas A. J., Callery R. T., McDowell J., Borel Y., Schlossman S. F. Direct evidence for loss of human suppressor cells during active autoimmune disease. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5150–5154. doi: 10.1073/pnas.75.10.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strelkauskas A. J., Schauf V., Wilson B. S., Chess L., Schlossman S. F. Isolation and characterization of naturally occurring subclasses of human peripheral blood T cells with regulatory functions. J Immunol. 1978 Apr;120(4):1278–1282. [PubMed] [Google Scholar]
- Suciu-Foca N., Buda J. A., Thiem T., Reemtsma K. Impaired responsiveness of lymphocytes in patients with systemic lupus erythematosus. Clin Exp Immunol. 1974 Nov;18(3):295–301. [PMC free article] [PubMed] [Google Scholar]
- Tadakuma T., Pierce C. W. Site of action of a soluble immune response suppressor (SIRS) produced by concanavalin A-activated spleen cells. J Immunol. 1976 Sep;117(3):967–972. [PubMed] [Google Scholar]
- Terasaki P. I., Mottironi V. D., Barnett E. V. Cytotoxins in disease. Autocytotoxins in lupus. N Engl J Med. 1970 Oct 1;283(14):724–728. doi: 10.1056/NEJM197010012831403. [DOI] [PubMed] [Google Scholar]
- Thomas D. B. Antibodies specific for human T lymphocytes in cold agglutinin and lymphocytotoxic sera. Eur J Immunol. 1973 Dec;3(12):824–828. doi: 10.1002/eji.1830031216. [DOI] [PubMed] [Google Scholar]
- Twomey J. J., Laughter A. H., Steinberg A. D. A serum inhibitor of immune regulation in patients with systemic lupus erythematosus. J Clin Invest. 1978 Sep;62(3):713–715. doi: 10.1172/JCI109180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan J. H., Chihara T. Lymphocyte function in rheumatic disorders. Arch Intern Med. 1975 Oct;135(10):1324–1328. [PubMed] [Google Scholar]
- Wernet P., Kunkel H. G. Antibodies to a specific surface antigen of T cells in human sera inhibiting mixed leukocyte culture reactions. J Exp Med. 1973 Oct 1;138(4):1021–1026. doi: 10.1084/jem.138.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Jr, Emmons J. D., Yunis E. J. Studies of human sera with cytotoxic activity. J Clin Invest. 1971 Jul;50(7):1514–1524. doi: 10.1172/JCI106637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Jr, Lies R. B., Messner R. P. Inhibition of mixed leukocyte culture responses by serum and gamma-globulin fractions from certain patients with connective tissue disorders. Arthritis Rheum. 1973 Sep-Oct;16(5):597–605. doi: 10.1002/art.1780160504. [DOI] [PubMed] [Google Scholar]
- Winchester R. J., Winfield J. B., Siegal F., Wernet P., Bentwich Z., Kunkel H. G. Analyses of lymphocytes from patients with rheumatoid arthritis and systemic lupus erythematosus. Occurrence of interfering cold-reactive antilymphocyte antibodies. J Clin Invest. 1974 Nov;54(5):1082–1092. doi: 10.1172/JCI107852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfield J. B., Winchester R. J., Wernet P., Fu S. M., Kunkel H. G. Nature of cold-reactive antibodies to lymphocyte surface determinants in systemic lupus erythematosus. Arthritis Rheum. 1975 Jan-Feb;18(1):1–8. doi: 10.1002/art.1780180101. [DOI] [PubMed] [Google Scholar]



