Abstract
Bone is a dynamic tissue, in which bone formation by osteoblasts and bone resorption by osteoclasts continue throughout life. In 1998, we molecularly cloned osteoclast differentiation factor (ODF), a long-thought factor responsible for osteoclast formation. This review article describes how Japanese scientists contributed to osteoclast biology before and after the discovery of ODF. This review article is based on the Louis V. Avioli Memorial Lecture of the American Society for Bone and Mineral Research (ASBMR) held in Honolulu in September, 2007.
Keywords: osteoclast formation and activation, M-CSF, ODF/RANKL, OCIF/OPG, DC-STAMP, NFATc1/NFAT2
Introduction
Bone is a dynamic tissue, in which bone formation and resorption continue throughout life. This process is called “bone remodeling”. The bone tissue contains various types of cells. Among them, the bone-forming osteoblasts and the bone-resorbing osteoclasts are two major types of cells responsible for bone remodeling. Osteoblasts are believed to be derived from undifferentiated mesenchymal cells present in bone marrow, which further differentiate into osteocytes and are embedded in calcified bone.1) In contrast, osteoclasts are multinucleated giant cells present only in bone.2) It is believed that osteoclasts are recruited from hemopoietic cells of the monocyte-macrophage lineage. Osteoclast progenitors then proliferate and differentiate into mononuclear pre-osteoclasts and fuse each other to form multinucleated osteoclasts. In ages under 50, the rate of bone resorption and formation is well balanced, thus bone maintains a level of peak bone mass.3) However, in ages over 50, humans lose bone mass annually at a rate of 1%. Thus, humans with 80 years old, contain only 70% of the peak bone mass. In addition, most women lose additional 10% of bone mass between 50 and 60 years old after menopause. This is due to the fact that osteoclastic bone resorption exceeds osteoblastic bone formation owing to estrogen deficiency. In patients with osteoporosis and rheumatoid arthritis, bone resorption also exceeds bone formation, which results in serious bone loss and bone damage. In Japan, the number of patients with osteoporosis has been estimated to be over 10 million, and the number of annual hip fracture has exceeded 120,000 cases.4)
We have been working in osteoclast biology for the past two decades.2) Two years ago, I had an e-mail from Harald Jueppner, the Chairman of the 2007 Program Committee of the American Society for Bone and Mineral Research (ASBMR), who asked me to deliver a Louis Avioli Memorial Lecture held in Honolulu in September, 2007. The ASBMR is the biggest society for bone research in the world. It is a domestic society for American bone community, but more than 50% of the members of the ASBMR come from outside of the US. The late Louis Avioli is the founder of the ASBMR. Louis’s dynamic nature has attracted us very much since we met him for the first time in 1974. His influence on our field over the past four decades resulted from his extraordinary leadership, intelligence, vision, energy and understanding of human relations. Therefore, we thought this is undoubtedly a great honor not only for us but also for all active Japanese members of the ASBMR. During that time we worked in the field of osteoclast biology, we had an acquaintance with many friends not only from Japan, but also from all over the world. In the molecular cloning of the osteoclast differentiation factor (ODF), we collaborated or competed with many foreign research groups. In the modern research on osteoclast biology, Japanese scientists discovered a number of important findings. Thus, in our Avioli lecture, we wanted to introduce the contributions to osteoclast biology not only from our own work but also from major work by Japanese scientists. This review article is based on the Avioli Memorial Lecture of the ASBMR held in Honolulu in September, 2007.
1. Characteristic features of osteoclasts
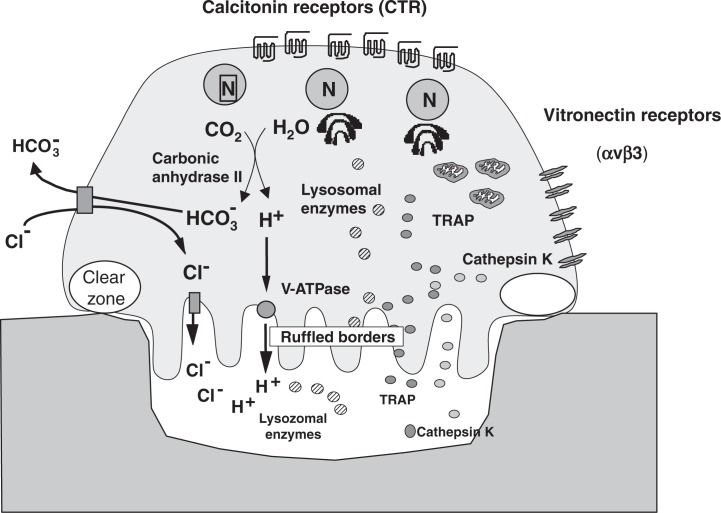
Osteoclasts exhibit typical ultra-structural features such as the presence of a number of nuclei, abundant pleomorphic mitochondria, and a large number of vacuoles and many stacks of Golgi membranes.5),6) The most characteristic features of osteoclasts are the presence of ruffled borders and clear zones (Fig. 1). The ruffled border is a complex structure of deeply infolded finger-like plasma membranes adjacent to the bone surface. The ruffled border is surrounded by a clear zone, which is totally devoid of cellular organelles, except for actin filaments. It is generally accepted that the clear zone serves for the attachment of osteoclasts to bone surface and for separating the bone resorbing area beneath the ruffled border from the unresorbed area.
Fig. 1.
Structure of osteoclasts.
Osteoclasts have several specific marker enzymes like tartrate-resistant acid phosphatase (TRAP) and cathepsin K in lysosomes, and vacuolar type proton ATPase (v-ATPase) in ruffled borders.5),6) Osteoclasts have a number of calcitonin receptors on the basolateral membranes. It was essential to generate osteoclasts with these characteristic features in vitro to understand how osteoclast differentiation and function are controlled.
2. Osteoblasts intervene in the process of osteoclastic bone resorption
The idea that osteoblasts or osteoblastic stromal cells may intervene in the process of osteoclastic bone resorption was first reported by Gideon Rodan and Jack Martin in 1981.7) Their argument for such a mechanism was based on the observations that first, bone-resorbing hormones and cytokines such as parathyroid hormone (PTH), interleukin 1 (IL-1) and 1α,25-dihydroxyvitamin D3 [1α, 25(OH)2D3] have their receptors in osteoblasts but not in osteoclasts, and second, the relative potencies of these bone-resorbing factors in osteoblasts resemble those in inducing bone resorption. The same conclusion was reached independently by Tim Chambers,8) who proposed that a factor called osteoclast activating factor (OAF) is produced by osteoblasts in response to bone-resorbing hormones and cytokines, which stimulates osteoclast activation.
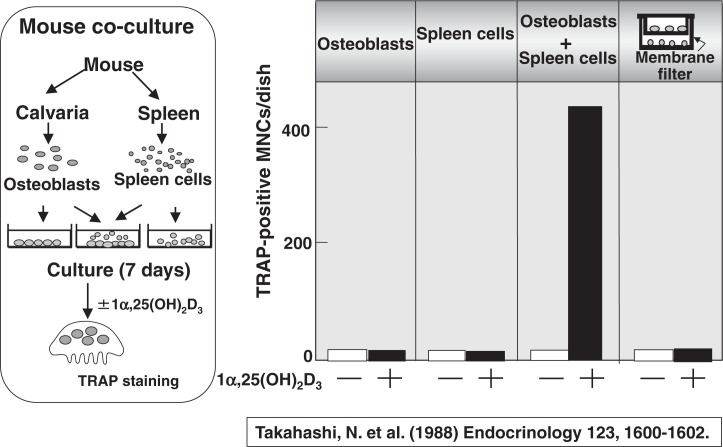
Inspired by those pioneering work, we established an efficient mouse co-culture system to recruit osteoclasts in vitro in 1988.9) Osteoblasts were isolated from mouse calvaria, and cells isolated from splenic tissues were used as osteoclast progenitors. Spleen cells represent osteoclast progenitors, in other word “seeds”, and osteoblasts represent supporting cells to provide a suitable microenvironment for osteoclast formation, in other words, “farm”. They were either separately cultured or co-cultured in the presence of 1α,25(OH)2D3. Figure 2 shows the number of TRAP-positive multinucleated osteoclasts formed. When osteoblasts alone or spleen cells alone were cultured, no osteoclasts were formed even in the presence of 1α,25(OH)2D3. Multinucleated cells which satisfy almost all of the osteoclast phenotype were formed only when spleen cells and osteoblasts were cocultured in the presence of 1α,25(OH)2D3. When osteoblasts and spleen cells were separately cultured in a trans-well through a membrane filter, no osteoclasts were formed, suggesting that cell-to-cell interaction between osteoblasts and spleen cells is important. Since this co-culture system was reproducible and efficient to recruit osteoclasts in vitro, most of the scientists working in osteoclast biology evaluated it highly useful.
Fig. 2.
Osteoclast formation in co-cultures of osteoblasts and spleen cells.
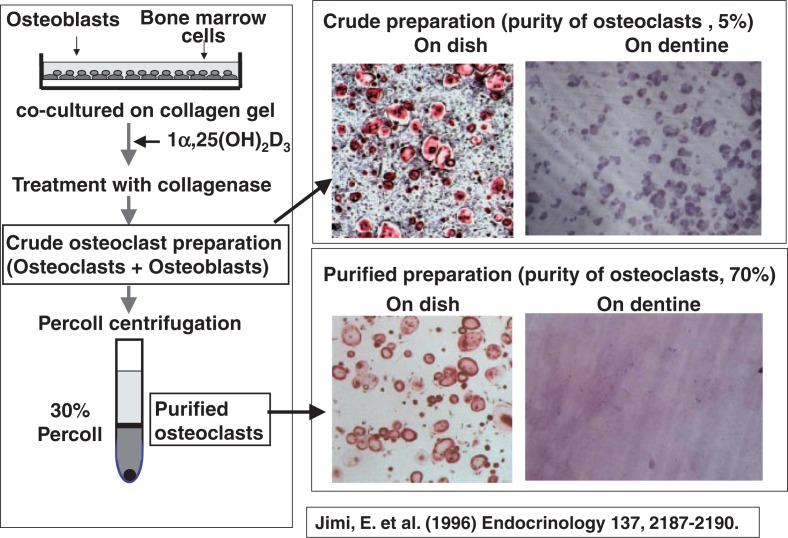
The next question was to examine whether osteoblasts are also needed for activating osteoclast function (in other words, for inducing bone-resorbing activity of osteoclasts). To explore this issue, Eijiro Jimi10) attempted to purify osteoclasts formed in our co-culture system. Since it was extremely difficult to detach osteoclasts formed on plastic dishes, he devised a new method, in which osteoblasts and bone marrow cells were co-cultured on collagen gel-coated dishes in the presence of 1 α,25(OH)2D3 to generate osteoclasts. The coculture was then treated with collagenase to recover most of the cells as a crude osteoclast preparation. The purity of osteoclasts in this preparation was only 5%. The crude osteoclast preparation was further subjected to a 30% Percoll solution and centrifuged to purify osteoclasts. The purity of osteoclasts increased up to 70%. The crude osteoclast preparation containing many osteoblasts did form resorption pits when they were cultured on dentine slices, whereas purified osteoclasts did not form resorption pits at all on the slices (Fig. 3). When osteoblasts were put onto purified osteoclasts, the pit-forming activity of the purified osteoclast preparation was strikingly recovered.10)
Fig. 3.
