Abstract
Phenotypic plasticity can be adaptive when phenotypes are closely matched to changes in the environment. In crickets, rhythmic fluctuations in the biotic and abiotic environment regularly result in diel rhythms in density of sexually active individuals. Given that density strongly influences the intensity of sexual selection, we asked whether crickets exhibit plasticity in signaling behavior that aligns with these rhythmic fluctuations in the socio-sexual environment. We quantified the acoustic mate signaling behavior of wild-caught males of two cricket species, Gryllus veletis and G. pennsylvanicus. Crickets exhibited phenotypically plastic mate signaling behavior, with most males signaling more often and more attractively during the times of day when mating activity is highest in the wild. Most male G. pennsylvanicus chirped more often and louder, with shorter interpulse durations, pulse periods, chirp durations, and interchirp durations, and at slightly higher carrier frequencies during the time of the day that mating activity is highest in the wild. Similarly, most male G. veletis chirped more often, with more pulses per chirp, longer interpulse durations, pulse periods, and chirp durations, shorter interchirp durations, and at lower carrier frequencies during the time of peak mating activity in the wild. Among-male variation in signaling plasticity was high, with some males signaling in an apparently maladaptive manner. Body size explained some of the among-male variation in G. pennsylvanicus plasticity but not G. veletis plasticity. Overall, our findings suggest that crickets exhibit phenotypically plastic mate attraction signals that closely match the fluctuating socio-sexual context they experience.
Introduction
Organisms exhibit phenotypic plasticity when individual genotypes produce different phenotypes in different environments [1]–[3]. Phenotypic plasticity is adaptive when phenotypes are matched to the environment and result in elevated fitness [1], [4]–[7]. Adaptive phenotypic plasticity can evolve provided that genetic variation in phenotypic traits is sufficient, plasticity is not constrained by pleiotropy or epistasis, and costs remain relatively low [6], [8]–[14]. Adaptive phenotypic plasticity is most likely to evolve when variable environments are predictable [15].
Fitness plays a key role in determining costs and benefits of phenotypic plasticity, making sexually selected traits ideal for use in plasticity studies [14] because males with the most exaggerated sexual traits usually have the highest fitness [16], [17]. Sexual traits also typically reflect condition [18] since only individuals in top condition should be able to bear the costs of advertising with the most exaggerated traits [19]. Sexually selected traits should, therefore, act as phenotypically plastic gauges of an individual’s condition [18], [20]–[23]. Several studies have revealed that sexually selected traits exhibit adaptive phenotypic plasticity. Adaptively plastic sexual traits can be fixed and irreversible at maturity or can be highly flexible and changeable throughout adulthood, changing as different environments are encountered [18], [24], [25]. For example, there are two male morphs in dung beetles (Onthophagus taurus), large males with elaborate weaponry that guard their mates, and small males with reduced weaponry that sneak copulate [26]. The social environment in which mothers are reared influences horn length in mate-guarding sons, although not in sneak copulating sons. Mothers reared with conspecifics produce sons with longer horns, while mothers reared in isolation produce sons with shorter horns [27]. In contrast, in the two-spotted field cricket (Gryllus bimaculatus), mating strategies are flexible. Males in the presence of rivals court females sooner, at higher rates, and transfer larger spermatophores than they do in control non-rival environments [28]. These examples reveal that changes in the socio-sexual environment can result in adaptively plastic mating strategies (reviewed by [29]).
