Abstract
Due to their chemical stability and nonallergic, nonirritant, and ultraviolet protective properties, titanium dioxide (TiO2) nanoparticles (NPs) have been widely used in industries such as electronics, optics, and material sciences, as well as architecture, medicine, and pharmacology. However, increasing concerns have been raised in regards to its ecotoxicity and toxicity on the aquatic environment as well as to humans. Although insights have been gained into the effects of TiO2 NPs on susceptible biological systems, there is still much ground to be covered, particularly in respect of our knowledge of the effects of the interaction of TiO2 NPs with other chemicals or physical factors. Studies suggest that interactions of TiO2 NPs with other chemicals or physical factors may result in an increase in toxicity or adverse effects. This review highlights recent progress in the study of the interactive effects of TiO2 NPs with other chemicals or physical factors.
Keywords: titanium dioxide, TiO2, nanoparticles, interaction, chemicals, physical factors
Introduction
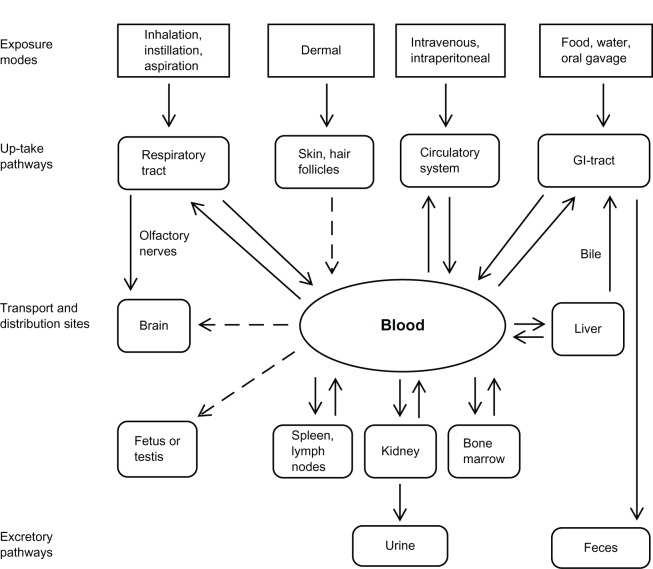
Nanoparticles (NPs) are raw materials used in nanotechnology, with a size range of 1–100 nm in no less than one of their three dimensions.1–3 Titanium dioxide (TiO2) NPs consists of three polymorphs, including anatase, rutile, and brookite.4 TiO2 NPs have been widely used in many products, such as toothpastes, sunscreens, cosmetics, food products, pharmaceuticals, and nanomedical reagents.5 TiO2 particles have been considered as nontoxic mineral particles and traditionally used in the fields of cosmetics, food, and drugs. They were even used as “dust negative control” in many in vitro and in vivo toxicological investigations for many years.3,6 However, research evidence suggests that TiO2 NPs may possess higher toxicity potential than their bulk materials. 5,7,8 Zhao et al8 found that TiO2 NPs caused higher cytotoxicity than fine particles in cell culture. Due to their very small size, NPs can penetrate basic biological structures, which may, in turn, disrupt their normal function.3,9 Recent research evidence shows that TiO2 NPs may induce cellular toxicity effects in cardiac tissue.10 The toxicity effects of TiO2 particles were also observed in cells of the circulatory system. Li et al6 found that the erythrocytes treated with TiO2 NPs underwent abnormal sedimentation, hemagglutination, and hemolysis, which were totally different from those treated with TiO2 fine particles. Lung tumors were also found in rats after lifetime exposure to high concentrations of TiO2 particles.11 Moreover, a recent study showed oxidative stress in mice brain as well as overproliferation of all glial cells.12 These occurred in mice that were exposed to 2.5 mg/kg, 5 mg/kg, and 10 mg/kg body weight TiO2 NPs through nasal administration for 90 days. The toxicokinetics (Figure 1) and toxic effects of TiO2 NPs13–15 alone have been well documented in many in vivo and in vitro studies, but reviews of the effects (or toxicities) of the interaction of TiO2 NPs with other chemicals or physical factors are currently unavailable. TiO2 NPs may coexist with other chemicals or physical factors in the surrounding environment and occupational settings. In the field of nanomedicine, TiO2 NPs are being used as drug carriers.3 Therefore, evaluating the interactive effects of TiO2 NPs with other chemicals or physical factors is vital for the safe application of TiO2 NPs. This review will mainly focus on the current knowledge concerning the effects of the interaction of TiO2 NPs with other chemicals or physical factors and will identify areas where further improvement is needed.
Figure 1.
Toxicokinetics and accumulation sites of titanium dioxide nanoparticles.
Note: Reprinted from Shi et al,13 Copyright 2013, with permission from BioMed Central Publishing.
Abbreviation: GI, gastrointestinal.
Effects of TiO2 NP interaction with metals and their compounds
With growing applications, TiO2 NPs are rapidly entering the aquatic environment,16 and thus the aquatic environment is expected ultimately to be a sink for the sedimentation of these NPs. Consequently, these NPs will inevitably mix and interact with other aquatic pollutants, including metals and their compounds.17 In addition, NPs have been found to be capable of absorbing and separating metals from aqueous or organic solutions.18,19
TiO2 NPs have been tested as a sorbent in the solid-phase extraction for preconcentrated lead (Pb) in river water and sea-water.20–22 Zhang et al20 investigated the potential acute toxicity of the interaction between TiO2 NPs (50 nm and 120 nm) and lead acetate (PbAC) in mice. Suspensions of TiO2 NPs (5 g/kg body weight) alone, PbAC (500 mg/kg) alone, and TiO2 NPs (5 g/kg) plus PbAC (500 mg/kg) were administered to mice via oral gavage, respectively. No synergistic acute toxicity in mice was found after treatment with the combination of TiO2 NPs and PbAC. However, Du et al23 found that, compared with the control (1% dimethyl sulfoxide), mixtures of TiO2 NPs of different doses (21 nm, 80% anatase, 20% rutile) plus PbAC (1 μg/mL) induced a significant increase in reactive oxygen species (ROS) generation (at 0.001 μg/mL, 0.01 μg/mL, 0.1 μg/mL, 1 μg/mL, and 10 μg/mL of TiO2), intracellular superoxide dismutase (SOD) activity (at 0.1 μg/mL and0.01 μg/mL of TiO2), glutathione (GSH) levels (at 0.01–1 μg/mL of TiO2), 8-hydroxydeoxyguanosine levels (at 1 μg/mL and 10 μg/mL of TiO2), 8-oxoguanine DNA glycosylase homologue 1 expression (at 0.001–1 μg/mL of TiO2), and cytotoxicity (at 0.1 μg/mL, 1 μg/mL, and 10 μg/mL of TiO2) in human embryo hepatocyte cells. These results suggest that interaction of TiO2 NPs and PbAC may result in an increase of oxidative stress in culture cells.
Arsenic (As) is a metalloid. Chronic As exposure could cause cancer, neuropathies, and bronchopulmonary, cardiovascular, and metabolic diseases.24 To reduce As pollution, TiO2 NPs have been used as photocatalytic oxidants and/or absorption materials to remove As from water,25 which implies an interaction between As and TiO2 NPs. Due to their small diameter, large surface area, and the ability to uptake –OH ions from solution, TiO2 NPs could adsorb metal ions through electrostatic interaction.26,27 Evidence shows that variables such as pH and temperature may affect the absorption and/or desorption of As (III) and As (V) by TiO2 NPs in the aqueous solution. Pena et al28 found that at 21°C–25°C and pH <8, TiO2 NPs could be used to remove As (V) from solution through adsorption, but the maximum removal for As (III) occurred at about pH 7.5. Additionally, they demonstrated that the competing anions such as silicate, carbonate, and phosphate had a low effect on the adsorption capacities of TiO2 NPs on As (III) and As (V) in a neutral pH range, which was in agreement with the results of Bang et al.29 Niu et al found that the adsorption of As (V) was more favored in acid solution at 25°C, whereas the uptake of As (III) was preferred in alkaline solution by TiO2 NPs at 25°C.30 The maximum uptake of As (V) and As (III) was 208 mg/g (pH =3.0) and 60 mg/g (pH =7.0). Their experiments also suggested that more than 80% of As (III) and 95% of As (V) adsorbed on TiO2 NPs could be desorbed with 1.0 M sodium hydroxide solution within 1 hour, as demonstrated by desorption tests, which was confirmed by Bang et al.29 Jegadeesan et al31 indicated that the capacity for sorption to As by TiO2 NP polymorphs might be affected by sorption site density, surface area (particle size), and crystalline structure. Wang et al32 stated that As toxicity on Ceriodaphnia dubia might increase when TiO2 NPs (5–10 nm) interact with As in the ecosystem. They found that TiO2 NPs less than 400 mg/L alone were nontoxic. The 24-hour median lethal concentration (LC50) of As alone on Ceriodaphnia dubia was 3.68±0.22 mg/L. In addition, the presence of low concentrations of 50 mg/L TiO2 NPs increased the toxicity of As significantly, and the LC50 of As was also lowered to 1.43 mg/L. In summary, available studies show that pH, temperature, composite method, and crystalline structure may all be important for the adsorption of As (III) and As (V) by TiO2 NPs, and an alkaline environment is suitable for the desorption of As (III) and As (V). More studies are needed to investigate the toxicity effects after combination of As with TiO2 NPs.