Purification of osteoclasts formed in co-cultures.
From these experimental results, we concluded that not only osteoclast differentiation but also osteoclast activation requires the presence of osteoblasts. Osteoblasts appeared to perform those actions through cell-to-cell interaction with osteoclast progenitors or mature osteoclasts.
3. Role of M-CSF in osteoclastogenesis
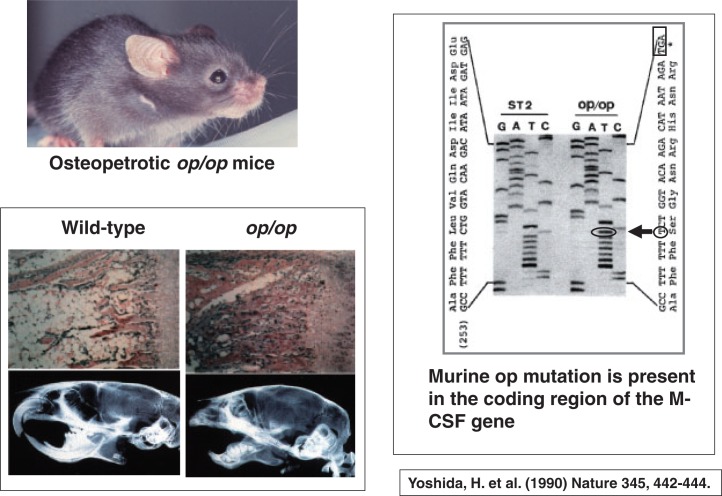
The next important discovery in osteoclast biology was the requirement of M-CSF (macrophage-colony stimulating factor)/CSF-1 (colony stimulating factor 1) in osteoclastogenesis. Shinichi Hayashi and his associates were interested in osteopetrotic op/op mice. Since the osteopetrotic phenotype of op/op mutant mice was not cured by transplantation of normal bone marrow cells, the defect was considered to be present in local microenvironment in bone. Hisa Yoshida11) found that there was an insertion of thymidine at bp 262 in the M-CSF gene of op/op mice, resulting in the formation of the stop codon TGA in the downstream (Fig. 4). This indicated that op/op mutant mice are unable to produce functionally active M-CSF.
Fig. 4.
op/op mice have a mutation in the cording region of the M-CSF gene. The radiographic features of op/op mice were provided by Dr. Shumpei Niida (National Center for Geriatrics and Gerontology, Aichi, Japan).
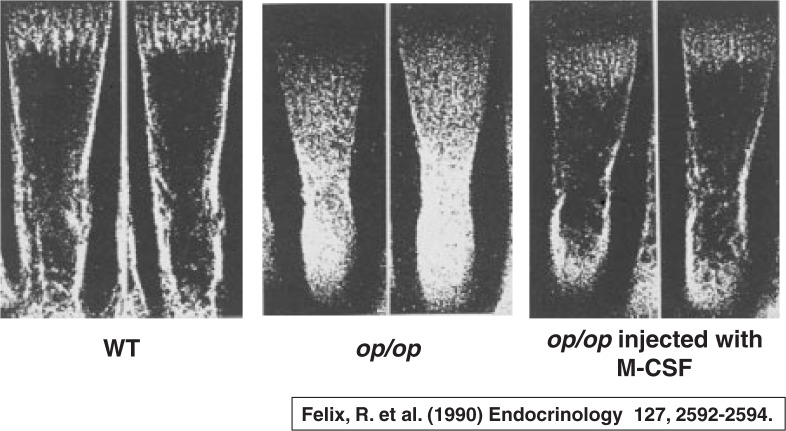
Almost simultaneously, Rolf Felix and Herbie Fleisch12) reported that administration of M-CSF into op/op mutant mice strikingly restored the osteopetrotic bone phenotype in vivo. The bone marrow cavity of op/op mutant mice was completely filled with bone mass without any appreciable osteoclasts, whereas the op/op mutant mice given M-CSF strikingly formed bone marrow cavity with appearance of a number of osteoclasts (Fig. 5). Thus, it is clear that M-CSF is essential for formation of bone marrow cavity. Hiroaki Kodama13) also reported that osteoclast deficiency in op/op mice is cured by injections of M-CSF. These results suggested that osteoclast deficiency in the op/op mutant mouse is due to the failure of the functionally active M-CSF. The op/op mouse is now designated as the Csf1op/Csf1op mouse.
Fig. 5.
Administration of M-CSF into op/op mice restores osteopetrotic bone phenotype.
4. Target cells of bone-resorbing hormones and cytokines
The next step in our study on osteoclast biology was to examine the target cells of osteotropic factors like PTH, 1α,25(OH)2D3 and IL-6 in osteoclastogenesis. Using vitamin D receptor (VDR) knockout mice, Shu Takeda14) clearly showed that co-culture of VDR-deficient osteoblasts and wild type (WT) spleen cells failed to form osteoclasts in response to 1α,25(OH)2D3, whereas VDR-deficient spleen cells differentiated into osteoclasts in response to 1α,25(OH)2D3, when co-cultured with WT osteoblasts. Similarly, B.Y. Liu15) showed that PTH receptor (PTHR)-deficient spleen cells differentiated into osteoclasts in response to PTH, when co-cultured with WT osteoblasts. These results suggested that the target cells of 1α,25(OH)2D3 and PTH in osteoclastogenesis are osteoblasts, but not osteoclast progenitors.
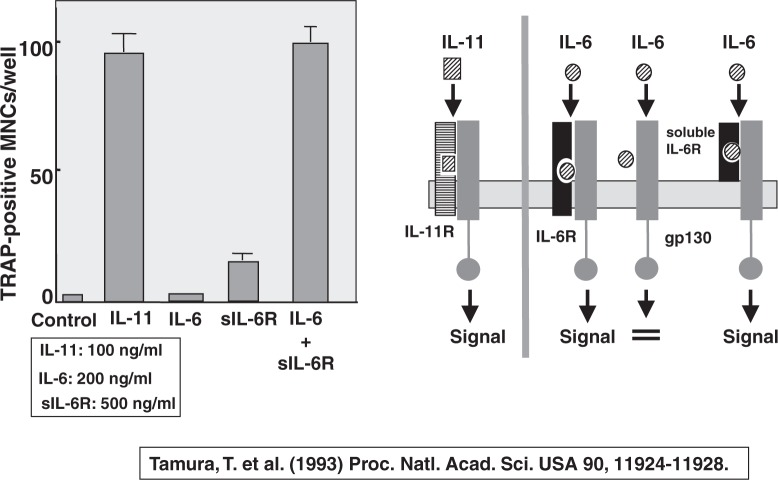
In 1992, Bob Jilka and Stavros Manolagas16) reported that IL-6 is involved in increased osteoclast development after estrogen loss. Their finding was important, but the role of IL-6 in osteoclastogenesis was rather complicated. IL-6 exerts its biological activity via a cell surface receptor, which consists of two components; membrane-bound IL-6 receptor and its signal transducing 130 Kd glycoprotein (gp130).17) When IL-6 receptor is occupied by IL-6, the ligand-receptor complex binds gp130, and IL-6 signals are transduced. IL-11 also belongs to the gp130 super-family. Tatsuya Tamura18) reported that IL-11 formed osteoclasts in co-cultures of WT osteoclasts and bone marrow cells, but IL-6 did not. IL-6 induced osteoclast formation only in the presence of soluble IL-6 receptors, which lack trans-membrane and cytoplasmic domains of the receptors. Soluble IL-6 receptors are found in the serum of healthy humans with a level of 40 to 50 ng/ml. This indicated that the lack of membranebound IL-6 receptors in either osteoblasts or bone marrow cells was critical for osteoclast formation.
Using IL-6 receptor overexpressing mice, Nobi Udagawa19) clearly showed that the presence of the membrane-bound IL-6 receptor in osteoblasts is critical for osteoclastogenesis induced by IL-6. Since WT osteoblasts do not appear to have enough numbers of membrane-bound IL-6 receptors, soluble IL-6 receptor could be substituted for the membrane-bound IL-6 receptors in osteoblasts (Fig. 6). Indeed, soluble IL-6 receptors in the synovial fluids are important for osteoclast formation induced by IL-6. Shigeru Kotake20) found that the levels of both soluble IL-6 receptors as well as IL-6 in the synovial fluids are much higher in patients with rheumatoid arthritis (RA) than those in osteoarthritis (OA) patients. Multinucleated cells found in the synovial tissues of RA patients expressed osteoclast-specific markers such as TRAP, carbonic anhydrase II, vacuolar type proton ATPase, and vitronectin receptors.20) These results indicated that soluble IL-6 receptors are involved in bone and joint destruction in RA patients.
Fig. 6.
Effects of mouse IL-6 and soluble IL-6 receptor (sIL-6R) on osteoclast formation in co-cultures of mouse osteoblastic cells and bone marrow cells.
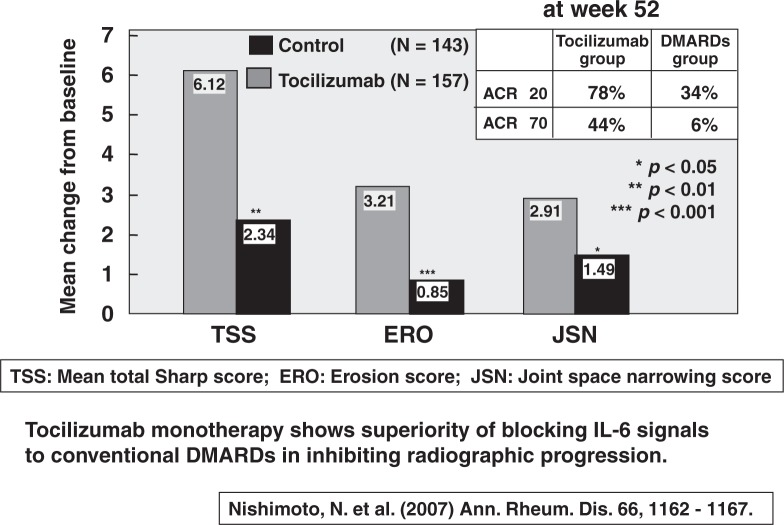
Based on these experimental findings, Norihiro Nishimoto and Tadamitsu Kishimoto21) prepared a humanized anti-IL-6 receptor monoclonal antibody (tocilizumab) and evaluated its ability to inhibit progression of structural joint damage in active RA patients. This clinical trial was called SAMURAI trial (Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor). In this clinical trial, 300 active RA patients were randomly allocated to receive either tocilizumab at 8 mg/kg iv every 4 weeks or conventional DMARDs including methotorexisate (MTX) for 52 weeks. No anti-TNF reagents were used in either group. At week 52, patients in the tocilizumab group showed significantly less radiographic progression, as measured by changes in TSS (total Sharp score), ERO (erosion score), and JSN (joint space narrowing score) (Fig. 7). The percentages of patients who achieved ACR20 were 78% in the tocilizumab group and 34% in the DMARDs group, and those who achieved ACR70 were 44% in the tocilizumab group and only 6% in the DMARDs group (Fig. 7).21) The tocilizumab monotherapy was generally tolerated. This is the first trial showing the superiority of blocking IL-6 signals with tocilizumab to conventional DMARDs therapy in inhibiting radiographic progression. The tocilizumab therapy for RA patients has now received permission from the Ministry of Health, Welfare, and Labor, which is expected to be useful for the treatment of bone and joint destruction of RA patients worldwide.