Here we explore whether rhythmic changes in the socio-sexual environment result in adaptively plastic mate attraction behaviors in North American spring (G. veletis) and fall (G. pennsylvanicus) field crickets. Male crickets signal acoustically to attract females using long distance mate attraction signals (chirps). They raise their forewings and rub them together; each closing stroke produces a pulse of sound, and males concatenate pulses into chirps [30]. Much is known about cricket long distance mate attraction signaling and mating behavior at the population level. Cricket density often changes in a rhythmic pattern as a result of predictable fluctuations in the biotic (predator and parasite density) and abiotic (light and temperature levels) environment (summarized in Table S1). For example, mate signaling in male Texas field crickets, G. texensis, aligns positively with female mating activity, but negatively with parasitoid tachinid fly (Ormia ochracea) host-searching activity [31], [32]. Given that the density of conspecific rivals and potential mates determines the intensity of sexual selection [33]–[38], the fitness of any given phenotype should also exhibit temporal rhythms that align with population rhythms. Males may therefore exhibit adaptive phenotypic plasticity in their mate attraction signals to match the temporally fluctuating competitive context they encounter [39]–[41].
Tantalizing evidence suggests that male crickets exhibit socio-sexual dependent differences in their signaling plasticity throughout the day, and that condition limits this plasticity. Wild G. campestris females exhibit natural rhythmic fluctuations in their mating behavior, with most mating occurring in the afternoon and early evening (12∶00–21∶00 h) and little occurring in the night and morning (21∶00–12∶00; [37]). Jacot et al. [37] provided supplemental food to wild G. campestris and revealed that better fed males align their signaling activity with mate availability. During times when female mate-searching activity was naturally reduced in the wild, the signaling effort of food-supplemented males did not differ from that of control males. However, during times when female mate-searching activity was naturally elevated in the wild, the signaling effort of food-supplemented males was significantly elevated compared to unfed conspecifics (controls). Food-supplemented males exhibit plastic signaling behaviors in the wild, optimizing their signaling effort to maximize their potential for mating success [37]. This phenotypic plasticity is likely adaptive, as female G. campestris preferentially mate with males that signal most often [42].
Here we quantify variation in field cricket mate signaling plasticity at the species and individual level. At the species level, we examine the temporal signaling rhythms of two cricket species. We captured spring and fall field crickets as adults in their natural environments and asked whether, under controlled abiotic and biotic conditions, males exhibited the same rhythmic signaling patterns as previously described in the wild. We focused on spring and fall field crickets for two reasons. First, the signaling and mating diel rhythms of wild G. pennsylvanicus and G. veletis have been described. Gryllus pennsylvanicus males have high mate signaling activity throughout the night that peaks just before sunrise; signaling activity is much lower during the day [32]. Male G. pennsylvanicus signaling diel rhythms are synchronized with female G. pennsylvanicus mating activity diel rhythms, with most mating (88%) occurring at night and around sunrise in the wild (highest activity: 22∶00–09∶59; lowest activity: 10∶00–21∶59; [32]). Conversely, in G. veletis the highest signaling activity starts just before sunrise and continues for several hours following sunrise; signaling activity is much lower in the late afternoon and early evening but starts to gradually increase following sunset. Male G. veletis signaling diel rhythms are synchronized with female G. veletis mating activity diel rhythms, with most mating (81%) occurring during the day, peaking around sunrise and for several hours following it in the wild (highest activity: 04∶00–15∶59; lowest activity: 16∶00–03∶59; [32]). Second, the geographic distributions and mating seasons of G. veletis and G. pennsylvanicus overlap spatially and temporally. In Ontario, Canada, temporal overlap in breeding seasons occurs as G. veletis breeds from May to early July, while G. pennsylvanicus breeds from June through October [32]. Spatial overlap also occurs, with both species preferring grassy fields and rocky crevices (SMB, SJH, IRT, and LPF personal observations). Hybridization is lethal between G. pennsylvanicus and G. veletis [43]. The temporal (within a day) differences in signaling activity described above have the potential to act as a behavioral barrier, preventing the potential mating that could occur when breeding seasons overlap. We compare and contrast signaling effort of field-captured G. pennsylvanicus and G. veletis monitored in the laboratory to reports of their signaling behavior in the wild.