Copper (Cu) is an important element in human physiological processes. Exposure to low doses of Cu33 is harmless because Cu is an essential trace element for the human body. Adverse immunotoxicological effects on human health could be caused only by an overexposure to Cu. Overexposure, especially a sublethal dose exposure of Cu, could induce immunotoxicity in mice, including cell-cycle arrest and cell death in the spleen and thymus.34 Fan et al35 found that TiO2 NPs (at a concentration generally considered to be safe in the environment) remarkably enhanced the toxicity of Cu on Daphnia magna by increasing the bioaccumulation of Cu. In addition, they found that the Cu was adsorbed on to the TiO2 NPs when ingested and was accumulated in the animals, thereby causing an increase in toxic effects.
Cadmium (Cd) is one of the most toxic elements to which human beings may be exposed.36–38 Cd compounds39 are widely used in rechargeable nickel-Cd batteries. Cigarette smoke, polluted foods, and batteries are the major sources of Cd pollution. Xia et al40 investigated the combined toxicity of cadmium chloride (CdCl2) and TiO2 NPs (25 nm) in human embryo kidney (HEK293T) cells. They found that cotreatment with 3.8 μm/L CdCl2 and 7.5 μg/mL TiO2 NPs exerted additive effects on the cellular oxidative damage by upregulation of heme oxygenase 1 gene expression, catalase activities, and malondialdehyde concentration. A combination of CdCl2 (5.12 μm/L) and TiO2 NPs (10.05 μg/mL) showed synergetic effects on activities of SOD and ROS concentrations. Zhang et al41 assessed the bioaccumulation of Cd in carp in the presence of TiO2 NPs (21 nm) and found that the presence of TiO2 NPs and the accumulation of Cd in carp was positively correlated.
Hu et al42 investigated the combined effects of TiO2 NPs (21 nm) and humic acid (HA) on the bioaccumulation of Cd in zebrafish. They found that the presence of TiO2 NPs at 5–20 mg/L in water containing HA could alter the exposure of Cd and other potential heavy metals to zebrafish. The presence of TiO2 NPs or HA alone with Cd slightly increased the uptake rate constants of Cd in fish. TiO2 NPs have a slightly higher uptake than HA, whereas mixtures of HA and TiO2 NPs with Cd slightly reduced the uptake rate constants. The mechanism underlying these combined effects is unclear.
Yang et al43 investigated Cd adsorption on polyacrylatecoated TiO2 NPs, which could decrease the concentration of Cd ion in an aquatic environment and its effect on the bioavailability as well as toxicity of Cd to green algae Chlamydomonas reinhardtii. They found that Cd absorbed quickly on to TiO2 NPs (anatase, 1–10 nm), reaching a steady state within 30 minutes. Interestingly they found that the presence of TiO2 NPs could alleviate the Cd toxicity to the green algae cells, which might be caused by TiO2 NP adsorption on Cd2+, resulting in a decrease of free Cd ion in the medium and further, its bioaccumulation in the algal cells. In addition, the electrostatic and potentially steric repulsions between TiO2 NPs and algal cells might hinder their direct contact with each other and then prevent the internalization of TiO2 NPs into the cells.
Taken together, toxicities of TiO2 NPs alone have been well documented.10,44,45 However, knowledge of the combined effects of TiO2 NPs with other chemicals is limited. The existing evidence suggests that TiO2 NPs can absorb metal ions, including Pb, As, Cu, and Cd, in the solution. Meanwhile, interaction of TiO2 NPs and metal compounds (see Table 1) may also result in the increased toxicity demonstrated by increased oxidative stress to cells and decreased LC50 to aquatic organisms.23,40 Therefore, more ecology investigations and biology experiments of their combined toxicity should be carried out.
Table 1.
Studies in vitro on the interactive effects of TiO2 NPs with chemicals or physical factors
| Reference | Supplier | Characteristics of TiO2NP
|
Dispersion method | Exposure concentration (μg/mL) | Exposure time | Cell line | Culture medium | Combined factors/exposure condition | Combined effects | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Particle size (nm) | Crystal structure | Surface area (m2/g) | |||||||||
| 23 | Degussa | 21 | 80% anatase, 20% rutile | 49.6 | 10 min ultrasonication and 30 s vortex mixing | 0.001, 0.01, 0.1, 1, 10 | 24 h | L-02 | DMEM | PbAC/1 μg/mL | Cell viability↓, ROS↑, GSH↑, SOD↓, 8-OHdG↑, OGG1 expression↑ |
| 40 | Degussa | 25 | 80% anatase, 20% rutile | 50 | Suspended fresh immediately before use | 0, 0.25, 0.5, 0.75, 1, 1.25 TU (1TU = 10.05 μg/mL) | 24 h | HEK293T | DMEM | CdCl2/0, 0.25, 0.5, 0.75, 1, 1.25 TU (1 TU = 5.12 μmol/L) | HO-1 gene express↑, OGG1 expression↑, SOD↓, ROS↑, MDA↔, CAT↑ |
| 49 | Degussa | 25–50 | 80% anatase, 20% rutile | 50 | vortexed for 2 min, ultrasonicated for 10 min | 0, 1, 5, 10 | 24 h | L-02 | DMEM | BPA/0, 0.1, 1, 10μmol/L | Cell viability↔, ROS↑, MDA↑, DNA double strand break↑, chromosomal damage↑ |
| 55 | Degussa | 25 | 80% anatase, 20% rutile | 50 | NA | 0, 0.01, 0.1, 1 | 12, 24, 36 h | L-02 | DMEM | p,p′-DDT/0, 0.001, 0.01, and 0.1 μmol/L | Cell viability↔, apoptosis test↔, ROS↑, MDA↑, 8-OHdG↑; DNA strand break↑; micronucleus frequency↑ |
| 57 | Degussa | 21 | 80% anatase, 20% rutile | NA | Ultrasonicated for 40 min | 0, 10 | 72 h | 16-HBE | RPMI-1640 | NaF/0, 10, 20, 30 mg/L | Cell viability↔, apoptosis test↑, SOD↓, MDA↑, NO↑ |
| 65 | Degussa | 20 | rutile and anatase | 50 | Ultrasonicated for 15 min | 50 | 1 h | HaCaT | NA | Nitrite, UVA/nitrite: 0, 0.1, 0.5, 1, 2 mM; UVA: 365 nm, 0.6 mW/cm2, 1 h | Cell viability↓, apoptosis test↑, protein tyrosine nitration↑ |
| 66 | Wanjin Material Corp; Degussa | 4, 10, 21 25, or 60 | anatase, rutile; anatase/rutile (3:1) | NA | Freshly prepared and diluted | 0, 10, 50, 100, 200 | 1 h | HaCaT | MEM | UVA/365 nm, 3.5 mW/cm2, 1 h | Cell viability↓, SOD↓, ROS↑, MDA↑ |