Fig. 7.
The Samurai trial: Clinical results.
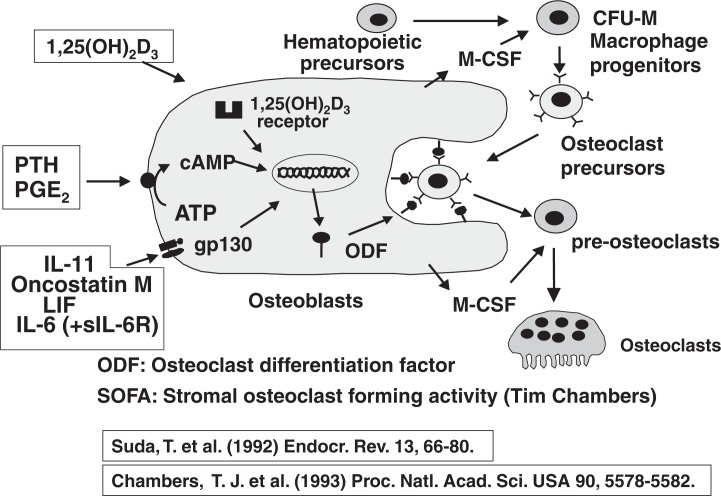
Figure 8 summarizes our working hypothesis on the possible involvement of osteoblasts in osteoclast formation induced by several bone-resorbing factors.22) It is important that the target cells of bone-resorbing hormones and cytokines are osteoblasts, but not osteoclast progenitors. The bone-resorbing factors were classified into three categories in terms of their signal transduction pathways. 1α,25(OH)2D3 induced osteoclast formation via vitamin D receptor (VDR) present in the nuclei. PTH, PGE2 and IL-1 induced osteoclast formation via a protein kinase A system. The third group included IL-6, IL-11, LIF and oncostatin M, all of which transduced their signals via gp130. These 3 diverse signals appeared to stimulate osteoclast formation independently, since osteoclasts were present in either VDR-deficient, PTHR-deficient or gp 130-deficient mice. In other words, there is redundancy in bone-resorbing factors to recruit osteoclasts. We proposed that osteoclast differentiation factor (ODF) is commonly induced on the plasma membrane of osteoblasts in response to these bone-resorbing factors.22) ODF appeared identical to SOFA (stromal osteoclast forming activity) proposed by Tim Chambers.23) Osteoclast precursors having ODF receptor recognize ODF in osteoblasts through cell-to-cell interaction and differentiate into osteoclasts. M-CSF produced by osteoblasts also appeared to play an important role in the proliferation and differentiation of osteoclast progenitors.
Fig. 8.
Roles of osteoblasts in osteoclast formation.
5. Discovery of OPG and OCIF
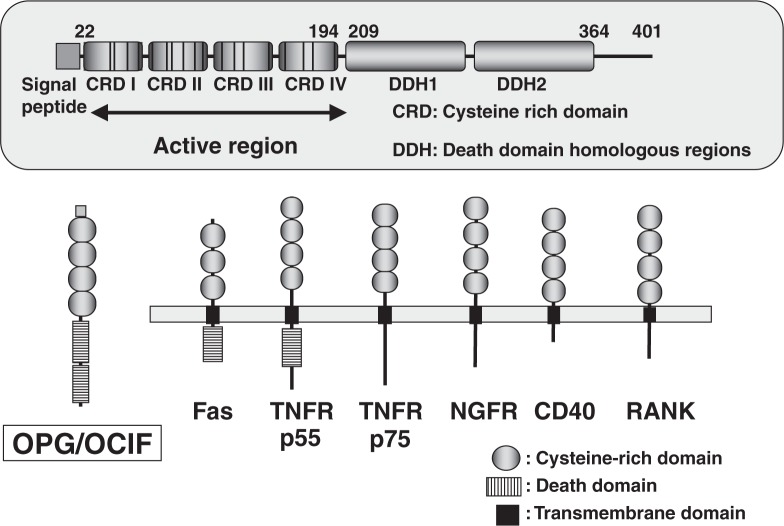
In the course of searching a new member of TNF receptor super-family, Bill Simonet of Amgen24) successfully isolated a novel secretory member of the TNF receptor super-family. Almost all of the members belonging to this super-family are trans-membrane proteins that elicit signal transduction in a wide variety of cells. However, the novel member isolated by Simonet lacked any apparent cell-association domain, indicating that it likely acts in the extra-cellular milieu (Fig. 9). This secretory protein contained four cysteine-rich domains and two death domain homologous regions, which mediate apoptotic signals (Fig. 9). Transgenic mice expressing this secretory protein exhibited a generalized increase in bone density like in osteopetrosis associated with a decreased number of osteoclasts.24) This protein was named osteoprotegerin (OPG) to protect bone.
Fig. 9.
Structure of OPG/OCIF.
Eisuke Tsuda of Snow Brand Milk Products25) independently and simultaneously isolated a novel protein termed osteoclastogenesis inhibitory factor (OCIF) as a heparin-binding glycoprotein from conditioned media of human embryonic lung fibroblasts (IMR-90). The molecular weight of this novel protein was 60 kDa for a monomer and 120 kDa for a homodimer. The cDNA sequence of OCIF was identical to that of OPG.
1α,25(OH)2D3 strikingly increased the specific binding sites for OPG/OCIF in stromal ST2 cells derived from mouse bone marrow.26) Scatchard analysis indicated the presence of a single class of high affinity binding sites for OPG/OCIF in response to 1α,25(OH)2D3. These observations suggested that a ligand specifically bound to OPG/OCIF is present in ST2 cells. We hypothesized that ODF could be a common ligand for both ODF receptor and OPG/OCIF. ODF receptor must be located on the plasma membrane of osteoclast progenitors, whereas OPG/OCIF should be a secretory protein of the TNF receptor super-family. In other words, OPG/OCIF could act as a decoy receptor for ODF to inhibit osteoclast formation.
6. Molecular cloning of ODF
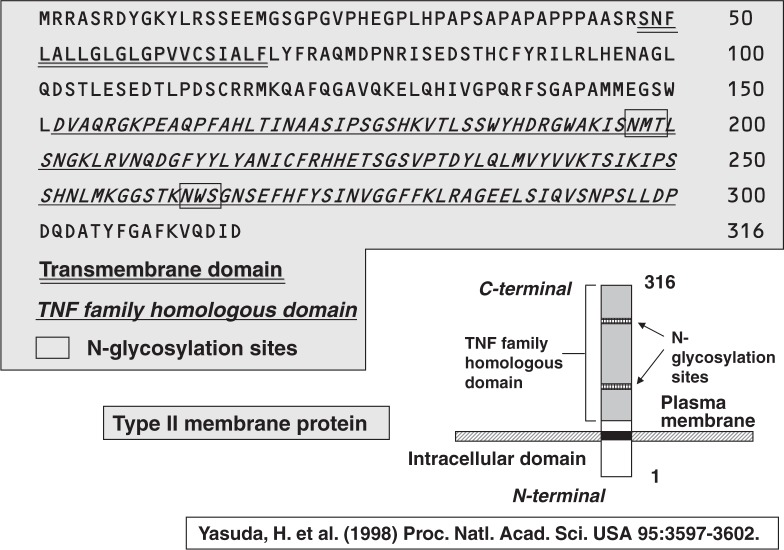
By screening a cDNA expression library of ST2 cells treated with 1α,25(OH)2D3 and dexamethasone, Hisataka Yasuda and Kanji Higashio27) finally cloned a single positive clone of ODF, which contained a 1.65 Kb insert with an open reading frame encoding 316 amino acid residues (Fig. 10). Hydropathy analysis showed that ODF had no signal sequence, but had an internal hydrophobic domain consisting of 24 amino acid residues, which presumably represents a trans-membrane domain. A homology search of the Gen Bank sequence database revealed that extra-cellular C-terminal 165 amino acid residues had a significant homology to the TNF ligand super-family. This structure was typical of a type II trans-membrane protein: its C-terminal sequence exposed outside the cell and the N-terminal sequence intra-cellularly located (Fig. 10).
Fig. 10.
Isolation of ODF as an OCIF-binding protein.
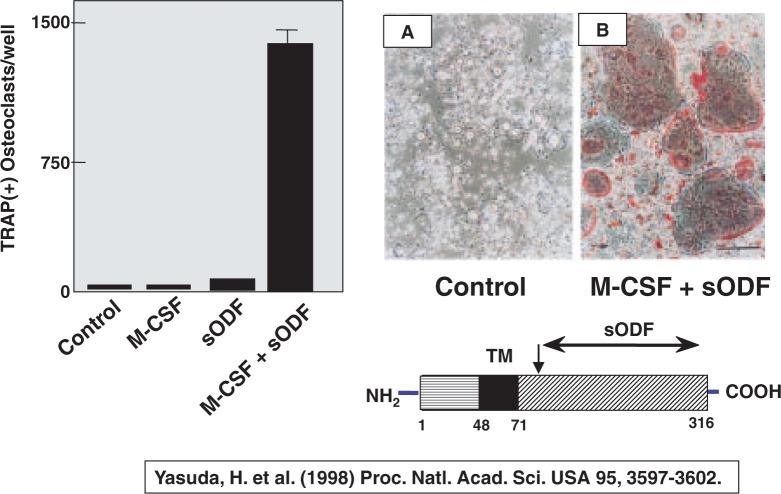
To examine whether ODF is indeed a longsought factor for osteoclastogenesis, Hisataka Yasuda27) produced a soluble form of ODF [ODF76–316], and examined its effects on osteoclast formation in the absence of osteoblasts. When mouse spleen cells were cultured with M-CSF and soluble ODF in the absence of osteoblasts, numerous TRAP-positive osteoclasts were formed (Fig. 11). Adding M-CSF alone or soluble ODF alone failed to form osteoclasts. Like in co-cultures with osteoblasts, many TRAP-positive multinucleated cells were formed in pure spleen cell cultures in response to soluble ODF and M-CSF. This indicates that the combination of M-CSF and ODF can be substituted for osteoblasts in recruiting osteoclasts.
Fig. 11.
Soluble ODF together with M-CSF induces osteoclasts in spleen cell cultures even in the absence of osteoblasts.
