Abstract
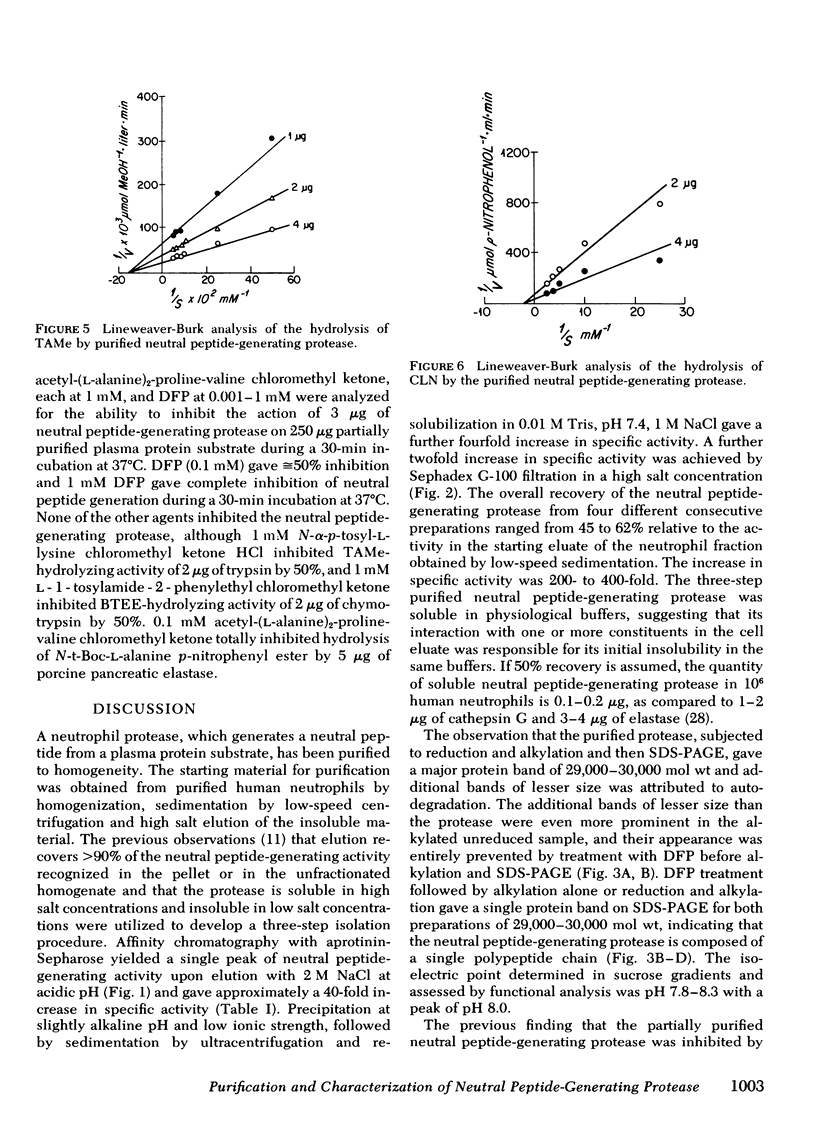
A human neutrophil neutral protease which generates a low molecular weight peptide from a plasma protein substrate and cleaves the basic amino acid ester substrates α-N-p-tosyl-l-arginine methyl ester HCl, α-N-benzoyl-l-arginine-methyl ester HCl, and α-N-carbobenzoxy-l-lysine-p-nitrophenyl ester has been purified to homogeneity and distinguished from the known lysosomal neutrophil proteases. The starting activity was obtained from purified human neutrophils by homogenization, sedimentation by low-speed centrifugation, and high salt elution of the insoluble material. Purification was achieved by aprotinin-affinity chromatography, precipitation at low ionic strength, and gel filtration. The overall recovery, relative to the activity in the starting eluate of the neutrophil fraction, was ≅50% with a 200- to 400-fold increase in specific activity. After treatment with diisopropylfluorophosphate to eliminate autodegradation, sodium dodecyl sulfate-polyacrylamide gel electrophoresis of reduced and unreduced protein gave a single protein band of 29,000-30,000 mol wt. The isoelectric point determined in sucrose gradients ranged from pH 7.8 to 8.3 with a peak at pH 8.0. This neutrophil protease, like cathepsin G and elastase, is composed of a single polypeptide chain of ≅30,000 mol wt, but differs from cathepsin G and elastase in its less cationic isoelectric point and its failure to cleave synthetic substrates presenting an aromatic amino acid ester linkage and alanyl peptide bonds, respectively.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bieth J., Spiess B., Wermuth C. G. The synthesis and analytical use of a highly sensitive and convenient substrate of elastase. Biochem Med. 1974 Dec;11(4):350–357. doi: 10.1016/0006-2944(74)90134-3. [DOI] [PubMed] [Google Scholar]
- Bretz U., Baggiolini M. Biochemical and morphological characterization of azurophil and specific granules of human neutrophilic polymorphonuclear leukocytes. J Cell Biol. 1974 Oct;63(1):251–269. doi: 10.1083/jcb.63.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman R. W., Mattler L., Sherry S. Studies on the prekallikrein (kallikreinogen)--kallikrein enzyme system of human plasma. I. Isolation and purification of plasma kallikreins. J Clin Invest. 1969 Jan;48(1):11–22. doi: 10.1172/JCI105959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewald B., Rindler-Ludwig R., Bretz U., Baggiolini M. Subcellular localization and heterogeneity of neutral proteases in neutrophilic polymorphonuclear leukocytes. J Exp Med. 1975 Apr 1;141(4):709–723. doi: 10.1084/jem.141.4.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein G., Janoff A. A rapid method for purification of human granulocyte cationic neutral proteases: purification and characterization of human granulocyte chymotrypsin-like enzyme. Biochim Biophys Acta. 1975 Oct 22;403(2):477–492. doi: 10.1016/0005-2744(75)90076-5. [DOI] [PubMed] [Google Scholar]