| 67 | Degussa | 20 | 70%–85% anatase and 30%–15% rutile | 48.08 | Sonicated for 30 min | 0, 1, 5 | 0, 24, 48 h | Human peripheral blood lymphocytes | RPMI-1640 | UVA/365 nm, 2.0 mW/cm2, 0, 24, 48 h | Cell viability↓, sub-G1 phase↑, caspase-9↑, caspase-3↑, and PARP↑ MMP↓, ROS↑DNA damage↑micronucleus formation↑ |
| 68 | Sigma Chemicals Degussa | <25, <100; 31 | anatase, rutile; 86% anatase, 14% rutile | NA | Sonicated for 30 min | 50, 100 | 4 h | HaCaT | DMEM | UvA/320–390 nm; 0, 2.5, 5.0, and 10 J/cm2 | MTS assay (A325, P25 and A25)↓, ROS↑ |
| 71 | Degussa | 21 | 25% rutile and 75% anatase | NA | Sonicated for 10–15 min | 200 | 24 h | HaCaT | MEM | NAC, UVA/NAC: 5 mM, 2 h UVA: 365 nm, 3.5 W/cm2, 1 h | Cell viability (UVA)↓ (UVA + NAC)↑, LDH (UvA+NAC)↓, apoptosis assay (UVA + NAC)↓, ROS (UVA + NAC)↓, MMP (UVA + NAC)↑, K6 mRNA (UVA + NAC)↑ |
| 75 | Sigma-Aldrich | NA | anatase and rutile | NA | Sonicated for 30 min | 200 | 12 h | U87-MG | DMEM | UVA/365 nm, 5 J/cm2, 20 min | Cell viability↓, BCL2↓, BAX↑ |
| 82 | Degussa | NA | NA | NA | NA | 61, 60 | 10 min | Leukemia K562 | RPMI-1640 | Daunorubicin, UVA/daunorubicin: 0.14 mM, 0.2 mM UVA: 100 s | Drug accumulation↑ |
Abbreviations: ↑ combined effect showed a significant increase than TiO2 NPs group and other factor group alone; ↓ combined effect showed a significant decrease than TiO2 NPs group and other factor group alone; ↔, combined effect showed no significant difference than TiO2 NPs group and other factor group alone; 8-OHdG, 8-hydroxydeoxyguanosine; GSH, glutathione; HO-1 gene, heme oxygenase 1 gene; MMP, mitochondrial membrane potential; NA, data not available; NPs, nanoparticles; OGG1, 8-oxoguanine DNA glycosylase homologue 1; ROS, reactive oxygen species; SOD, superoxide dismutase; TiO2, titanium dioxide; UVA, ultraviolet A ; DNA, deoxyriboNucleic acid; MDA, malondialdehyde; CAT, catalase activities; NO, nitric oxide; PARP, poly (ADP-ribose) polymerase ; MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; NAC, N-acetyl cysteine; LDH, lactic acid dehydrogenase; mRNA, messenger ribonucleic acid; BAX, Bcl-2-associated X protein; DMEM, Dulbecco’s Modified Eagle’s Medium; TU, toxic unit; MEM, Minimum Essential Medium; ADP, adenosine diphosphate; min, minutes; PbAC, Plumbi Acetatis; BPA, bisphenol A; HBE, human bronchial epithelial cell; NaF, sodium fluoride; h, hours; HaCaT, a cell type belonging to an immortal human keratinocyte line; RPMI, Roswell Park Memorial Institute; BCL2, B-cell lymphoma 2 protein.
Effects of TiO2 NP interaction with organic and inorganic compounds
Bisphenol A (BPA) is an endocrine disruptor that can mimic estrogen and may lead to adverse health effects.46,47 Woodruff et al48 analyzed data for 163 chemical analytes in 12 chemical classes in subsamples of 268 pregnant women from the National Health and Nutrition Examination Survey2003–04, a nationally representative sample of the US population. They found that BPA was detectable in 96% of pregnant women. Therefore, interactions between TiO2 NPs and BPA may occur when TiO2 NPs are used as drug carriers in the human body. Zheng et al49 evaluated the interactive effects of TiO2 NPs (25–50 nm) with BPA on their physiochemical properties and in vitro toxicity in human embryo hepatocytes (L-02 cells). They found that TiO2 NPs alone (0 mg/L, 0.1 mg/L, 1 mg/L, and 10 mg/L) or BPA alone (0 μmol/L, 0.1 μmol/L, 1 μmol/L, and 10 μmol/L) did not exert significant DNA and chromosomal damage, whereas the mixture of TiO2 NPs and BPA induced a significant increase in oxidative stress, DNA double-strand breaks, and micronuclei formation in a weak synergistic manner. An increase in intracellular levels of BPA bound by TiO2 NPs was hypothesized to be the reason behind the synergistic toxicity. This could have been determined if the investigators in this study used varying concentrations of TiO2 NPs in the combination.
Dichlorodiphenyltrichloroethane (p,p′-DDT) was widely used as an effective insecticide, and had been proven to have genotoxicity, developmental toxicity, and endocrine-disruptive effects in human beings and a wide range of living organisms.50–52 Therefore, the degradation of DDT had attracted great attention.53,54 Recently, TiO2 NPs have been tested to degrade the p,p′-DDT, which increases the risk of exposure to mixtures of TiO2 NPs and p,p′-DDT Shi et al55 examined the interactive toxicities of p,p′-DDT and TiO2 NPs (25 nm) at low concentrations in L-02 cells. The mixtures induced higher toxicity than TiO2 NPs or p,p′-DDT alone. Combination of traces of TiO2 NPs (0 μg/mL, 0.01 μg/mL, 0.1 μg/mL, and 1 μg/mL) and traces of p,p′-DDT (0 μmol/L, 0.001 μmol/L, 0.01 μmol/L, and 0.1 μmol/L) synergistically enhanced genotoxicity, as demonstrated by an increase in oxidative stress, oxidative DNA adducts, DNA breaks, and chromosomal damage in L-02 cells. The adsorption of p,p′-DDT by TiO2 NPs was approximately 0.3 mmol/g. Synergistic genotoxicity induced by a combination of traces of p,p′-DDT and TiO2 NPs may be a potential environmental risk factor. Sodium fluoride (NaF) and TiO2 NPs are useful additives in household products such as toothpastes.3,56 Xie et al57 examined the combined effects of NaF and TiO2 NPs (21 nm) in human bronchial epithelial cells (16-HBE) and found that combined exposure of NaF and TiO2 NPs could enhance the oxidative stress in 16-HBE cells. In another study, Xu et al58 investigated the interactions of TiO2 NPs with functional biomolecule lysozymes in culture cells. They found that lysozymes were adsorbed on to the surface of TiO2 NPs (60 nm) via electrostatic attraction and hydrogen bonds. They therefore suggested that TiO2 NPs might have some toxic impacts on biomolecules after interacting with biomolecule lysozymes. In summary, studies on the effects of TiO2 NP interaction with organic or inorganic compounds are limited. The existing evidence indicates that interaction of TiO2 NPs with BPA, p,p′-DDT, or NaF may result in enhancement of oxidative stress and further cyto or ecotoxicity.