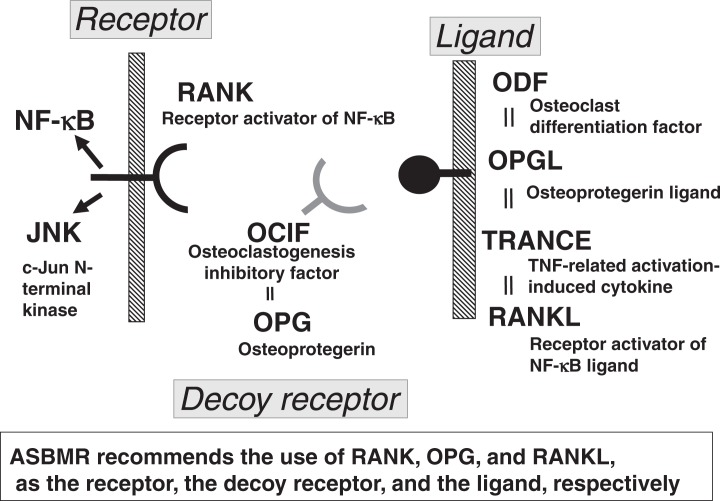
Our paper on the molecular cloning of ODF appeared in the 1998 March issue of the PNAS, USA,27) and it was ranked as the 32nd of the Red Hot Papers in that year, which were selected and ranked in order by the citation numbers within the same year of publication. David Lacy of Amgen28) reported the molecular cloning of OPG ligand (OPGL) in the April issue of the Cell. ODF and OPGL were the same molecule, which were also identical to TNF-related activation-induced cytokine (TRANCE) reported by Yangwon Choi of Rockefeller University29) and receptor activator of NF-kB ligand (RANKL) reported by DM Anderson of Immunex (Fig. 12).30) The ad hoc committee of the ASBMR chaired by Larry Riggs recommended the use of RANK, OPG and RANKL as the receptor, the decoy receptor and the ligand, respectively.31)
Fig. 12.
RANKL-RANK interaction in osteoclastogenesis.
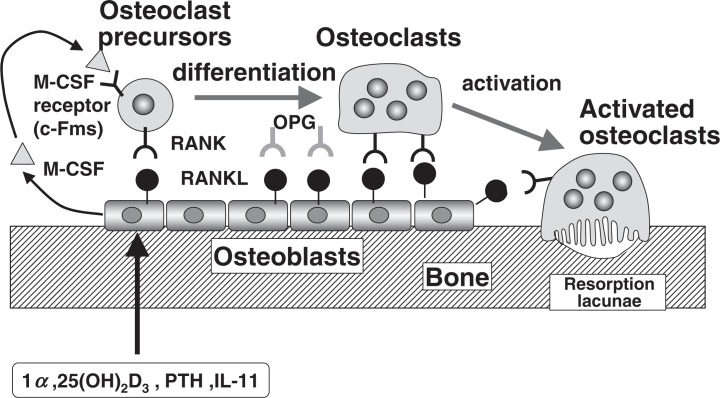
Figure 13 summarizes the molecular mechanisms of osteoclast differentiation and activation. All bone-resorbing factors like 1α,25(OH)2D3, PTH and IL-11 act on osteoblasts to induce the membrane-associated factor called RANKL, which recognizes RANK present in osteoclast progenitors and osteoclasts by a mechanism involving cell-to-cell interaction. M-CSF is also an essential factor for osteoclast proliferation and differentiation, which is produced by osteoblasts in bone tissue. Osteoclast precursors differentiate into osteoclasts, then activated, both mechanisms are controlled by the same molecule RANKL. When OPG covers RANKL, osteoclast precursors and osteoclasts are unable to bind RANKL, thus OPG acts as a decoy receptor in the RANKL/RANK interaction.
Fig. 13.
Molecular mechanism of osteoclast differentiation and activation.
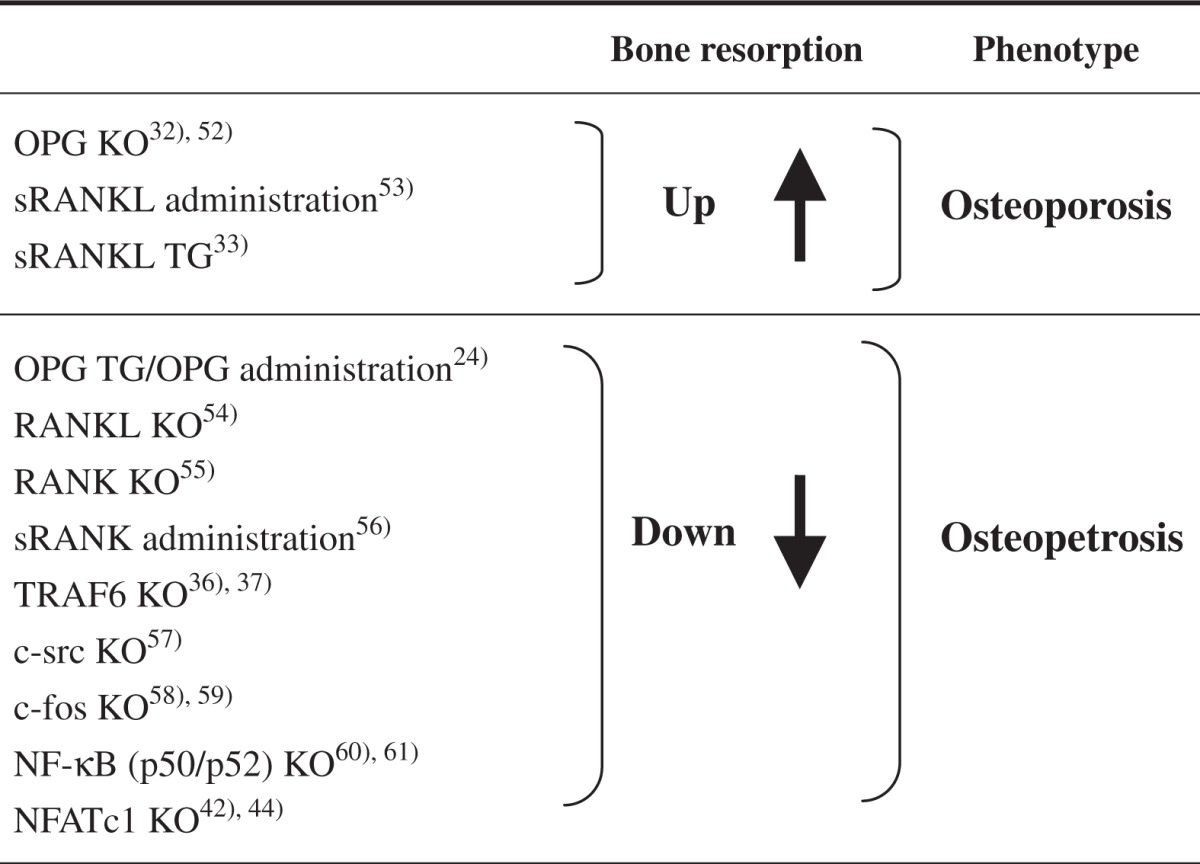
The molecular mechanism of osteoclast formation and activation proposed by in vitro studies has now been confirmed by a number of in vivo studies using engineered gene-mutated transgenic and knockout mice of the RANKL-RANK-OPG system. Table 1 summarizes that OPG knockout mice induced osteoporotic phenotype with many osteoclasts. 32) Soluble RANKL (sRANKL) transgenic mice as well as administration of recombinant sRANKL also exhibited osteoporosis.33) In contrast, OPG transgenic mice as well as administration of OPG induced osteopetrosis with no osteoclasts. Knockout mice of RANK or RANKL induced osteopetrosis as well. These results indicate that the proposed mechanism of osteoclast differentiation and activation occurs in vivo as well.
Table 1.
Bone resorption and skeletal phenotype of genemutated mice of RANKL-RANK signals
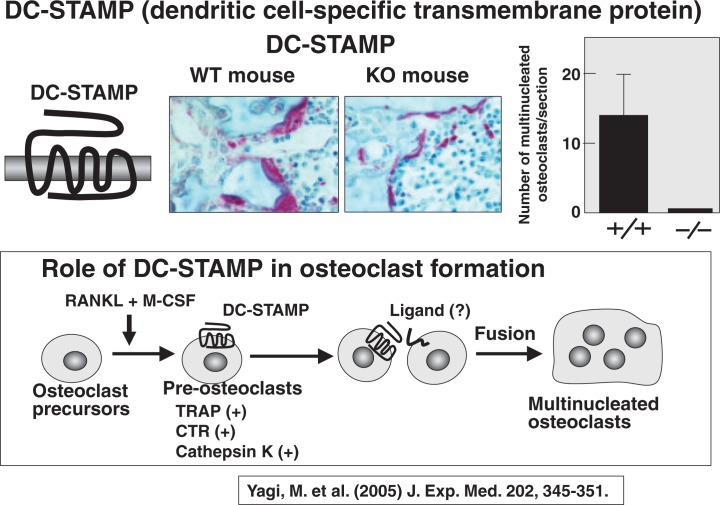
7. DC-STAMP is involved in cell-to-cell fusion in osteoclastogenesis
Osteoclasts are multinucleated cells. Very recently, two Japanese research groups discovered DC-STAMP (a dendritic cell specific trans-membrane protein) as an essential factor involved in cell fusion of mononuclear pre-osteoclasts. Using the osteoclastogenic RAW cell line, Toshio Kukita of Kyushu University34) identified an interesting protein with a seven trans-membrane domain, called DC-STAMP. Mitsuru Yagi and Toshio Suda of Keio University35) generated DC-STAMP knockout mice. Figure 14 shows TRAP staining of tibia of 8-week-old wild type (WT) and DC-STAMP-deficient mice. To our surprise, no multinucleated osteoclasts were detected in DC-STAMP-deficient mice, though many TRAP-positive mononuclear cells were found. Osteoclast progenitors from DCSTAMP-deficient mice differentiated into TRAPpositive mononuclear cells but did not form multinucleated cells in vitro.35) These results confirm that DC-STAMP is essential for inducing cell-to-cell fusion of mononuclear pre-osteoclasts.
Fig. 14.
DC-STAMP is involved in fusion of pre-osteoclasts.
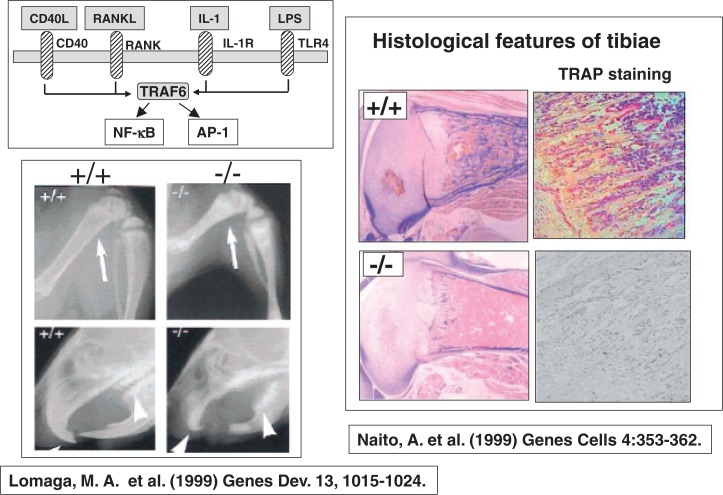
8. Involvement of TRAF6 in RANKL-induced osteoclast formation
The next mile-stone in the research on osteoclast biology was the discovery of involvement of TRAF6 (TNF receptor-associated factor 6) in RANKL-induced osteoclast formation. Asuka Naito and Jun-ichiro Inoue of the University of Tokyo,36) and Mark Lomaga of Amgen at the University of Toronto37) contributed a lot to our understanding of the role of TRAF6 in osteoclastogenesis. TRAF6 is involved in signaling pathways of not only RANK, but also CD40, IL1 receptor (IL-1R), and toll-like receptor (TLR4), the receptor for LPS (Fig. 15). TRAF6 signals activate both NF-κB and AP1. Mark Lomaga in Toronto and Asuka Naito in Tokyo independently generated TRAF6-deficient mice, which showed severe osteopetrotic phenotype with defects in tooth eruption due to impaired bone resorption.36),37) Histological features of TRAF6-deficient mice produced by the Tokyo group showed severe osteopetrosis with fewer numbers of osteoclasts (Fig. 15). These results suggest that TRAF6 plays an important role not only in osteoclast function but also in osteoclast formation.