We also provide the first species level investigation into how the fine scale structure of long distance mate attraction signals (Figure 1) changes over the course of the day. Plasticity in signal structure may be important because small differences in sound structure can influence female mating decisions. For example, females tend to prefer males that produce loud chirps (G. lineaticeps: [44]; G. bimaculatus: [45]) at low carrier frequencies (G. bimaculatus: [46]; G. campestris: [47], [48]; note G. pennsylvanicus prefer 5 kHz over 4 kHz signals [49]). Female crickets also prefer either average or elevated number of pulses per chirp (G. campestris: [46], [50]; G. texensis: [51]), species specific pulse periods (e.g. G. campestris: 40–60 msec [52]; G. bimaculatus: 40–60 msec [53]; G. pennsylvanicus: 35–60 msec [49], [54], G. veletis: 40–70 msec [54]), short chirp intervals (G. integer: [51]), high chirp rates (G. lineaticeps: [55]), long chirp durations (G. lineaticeps: [44]), and long calling bouts (G. integer: [56]). We therefore examine how G. pennsylvanicus and G. veletis alter the fine scale components of their signals through the course of the day.
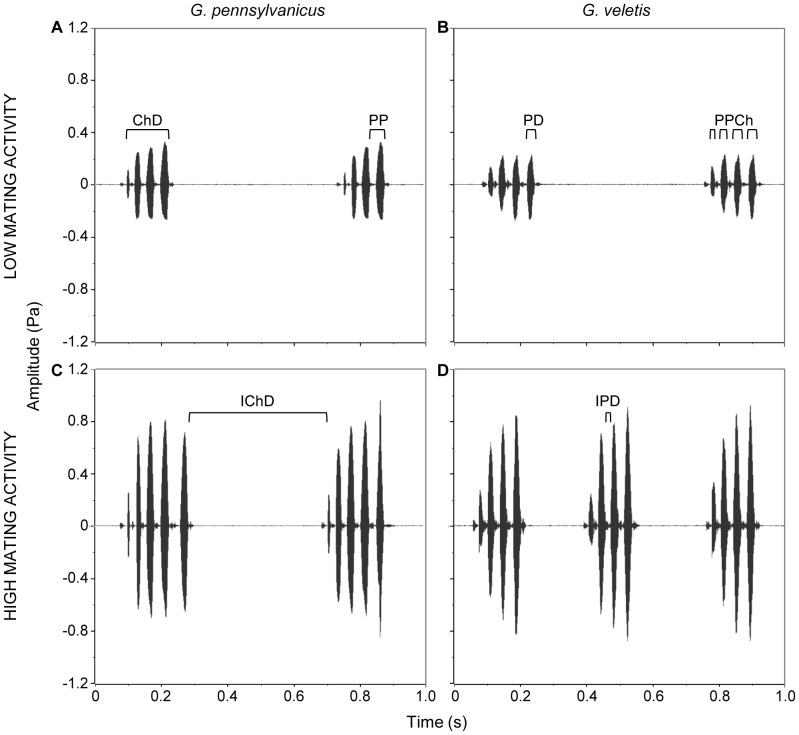
Figure 1. Waveforms of long-distance mate attraction signals of one G. pennsylvanicus and one G. veletis male.
Figures show typical long-distance mate attraction signal for each species and how signaling typically changes during time periods indicative of low (A & B) and high (C & D) mating activity in the wild. Signal fine-scale properties are indicated as follows: ChD = chirp duration; IChD = interchirp duration; PPCh = pulses per chirp; PD = pulse duration; IPD = interpulse duration; and PP = pulse period, which combines PD and IPD.
