Abstract
Endothelial cells in tissue culture degrade bradykinin and convert angiotensin I to angiotensin II. These are both functions of a single dipeptidyl hydrolase, angiotensin converting enzyme. Monolayer cultures were prepared from human, rabbit, pig, and calf vessels. Angiotensin converting enzyme activity was assessed by adding either bradykinin or angiotensin I to the cells in culture flasks, and measuring residual peptide over time by radioimmunoassay. Peptide degradation was inhibited by the specific converting enzyme inhibitor, SQ 20881. The flasks were equilibrated with varying hypoxic gas mixtures: hypoxia rapidly (less than 2 min) decreased enzyme activity and room air restored it as rapidly. The extent to which activity was reduced was a direct function of PO2 (r = 0.93, P less than 0.001), and there was no enzyme activity below a PO2 of 30 mm Hg. Four preparations were studied with respect to decrease in enzyme activity by hypoxia: (a) intact cells in monolayer, (b) sonicated cells, (c) sonicated cells from which converting enzyme was partially dissolved by a detergent, and (d) purified converting enzyme. Hypoxia had progressively less of an inhibiting effect on the enzyme activity of the preparations as the degree of cell integrity decreased. Hypoxia inhibits angiotensin converting enzyme activity in cultured endothelial cells, but the effect of hypoxia is not on the enzyme per se, but appears to be a unique characteristic of the endothelial cell.
Full text
PDF


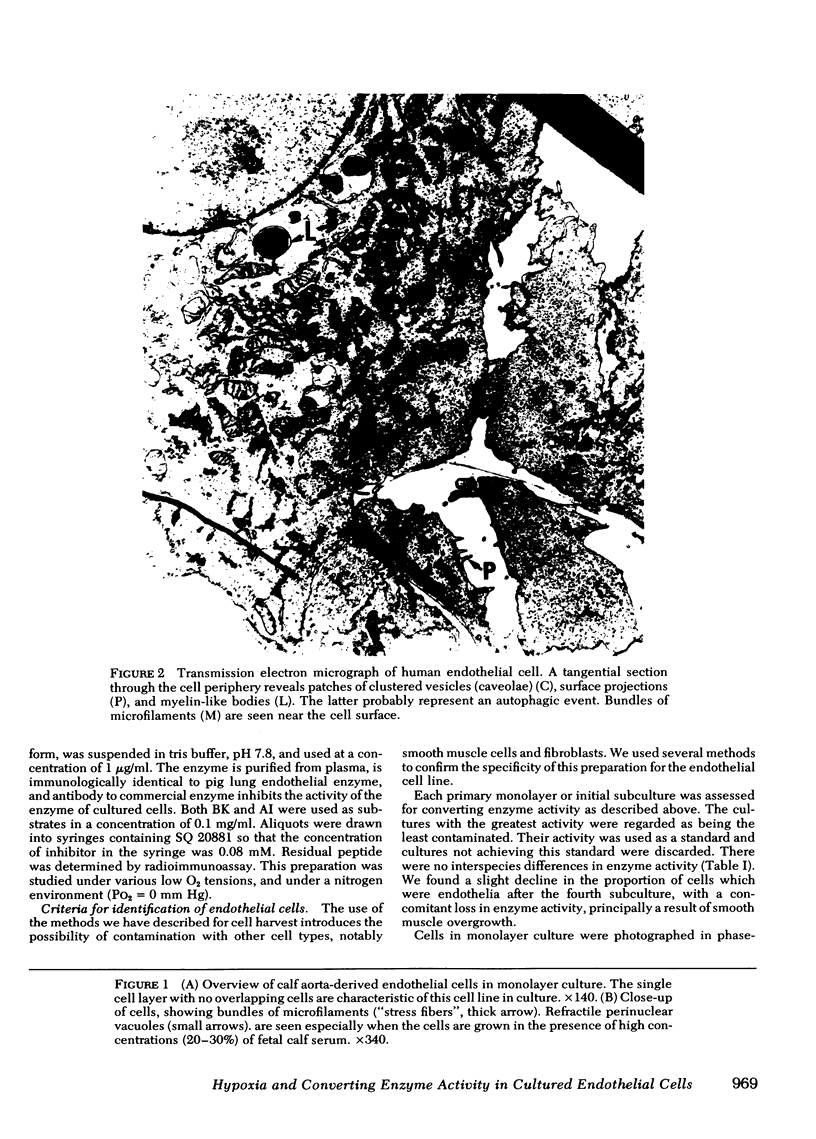


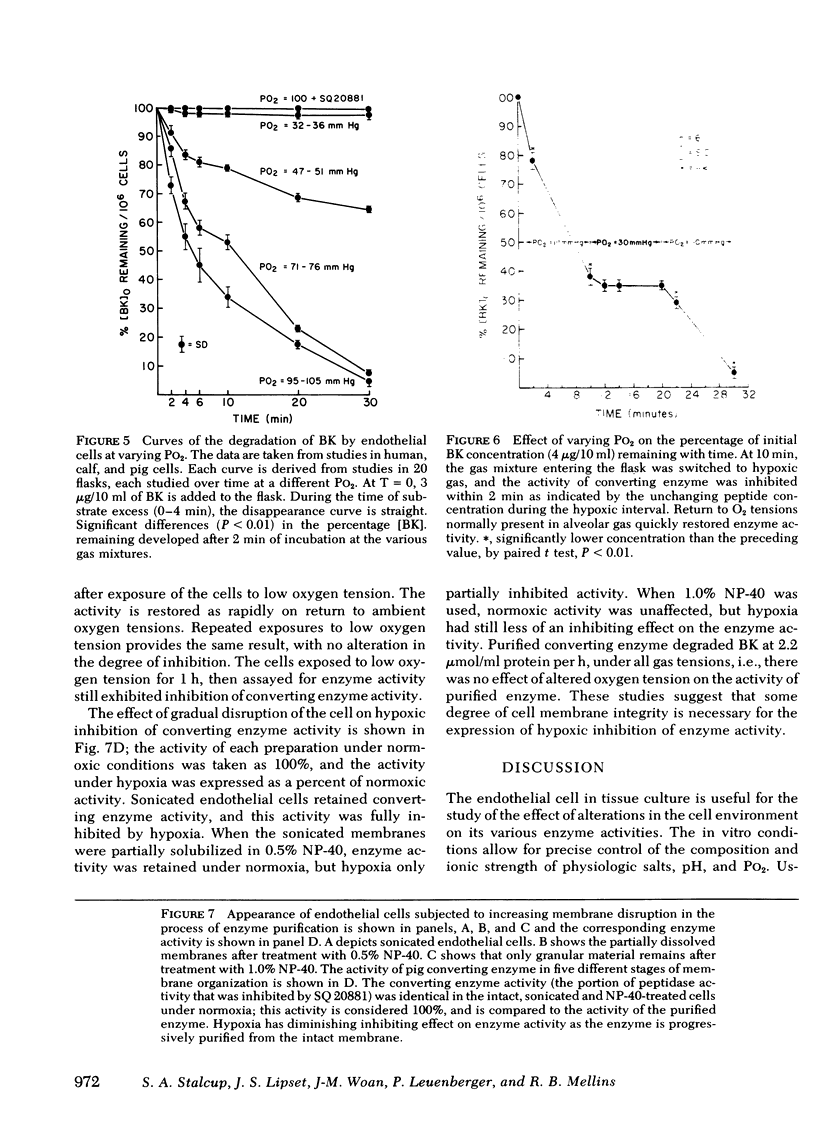




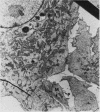
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COOK C. D., DRINKER P. A., JACOBSON H. N., LEVISON H., STRANG L. B. CONTROL OF PULMONARY BLOOD FLOW IN THE FOETAL AND NEWLY BORN LAMB. J Physiol. 1963 Nov;169:10–29. doi: 10.1113/jphysiol.1963.sp007238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coceani F., Olley P. M. The response of the ductus arteriosus to prostaglandins. Can J Physiol Pharmacol. 1973 Mar;51(3):220–225. doi: 10.1139/y73-031. [DOI] [PubMed] [Google Scholar]
- Fishman A. P. Hypoxia on the pulmonary circulation. How and where it acts. Circ Res. 1976 Apr;38(4):221–231. doi: 10.1161/01.res.38.4.221. [DOI] [PubMed] [Google Scholar]
- GOODFRIEND T. L., LEVINE L., FASMAN G. D. ANTIBODIES TO BRADYKININ AND ANGIOTENSIN: A USE OF CARBODIIMIDES IN IMMUNOLOGY. Science. 1964 Jun 12;144(3624):1344–1346. doi: 10.1126/science.144.3624.1344. [DOI] [PubMed] [Google Scholar]