Fig. 15.
Severe osteopetrosis in TRAF6-deficient mice.
To understand the molecular mechanism of TRAF6-mediated osteoclastogenesis, Jin Gohda38) examined RANKL-induced osteoclast formation in splenocyte cultures prepared from TRAF6-deficient mice. Osteoclasts were formed in wild type splenocyte cultures, but no osteoclasts were formed in TRAF6-deficient splenocyte cultures. Transfection of TRAF6 into splenocytes induced osteoclast formation, but TRAF2 transfection did not.38) These results suggest that the RANK-TRAF6 pathway leads to osteoclast differentiation and function.
9. Discovery of NFATc1 (also called NFAT2) as an essential transcription factor in RANKL-induced osteoclastogenesis
The next breakthrough in osteoclast research was the identification of NFATc1, also called NFAT2, a transcription factor, essentially involved in RANKL-induced osteoclastogenesis. NFAT was originally identified as a transcription factor responsible for inducing IL-2 after T-cell activation. Japanese scientists including Tatsuo Takeya and Hiro Takayanagi played major roles on this matter as well.
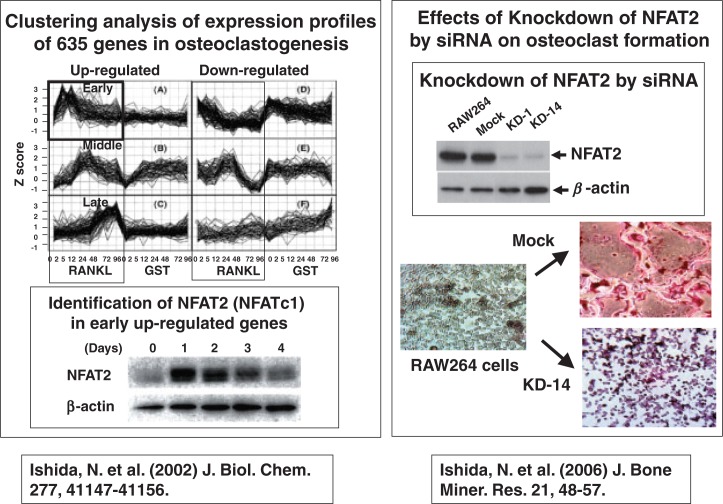
Norihiro Ishida of Takeya’s group39) selected 635 genes that showed marked changes in expression after RANKL stimulation in RAW 264 cells. They were classified into 6 groups: up-regulated genes and down-regulated genes at early, middle, and late stages after RANKL stimulation (Fig. 16). Norihiro Ishida focused on a group of up-regulated early genes, and identified NFAT2 as an important transcription factor for osteoclast differentiation. Down-regulation by siRNA of NFAT2 mRNA in RAW264 cells (clone KD1 and KD14) suppressed the differentiation of RAW cells into osteoclasts (Fig. 16).40) These results suggest that NFAT2 plays a key role in osteoclastogenesis.
Fig. 16.
Identification of NFAT2 in early up-regulated genes during oteoclastogenesis.
(The upper right panel adapted from J. Bone Miner. Res. 2006; 21:48–57 with permission of the American Society for Born and Mineral Research.)
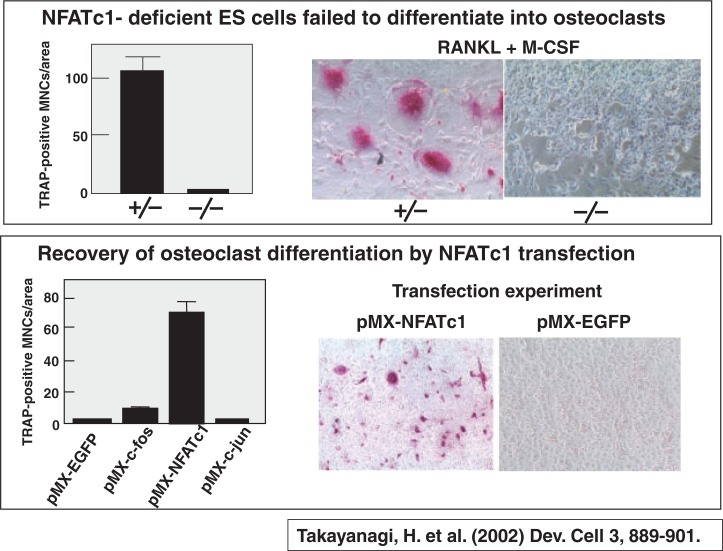
Hiro Takayanagi41) independently identified NFATc1 as an essential transcription factor for osteoclastogenesis. NFATc1 was the same molecule as NFAT2, which Takeya identified as an early upregulated gene after RANKL stimulation.39) Hiro Takayanagi investigated the role of NFATc1 in osteoclastogenesis, using NFATc1-deficient ES cells. Heterogeneously NFATc1-deficient ES cells underwent differentiation into TRAP-positive osteoclasts, but ES cells totally lacking NFATc1 was defective in osteoclast formation (Fig. 17). Osteoclast differentiation of NFATc1-deficient ES cells was recovered specifically by NFATc1 transfection (Fig. 17). Neither c-Fos nor c-Jun transfection failed to generate osteoclast differentiation of NFATc1-deficient ES cells. These results suggest that NFATc1 (NFAT2) plays a key role in osteoclastogenesis. However, in vivo analysis of the role of NFATc1 was hampered at that time, because NFATc1-deficient embryos died of defects in heart valve development. Asagiri et al.42) provided genetic evidence for the first time that NFATc1 is essential for osteoclast differentiation in vivo using adoptive transfer of NFATc1-deficient hematopoietic stem cells to c-Fos-deficient mice, which lack osteoclast differentiation, originally reported by Agi Grigoriadis in 1994.43) When hematopoietic stem cells obtained from WT mice were injected into c-Fos-deficient newborn mice, osteopetrosis was markedly rescued. In contrast, when NFATc1-deficient hematopoietic stem cells were transferred to c-Fos-deficient mice, they still exhibited severe osteopetrosis, and bone marrow cavities remained occupied with unresorbed bone.42) Winslow et al.44) generated viable NFATc1-deficient mice by expression of an NFATc1 transgene under the control of the endothelial-specific Ti-2 promoter in NFATc1-deficient embryos (Tie-2-NFATc1+ embryos). Tie-2-NFATc1+ mice appeared normal at birth, but gained much weight slower than their littermates and died before adulthood. These mice showed greatly reduced osteoclastogenesis as revealed by the failure of tooth eruption, osteopetrosis, and very few small TRAP-positive cells.44) These results confirm that NFATc1 is indispensable for osteoclast formation in vivo as well.
Fig. 17.
NFATc1 is essential for differentiation of ES cells into osteoclasts.
10. Relationship between NFATc1 (NFAT2) with other transcription factors in RANKL-induced osteoclastogenesis
Investigation of the relationship between NFATc1 and other transcription factors like c-Fos and c-Jun was another important issue in osteoclast biology. AP-1 refers to a family of dimeric transcription factors composed of Fos and Jun. Koichi Matsuo and Toshi Yoneda also contributed a lot to our understanding of the relationship between NFATc1 and other transcription factors.
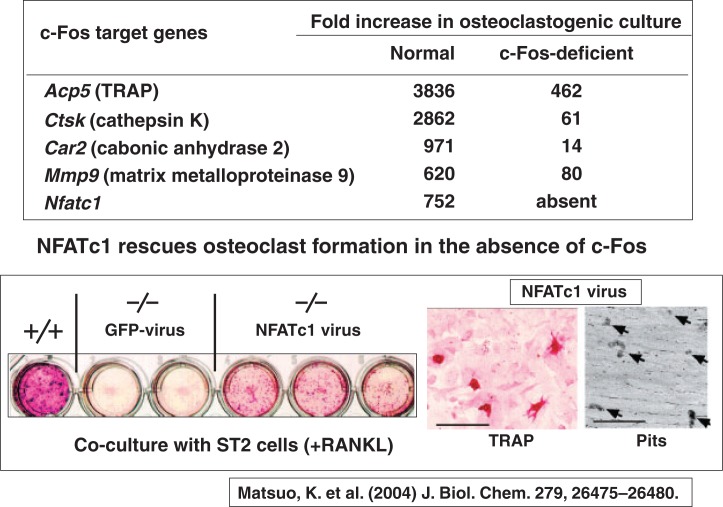
Koichi Matsuo of Keio University was interested in the osteopetrotic phenotype of c-Fosknockout mice, which lack osteoclast differentiation. Using microarray analysis, Koichi45) clearly showed that NFATc1 was down-regulated in c-Fosdeficient osteoclast precursors, like other osteoclastspecific genes encoding TRAP, cathepsin K, carbonic anhydrase 2, and MMP9 (Fig. 18). Moreover, TRAP staining as well as pit-forming activity of c-Fos-deficient splenocytes was rescued by transfection of NFATc1 virus, but not by GFP virus in the presence of RANKL (Fig. 18). These results clearly indicate that the lack of NFATc1 expression in c-Fos-deficient osteoclast precursors is the cause of the block of osteoclast differentiation.
Fig. 18.
NFATc1 is down-regulated in c-Fos-deficient osteoclast precursors.
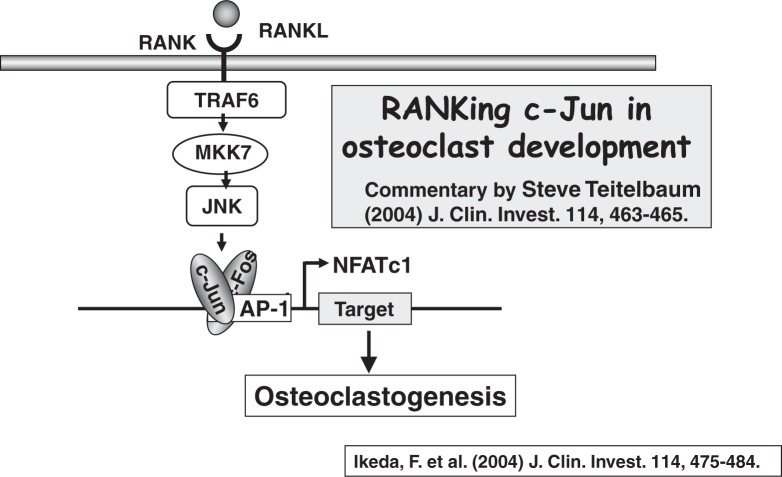
To investigate the role of c-Jun in osteoclastogenesis, Fumiyo Ikeda of Toshi Yoneda’s laboratory at Osaka University46) generated transgenic mice expressing dominant negative c-Jun in the osteoclast lineage cells. They clearly showed that the transgenic mice of dominant negative c-Jun exhibited severe osteopetrosis due to impaired osteoclastogenesis. 46) The tooth eruption was missing in the transgenic mice, despite of the formation of tooth germs. The transgenic mice also showed increased radiodensity of long bones. TRAP staining of long bones showed the absolute lack of TRAP-positive osteoclasts in dominant negative c-Jun transgenic mice.
