Abstract
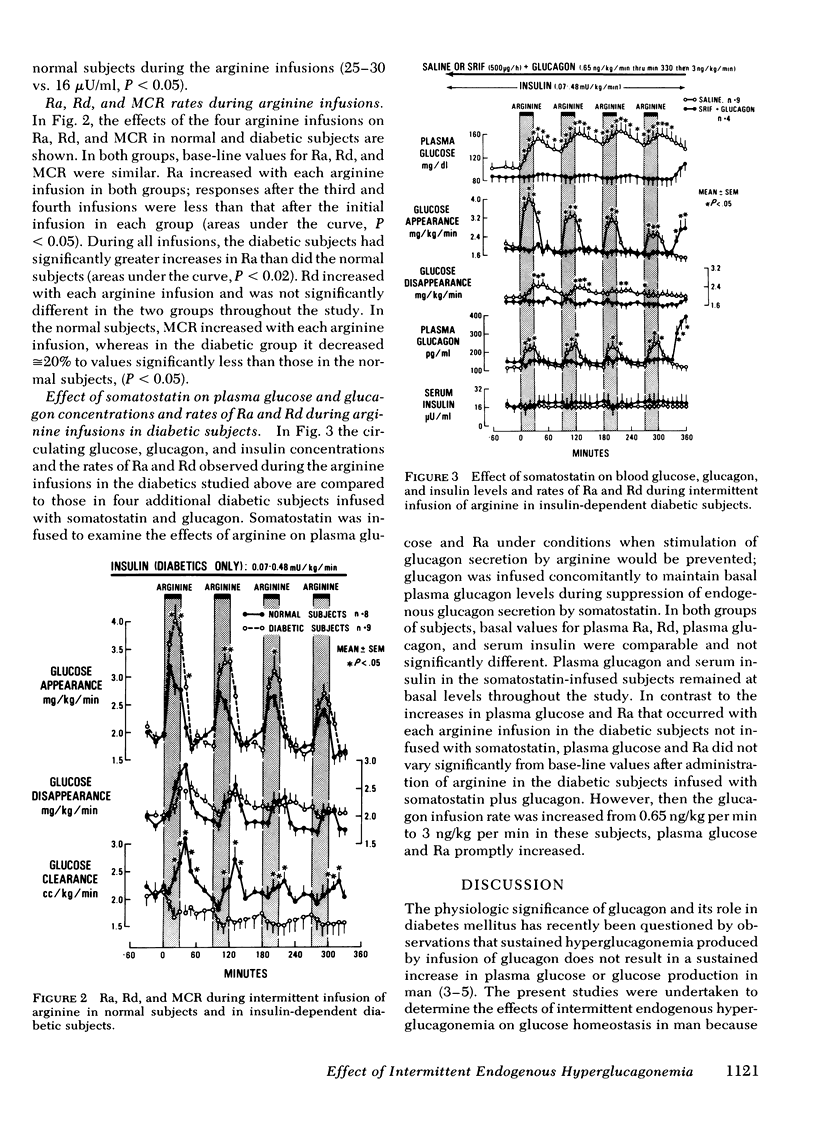
Infusion of glucagon causes only a transient increase in glucose production in normal and diabetic man. To assess the effect of intermittent endogenous hyperglucagonemia that might more closely reflect physiologic conditions, arginine (10 g over 30 min) was infused four times to 8 normal subjects and 13 insulin-dependent diabetic subjects (4 of whom were infused concomitantly with somatostatin to examine effects of arginine during prevention of hyperglucagonemia). Each arginine infusion was separated by 60 min. Diabetic subjects were infused throughout the experiments with insulin at rates (0.07-0.48 mU/kg per min) that had normalized base-line plasma glucose and rates of glucose appearance (Ra) and disappearance (Rd). Basal plasma glucagon and arginine-induced hyperglucagonemia were similar in both groups; basal serum insulin in the diabetics (16±1 μU/ml, P < 0.05) exceeded those of the normal subjects (10±1 μU/ml, P < 0.05) but did not increase with arginine. Serum insulin in normal subjects increased 15-20 μU/ml with each arginine infusion. In both groups each arginine infusion increased plasma glucose and Ra. Increments of Ra in the diabetics exceeded those of normal subjects, (P < 0.02); Rd was similar in both groups. In normal subjects, plasma glucose returned to basal levels after each arginine infusion, whereas in the diabetics hyperglycemia persisted reaching 151±15 mg/dl after the last arginine infusion. When glucagon responses were prevented by somatostatin, arginine infusions did not alter plasma glucose or Ra.