- HUMMEL B. C. A modified spectrophotometric determination of chymotrypsin, trypsin, and thrombin. Can J Biochem Physiol. 1959 Dec;37:1393–1399. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ohlsson K., Olsson I. The neutral proteases of human granulocytes. Isolation and partial characterization of two granulocyte collagenases. Eur J Biochem. 1973 Jul 16;36(2):473–481. doi: 10.1111/j.1432-1033.1973.tb02932.x. [DOI] [PubMed] [Google Scholar]
- Plow E. F., Edgington T. S. An alternative pathway for fibrinolysis. I. The cleavage of fibrinogen by leukocyte proteases at physiologic pH. J Clin Invest. 1975 Jul;56(1):30–38. doi: 10.1172/JCI108076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers J. C., Gupton B. F., Harley A. D., Nishino N., Whitley R. J. Specificity of porcine pancreatic elastase, human leukocyte elastase and cathepsin G. Inhibition with peptide chloromethyl ketones. Biochim Biophys Acta. 1977 Nov 23;485(1):156–166. doi: 10.1016/0005-2744(77)90203-0. [DOI] [PubMed] [Google Scholar]
- Pryce-Jones R. H., Saklatvala J., Wood G. C. Neutral protease from the polymorphonuclear leucocytes of human rheumatoid synovial fluid. Clin Sci Mol Med. 1974 Nov;47(5):403–414. doi: 10.1042/cs0470403. [DOI] [PubMed] [Google Scholar]
- Rindler-Ludwig R., Braunsteiner H. Cationic proteins from human neutrophil granulocytes. Evidence for their chymotrypsin-like properties. Biochim Biophys Acta. 1975 Feb 27;379(2):606–617. doi: 10.1016/0005-2795(75)90167-1. [DOI] [PubMed] [Google Scholar]
- Ruddy S., Austen K. F. C3 inactivator of man. I. Hemolytic measurement by the inactivation of cell-bound C3. J Immunol. 1969 Mar;102(3):533–543. [PubMed] [Google Scholar]
- SACHAR L. A., WINTER K. K., SICHER N., FRANKEL S. Photometric method for estimation of elastase activity. Proc Soc Exp Biol Med. 1955 Nov;90(2):323–326. doi: 10.3181/00379727-90-22022. [DOI] [PubMed] [Google Scholar]
- SIEGELMAN A. M., CARLSON A. S., ROBERTSON T. Investigation of serum trypsin and related substances. I. The quantitative demonstration of trypsinlike activity in human blood serum by a micromethod. Arch Biochem Biophys. 1962 Apr;97:159–163. doi: 10.1016/0003-9861(62)90058-9. [DOI] [PubMed] [Google Scholar]