Effects of TiO2 NP interaction with the physical factor UVA
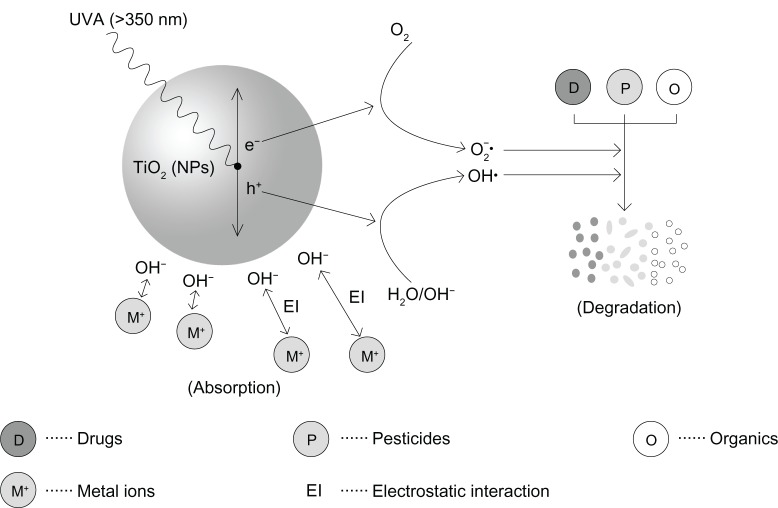
TiO2 NPs possess excellent optical and electrical properties.59–61 Due to photocatalysis, TiO2 NPs62,63 are used to degrade formaldehyde and thus improve air quality in the indoor environment. However, some studies suggest that TiO2 NPs might be toxic under ultraviolet A (UVA), an electromagnetic radiation with wavelength range from 315 nm to 400 nm, ISO-21348. Lu et al64 found that TiO2 NPs (20 nm) could induce photocatalytic nitration of the protein tyrosine, which could lead to prevalent post-translational modification by TiO2 NPs as a result of oxidative and nitrative stress. In another study, Tu et al65 found that nitrative stress induced by TiO2 NPs (20 nm) under UVA radiation triggered apoptotic cell death in human keratinocyte cells. This result suggests that skincare products with TiO2 NP components may cause damage to human keratinocyte cells under UVA radiation. Xue et al66 investigated the oxidative stress and cytotoxicity induced by different crystalline forms (anatase, rutile, and anatase/rutile) and sizes (4 nm, 10 nm, 21 nm, 25 nm, or 60 nm) of TiO2 NPs in HaCaT cells under UVA irradiation. They found that TiO2 NPs could induce ROS generation and toxicity in cells under U VA irradiation. Kang et al67 also showed that TiO2 NPs (20 nm) and UVA (0.6 mW/cm2 for 1 hour) synergistically promoted ROS generation and triggered cell apoptosis. Yin et al68 examined the phototoxicity of TiO2 NPs with different sizes and crystal forms (anatase and rutile) in human skin keratinocytes under UVA irradiation. They found that TiO2 NPs are phototoxic to human skin keratinocytes, and that the phototoxicity is mediated by ROS generated during UVA radiation. Moreover, the phototoxicity of TiO2 NPs was less with larger particle size and surface areas. Zhang et al69 investigated the combined effects of TiO2 NPs and UVA exposure on African clawed frogs (Xenopus laevis). They found that, regardless of UVA exposure, the rate of X. laevis survival decreased with increased concentrations of TiO2 NPs. Exposure to 10 nm TiO2 NPs and UVA significantly decreased the rate of X. laevis survival. However, exposure to 32 nm TiO2 NPs and UVA had no statistical effect on the rate of X. laevis survival. This experiment suggests that toxicity is related to the size of TiO2 NPs to some extent. Bar-Ilan et al70 also found that TiO2 NPs under UV produced ROS as the major phototoxic agent to the development of zebrafish. Xue et al71 examined the chemoprotective effects of N-acetylcysteine (NAC) (5 mM pretreated for 2 hours) on TiO2 NP-induced (21 nm, 200 μg/mL for 24 hours) oxidative stress and apoptosis in human keratinocytes under UVA (3.5 mW/cm2 for 1 hour). NAC,72–74 a nutritional supplement for cysteine donating, is widely used as an antioxidant. They found that NAC could prevent TiO2 NP-induced oxidative stress and apoptosis in cells. The protective effects of NAC on TiO2 NP-induced apoptosis were related to modulation of ROS and intracellular nitric oxide levels. It is worth noting that the combined effect of TiO2 NPs and UVA may be a double-edged sword. Wang et al75 investigated the antitumor effects of TiO2 NPs excited with UVA irradiation both in vitro and in vivo. Their results revealed that TiO2 NPs alone had no effect on glioma cell proliferation. However, when TiO2 NPs were combined with UVA irradiation, the proliferation rate of cells was decreased significantly compared with controls (TiO2 NPs alone or UVA alone). They further investigated the in vivo antitumor effects of combined TiO2 NPs plus UVA on established glioma tumors. TiO2 NPs plus UVA led to pronounced areas of necrosis, elevated indices of apoptosis, delayed tumor growth, and increased survival compared with the TiO2 NPs alone or UVA alone. Moreover, the log-rank test for trend in survival analysis of tumors implanted in animals showed that the average survival duration was prolonged. Additionally, degradation, detoxification, and/or bactericidal effects of TiO2 NPs under UVA to some pesticides or drugs in the ecosystem have been widely investigated.76,77 Under UV-irradiated conditions (λ >350 nm), a TiO2 electron excites an electron from the valance band to the conduction band and electron hole pairs are generated in the surface of TiO2, which consists of the positive hole (h+) and electron (e−). The hole in the valence band has a positive redox potential and is capable of oxidizing organics, H2O, and hydroxide ions on the surface of TiO2 NPs, and eventually to generate hydroxyl radicals (OH). At the same time, the electron promoted from the valence band to the conduction band reduces oxygen into a superoxide radical (O2−). OH, O2− and other perhydroxyl radicals have a strong oxidability to degradate pesticides, drugs, and organic substances (Figure 2). The photocatalytic effects of TiO2 NPs have been indicated to degrade pesticides such as endosulfan and organochlorine.78 Seitz et al79 found that TiO2 NPs (~100 nm; 0.2 mg/L) could reduce nearly 30% of the pirimicarb concentration under UV irradiation (40 W/m2 for 15 minutes), which resulted in an almost complete removal of pirimicarb toxicity to D. magna. Zhao et al80 indicated that TiO2 NPs under UV irradiation could degrade oxytetracycline, which is widely used in both human and veterinary medicine. Higher degraded toxic byproducts were detected by a standardized bioluminescence assay of inhibition rate on Vibrio qinghaiensis sp.–Q67 (Q67), which indicates that a possible enhancement in ecological toxicity may occur after combination of TiO2 NPs with oxytetracycline under UVA irradiation. These results indicate that the photocatalytic effect of TiO2 NPs on pesticides and drugs under UVA irradiation may result in either increased or decreased toxicity, depending on the characteristics of the byproducts.
Figure 2.
Interactive effects (degradation and absorption) of titanium dioxide (TiO2) nanoparticles (NPs) with chemicals under ultraviolet A (UVA) radiation.
Abbreviations: OH, hydroxyl radicals; O2, oxygen; O2–, superoxide radical; H2O, water; e−, electron; h+, the positive hole.
Li et al81 demonstrated that Ag–TiO2 NPs showed a greater synergistic bactericidal activity under UV than TiO2 (inert) or pure Ag NPs alone to Gram-positive bacteria bacillus subtilis and Gram-negative bacteria pseudomonas putida at 25°C. Additionally, Song et al82 found that the synergistic effect of TiO2 NPs under UV irradiation could enhance the drug accumulation dose in targeted leukemia K562 cells and inhibit multidrug resistance. Their findings suggest that combined effects of TiO2 NPs and UVA irradiation may be beneficial for tumor treatment. TiO2 NPs have been well investigated in recent years for enhancing the photocatalytic activity, coating, and doping of zinc oxide (ZnO) on to its surface.83,84 Liao et al83 found that TiO2/ZnO composite NPs had a higher photocatalytic activity in the degradation of methyl orange than both TiO2 and shape-controlled NPs alone. Additionally, Jiang et al85 demonstrated that a combination of the nanosized TiO2 and ZnO powders displayed high photocatalytic activity toward the decolorization of C.I. Basic Blue 41 in water under solar radiation, and a Ti/Zn molar ratio of 1:1 showed highest photocatalytic activity.
In conclusion, TiO2 NPs excited with UVA irradiation could induce ROS generation and thus trigger photocatalytic nitration of the protein tyrosine, oxidative stress, and eventually cell apoptosis. Increased oxidative stress damage may be the principal toxic mechanism. Combined toxicity of TiO2 NPs under U VA may be through ROS-mediated upregulation of the death receptor Fas, and activation of the preapoptotic protein Bax.86 Meanwhile, ROS-induced p53-mediated DNA damage can lead to G2/M cell-cycle arrest or delay and mitochondrial DNA damage.67,69,88 These procedures can ultimately lead to cell apoptosis. This combined toxicity could be alleviated by antioxidant NAC. The combined effects of TiO2 NPs and UVA are a double-edged sword. Synergistic effects in ROS generation and cytotoxicity after a combination of TiO2 NPs and U VA irradiation may be beneficial in the treatment of tumors. A combination of TiO2 NPs and UVA irradiation may also be helpful in degradation of pesticide or drug content in the environment. The beneficial effects also include enhancement in bactericidal activity. However, degradation may also be a possible contributor. An enhancement in toxicity in the ecosystem occurs when higher toxics are degraded and byproducts are generated. Therefore, more studies are necessary to elucidate the effects of the interaction of TiO2 NPs with different drugs or pesticides in ecosystems under UV radiation.