Conclusions: Infusion of arginine acutely increases plasma glucose and glucose production in man solely by stimulating glucagon secretion; physiologic increments in plasma glucagon (100-150 pg/ml) can result in sustained hyperglycemia when pancreatic beta cell function is limited.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altszuler N., Barkai A., Bjerknes C., Gottlieb B., Steele R. Glucose turnover values in the dog obtained with various species of labeled glucose. Am J Physiol. 1975 Dec;229(6):1662–1667. doi: 10.1152/ajplegacy.1975.229.6.1662. [DOI] [PubMed] [Google Scholar]
- Bhathena S. J., Voyles N. R., Smith S., Recant L. Decreased glucagon receptors in diabetic rat hepatocytes. Evidence for regulation of glucagon receptors by hyperglucagonemia. J Clin Invest. 1978 Jun;61(6):1488–1497. doi: 10.1172/JCI109069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomboy J. D., Jr, Lewis S. B., Lacy W. W., Sinclair-Smith B. C., Liljenquist J. E. Transient stimulatory effect of sustained hyperglucagonemia on splanchnic glucose production in normal and diabetic man. Diabetes. 1977 Mar;26(3):177–174. doi: 10.2337/diab.26.3.177. [DOI] [PubMed] [Google Scholar]
- Cherrington A. D., Kawamori R., Pek S., Vranic M. Arginine infusion in dogs. Model for the roles of insulin and glucagon in regulating glucose turnover and free fatty acid levels. Diabetes. 1974 Oct;23(10):805–815. doi: 10.2337/diab.23.10.805. [DOI] [PubMed] [Google Scholar]
- Cherrington A. D., Lacy W. W., Chiasson J. L. Effect of glucagon on glucose production during insulin deficiency in the dog. J Clin Invest. 1978 Sep;62(3):664–677. doi: 10.1172/JCI109174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrington A. D., Vranic M. Effect of interaction between insulin and glucagon on glucose turnover and FFA concentration in normal and depancreatized dogs. Metabolism. 1974 Aug;23(8):729–744. doi: 10.1016/0026-0495(74)90005-5. [DOI] [PubMed] [Google Scholar]
- Cowan J. S., Hetenyi G., Jr Glucoregulatory responses in normal and diabetic dogs recorded by a new tracer method. Metabolism. 1971 Apr;20(4):360–372. doi: 10.1016/0026-0495(71)90098-9. [DOI] [PubMed] [Google Scholar]
- DeRubertis F. R., Craven P. Reduced sensitivity of the hepatic adenylate cyclase-cyclic AMP system to glucagon during sustained hormonal stimulation. J Clin Invest. 1976 Feb;57(2):435–443. doi: 10.1172/JCI108294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dencker H., Hedner P., Holst J., Tranberg K. G. Pancreatic glucagon response to an ordinary meal. Scand J Gastroenterol. 1975;10(5):471–474. [PubMed] [Google Scholar]
- Felig P., Wahren J., Hendler R. Influence of physiologic hyperglucagonemia on basal and insulin-inhibited splanchnic glucose output in normal man. J Clin Invest. 1976 Sep;58(3):761–765. doi: 10.1172/JCI108523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbo H., Holst J. J., Christensen N. J. Glucagon and plasma catecholamine responses to graded and prolonged exercise in man. J Appl Physiol. 1975 Jan;38(1):70–76. doi: 10.1152/jappl.1975.38.1.70. [DOI] [PubMed] [Google Scholar]
- Gerich J. E., Lorenzi M., Bier D. M., Schneider V., Tsalikian E., Karam J. H., Forsham P. H. Prevention of human diabetic ketoacidosis by somatostatin. Evidence for an essential role of glucagon. N Engl J Med. 1975 May 8;292(19):985–989. doi: 10.1056/NEJM197505082921901. [DOI] [PubMed] [Google Scholar]
