Abstract
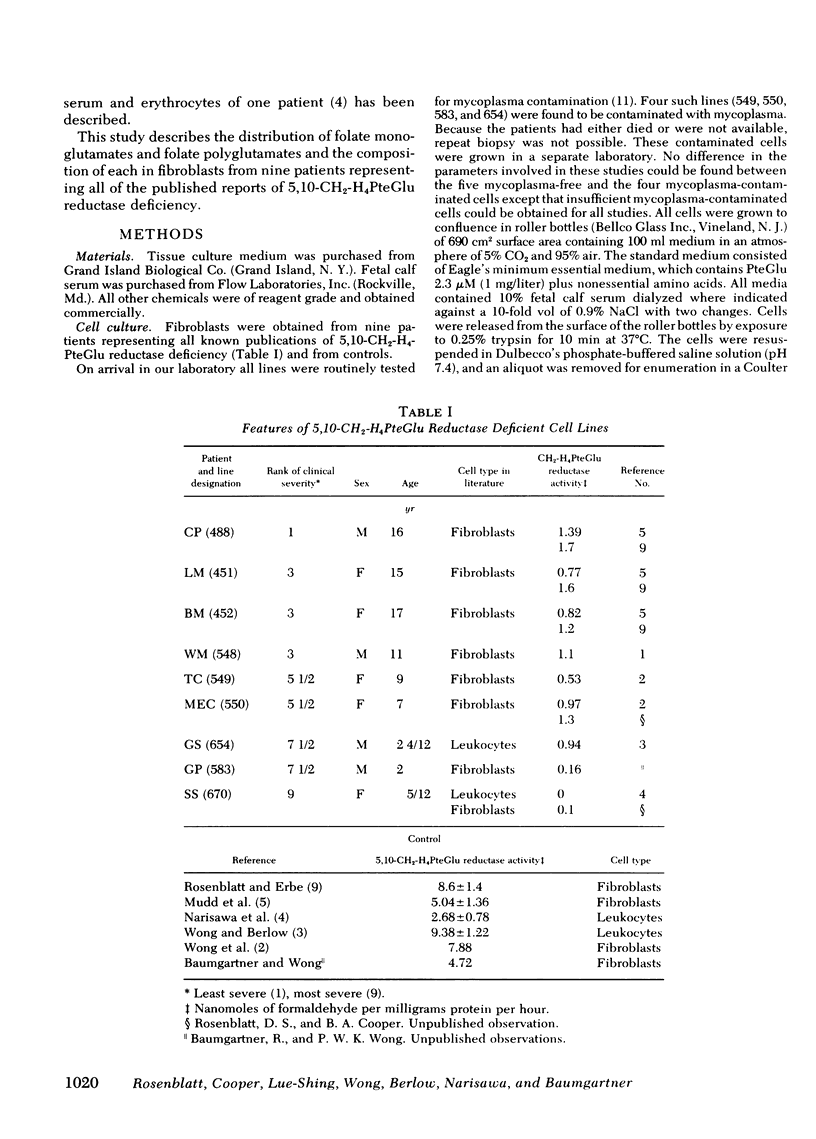
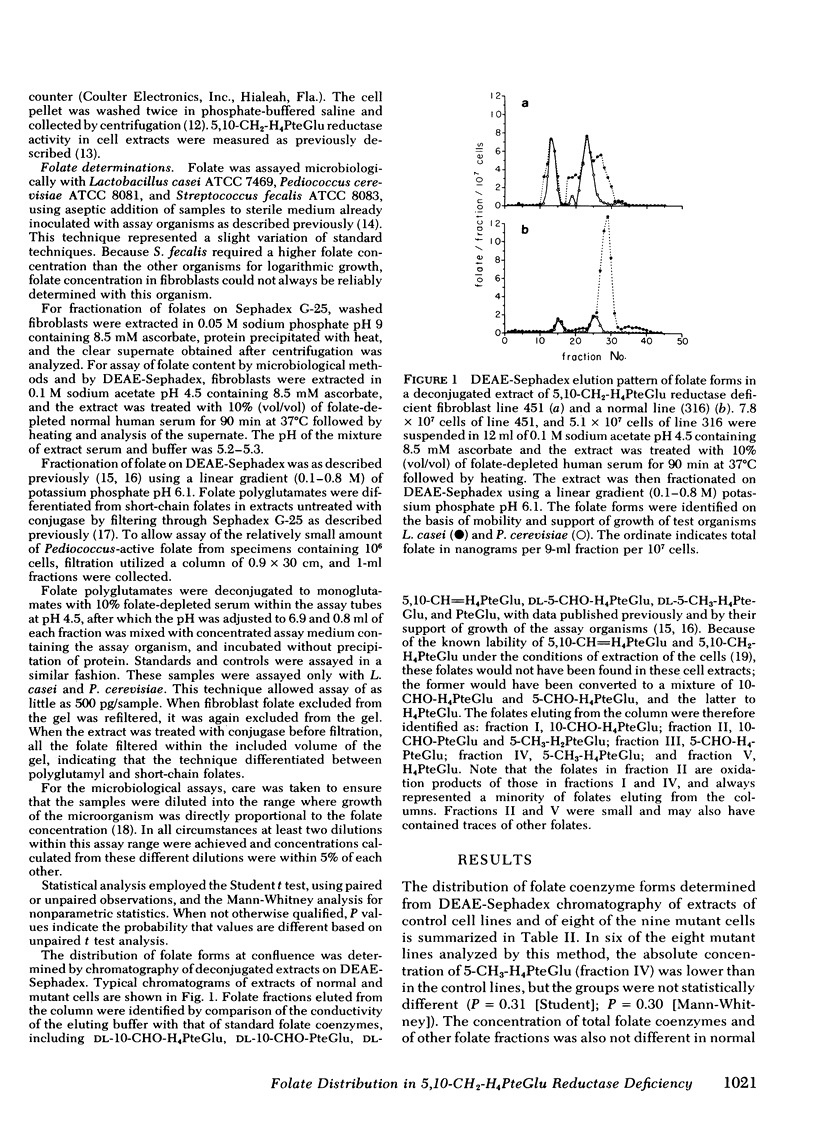
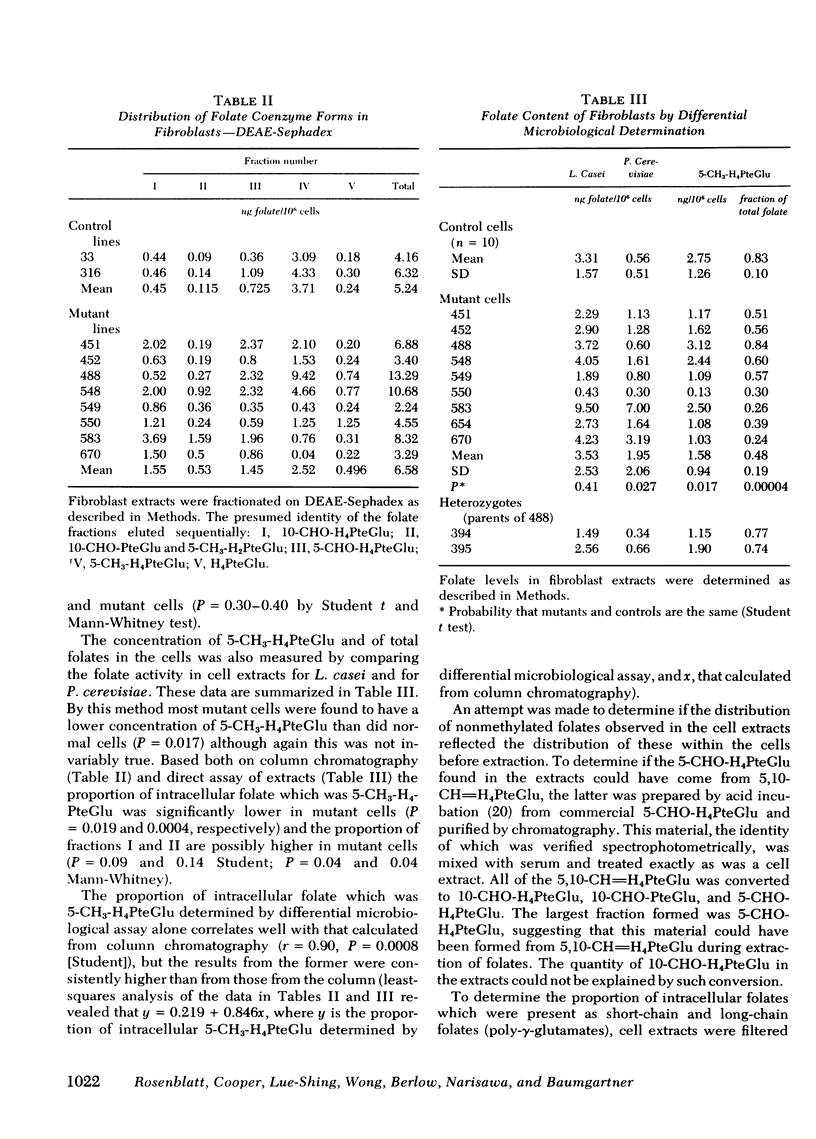
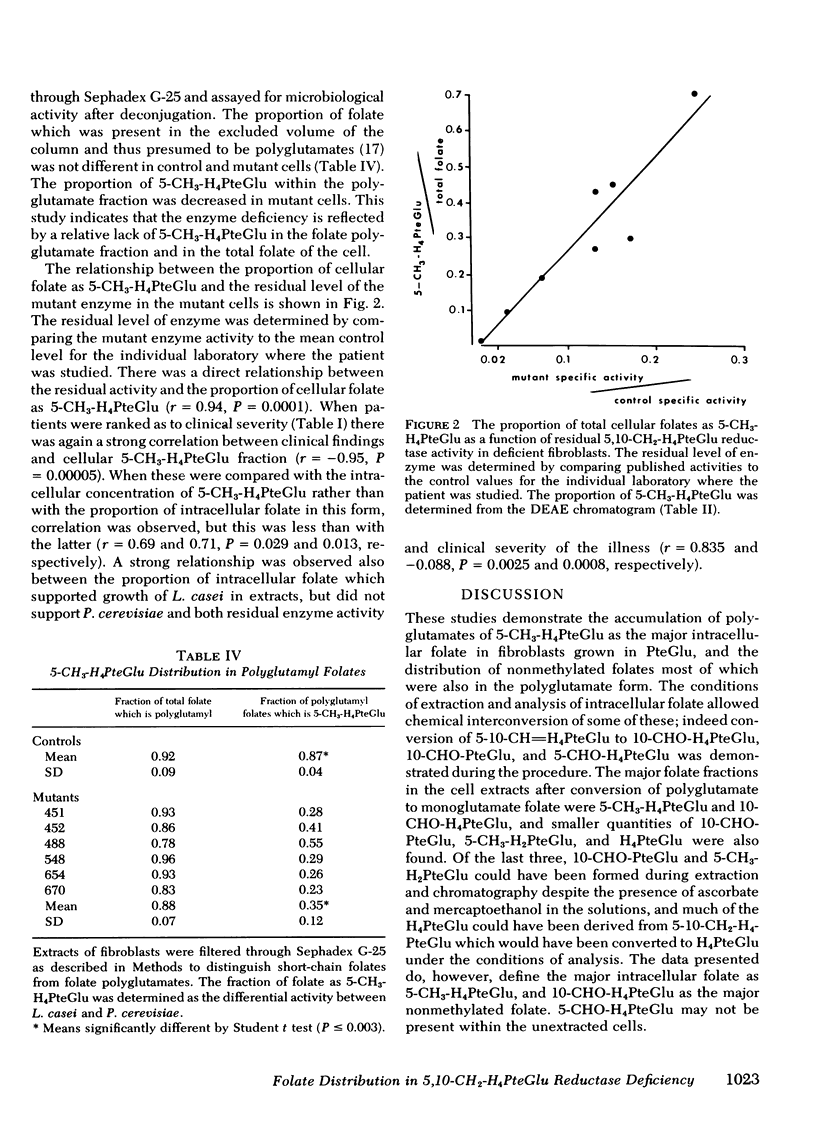
We have studied the distribution of folate coenzyme forms in cultured human fibroblasts from control lines and from lines derived from nine patients representing all of the published reports of 5,10-CH2-H4PteGlu reductase deficiency. Based on mobility on DEAE-Sephadex and differential microbiological assay the major folate fractions in extracts of human fibroblasts were 5-CH3-H4PteGlu, 10-CHO-H4PteGlu, and 5-CHO-H4PteGlu with smaller fractions, which included 5-CH3-H2PteGlu, 10-CHO-PteGlu, and H4PteGlu. Evidence that the 5-CHO-H4PteGlu may have been derived from 5,10-CH=H4PteGlu during extraction is presented. In most of the mutant fibroblasts the absolute concentration of 5-CH3-H4PteGlu was lower than in control cells but the proportion of intracellular folate which was 5-CH3-H4PteGlu was strikingly lower in mutant cells when determined by chromatography or differential microbiological assay. In both control and mutant cells most of the 5-CH3-H4-PteGlu was polyglutamate. The proportion of intracellular folate which was polyglutamate was similar in control and mutant cells. A direct relationship was observed between the proportion of cellular folate which was 5-CH3-H4PteGlu, and both the clinical severity of this disorder and the residual enzyme activity indicating that the distribution of different folates