Taken together, these results indicate that the RANKL-RANK signals are transported into TRAF6, MKK7, JNK, AP1 and NFATc1, in that order. Steve Teitelbaum47) has named this signalling cascade “RANKing c-Jun in osteoclast development” in the commentary in the same issue of the J. Clin. Invest. (Fig. 19).
Fig. 19.
Activation of c-Jun is essential for RANKL-induced osteoclastogenesis.
11. Establishment of a new research field: Osteoimmunology
One of the most exciting findings in osteoclast biology for the past few years would be the establishment of a new research field called “Osteoimmunology.” The crosstalk between the immune and bone systems has long been appreciated, but only recent work on bone destruction associated with inflammation as well as bone phenotype found in knockout mice of immune-related molecules has attracted attention to the interdisciplinary field called “Osteoimmunology”.48)
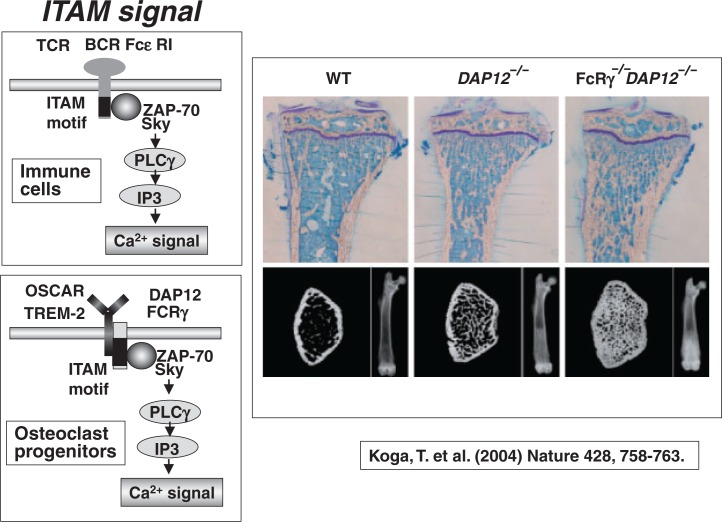
Takako Koga of Takayanagi’s laboratory49) found that mice lacking immune receptor tyrosine-based activation motif (ITAM)-harbouring adaptors, Fc receptor common gamma (FcR gamma) subunit and DNAX-activating protein 12 (DAP12) exhibit severe osteopetrosis owing to impaired osteoclast differentiation. Double knockout mice of FcR gamma and DAP12 developed much severer osteopetrosis (Fig. 20). This indicates that, in addition to RANKL- and M-CSF-induced signals, ITAM signals are also essential for osteoclast formation. It is known that FcR gamma and DAP12 are associated with multiple immunoreceptors and activate calcium signaling through phospholipase C gamma. Takako Koga49) proved that FcR gamma and DAP12 activate calcium signaling through PLC gamma in osteoclast progenitors as well.
Fig. 20.
Severe osteopetrosis in mice lacking both DAP12 and FcRγ.
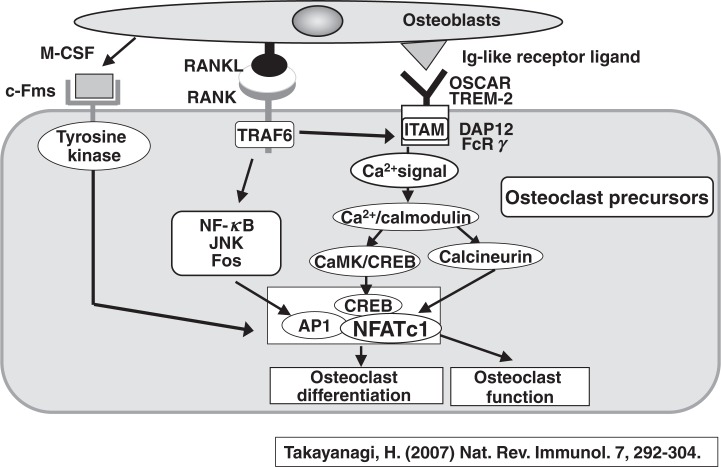
Figure 21 schematically describes an up-to-date model of signal transduction pathways in osteoclastogenesis.50) There are three major signal transduction pathways between osteoblasts and osteoclast progenitors. The first one is the M-CSF signal, which is transduced through tyrosine kinase. The second one is the most important RANKL signal, which is transduced through RANK and TRAF6 in osteoclast progenitors. The induction of NFATc1 is dependent on NF-kB and AP1. The third essential signal is transduced through immunoglobulin-like receptors such as osteoclast-associated receptor (OSCAR) and triggering receptor 2 expressed on myeloid cells (TREM2) with ITAM-harboring adaptors, DAP12, and FcR gamma. The ITAM signal acts as a co-stimulatory signal for RANKL, and activates calcium signaling in osteoclast precursors. Recent studies have shown that the CAMK/CREB pathways play important roles in osteoclast differentiation and function.51)
Fig. 21.
Signaling pathways required for osteoclast differentiation.
Summary and Acknowledgments
Finally, we would like to summarize the major contributions to osteoclast biology from Japan by thanking all of the Japanese scientists who allowed us to introduce their excellent scientific achievements.
Establishment of the mouse co-culture system to examine osteoclast differentiation brought about a new era to modern osteoclast biology research. This system was devised by Takuhiko Akatsu and Nobi Udagawa and ourselves (Showa University), based on the pioneering work by Gideon Rodan (Merck Research Laboratories, West Point, PA.) and Jack Martin (St. Vincent’s Institute of Medical Research, Melbourne), and also by Tim Chambers (St. George’s Hospital Medical School, London). We never forget the great contributions of Eijiro Jimi (Showa University), who proved the essential roles of osteoblasts in activating osteoclast function as well.
The next big contribution from Japan was the establishment of requirement of M-CSF in osteoclast differentiation. Hisa Yoshida, Shin-ichi Hayashi (Kumamoto University), Hiro Kodama (Ohu University), and Sakae Tanaka (University of Tokyo) established the role of M-CSF in osteoclastogenesis. Nobi Udagawa also showed that even mature macrophages can differentiate into osteoclasts. Their contributions confirmed that the origin of osteoclasts is indeed haemopoietic cells of the monocyte-macrophage lineage.
The next step in the understanding of the mechanism of osteoclast formation was the proposal of osteoclast differentiation factor “ODF” by Jack Martin and ourselves (Showa University). Tim Chambers also proposed the presence of stromal osteoclast forming activity “SOFA” in osteoblasts. We believe that the proposal of ODF and SOFA made a driving force to clone the RANK ligand.
The discovery of the identification of osteoblasts as the target cells of vitamin D is due to the excellent work by Shu Takeda, Toshio Matsumoto and Shige-aki Kato (University of Tokyo).
Isolation of OCIF and molecular cloning of ODF by Snow Brand Milk Products chaired by Kanji Higashio was one of the highlights in modern osteoclast biology. His excellent research group consists of Eisuke Tsuda, Hisataka Yasuda, Nobuyuki Shima, and many other brilliant collaborators.
The pioneering work of Bob Jilka and Stavros Manolagas (University of Arkansas for Medical Science) that IL-6 is involved in osteoclastic bone resorption was further extended by Tatsuya Tamura and Shigeru Kotake (Showa University). Based on these basic findings, Norihiro Nishimoto and Tadamitsu Kishimoto (Osaka University) prepared a humanized anti-IL-6 receptor monoclonal antibody called “Tocilizumab” and evaluated its ability to inhibit progression of structural joint damage in active RA patients. This clinical study called the SAMURAI trial has recently established a new therapeutic strategy for RA patients.
Two research groups have successfully identified DC-STAMP (dendritic cell specific transmembrane protein) as a key molecule for cell fusion of mononuclear pre-osteoclasts. Toshio Kukita, Akiko Kukita, and Naohisa Wada (Kyushu University), and Mitsuru Yagi, Takeshi Miyamoto and Toshio Suda (Keio University) contributed to this exciting discovery.
The discovery of the importance of TRAP6 signals for osteoclastogenesis by Jun-ichiro Inoue, Asuka Naito, and Jin Gohda (Institute for Medical Sciences, University of Tokyo) contributed a lot to establish the RANK-mediated signaling pathway.
NFATc1 (also called NFAT2), a key transcription factor for osteoclastogenesis, was independently discovered by two research groups in Japan. Tatsuo Takeya, Norihiro Ishida (Nara Institute of Science and Technology), and Hiro Takayanagi, and Masataka Asagiri (Tokyo Medical and Dental University) were involved in the establishment of this key transcription factor for osteoclastogenesis. Koichi Matsuo (Keio University) discovered a close relation between c-Fos and NFATc1 in osteoclastogenesis.
Discovery of the ITAM motif-mediated costimulatory signals in the RANKL-induced osteoclastogenesis by Takako Koga, Kajiro Sato, and Hiro Takayanagi (Tokyo Medical and Dental University) was another breakthrough in understanding the signal transduction pathways in osteoclastogenesis. This discovery brought about the establishment of a new research field called “Osteoimmunology”, which was originally proposed by Yangwon Choi and Masamichi Takami (Rockefeller University).
Toshi Yoneda and his colleagues (Osaka University) also greatly contributed to osteoclast biology. Fumiyo Ikeda, Riko Nishimura of Toshi’s laboratory (Osaka University) established essential roles of c-Jun signals in RANKL-induced osteoclastogenesis.
We would like to congratulate all of these great contributions to osteoclast biology by Japanese scientists. We also deeply appreciate their encouragement and friendship to allow us to include their exciting work in this review article.