- Haddy F. J., Scott J. B. Metabolically linked vasoactive chemicals in local regulation of blood flow. Physiol Rev. 1968 Oct;48(4):688–707. doi: 10.1152/physrev.1968.48.4.688. [DOI] [PubMed] [Google Scholar]
- Heymann M. A., Rudolph A. M. Control of the ductus arteriosus. Physiol Rev. 1975 Jan;55(1):62–78. doi: 10.1152/physrev.1975.55.1.62. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. R., Erdös E. G. Metabolism of vasoactive peptides by human endothelial cells in culture. Angiotensin I converting enzyme (kininase II) and angiotensinase. J Clin Invest. 1977 Apr;59(4):684–695. doi: 10.1172/JCI108687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellermeyer R. W., Graham R. C., Jr Kinins--possible physiologic and pathologic roles in man. N Engl J Med. 1968 Oct 17;279(16):859–concl. doi: 10.1056/NEJM196810172791605. [DOI] [PubMed] [Google Scholar]
- Leuenberger P. J., Stalcup S. A., Mellins R. B., Greenbaum L. M., Turino G. M. Decrease in angiotensin I conversion by acute hypoxia in dogs. Proc Soc Exp Biol Med. 1978 Sep;158(4):586–589. doi: 10.3181/00379727-158-40252. [DOI] [PubMed] [Google Scholar]
- MAJNO G., PALADE G. E., SCHOEFL G. I. Studies on inflammation. II. The site of action of histamine and serotonin along the vascular tree: a topographic study. J Biophys Biochem Cytol. 1961 Dec;11:607–626. doi: 10.1083/jcb.11.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melmon K. L., Cline M. J., Hughes T., Nies A. S. Kinins: possible mediators of neonatal circulatory changes in man. J Clin Invest. 1968 Jun;47(6):1295–1302. doi: 10.1172/JCI105821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher D. F., Saksela O., Vaheri A. Synthesis and secretion of alpha-2-macroglobulin by cultured adherent lung cells. Comparison with cell strains derived from other tissues. J Clin Invest. 1977 Nov;60(5):1036–1045. doi: 10.1172/JCI108854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., Willingham M., Anderson W., Gallo M. Localization of serum-derived alpha 2 macroglobulin in cultured cells and decrease after Moloney sarcoma virus transformation. Cell. 1977 Nov;12(3):609–617. doi: 10.1016/0092-8674(77)90261-6. [DOI] [PubMed] [Google Scholar]
- Pinto da Silva P., Branton D. Membrane intercalated particles: the plasma membrane as a planar fluid domain. Chem Phys Lipids. 1972 May;8(4):265–278. doi: 10.1016/0009-3084(72)90054-0. [DOI] [PubMed] [Google Scholar]