may be an important control of intracellular folate metabolism. These studies indicate that 5,10-CH2-H4PteGlu reductase is the only significant intracellular pathway for the generation of 5-CH3-H4PteGlu, that the activity of this enzyme regulates the level of this folate in control and mutant cells under conditions of culture used here, that the majority of intracellular folate is in the polyglutamate form, and that the relative distribution of folates may control folate metabolism by interaction in the various folate reactions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buehring K. U., Tamura T., Stokstad E. L. Folate coenzymes of Lactobacillus casei and Streptococcus faecalis. J Biol Chem. 1974 Feb 25;249(4):1081–1089. [PubMed] [Google Scholar]
- Cooper B. A., Rosenblatt D. Folate coenzyme forms in fibroblasts from patients deficient in 5,10-methylenetetrahydrofolate reductase. Biochem Soc Trans. 1976;4(5):921–922. doi: 10.1042/bst0040921. [DOI] [PubMed] [Google Scholar]
- Cooper B. A. Studies of (3H)folic acid uptake by Lactobacillus casei. Biochim Biophys Acta. 1970 Apr 14;208(1):99–109. doi: 10.1016/0304-4165(70)90052-8. [DOI] [PubMed] [Google Scholar]
- Cooper B. A. Superiority of simplified assay for folate with Lactobacillus casei ATCC 7469 over assay with chloramphenicol-adapted strain. J Clin Pathol. 1973 Dec;26(12):963–967. doi: 10.1136/jcp.26.12.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbe R. W. Inborn errors of folate metabolism (second of two parts). N Engl J Med. 1975 Oct 16;293(16):807–812. doi: 10.1056/NEJM197510162931606. [DOI] [PubMed] [Google Scholar]