At the individual level, we quantify phenotypic plasticity in mate attraction behavior. Given that the density of competitive rivals and female mates exhibit diel rhythms [32], the potential fitness payoffs of any given phenotype should also exhibit diel rhythms that align with species level rhythms. We therefore asked whether individual males exhibit phenotypic plasticity in their mate attraction signals that match the temporally fluctuating competitive and mating context they experience. We examined plasticity by comparing signaling behavior during two daily time periods: when mating activity is typically high in the wild versus when it is typically low. Higher mating activity means higher density of conspecific rival males and potential mates. We also examined whether body size (pronotum area) or residual mass (on body size) influenced signaling plasticity. We define condition as variation in resource acquisition and assimilation ability [57], which may result from differences in resource availability in the environment and/or individual physiological or genetic differences in the ability to locate, assimilate and utilize resources. Wild-caught crickets that naturally vary in body size and residual mass should allow us to explore the effect of natural variation in resource abundance and acquisition ability experienced during development in the wild. Bretman et al. [29] hypothesized males in high and low condition should exhibit minimal plasticity, whereas males in intermediate condition should display high plasticity, because intermediate condition males should have the stores available to amplify their signals when conditions are best but not enough stores to constantly maintain amplified signals. We therefore followed Bretman et al. [29] and predicted that males in average condition should display more adaptive signaling plasticity than males in high or low condition. Our correlational study exploring variation in plasticity is the first step towards determining whether crickets exhibit genotype by environment interactions in their signaling behaviors (eg. [58]–[60]).
Methods
Ethics Statement
Our study was conducted in accordance with the guidelines of the Canadian Council on Animal Care. We thank Art Weis and Koffler Scientific Reserve (Jokers Hill - University of Toronto) for allowing us to collect crickets on their property and for hosting our laboratory.
Collection and Husbandry
We captured wild adult Gryllus veletis in Mississippi Mills, ON in May and June 2010, and G. pennsylvanicus at the Koffler Scientific Reserve (Jokers Hill - University of Toronto) near King City, ON in August 2010 (no collecting permits required). Crickets were housed individually in 520 mL clear plastic containers with a screened lid and crumpled unbleached paper towel as shelter. They were provided with unlimited water and food (powdered Harlan Teklad Inc. Rodent diet no. 8604 M, Harlan Laboratories, Madison, WI, USA). Crickets were transferred to Carleton University where they were housed in a temperature and photoperiod controlled greenhouse at 28±2°C on a 14∶10 h light:dark cycle.
Acoustic Recording
We recorded the long distance mate attraction signals of wild-caught adult male G. veletis and G. pennsylvanicus by placing each male’s container in an electronic acoustic recording system (EARS-II; designed and developed for our laboratory by Cambridge Electronic Design, Cambridge, UK). Gryllus veletis males were caught locally so their acoustic mate attraction signals could be recorded in the EARS-II starting the morning immediately following capture. Following capture, G. pennsylvanicus had a 2–6 day delay before they could be recorded, resulting from our having to complete all field collections prior to transporting the crickets back to Carleton University. All males had their acoustic mate attraction signals recorded for 2–4 days in the EARS-II. We recorded a total of 63 G. pennsylvanicus and 32 G. veletis. Of these, almost all males signaled for mates, with the exception of 1 G. pennsylvanicus and 3 G. veletis.
The EARS-II consists of three units, each capable of recording the acoustic mate attraction signals of 32 males in real time (96 males in total; refer to [61] for detailed descriptions). Microphones (electret condenser type KECG2742PBL-A; Kingstate Electronics, Tamshui, Taipei, Taiwan) are positioned next to LED lights which are set to a 14∶10 h light:dark cycle. Each microphone and light combination is held 6.6-cm above the top of the male’s container. Males are separated from their neighbors by acoustically isolated enclosures (7-cm thick Styrofoam box lined with 3.5-cm thick acoustic foam) that contain both microphone and LED light. This design minimizes the likelihood of individuals detecting their neighbors’ signals. The Styrofoam boxes are configured in a 4×4 array on six shelving units within our greenhouse.