- Showell H. J., Freer R. J., Zigmond S. H., Schiffmann E., Aswanikumar S., Corcoran B., Becker E. L. The structure-activity relations of synthetic peptides as chemotactic factors and inducers of lysosomal secretion for neutrophils. J Exp Med. 1976 May 1;143(5):1154–1169. doi: 10.1084/jem.143.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein R. M. The determination of human plasminogen using Nalpha-CBZ-L-lysin p-nitrophenyl ester as substrate. Anal Biochem. 1975 May 12;65(1-2):500–506. doi: 10.1016/0003-2697(75)90535-7. [DOI] [PubMed] [Google Scholar]
- Spragg J. Specific functional and immunologic assay of plasma plasminogen in hereditary angioedema, in hereditary angioedema treated with tranexamic acid, and in normal subjects. J Immunol. 1978 Feb;120(2):592–596. [PubMed] [Google Scholar]
- Starkey P. M., Barrett A. J. Human lysosomal elastase. Catalytic and immunological properties. Biochem J. 1976 May 1;155(2):265–271. doi: 10.1042/bj1550265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack B. D., Prahl J. W. Third component of human complement: purification from plasma and physicochemical characterization. Biochemistry. 1976 Oct 5;15(20):4513–4521. doi: 10.1021/bi00665a028. [DOI] [PubMed] [Google Scholar]
- Visser L., Blout E. R. The use of p-nitrophenyl N-tert-butyloxycarbonyl-L-alaninate as substrate for elastase. Biochim Biophys Acta. 1972 Apr 7;268(1):257–260. doi: 10.1016/0005-2744(72)90223-9. [DOI] [PubMed] [Google Scholar]
- Wasserman S. I., Austen K. F. Identification and characterization of arylsulfatase A and B of the rat basophil leukemia tumor. J Biol Chem. 1977 Oct 25;252(20):7074–7080. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weissmann G., Dukor P., Zurier R. B. Effect of cyclic AMP on release of lysosomal enzymes from phagocytes. Nat New Biol. 1971 Jun 2;231(22):131–135. doi: 10.1038/newbio231131a0. [DOI] [PubMed] [Google Scholar]
- Wintroub B. U., Goetzl E. J., Austen K. F. A neutrophil-dependent pathway for the generation of a neutral peptide mediator. II. Subcellular localization of the neutrophil protease. Immunology. 1977 Jul;33(1):41–49. [PMC free article] [PubMed] [Google Scholar]
- Wintroub B. U., Goetzl E. J., Austen K. F. A neutrophil-dependent pathway for the generation of a neutral peptide mediator: partial characterization of components and control by alpha-1-antitrypsin. J Exp Med. 1974 Sep 1;140(3):812–824. doi: 10.1084/jem.140.3.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman M., Ashe B. M. Sbustrate specificity of the elastase and the chymotrypsin-like enzyme of the human granulocyte. Biochim Biophys Acta. 1977 Jan 11;480(1):241–245. doi: 10.1016/0005-2744(77)90337-0. [DOI] [PubMed] [Google Scholar]
- ole-MoiYoi O. K., Spragg J., Austen K. F. Inhibition of human urinary kallikrein (urokallikrein) by anti-enzyme FAB. J Immunol. 1978 Jul;121(1):66–71. [PubMed] [Google Scholar]