- Freeman J. M., Finkelstein J. D., Mudd S. H. Folate-responsive homocystinuria and "schizophrenia". A defect in methylation due to deficient 5,10-methylenetetrahydrofolate reductase activity. N Engl J Med. 1975 Mar 6;292(10):491–496. doi: 10.1056/NEJM197503062921001. [DOI] [PubMed] [Google Scholar]
- Kanwar Y. S., Manaligod J. R., Wong P. W. Morphologic studies in a patient with homocystinuria due to 5, 10-methylenetetrahydrofolate reductase deficiency. Pediatr Res. 1976 Jun;10(6):598–609. doi: 10.1203/00006450-197606000-00008. [DOI] [PubMed] [Google Scholar]
- Lavoie A., Cooper B. A. Rapid transfer of folic acid from blood to bile in man, and its conversion into folate coenzymes and into a pteroylglutamate with little biological activity. Clin Sci Mol Med. 1974 Jun;46(6):729–741. doi: 10.1042/cs0460729. [DOI] [PubMed] [Google Scholar]
- Mudd S. H., Uhlendorf B. W., Freeman J. M., Finkelstein J. D., Shih V. E. Homocystinuria associated with decreased methylenetetrahydrofolate reductase activity. Biochem Biophys Res Commun. 1972 Jan 31;46(2):905–912. doi: 10.1016/s0006-291x(72)80227-4. [DOI] [PubMed] [Google Scholar]
- Narisawa K., Wada Y., Saito T., Suzuki H., Kudo M. Infantile type of homocystinuria with N5,10-methylenetetrahydrofolate reductase defect. Tohoku J Exp Med. 1977 Feb;121(2):185–194. doi: 10.1620/tjem.121.185. [DOI] [PubMed] [Google Scholar]