Abbreviations
- 1α,25(OH)2D3
1α,25-dihydroxyvitamin D3
- DMARDs
disease-modifying anti-rheumatic drugs
- ERO
erosion score
- FcRγ
Fc receptor common γ
- CRD
cysteine-rich domain
- CTR
calcitonin receptor
- DC-STAMP
dendritic cell specific trans-membrane protein
- DDH
death domain homologous region
- DAP12
DNAX-activating protein 12
- gp130
130 kDa glycoprotein
- OAF
osteoclast activating factor
- OCIF
osteoclastogenesis inhibitory factor
- ODF
osteoclast differentiation factor
- OPG
osteoprotegerin
- OPGL
OPG ligand
- OSCAR
osteoclast-associated receptor
- IL-6
interleukin 6
- IL-6R
IL-6 receptor
- sIL-6R
soluble IL-6 receptor
- IL-11
interleukin 11
- IL-11R
IL-11 receptor
- ITAM
immune receptor tyrosine-based activation motif
- JSN
joint space narrowing score
- M-CSF
macrophage colony stimulating factor
- MNC
multinucleated cell
- MMP9
matrix metalloproteinase 9
- MKK7
mitogen-activated protein kinase kinase 7
- MTX
methotorexisate
- NFAT
nuclear factor for activating T-cells
- OSCAR
osteoclast-associated receptor
- RA
rheumatoid arthritis
- SAMURAI
Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor
- RANKL
receptor activator of NF-κB ligand
- sRANKL
soluble RANKL
- SOFA
stromal osteoclast forming activity
- TLR4
toll-like receptor 4
- TNF
tumor necrosis factor
- TNFR
TNF receptor
- TRAF6
TNF receptor-associated factor 6
- TRANCE
TNF-related activation-induced cytokine
- TRAP
tartrate-resistant acid phosphatase
- TREM
triggering receptor expressed on myeloid cells
- TSS
total sharp score
- v-ATPase
vacuolar type proton ATPase
- VDR
vitamin D receptor
Biographies
Profile
Tatsuo Suda was born in Tokyo, Japan, in 1935. After graduated from the School of Dentistry, Tokyo Medical and Dental University in 1960, he started his research career on bone biology as a graduate student of the alma mater, inspired by the monograph entiteled “The Chemical Dynamics of Bone Mineral” written by William F. and Margaret W. Neuman. He was a postdoctoral fellow from 1968 to 1971 at the University of Wisconsin, where he was involved in the isolation and identification of vitamin D metabolites including 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3] and 24,25-dihydroxyvitamin D3 under the guidance of Professor Hector. F. DeLuca. After he came back to Tokyo, he developed 1α-hydroxyvitamin D3 [1α (OH)D3] as a synthetic analog of 1α,25(OH)2D3 for the treatment of renal osteodystrophy and osteoporosis in collaboration with Yasuho Nishii of Chugai. In Japan, over one million patients with osteoporosis have been taking 1α (OH)D3 every day since 1983. In collaraboration with Naoyuki Takahashi and many other colleagues at Showa University, he proposed a factor responsible for osteoclast differentiation called osteoclast differentiation factor (ODF) induced by 1α,25(OH)2D3 in 1992. ODF was finally molecularly cloned in 1998 in collaboration with Hisataka Yasuda and Kanji Higashio of Snow Brand Milk Products. He received numerous awards and recognition for his basic research including the William F. Neuman Award from the American Society for Bone and Mineral Research (ASBMR) (1997), the Purple Ribbon Medal (1998), the Asahi Prize (2000), and the Japan Academy Prize (2001). He was elected as a member of the Japan Academy in 2007.

Profile
Naoyuki Takahashi was born in Kanagawa, Japan, in 1952. After finished the master course of the Graduate School of Iwate University (specialized in Agricultural Chemistry), he joined the Department of Biochemistry (Professor: Tatsuo Suda) of Showa University Dental School as a research associate in 1978. In 1984, he took Ph.D. degree from Tokyo Medical and Dental University for the study on the role of vitamin D in bone metabolism in Japanese quail under the guidance of Professor Suda. Nao joined the bone research group in the University of Texas Health Science Center at San Antonio in 1985 as a postdoctoral fellow. He started his life work on osteoclast biology there under the guidance of Professor G. R. Mundy and G. D. Roodman. After Nao returned to Showa University, he organized his research group on osteoclast biology in 1987. In 1988, he established a co-culture system of osteoblasts and hematopoietic cells to study osteoclast formation, and proposed an important concept that osteoblasts express osteoclast differentiation factor (ODF) in response to several bone resorbing factors. The concept was finally proved by the molecular cloning of ODF (known as RANKL) in collaboration with Drs. Hisataka Yasuda and Kanji Higashio of Snow Brand Milk Products. Nao moved to Matsumoto Dental University Institute for Oral Science as Professor of Hard Tissue Research Division in 2001. Since then, he is a leader of the hard tissue research group in Matsumoto Dental University.

References
- 1).Yamaguchi, A., Komori, T. and Suda, T. (2000) Regulation of osteoblast differentiation mediated by bone morphogenetic proteins, hedgehogs, and Cbfa1. Endocr. Rev. 21, 393–411 [DOI] [PubMed] [Google Scholar]
- 2).Suda, T., Udagawa, N. and Takahashi, N. (1996) InOsteoclast generation. In Principles of bone biology (eds. Raisz L.G., Rodan G.A., Bilezikian J.P.). Academic Press, San Diego, pp 87–102 [Google Scholar]
- 3).Seino, Y. (2006) Importance of bone growth during childhood. The Bone 20, 411–415 (in Japanese). [Google Scholar]
- 4).Hosoi, T. (2007) Diagnostic and therapeutic criteria for osteoporosis. The Bone 21, 311–313 (in Japanese). [Google Scholar]
- 5).Väänänen, H. K., Zhao, H., Mulari, M. and Halleen, J. M. (2000). The cell biology of osteoclast function. J. Cell Sci. 113, 377–381 [DOI] [PubMed] [Google Scholar]
- 6).Suda, T., Nakamura, I., Jimi, E. and Takahashi, N. (1997) Regulation of osteoclast function. J. Bone Miner. Res. 12, 869–879 [DOI] [PubMed] [Google Scholar]
- 7).Rodan. G. A. and Martin, T. J. (1981) Role of osteoblasts in hormonal control of bone resorption— a hypothesis. Calcif. Tissue Int. 33, 349–351 [DOI] [PubMed] [Google Scholar]
- 8).McSheehy, P. M. and Chambers, T. J. (1986) Osteoblastic cells mediate osteoclastic responsiveness to parathyroid hormone. Endocrinology 118, 824–828 [DOI] [PubMed] [Google Scholar]
- 9).Takahashi, N., Akatsu, T., Udagawa, N., Sasaki, T., Yamaguchi, A., Moseley, J. M., Martin, T. J. and Suda, T. (1988) Osteoblastic cells are involved in osteoclast formation. Endocrinology 123, 2600–2602 [DOI] [PubMed] [Google Scholar]
- 10).Jimi, E., Nakamura, I., Amano, H., Taguchi, Y., Tsurukai, T., Tamura, M., Takahashi, N. and Suda, T. (1996) Osteoclast function is activated by osteoblastic cells through a mechanism involving cell-to-cell contact. Endocrinology 137, 2187–2190 [DOI] [PubMed] [Google Scholar]
- 11).Yoshida, H., Hayashi, S., Kunisada, T., Ogawa, M., Nishikawa, S., Okamura, H., Sudo, T. and Shultz, L. D. (1990) The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature 345, 442–444 [DOI] [PubMed] [Google Scholar]
- 12).Felix, R., Cecchini, M. G. and Fleisch, H. (1990) Macrophage colony stimulating factor restores in vivo bone resorption in the op/op osteopetrotic mouse. Endocrinology 127, 2592–2594 [DOI] [PubMed] [Google Scholar]
- 13).Kodama, H., Yamasaki, A., Nose, M., Niida, S., Ohgame, Y., Abe, M., Kumegawa, M. and Suda, T. (1991) Congenital osteoclast deficiency in osteopetrotic (op/op) mice is cured by injections of macrophage colony-stimulating factor. J. Exp. Med. 173, 269–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Takeda, S., Yoshizawa, T., Nagai, Y., Yamato, H., Fukumoto, S., Sekine, K., Kato, S., Matsumoto, T. and Fujita, T. (1999) Stimulation of osteoclast formation by 1,25-dihydroxyvitamin D3 requires its binding to vitamin D receptor (VDR) in osteoblastic cells: Studies using VDR knockout mice. Endocrinology 140, 1005–1008 [DOI] [PubMed] [Google Scholar]
- 15).Liu, B. Y., Guo, J., Lanske, B., Divieti, P., Kronenberg, H. M. and Bringhurst, F. R. (1998) Conditionally immortalized murine bone marrow stromal cells mediate parathyroid hormone-dependent osteoclastogenesis in vitro. Endocrinology 139, 1952–1964 [DOI] [PubMed] [Google Scholar]
- 16).Jilka, R. L., Hangoc, G., Girasole, G., Passeri, G., Williams, D. C., Abrams, J. S., Boyce, B., Broxmeyer, H. and Manolagas, S. C. (1992) Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science 257, 88–91 [DOI] [PubMed] [Google Scholar]
- 17).Kishimoto, T. (2005) Interleukin-6: from basic science to medicine—40 years in immunology. Annu. Rev. Immunol. 23, 1–21 [DOI] [PubMed] [Google Scholar]
- 18).Tamura, T., Udagawa, N., Takahashi, N., Miyaura, C., Tanaka, S., Ohsugi, Y., Kumaki, K., Taga, T., Kishimoto, T. and Suda, T. (1993) Soluble interleukin-6 receptor triggers osteoclast formation by interleukin 6. Proc. Natl. Acad. Sci. USA 90, 11924–11928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Udagawa, N., Takahashi, N., Katagiri, T., Tamura, T., Wada, S., Findlay, D. M., Martin, T. J., Hirota, H., Taga, T., Kishimoto, T. and Suda, T. (1995) Interleukin (IL)-6 induction of osteoclast differentiation depends on IL-6 receptors expressed on osteoblastic cells but not on osteoclast progenitors. J. Exp. Med. 182, 1461–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Kotake, S., Sato, K., Kim, K. J., Takahashi, N., Udagawa, N., Nakamura, I., Yamaguchi, A., Kishimoto, T., Suda, T. and Kashiwazaki, S. (1996) Interleukin-6 and soluble interleukin-6 receptors in the synovial fluids from rheumatoid arthritis patients are responsible for osteoclastlike cell formation. J. Bone Miner. Res. 11, 88–95 [DOI] [PubMed] [Google Scholar]
- 21).Nishimoto, N., Hashimoto, J., Miyasaka, N., Yamamoto, K., Kawai, K., Takeuchi, T., Murata, N., van der Hejide, D. and Kishimoto, T. (2007) Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an X-ray reader-blinded randomised controlled trial of tocilizumab, Ann. Rheum. Dis. 66, 1162–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Suda, T., Takahashi, N. and Martin, T. J. (1992) Modulation of osteoclast differentiation. Endocr. Rev. 13, 66–80 [DOI] [PubMed] [Google Scholar]