- Gerich J. E., Schultz T. A., Lewis S. B., Karam J. H. Clinical evaluation of somatostatin as a potential ajunct to insulin in the management of diabetes mellitus. Diabetologia. 1977 Sep;13(5):537–544. doi: 10.1007/BF01234510. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Kuzuya H., Blix P. M., Horwitz D. L., Steiner D. F., Rubenstein A. H. Determination of free and total insulin and C-peptide in insulin-treated diabetics. Diabetes. 1977 Jan;26(1):22–29. doi: 10.2337/diab.26.1.22. [DOI] [PubMed] [Google Scholar]
- Nakagawa S., Nakayama H., Sasaki T., Yoshino K., Yu Y. Y. A simple method for the determination of serum free insulin levels in insulin-treated patients. Diabetes. 1973 Aug;22(8):590–600. doi: 10.2337/diab.22.8.590. [DOI] [PubMed] [Google Scholar]
- Plas C., Nunez J. Glycogenolytic response to glucagon of cultured fetal hepatocytes. Refractoriness following prior exposure to glucagon. J Biol Chem. 1975 Jul 25;250(14):5304–5311. [PubMed] [Google Scholar]
- Radziuk J., Norwich K. H., Vranic M. Measurement and validation of nonsteady turnover rates with applications to the inulin and glucose systems. Fed Proc. 1974 Jul;33(7):1855–1864. [PubMed] [Google Scholar]
- Raskin P., Unger R. H. Effect of insulin therapy on the profiles of plasma immunoreactive glucagon in juvenile-type and adult-type diabetics. Diabetes. 1978 Apr;27(4):411–419. doi: 10.2337/diab.27.4.411. [DOI] [PubMed] [Google Scholar]
- Raskin P., Unger R. H. Hyperglucagonemia and its suppression. Importance in the metabolic control of diabetes. N Engl J Med. 1978 Aug 31;299(9):433–436. doi: 10.1056/NEJM197808312990901. [DOI] [PubMed] [Google Scholar]
- Sherwin R. S., Fisher M., Hendler R., Felig P. Hyperglucagonemia and blood glucose regulation in normal, obese and diabetic subjects. N Engl J Med. 1976 Feb 26;294(9):455–461. doi: 10.1056/NEJM197602262940901. [DOI] [PubMed] [Google Scholar]
- Sherwin R. S., Tamborlane W., Hendler R., Saccá L., DeFronzo R. A., Felig P. Influence of glucagon replacement on the hyperglycemic and hyperketonemic response to prolonged somatostatin infusion in normal man. J Clin Endocrinol Metab. 1977 Nov;45(5):1104–1107. doi: 10.1210/jcem-45-5-1104. [DOI] [PubMed] [Google Scholar]
- Soman V., Felig P. Glucagon binding and adenylate cyclase activity in liver membranes from untreated and insulin-treated diabetic rats. J Clin Invest. 1978 Mar;61(3):552–560. doi: 10.1172/JCI108966. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Soman V., Felig P. Regulation of the glucagon receptor by physiological hyperglucagonaemia. Nature. 1978 Apr 27;272(5656):829–832. doi: 10.1038/272829a0. [DOI] [PubMed] [Google Scholar]
- Srikant C. B., Freeman D., McCorkle K., Unger R. H. Binding and biologic activity of glucagon in liver cell membranes of chronically hyperglucagonemic rats. J Biol Chem. 1977 Nov 10;252(21):7434–7438. [PubMed] [Google Scholar]
- Unger R. H., Orci L. Physiology and pathophysiology of glucagon. Physiol Rev. 1976 Oct;56(4):778–826. doi: 10.1152/physrev.1976.56.4.778. [DOI] [PubMed] [Google Scholar]
- WALL J. S., STEELE R., DE BODO R. C., ALTSZULER N. Effect of insulin on utilization and production of circulating glucose. Am J Physiol. 1957 Apr;189(1):43–50. doi: 10.1152/ajplegacy.1957.189.1.43. [DOI] [PubMed] [Google Scholar]
- Wahren J., Felig P., Hagenfeldt L. Effect of protein ingestion on splanchnic and leg metabolism in normal man and in patients with diabetes mellitus. J Clin Invest. 1976 Apr;57(4):987–999. doi: 10.1172/JCI108375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren J., Hagenfeldt L., Felig P. Splanchnic and leg exchange of glucose, amino acids, and free fatty acids during exercise in diabetes mellitus. J Clin Invest. 1975 Jun;55(6):1303–1314. doi: 10.1172/JCI108050. [DOI] [PMC free article] [PubMed] [Google Scholar]