Summary
Research data on the effects of the interaction of TiO2 NPs with chemicals or physical factors are emerging slowly. Experimental factors such as agglomeration/aggregation of NPs, cell type, dispersion method, or culture medium may all affect the experiment results,15,88–90 which may inevitably cause difficulties in interpreting these results. To give the whole picture of the experiments, we summarize in detail the combined toxicities of TiO2 and chemicals or physical factors for all available studies in Tables 1 and 2.
Table 2.
Studies in vivo on the interactive effects of TiO2 NPs with chemicals or physical factors
| Reference | Supplier | Characteristics of TiO2 NP
|
Dispersion method | Living organism | Exposure concentration | Exposure method | Exposure time | Exposure condition of combined factors and experiment | Combined effectsa | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Particle size (nm) | Crystal structure | Surface area (m2/g) | |||||||||
| 20 | Zhejiang Hongsheng Nanotechnology | 50, 120 | NA | NA | Sonicated for 20 min | Kun Ming mice | 5 g/kg | Oral gavage | 7 d | PbAC: 500 mg/kg | Liver and kidney function↓; ROS: liver↑, kidney/cortex/hippocampus↔; MDA↔; liver/kidney: SOD↔, GSH-Px↔, cortex and hippocampus: SOD↓, GSH-Px↓ |
| 32 | Skyspring Nanomaterials Inc | 5–10 | NA | NA | Mixed in a shaker for 24 h | Ceriodaphnia dubia | 200 mg/L | In food | 24 h | NaH2 AsO4 : 0, 0.45, 0.75, 1.5, 2.25, 2.5, 3, and 4.5 mg/L; pH = 7.8, T = 20°C | TiO2 NPs concentration: mortality at low dose↑; mortality at high dose↓ |
| 35 | Nanjing High Technology Material | 13.5 | Anatase | NA | Sonicated for at least 30 min | Daphnia magna | 2 mg/L | In water | 3 d | Copper nitrate: 10, 20, 30, 40, 50, 70 and 100 μg/L; pH = 7.6, T = 23°C | Cu2+ LC50↓, metallothionein level↓ |
| 41 | Degussa | 21 | NA | 50 | NA | Cyprinus carpio | 10.0 ± 1.3 mg/L | In water | 0, 5, 10, 15, 20, 25 d | Cd: 3 4.4 ± 4.8 μg/l, 97.3 ± 6.9 μg/L; pH = 7.8, T = 23°C ± 2°C | Cd accumulation↑ |
| 42 | Evonik Degussa | 21 | NA | 50 ± 15 | Pre-equilibrated for at least 24 h | Zebrafish | 5, 10, 20 mg/L | In water | 0, 1, 2, 5, 8, 12, 16, 20 d | HA: 5, 10, 20 mg/L; Cd: 50 μg/L; pH = 7.0, T = 25°C ± 2°C | Uptake rate constants of Cd bioaccumulation: HA/TiO2↑, HA & TiO2↓ |
| 43 | vivo Nano | 1–10 | Anatase | NA | Coated with hydrophilic sodium polyacrylate | Chlamydomonas reinhardtii | 1, 3, 10, 30, and 100 mg/L | In water | 0, 0.25, 0.5, 0.75, 1, 2, 6 h | Cd: 0, 0.1, 0.3, 0.5, 0.8, 1.0, and 3.0 mg/L; pH = 7.5 ± 0.1, T = 25°C | Free Cd2+ concentration in media↓ |
| 58 | Degussa | 21 | NA | NA | Ultrasonicated for 10 min | Micrococcus lysodeikticus | 0, 2, 4, 6, 8, 10, 15, 20, 25, 30, 40, 50, 60, 80, and 100 mg/L | In water | 10 min | Lysozymes: 0, 0.42, 0.97, 1.39, 2.08 μM; pH = 7.4 | Bacteriolysis activity of lysozyme↓ |
| 69 | Alfa Aesar | 5, 10, 32 | NA | 210, 115, 45 | NA | Xenopus laevis 0 | 31, 1, 3.1, 10, 31, 100, 310, and 1000 mg/L | In water | 14 d | UVA: 400 mW/m2; pH = 7.0~7.8 | X. laevis survival↓; tadpole: body length↓, development stages↓, |
| 75 | Sigma-Aldrich | NA | Anatase and rutile | NA | Sonicated for 30 min | Female BALB/c nude mice | 200 μg per tumor | Air pouch | 12 h | UVA: 365 nm, 5 J/cm2, 30 min | Necrosis↑, apoptosis↑, tumor growth↓, survival↑ |
| 79 | Degussa | 21 | 80% anatase, 20% rutile | 50 ± 15 | Sonicated for 10 min | D. magna | 0.02, 0.2, and 2.0 mg/L | In water | 72 h | Pirimicarb: 20 μg/L; UVA: 300–400 nm, 40 ± 5 W/m2, 15 min; T = 20°C ± 1 °C | UVA and nTiO2: pirimicard concentration↓ |
| 80 | Degussa | 27 | NA | 50 | Dispersed on 5 A or 13X surface | Vibrio qinghaiensis sp.-Q67 | 1, 5, 10, and 15 wt% | In solution | 30, 60, 270 min | OTC: 50 mg/L; UVA: 254 nm, 845 μW/cm2, 2 h; pH = 7 | (TiO2/5A or TiO2/13X) and UVA: inhibition rate↑ |
Note:
The combined effects are effects with TiO2 NPs and combined factors, comparing with effects of nTiO2 or combined factor alone.
Abbreviations: ↓, inhibit, decrease, suppress, or delay; ↑, increase; ↔, no significant changes; GSH, glutathione; NA, data not available; NPs, nanoparticles; ROS, reactive oxygen species; SOD, superoxide dismutase; T, temperature; TiO2, titanium dioxide; UVA, ultraviolet A; MDA, malondialdehyde; CD, cadmium; HA, humic acid; NaH2 AsO4, sodiumarsenate; OTC, oxytetracycline; min, minutes; PbAC, Plumbi Acetatis; h, hours; d, days; pH, the acidity or basicity of an aqueous solution; nTiO2, nano-TiO2.
The existing evidence suggests that TiO2 NPs can absorb metals, including Pb, As, Cu, and Cd, or their ions in solution. A combination of TiO2 NPs with metals, organic or inorganic compounds such as BPA, p,p-DDT, or NaF, and physical factors such as UVA light can result in an increase in oxidative stress and toxicity in culture cells or in aquatic animals. Oxidative stress may further induce tumor gene expression or lead to nuclear and mitochondrial DNA damage in mammalian cells. Further well-designed studies are necessary to elucidate possible mechanisms of these combined effects. In addition, combined toxicity of TiO2 NPs with ZnO/Fe3O4 is yet to be investigated by molecular biological and toxicological experiments. Meanwhile, studies on the combined toxicity of TiO2 NPs with other nanosized particles in toxicology and nanomedicine are also urgently needed. Human epidemiological investigation on the combination of TiO2 NPs with other chemicals is urgently encouraged because TiO2 NPs are increasingly being used as drug carriers in nanomedicine. Studies on the combined effects of TiO2 NPs with chemicals or physical factors on aquatic animals are also urgently needed. In addition, the combined effects of TiO2 NPs with chemicals or physical factors may serve as a double-edged sword. Therefore, studies are also encouraged for TiO2 NP application in heavy metal pollution prevention and tumor treatment.