We recorded the long distance mate attraction calls of each male for 2–4 days. Sounds were recorded at 31.25 kHz. The microphones are continuously monitored and analyzed using CricketSong software (designed by Cambridge Electronic Design Ltd., Cambridge, UK; for details see [61]). The EARS II CricketSong software automatically filters out background noise and auto-adjusts the amplitude threshold for quiet or loud individuals. CricketSong software creates an acoustic file for each male for every hour he spends in the EARS II unit. We analyzed each acoustic file using Spike2 v6.12 (Cambridge Electronic Design, Cambridge, UK) to produce an hourly summary of the nine signaling parameters quantified: signaling time (# min in the hour), pulse duration (ms), interpulse duration (ms), pulse period (ms), pulses per chirp, chirp duration (ms), interchirp duration (ms), amplitude (dB), and carrier frequency (Hz) (Figure 1). Note, signaling time represents the total amount of time the individual spent signaling in that hour, whereas the other eight signaling parameters are represented by mean parameter values for that hour. We included pulse period, even though it is not a separate trait but instead combines pulse duration and interpulse duration, because females are known to prefer species specific pulse periods (G. pennsylvanicus: 35–60 msec [49], [54], G. veletis: 40–70 msec [54]). We included pulse duration and interpulse duration so that we could know what component of the signal males were changing to change pulse period.
Body Size and Weight Measurements
Following acoustic recording we photographed live crickets in a dorsal position using a Zeiss Discovery V12 stereo dissecting microscope (AxioVision v4.8, Carl Zeiss; magnification: 5×, resolution: ∼1.60 µm). We used these photographs to quantify each male’s pronotum area (mm2). Males were weighed using a Denver Instruments balance (Pinnacle Series model PI-314; precision ±0.1 mg). Males were then added to the stock population to mate with collected females and build a laboratory culture.
Statistical Methods
We performed statistical analyses in JMP v10.0.0 (SAS Institute Incorporated, Cary, NC, USA). Residual mass was quantified using a logistic regression of mass on pronotum area. Males were categorized as being in high, average, or low condition depending on whether they fell within the upper, middle, or bottom third of rank ordered residual mass scores. Similarly, males were categorized as high, average, or low condition depending on their rank ordered body size (pronotum area) scores.
We ensured that all parameters were normally distributed using Shapiro-Wilk Goodness of Fit tests. All parameters were normally distributed except signaling time and inter-chirp duration; we Box-Cox transformed these two parameters for both species. To quantify species-level differences in signaling behavior we used ANOVA. We compared nine different signaling parameters, and we corrected for multiple tests using Benjamini and Yekutieli’s [62] false discovery rate (FDRB-Y) method; our FDRB-Y corrected alpha was P<0.0177. To visualize temporal signaling rhythms at the species level we quantified signaling behavior across 24 hours using six different time periods (period 1 = 00∶00–03∶59; 2 = 04∶00–07∶59; 3 = 08∶00–11∶59; 4 = 12∶00–15∶59; 5 = 16∶00–19∶59; 6 = 20∶00–23∶59). We visually compared our temporal rhythm plots that quantified wild-caught crickets signaling in the laboratory to previously published temporal rhythm plots that quantified wild-crickets signaling over a 24-hour period [32].
To investigate phenotypic plasticity in signaling behavior at the individual level we employed repeated measures general linear mixed models (GLMM) using restricted maximum likelihood (REML). We used the restricted maximum likelihood approach because we had missing cells (some males did not signal in a period on some days). The models included individual as a random effect, two time periods (high and low mating activity, see below for details) as a fixed effect, male condition (body size and residual mass) as fixed effects, and mating activity * residual mass and mating activity * body size interaction terms. Each male’s signals were repeatedly measured across the two mating activity levels previously defined by French and Cade [32] for wild populations: G. pennsylvanicus high = 22∶00–09∶59, low = 10∶00–21∶59; G. veletis high = 04∶00–15∶59, low = 16∶00–03∶59. We ran each of these models twice, once using continuous condition measures and once using categorical (high, medium, or low) condition variables. Our overall findings did not differ between the two model types (continuous or categorical condition measures), so we only present our categorical findings. We ran 36 different mixed effect models: 9 parameters×2 species×2 condition runs, and our FDRB-Y corrected alpha level of significance was P<0.0120.