- Ryan J. W., Ryan U. S. Pulmonary endothelial cells. Fed Proc. 1977 Dec;36(13):2683–2691. [PubMed] [Google Scholar]
- Ryan U. S., Ryan J. W., Whitaker C., Chiu A. Localization of angiotensin converting enzyme (kininase II). II. Immunocytochemistry and immunofluorescence. Tissue Cell. 1976;8(1):125–145. doi: 10.1016/0040-8166(76)90025-2. [DOI] [PubMed] [Google Scholar]
- Sancho J., Re R., Burton J., Barger A. C., Haber E. The role of the renin-angiotensin-aldosterone system in cardiovascular homeostasis in normal human subjects. Circulation. 1976 Mar;53(3):400–405. doi: 10.1161/01.cir.53.3.400. [DOI] [PubMed] [Google Scholar]
- Smith U., Ryan J. W., Smith D. S. Freeze-etch studies of the plasma membrane of pulmonary endothelial cells. J Cell Biol. 1973 Feb;56(2):492–499. doi: 10.1083/jcb.56.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soffer R. L., Reza R., Caldwell P. R. Angiotensin-converting enzyme from rabbit pulmonary particles. Proc Natl Acad Sci U S A. 1974 May;71(5):1720–1724. doi: 10.1073/pnas.71.5.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalcup S. A., Lipset J. S., Legant P. M., Leuenberger P. J., Mellins R. B. Inhibition of converting enzyme activity by acute hypoxia in dogs. J Appl Physiol Respir Environ Exerc Physiol. 1979 Feb;46(2):227–234. doi: 10.1152/jappl.1979.46.2.227. [DOI] [PubMed] [Google Scholar]
- Starling M. B., Elliott R. B. The effects of prostaglandins, prostaglandin inhibitors, and oxygen on the closure of the ductus arteriosus, pulmonary arteries and umbilical vessels in vitro. Prostaglandins. 1974 Nov 10;8(3):187–203. doi: 10.1016/0090-6980(74)90042-2. [DOI] [PubMed] [Google Scholar]
- Walker W. G., Moore M. A., Horvath J. S., Whelton P. K. Arterial and venous angiotensin II in normal subjects. Relation to plasma renin activity and plasma aldosterone concentration, and response to posture and volume changes. Circ Res. 1976 Jun;38(6):477–483. doi: 10.1161/01.res.38.6.477. [DOI] [PubMed] [Google Scholar]
- Wigger H. J., Stalcup S. A. Distribution and development of angiotensin converting enzyme in the fetal and newborn rabbit. An immunofluorescence study. Lab Invest. 1978 May;38(5):581–585. [PubMed] [Google Scholar]
- Williams G. H., Hollenberg N. K. Accentuated vascular and endocrine response to SQ 20881 in hypertension. N Engl J Med. 1977 Jul 28;297(4):184–188. doi: 10.1056/NEJM197707282970404. [DOI] [PubMed] [Google Scholar]