Acknowledgments
The excellent assistance of Miss Ruth Magaye, Mrs Linda Bowman, and Mr Yaseen Habeeb in the preparation of this article is greatly appreciated. This work was partly supported by the National Nature Science Foundation of China (Grant No 81273111), the Foundations of Innovative Research Team of Educational Commission of Zhejiang Province (T200907), the Nature Science Foundation of Ningbo City (Grant No 2012A610185), the Ningbo Scientific Project (Grant Nos 2012C5019 and SZX11073), the Scientific Innovation Team Project of Ningbo (No 2011B82014), Innovative Research Team of Ningbo (2009B21002), and KC Wong Magna Fund in Ningbo University.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hagens WI, Oomen AG, de Jong WH, Cassee FR, Sips AJ. What do we (need to) know about the kinetic properties of nanoparticles in the body? Regul Toxicol pharmacol. 2007;49:217–229. doi: 10.1016/j.yrtph.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 2.Magaye R, Zhao J, Bowman L, Ding M. Genotoxicity and carcinogenicity of cobalt-, nickel- and copper-based nanoparticles. Exp Ther Med. 2012;4:551–561. doi: 10.3892/etm.2012.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao J, Castranova V. Toxicology of nanomaterials used in nanomedicine. Toxicol Environ Health B Crit Rev. 2011;14:593–632. doi: 10.1080/10937404.2011.615113. [DOI] [PubMed] [Google Scholar]
- 4.Cho WS, Kang BC, Lee JK, Jeong J, Che JH, et al. Comparative absorption, distribution, and excretion of titanium dioxide and zinc oxide nanoparticles after repeated oral administration. Particle and fibre toxicology. 2013;10:9. doi: 10.1186/1743-8977-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Long TC, Tajuba J, Sama P, Saleh N, Swartz C, et al. Nanosize titanium dioxide stimulates reactive oxygen species in brain microglia and damages neurons in vitro. Environ Health perspect. 2007;115:1631–1637. doi: 10.1289/ehp.10216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li SQ, Zhu RR, Zhu H, Xue M, Sun XY, et al. Nanotoxicity of TiO2 nanoparticles to erythrocyte in vitro. Food Chem Toxicol. 2008;46:3626–3631. doi: 10.1016/j.fct.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Magaye R, Zhao J. Recent progress in studies of metallic nickel and nickel-based nanoparticles’ genotoxicity and carcinogenicity. Environ Toxicol pharmacol. 2012;34:644–650. doi: 10.1016/j.etap.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Zhao J, Bowman L, Zhang X, Vallyathan V, Young SH, et al. Titanium dioxide (TiO2) nanoparticles induce JB6 cell apoptosis through activation of the caspase-8/Bid and mitochondrial pathways. J Toxicol Environ Health A. 2009;72:1141–1149. doi: 10.1080/15287390903091764. [DOI] [PubMed] [Google Scholar]
- 9.Buzea C, Pacheco II, Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2007;2:17–71. doi: 10.1116/1.2815690. [DOI] [PubMed] [Google Scholar]
- 10.Jawad H, Boccaccini AR, Ali NN, Harding SE. Assessment of cellular toxicity of TiO2 nanoparticles for cardiac tissue engineering applications. Nanotoxicology. 2011;5:372–380. doi: 10.3109/17435390.2010.516844. [DOI] [PubMed] [Google Scholar]
- 11.Lee KP, Trochimowicz HJ, Reinhardt CF. Pulmonary response of rats exposed to titanium dioxide (TiO2) by inhalation for two years. Toxicol Appl pharmacol. 1985;79:179–192. doi: 10.1016/0041-008x(85)90339-4. [DOI] [PubMed] [Google Scholar]
- 12.Ze Y, Hu R, Wang X, Li B, Su J, et al. Neurotoxicity and gene-expressed profile in brain injured mice caused by exposure to titanium dioxide nanoparticles. J Biomed Mater Res A. 2013 Mar 27;00A:3–9. doi: 10.1002/jbm.a.34705. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 13.Iavicoli I, Leso V, Bergamaschi A. Toxicological effects of titanium dioxide nanoparticles: a review of in vivo studies. J Nanomater. 2012;2012:5. [PubMed] [Google Scholar]
- 14.Magdolenova Z, Collins AR, Kumar A, Dhawam A, Stone V, et al. Mechanisms of genotoxicity: review of recent in vitro and in vivo studies with engineered nanoparticles. Nanotoxicology. 2013:1–73. doi: 10.3109/17435390.2013.773464. [DOI] [PubMed] [Google Scholar]
- 15.Shi H, Magaye R, Castranova V, Zhao J. Titanium dioxide nanoparticles: a review of current toxicological data. Part Fibre Toxicol. 2013;10:15. doi: 10.1186/1743-8977-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma VK. Aggregation and toxicity of titanium dioxide nanoparticles in aquatic environment: a review. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2009;44:1485–1495. doi: 10.1080/10934520903263231. [DOI] [PubMed] [Google Scholar]
- 17.Hartmann NB, Legros S, Von der Kammer F, Hofmann T, Baun A. The potential of TiO2nanoparticles as carriers for cadmium uptake in Lumbriculus variegatus and Daphnia magna. Aquat Toxicol. 2012:118–119. 1–8. doi: 10.1016/j.aquatox.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Tavallali H. Alumina-coated magnetite nanoparticles for solid phase extraction of Cd in water samples. Chem Tech. 2011;3:1647–1651. [Google Scholar]
- 19.Mashhadizadeh MH, Karami Z. Solid phase extraction of trace amounts of Ag, Cd, Cu, and Zn in environmental samples using magnetic nanoparticles coated by 3-(trimethoxysilyl)-1-propantiol and modified with 2-amino-5-mercapto-1,3,4-thiadiazole and their determination by ICP-OES. J Hazard Mater. 2011;190:1023–1029. doi: 10.1016/j.jhazmat.2011.04.051. [DOI] [PubMed] [Google Scholar]
- 20.Zhang R, Niu Y, Li Y, Zhao C, Song B, et al. Acute toxicity study of the interaction between titanium dioxide nanoparticles and lead acetate in mice. Environ Toxicol pharmacol. 2010;30:52–60. doi: 10.1016/j.etap.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 21.Bakircioglu Y, Bakircioglu D, Akman S. Biosorption of lead by filamentous fungal biomass-loaded TiO2 nanoparticles. J Hazard Mater. 2010;178:1015–1020. doi: 10.1016/j.jhazmat.2010.02.040. [DOI] [PubMed] [Google Scholar]
- 22.Kalfa OM, Yalçınkaya Ö, Türker AR. Synthesis of nano B2O3/TiO2 composite material as a new solid phase extractor and its application to preconcentration and separation of cadmium. J Hazard Mater. 2009;166:455–461. doi: 10.1016/j.jhazmat.2008.11.112. [DOI] [PubMed] [Google Scholar]
- 23.Du H, Zhu X, Fan C, Xu S, Wang Y, et al. Oxidative damage and OGG1 expression induced by a combined effect of titanium dioxide nanoparticles and lead acetate in human hepatocytes. Environmental Toxicology. 2011;27:590–597. doi: 10.1002/tox.20682. [DOI] [PubMed] [Google Scholar]
- 24.Barchowsky A, Cartwright IL, Reichard JF, Futscher BW, Lantz RC. Arsenic toxicology: translating between experimental models and human pathology. Environ Health perspect. 2011;119:1356. doi: 10.1289/ehp.1103441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guan X, Du J, Meng X, Sun Y, Sun B, et al. Application of titanium dioxide in arsenic removal from water: a review. J Hazard Mater. 2012:215–216. 1–16. doi: 10.1016/j.jhazmat.2012.02.069. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Liang P, Guo L, Lu HB. Study on the adsorption behavior of heavy metal ions on nanometer TiO2 supported on silica gel [in Chinese] Acta Chim Sin. 2005;63:312–316. [Google Scholar]