- 23).Chambers, T. J., Owens, J.M., Hattersley, G., Jat, P. S. and Noble, M. D. (1993) Generation of osteoclast-inductive and osteoclastogenic cell lines from the H-2KbtsA58 transgenic mouse. Proc. Natl. Acad. Sci. USA 90, 5578–5582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Simonet, W. S., Lacey, D. L., Dunstan, C. R., Kelley, M., Chang, M. S., Luthy, R., Nguyen, H. Q., Wooden, S., Bennett, L., Boone, T.et al. (1997) Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell 89, 309–319 [DOI] [PubMed] [Google Scholar]
- 25).Tsuda, E., Goto, M., Mochizuki, S., Yano, K., Kobayashi, F., Morinaga, T. and Higashio, K. (1997) Isolation of a novel cytokine from human fibroblasts that specifically inhibits osteoclastogenesis. Biochem. Biophys. Res. Commun. 234, 137–142 [DOI] [PubMed] [Google Scholar]
- 26).Yasuda, H., Shima, N., Nakagawa, N., Mochizuki, S., Yano, K., Fujise, N., Sato, Y., Goto, M., Yamaguchi, K., Kuriyama, M.et al. (1998) Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): A mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology 139, 1329–1337 [DOI] [PubMed] [Google Scholar]
- 27).Yasuda, H., Shima, N., Nakagawa, N., Yamaguchi, K., Kinosaki, M., Mochizuki, S., Tomoyasu, A., Yano, K., Goto, M., Murakami, A.et al. (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. USA 95, 3597–3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Lacey, D. L., Timms, E., Tan, H. L., Kelley, M. J., Dunstan, C. R., Burgess, T., Elliott, R., Colombero, A., Elliott, G., Scully, S.et al. (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93, 165–176 [DOI] [PubMed] [Google Scholar]
- 29).Wong, B. R., Rho, J., Arron, J., Robinson, E., Orlinick, J., Chao, M., Kalachikov, S., Cayani, E., Bartlett, F. S.III, Frankel, W. N.et al. (1997). TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. J. Biol. Chem. 272, 25190–25194 [DOI] [PubMed] [Google Scholar]
- 30).Anderson, D. M., Maraskovsky, E., Billingsley, W. L., Dougall, W. C., Tometsko, M. E., Roux, E. R., Teepe, M. C., DuBose, R. F., Cosman, D. and Galibert, L. (1997) A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 390, 175–179 [DOI] [PubMed] [Google Scholar]
- 31).Riggs, B.L., Baron, R., Boyle, W.J., Drezner, M., Manolagas, S., Martin, T. J., Stewart, A. F., Suda, T., Yasuda, H., Aubin, J. and Goltzman, D. (The American Society for Bone and Mineral Research, President’s Committee on Nomenclature) (2000). Proposed standard nomenclature for new tumor necrosis factor family members involved in the regulation of bone resorption. J. Bone Miner. Res. 15, 2293–2296 [DOI] [PubMed] [Google Scholar]
- 32).Mizuno, A., Amizuka, N., Irie, K., Murakami, A., Fujise, N., Kanno, T., Sato, Y., Nakagawa, N., Yasuda, H., Mochizuki, S.et al. (1998). Severe osteoporosis in mice lacking osteoclastogenesis inhibitory factor/osteoprotegerin. Biochem. Biophys. Res. Commun. 247, 610–615 [DOI] [PubMed] [Google Scholar]
- 33).Mizuno, A., Kanno, T., Hoshi, M., Shibata, O., Yano, K., Fujise, N., Kinosaki, M., Yamaguchi, K., Tsuda, E., Murakami, A.et al. (2002) Transgenic mice overexpressing soluble osteoclast differentiation factor (sODF) exhibit severe osteoporosis. J. Bone Miner. Metab. 20, 337–344 [DOI] [PubMed] [Google Scholar]
- 34).Kukita, T., Wada, N., Kukita, A., Kakimoto, T., Sandra, F., Toh, K., Nagata, K., Iijima, T., Horiuchi, M., Matsusaki, H.et al. (2004) RANKL-induced DC-STAMP is essential for osteoclastogenesis. J. Exp. Med. 200, 941–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Yagi, M., Miyamoto, T., Sawatani, Y., Iwamoto, K., Hosogane, N., Fujita, N., Morita, K., Ninomiya, K., Suzuki, T., Miyamoto, K.et al. (2005) DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J. Exp. Med. 202, 345–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Naito, A., Azuma, S., Tanaka, S., Miyazaki, T., Takaki, S., Takatsu, K., Nakao, K., Nakamura, K., Katsuki, M., Yamamoto, T. and Inoue, J. (1999) Severe osteopetrosis, defective interleukin-1 signalling and lymph node organogenesis in TRAF6-deficient mice. Genes Cells 4, 353–362 [DOI] [PubMed] [Google Scholar]
- 37).Lomaga, M. A., Yeh, W. C., Sarosi, I., Duncan, G. S., Furlonger, C., Ho, A., Morony, S., Capparelli, C., Van, G., Kaufman, S.et al. (1999) TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 13, 1015–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Gohda, J., Akiyama, T., Koga, T., Takayanagi, H., Tanaka, S. and Inoue, J. (2005) RANK-mediated amplification of TRAF6 signaling leads to NFATc1 induction during osteoclastogenesis. EMBO J. 24, 790–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Ishida, N., Hayashi, K., Hoshijima, M., Ogawa, T., Koga, S., Miyatake, Y., Kumegawa, M., Kimura, T. and Takeya, T. (2002) Large scale gene expression analysis of osteoclastogenesis in vitro and elucidation of NFAT2 as a key regulator. J. Biol. Chem. 277, 41147–41156 [DOI] [PubMed] [Google Scholar]
- 40).Ishida, N., Hayashi, K., Hattori, A., Yogo, K., Kimura, T. and Takeya, T. (2006) CCR1 acts downstream of NFAT2 in osteoclastogenesis and enhances cell migration. J. Bone Miner. Res. 21, 48–57 [DOI] [PubMed] [Google Scholar]
- 41).Takayanagi, H., Kim, S., Koga, T., Nishina, H., Ishiki, M., Yoshida, H., Saiura, A., Isobe, M., Yokochi, T., Inoue, J.et al. (2002) Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 3, 889–901 [DOI] [PubMed] [Google Scholar]
- 42).Asagiri, M., Sato, K., Usami, T., Ochi, S., Nishina, H., Yoshida, H., Morita, I., Wagner, E.F., Mak, T. W., Serfling, E. and Takayanagi, H. (2005) Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J. Exp. Med. 202, 1261–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Grigoriadis, A. E., Wang, Z. Q., Cecchini, M. G., Hofstetter, W., Felix, R., Fleisch, H. A. and Wagner, E. F. (1994) c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science 266, 443–448 [DOI] [PubMed] [Google Scholar]
- 44).Winslow, M. M., Pan, M., Starbuck, M., Gallo, E. M., Deng, L., Karsenty, G. and Crabtree, G. R. (2006) Calcineurin/NFAT signaling in osteoblasts regulates bone mass. Dev. Cell 10, 771–782 [DOI] [PubMed] [Google Scholar]
- 45).Matsuo, K., Galson, D. L., Zhao, C., Peng, L., Laplace, C., Wang, K. Z., Bachler, M. A., Amano, H., Aburatani, H., Ishikawa, H. and Wagner, E. F. (2004) Nuclear factor of activated T-cells (NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J. Biol. Chem. 279, 26475–26480 [DOI] [PubMed] [Google Scholar]
- 46).Ikeda, F., Nishimura, R., Matsubara, T., Tanaka, S., Inoue, J., Reddy, S. V., Hata, K., Yamashita, K., Hiraga, T., Watanabe, T.et al. (2004) Critical roles of c-Jun signaling in regulation of NFAT family and RANKL-regulated osteoclast differentiation. J. Clin. Invest. 114, 475–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47).Teitelbaum, S. L. (2004) RANKing c-Jun in osteoclast development. J. Clin. Invest. 114, 463–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48).Rho, J., Takami, M. and Choi, Y. (2004) Osteoimmunology: interactions of the immune and skeletal systems. Mol. Cells. 17, 1–9 [PubMed] [Google Scholar]
- 49).Koga, T., Inui, M., Inoue, K., Kim, S., Suematsu, A., Kobayashi, E., Iwata, T., Ohnishi, H., Matozaki, T., Kodama, T.et al. (2004) Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature 428, 758–763 [DOI] [PubMed] [Google Scholar]
- 50).Takayanagi, H. (2007) Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 7, 292–304 [DOI] [PubMed] [Google Scholar]
- 51).Sato, K., Suematsu, A., Nakashima, T., Takemoto-Kimura, S., Aoki, K., Morishita, Y., Asahara, H., Ohya, K., Yamaguchi, A., Takai, T.et al. (2006) Regulation of osteoclast differentiation and function by the CaMK-CREB pathway. Nat. Med. 12, 1410–1416 [DOI] [PubMed] [Google Scholar]
- 52).Bucay, N., Sarosi, I., Dunstan, C. R., Morony, S., Tarpley, J., Capparelli, C., Scully, S., Tan, H. L., Xu, W., Lacey, D. L.et al. (1998) Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 12, 1260–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53).Yasuda, H., Mori, K., Nakamichi, Y., Koide, M., Udagawa, N. and Tomimori, Y. (2007) One week evaluation of pharmaceuticals in sRANKL-injected osteopenia model mice. J. Bone Miner Res. 22, Suppl 1, S445. [DOI] [PubMed] [Google Scholar]
- 54).Kong, Y. Y., Yoshida, H., Sarosi, I., Tan, H. L., Timms, E., Capparelli, C., Morony, S., Oliveirados-Santos, A. J., Van, G., Itie, A.et al. (1999) OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397, 315–323 [DOI] [PubMed] [Google Scholar]
- 55).Li, J., Sarosi, I., Yan, X. Q., Morony, S., Capparelli, C., Tan, H. L., McCabe, S., Elliott, R., Scully, S., Van, G.et al. (2000) RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc. Natl. Acad. Sci. USA 97, 1566–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56).Oyajobi, B. O., Anderson, D. M., Traianedes, K., Williams, P. J., Yoneda, T. and Mundy, G. R. (2001) Therapeutic efficacy of a soluble receptor activator of nuclear factor κB-IgG Fc fusion protein in suppressing bone resorption and hypercalcemia in a model of humoral hypercalcemia of malignancy. Cancer Res. 61, 2572–2578 [PubMed] [Google Scholar]
- 57).Soriano, P., Montgomery, C., Geske, R. and Bradley, A. (1991) Targeted disruption of the csrc proto-oncogene leads to osteopetrosis in mice. Cell 64, 693–702 [DOI] [PubMed] [Google Scholar]
- 58).Wang, Z. Q., Ovitt, C., Grigoriadis, A. E., Möhle-Steinlein, U., Rüther, U. and Wagner, E. F. (1992) Bone and haematopoietic defects in mice lacking c-fos. Nature 360, 741–745 [DOI] [PubMed] [Google Scholar]
- 59).Johnson, R. S., Spiegelman, B. M. and Papaioannou, V. (1992) Pleiotropic effects of a null mutation in the c-fos proto-oncogene. Cell 71, 577–586 [DOI] [PubMed] [Google Scholar]
- 60).Iotsova, V., Caamano, J., Loy, J., Yang, Y., Lewin, A. and Bravo, R. (1997) Osteopetrosis in mice lacking NF-κB1 and NF-κB2. Nat. Med. 3, 1285–1289 [DOI] [PubMed] [Google Scholar]
- 61).Franzoso, G., Carlson, L., Xing, L., Poljak, L., Shores, E.W., Brown, K.D., Leonardi, A., Tran, T., Boyce, B.F. and Siebenlist, U. (1997) Requirement for NF-κB in osteoclast and B-cell development. Genes Dev. 11, 3482–3496 [DOI] [PMC free article] [PubMed] [Google Scholar]