- Rosenblatt D. S., Erbe R. W. Methylenetetrahydrofolate reductase in cultured human cells. I. Growtha and metabolic studies. Pediatr Res. 1977 Nov;11(11):1137–1141. doi: 10.1203/00006450-197711000-00004. [DOI] [PubMed] [Google Scholar]
- Rosenblatt D. S., Erbe R. W. Methylenetetrahydrofolate reductase in cultured human cells. II. Genetic and biochemical studies of methylenetetrahydrofolate reductase deficiency. Pediatr Res. 1977 Nov;11(11):1141–1143. doi: 10.1203/00006450-197711000-00005. [DOI] [PubMed] [Google Scholar]
- Rosenblatt D. S., Erbe R. W. Reciprocal changes in the levels of functionally related folate enzymes during the culture cycle in human fibroblasts. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1627–1633. doi: 10.1016/0006-291x(73)91173-x. [DOI] [PubMed] [Google Scholar]
- Schneider E. L., Stanbridge E. J., Epstein C. J. Incorporation of 3H-uridine and 3H-uracil into RNA: a simple technique for the detection of mycoplasma contamination of cultured cells. Exp Cell Res. 1974 Mar 15;84(1):311–318. doi: 10.1016/0014-4827(74)90411-x. [DOI] [PubMed] [Google Scholar]
- Wong P. W., Justice P., Berlow S. Detection of homozygotes and heterozygotes with methylenetetrahydrofolate reductase deficiency. J Lab Clin Med. 1977 Aug;90(2):283–288. [PubMed] [Google Scholar]
- Wong P. W., Justice P., Hruby M., Weiss E. B., Diamond E. Folic acid nonresponsive homocystinuria due to methylenetetrahydrofolate reductase deficiency. Pediatrics. 1977 May;59(5):749–756. [PubMed] [Google Scholar]