- 27.Bleam W F, McBride MB. The chemistry of adsorbed Cu (II) and Mn (II) in aqueous titanium dioxide suspensions. J Colloid Interface Sci. 1986;110:335–346. [Google Scholar]
- 28.Pena ME, Korfiatis G P, Patel M, Lippincott L, Meng X. Adsorption of As (V) and As (III) by nanocrystalline titanium dioxide. Water Research. 2005;39:2327–2337. doi: 10.1016/j.watres.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 29.Bang S, Patel M, Lippincott L, Meng X. Removal of arsenic from groundwater by granular titanium dioxide adsorbent. Chemosphere. 2005;60:389–397. doi: 10.1016/j.chemosphere.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Niu HY, Wang JM, Shi YL, et al. Adsorption behavior of arsenic onto protonated titanate nanotubes prepared via hydrothermal method. Micropor Mesopor Mat. 2009;122:28–35. [Google Scholar]
- 31.Jegadeesan G, Al-Abed SR, Sundaram V, Choi H, Scheckel KG, et al. Arsenic sorption on TiO2 nanoparticles: size and crystallinity effects. Water Research. 2010;44:965–973. doi: 10.1016/j.watres.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 32.Wang D, Hu J, Irons DR, Wang J. Synergistic toxic effect of nano-TiO2 and As (V) on Ceriodaphnia dubia. STOTEN. 2011;409:1351–1356. doi: 10.1016/j.scitotenv.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 33.Malkin R, Malmström BG. The state and function of copper in biological systems. Adv Enzymol Relat Areas Mol Biol. 1970:177–244. doi: 10.1002/9780470122785.ch4. [DOI] [PubMed] [Google Scholar]
- 34.Mitra S, Keswani T, Dey M, Bhattacharya S, Sarkar S, et al. Copper-induced immunotoxicity involves cell cycle arrest and cell death in the spleen and thymus. Toxicology. 2012;293:1–3. doi: 10.1016/j.tox.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 35.Fan W, Cui M, Liu H, Wang C, Shi Z, et al. NanoTiO2 enhances the toxicity of copper in natural water to Daphnia magna. Environ pollut. 2011;159:729–734. doi: 10.1016/j.envpol.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 36.Kjellström T. IARC Scientific publications. 1992. Mechanism and epidemiology of bone effects of cadmium; p. 301. [PubMed] [Google Scholar]
- 37.Nordberg GF, Herber RFM, Alessio L. Cadmium in the human environment: toxicity and carcinogenicity. International Agency for Research on Cancer; 1992. [Google Scholar]
- 38.Flick D, Kraybill H, Dlmitroff J. Toxic effects of cadmium: a review. Environmental Research. 1971;4:71–85. doi: 10.1016/0013-9351(71)90036-3. [DOI] [PubMed] [Google Scholar]
- 39.Järup L. Hazards of heavy metal contamination. British Medical bulletin. 2003;68:167–182. doi: 10.1093/bmb/ldg032. [DOI] [PubMed] [Google Scholar]
- 40.Xia B, Chen J, Zhou Y. Cellular oxidative damage of HEK293T cells induced by combination of CdCl2 and Nano-TiO2. Journal of Huazhong University of Science and Technology Medical Sciences. 2011;31:290–294. doi: 10.1007/s11596-011-0369-4. [DOI] [PubMed] [Google Scholar]
- 41.Zhang X, Sun H, Zhang Z, Niu Q, Chen Y, et al. Enhanced bioaccumulation of cadmium in carp in the presence of titanium dioxide nanoparticles. Chemosphere. 2007;67:160–166. doi: 10.1016/j.chemosphere.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Hu X, Chen Q, Jiang L, Yu Z, Jiang D, et al. Combined effects of titanium dioxide and humic acid on the bioaccumulation of cadmium in zebrafish. Environ pollut. 2011;159:1151–1158. doi: 10.1016/j.envpol.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 43.Yang WW, Miao AJ, Yang LY. Cd2+ Toxicity to a green alga Chlamydomonas reinhardtii as influenced by its adsorption on TiO2 engineered nanoparticles. PLoS One. 2012;7:e32300. doi: 10.1371/journal.pone.0032300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shukla RK, Kumar A, Gurbani D, Pandey AK, Singh S, et al. TiO2nanoparticles induce oxidative DNA damage and apoptosis in human liver cells. Nanotoxicology. 2011:1–13. doi: 10.3109/17435390.2011.629747. [DOI] [PubMed] [Google Scholar]
- 45.Shukla RK, Sharma V, Pandey AK, Singh S, Sultana S, et al. ROSmediated genotoxicity induced by titanium dioxide nanoparticles in human epidermal cells. Toxicol In Vitro. 2011;25:231–241. doi: 10.1016/j.tiv.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 46.Huang Y, Wong C, Zheng J, Bouwman H, Barra R, et al. Bisphenol A (BPA) in China: a review of sources, environmental levels, and potential human health impacts. Environment International. 2011;42:91–99. doi: 10.1016/j.envint.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 47.Alexander HC, Dill DC, Smith LW, Guiney PD, Dorn P. Bisphenol A: acute aquatic toxicity. Environ Toxicol Chem. 1988;7:19–26. [Google Scholar]
- 48.Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health perspect. 2011;119:878–885. doi: 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng D, Wang N, Wang X, Tang Y, Zhu L, et al. Effects of the interaction of TiO2nanoparticles with bisphenol A on their physicochemical properties and in vitro toxicity. J Hazard Mater. 2012:199–200. 426–432. doi: 10.1016/j.jhazmat.2011.11.040. [DOI] [PubMed] [Google Scholar]
- 50.Cohn BA, Wolff MS, Cirillo PM, Sholtz RI. DDT and breast cancer in young women: new data on the significance of age at exposure. Environ Health perspect. 2007;115:1406. doi: 10.1289/ehp.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rogan WJ, Chen A. Health risks and benefits of bis (4-chlorophenyl)-1,1, 1-trichloroethane (DDT) Lancet. 2005;366:763–773. doi: 10.1016/S0140-6736(05)67182-6. [DOI] [PubMed] [Google Scholar]
- 52.Rogan WJ, Ragan NB. Evidence of effects of environmental chemicals on the endocrine system in children. Pediatrics. 2003;112:247–252. [PubMed] [Google Scholar]
- 53.Zoro J, Hunter JM, Eglinton G, Ware G. Degradation of p,p′-DDT in reducing environments. Nature. 1974;247:235–237. doi: 10.1038/247235a0. [DOI] [PubMed] [Google Scholar]
- 54.Garrison AW, Nzengung VA, Avants JK, Ellington JJ, Jones WJ, et al. Phytodegradation of p,p′-DDT and the enantiomers of o,p′-DDT. Environmental Science and Technology. 2000;34:1663–1670. [Google Scholar]
- 55.Shi Y, Zhang JH, Jiang M, Zhu LH, Tan HQ, et al. Synergistic genotoxicity caused by low concentration of titanium dioxide nanoparticles and p, p′-DDT in human hepatocytes. Environ Mol Mutagen. 2010;51:192–204. doi: 10.1002/em.20527. [DOI] [PubMed] [Google Scholar]
- 56.O’Mullane DM, Kavanagh D, Ellwood R P, Chesters RK, Schafer F, et al. A three-year clinical trial of a combination of trimetaphosphate and sodium fluoride in silica toothpastes. J Dent Res. 1997;76:1776–1781. doi: 10.1177/00220345970760110901. [DOI] [PubMed] [Google Scholar]
- 57.Xie C, Liang G Y, Ye B, Pu Y P. Combined effects of sodium fluoride and nano-TiO2 on human bronchial epithelial cells. Journal of Environmental and Occupational Medicine. 2009;26:242–244. [Google Scholar]
- 58.Xu Z, Liu XW, Ma YS, Gao HW. Interaction of nano-TiO2 with lysozyme: insights into the enzyme toxicity of nanosized particles. Environ Sci pollut Res Int. 2010;17:798–806. doi: 10.1007/s11356-009-0153-1. [DOI] [PubMed] [Google Scholar]
- 59.Hui YLS. Research progress and application of photocatalysis of TiO2. Materials Review. 2000;12:23–25. [Google Scholar]
- 60.Kwon S, Fan M, Cooper AT, Yang H. Photocatalytic applications of micro-and nano-TiO2 in environmental engineering. Crit Rev Environ Sci Technol. 2008;38:197–226. [Google Scholar]
- 61.Aarthi T, Madras G. Photocatalytic degradation of rhodamine dyes with nano-TiO2. Ind Eng Chem Res. 2007;46:7–14. [Google Scholar]
- 62.Yu H, Zhang K, Rossi C. Experimental study of the photocatalytic degradation of formaldehyde in indoor air using a nano-particulate titanium dioxide photocatalyst. Indoor and built Environment. 2007;16:529–537. [Google Scholar]
- 63.Wu YP, Zhang WM, Ma CF, Lu YW, Liu L. Photocatalytic degradation of formaldehyde by diffuser of solar light pipe coated with nanometer titanium dioxide thin films. Science China Technological Sciences. 2010;53:150–154. [Google Scholar]
- 64.Lu N, Zhu Z, Zhao X, Tao R, Yang X, et al. Nano titanium dioxide photocatalytic protein tyrosine nitration: a potential hazard of TiO2 on skin. Biochem Biophys Res Commun. 2008;370:675–680. doi: 10.1016/j.bbrc.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 65.Tu M, Huang Y, Li HL, Gao ZH. The stress caused by nitrite with titanium dioxide nanoparticles under UVA irradiation in human keratinocyte cell. Toxicology. 2012;299:60–68. doi: 10.1016/j.tox.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 66.Xue C, Wu J, Lan F, Liu W, Yang X, et al. Nano titanium dioxide induces the generation of ROS and potential damage in HaCaT cells under UVA irradiation. J Nanosci Nanotechnol. 2010;10:8500–8507. doi: 10.1166/jnn.2010.2682. [DOI] [PubMed] [Google Scholar]
- 67.Kang SJ, Lee YJ, Kim BM, Choi YJ, Chung H W. Cytotoxicity and genotoxicity of titanium dioxide nanoparticles in UVA-irradiated normal peripheral blood lymphocytes. Drug Chem Toxicol. 2011;34:277–284. doi: 10.3109/01480545.2010.546800. [DOI] [PubMed] [Google Scholar]
- 68.Yin JJ, Liu J, Ehrenshaft M, Roberts JE, Fu PP, et al. Phototoxicity of nano titanium dioxides in HaCaT keratinocytes – generation of reactive oxygen species and cell damage. Toxicol Appl pharmacol. 2012;263:81–88. doi: 10.1016/j.taap.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang J, Wages M, Cox SB, Maul JD, Li Y, et al. Effect of titanium dioxide nanomaterials and ultraviolet light coexposure on African clawed frogs (Xenopus laevis) Environ Toxicol Chem. 2012;31:176–183. doi: 10.1002/etc.718. [DOI] [PubMed] [Google Scholar]
- 70.Bar-Ilan O, Louis KM, Yang S P, Pedersen JA, Hamers RJ, et al. Titanium dioxide nanoparticles produce phototoxicity in the developing zebrafish. Nanotoxicology. 2012;6:670–679. doi: 10.3109/17435390.2011.604438. [DOI] [PubMed] [Google Scholar]
- 71.Xue C, Liu W, Wu J, Yang X, Xu H. Chemoprotective effect of N-ace-tylcysteine (NAC) on cellular oxidative damages and apoptosis induced by nano titanium dioxide under UVA irradiation. Toxicology In Vitro. 2011;25:110–116. doi: 10.1016/j.tiv.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 72.Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985) N Engl J Med. 1988;319:1557. doi: 10.1056/NEJM198812153192401. [DOI] [PubMed] [Google Scholar]
- 73.Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radical Biology and Medicine. 1989;6:593–597. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- 74.Pannu N, Manns B, Lee H, Tonelli M. Systematic review of the impact of N-acetylcysteine on contrast nephropathy. Kidney Int. 2004;65:1366–1374. doi: 10.1111/j.1523-1755.2004.00516.x. [DOI] [PubMed] [Google Scholar]
- 75.Wang C, Cao S, Tie X, Qiu B, Wu A, et al. Induction of cytotoxicity by photoexcitation of TiO2 can prolong survival in glioma-bearing mice. Mol Biol Rep. 2011;38:523–530. doi: 10.1007/s11033-010-0136-9. [DOI] [PubMed] [Google Scholar]
- 76.Moon J, Yun CY, Chung K-W, Kang M-S, Yi J. Photocatalytic activation of TiO2 under visible light using Acid Red 44. Catalysis Today. 2003;87:77–86. [Google Scholar]
- 77.Srinivasan C, Somasundaram N. Bactericidal and detoxification effects of irradiated semiconductor catalyst, TiO2. Current Science-bangalore. 2003;85:1431–1438. [Google Scholar]
- 78.Thomas J, Kumar KP, Chitra K. Synthesis of Ag doped nano TiO2 as efficient solar photocatalyst for the degradation of endosulfan. Adv Sci Lett. 2011;4:108–114. [Google Scholar]
- 79.Seitz F, Bundschuh M, Dabrunz A, Bandow N, Schaumann GE, et al. Titanium dioxide nanoparticles detoxify pirimicarb under UV irradiation at ambient intensities. Environ Toxicol Chem. 2012;31:518–523. doi: 10.1002/etc.1715. [DOI] [PubMed] [Google Scholar]
- 80.Zhao C, Deng H, Li Y, Liu Z. Photodegradation of oxytetracycline in aqueous by 5A and 13X loaded with TiO2 under UV irradiation. J Hazard Mater. 2010;176:884–892. doi: 10.1016/j.jhazmat.2009.11.119. [DOI] [PubMed] [Google Scholar]
- 81.Li M, Noriega-Trevino ME, Nino-Martinez N, Marambio-Jones C, Wang J, et al. Synergistic bactericidal activity of Ag-TiO2 nanoparticles in both light and dark conditions. Environ Sci Technol. 2011;45:8989–8995. doi: 10.1021/es201675m. [DOI] [PubMed] [Google Scholar]
- 82.Song M, Zhang R, Dai Y, Gao F, Chi H, et al. The in vitro inhibition of multidrug resistance by combined nanoparticulate titanium dioxide and UV irradition. Biomaterials. 2006;27:4230–4238. doi: 10.1016/j.biomaterials.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 83.Liao D, Badour C, Liao B. Preparation of nanosized TiO2/ZnO composite catalyst and its photocatalytic activity for degradation of methyl orange. J photochem photobiol A Chem. 2008;194:11–19. [Google Scholar]
- 84.Panigrahi S, Basak D. Core-shell TiO2@ ZnO nanorods for efficient ultraviolet photodetection. Nanoscale. 2011;3:2336–2341. doi: 10.1039/c1nr10064e. [DOI] [PubMed] [Google Scholar]
- 85.Jiang Y, Sun Y, Liu H, Zhu F, Yin H. Solar photocatalytic decolorization of CI Basic Blue 41 in an aqueous suspension of TiO2–ZnO. Dyes pigm. 2008;78:77–83. [Google Scholar]
- 86.Yoo K-C, Yoon C-H, Kwon D, Hyun K-H, Woo SJ, et al. Titanium dioxide induces apoptotic cell death through reactive oxygen species-mediated Fas upregulation and Bax activation. Int J Nanomedicine. 2012;7:1203. doi: 10.2147/IJN.S28647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sanders K, Degn LL, Mundy WR, Zucker RM, Dreher K, et al. in vitro phototoxicity and hazard identification of nano-scale titanium dioxide. Toxicol Appl pharmacol. 2012;258:226–236. doi: 10.1016/j.taap.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 88.Lankoff A, Sandberg WJ, Wegierek-Ciuk A, Lisowska H, Refsnes M, et al. The effect of agglomeration state of silver and titanium dioxide nanoparticles on cellular response of HepG2, A549 and THP-1 cells. Toxicol Lett. 2012;208:197–213. doi: 10.1016/j.toxlet.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 89.Ekstrand-Hammarström B, Akfur CM, Andersson PO, Lejon C, Österlund L, et al. Human primary bronchial epithelial cells respond differently to titanium dioxide nanoparticles than the lung epithelial cell lines A549 and BEAS-2B. Nanotoxicology. 2012;6:623–634. doi: 10.3109/17435390.2011.598245. [DOI] [PubMed] [Google Scholar]
- 90.Magdolenova Z, Bilaničová D, Pojana G, Fjellsbø LM, Hudecova A, et al. Impact of agglomeration and different dispersions of titanium dioxide nanoparticles on the human related in vitro cytotoxicity and genotoxicity. J Environ Monit. 2012;14:455–464. doi: 10.1039/c2em10746e. [DOI] [PubMed] [Google Scholar]