Results
Species Differences
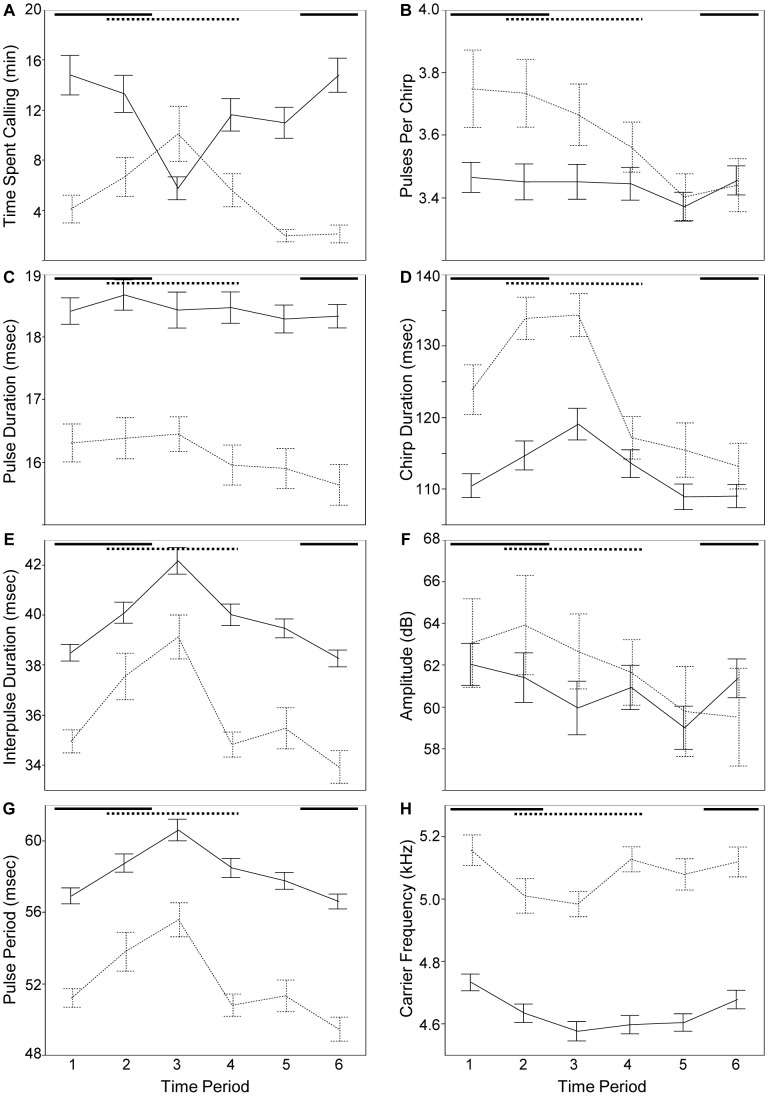
Male crickets exhibited strong temporal rhythms in their signaling behavior that differed across species (Figure 2). During times of the day that G. pennsylvanicus mating activity is highest in the wild (22∶00–09∶59; [32]) males chirped more often and louder, with shorter interpulse, pulse period, chirp, and interchirp durations, and at slightly higher carrier frequencies. During times of the day that G. veletis mating activity is highest in the wild (04∶00–15∶59; [32]) males chirped more often, with more pulses per chirp, longer interpulse, pulse period, and chirp durations, shorter interchirp durations, and at lower carrier frequencies. Note: time effects were significant (Table S2). Gryllus pennsylvanicus and G. veletis also differed in other ways, with G. pennsylvanicus males signaling more often, at slightly lower carrier frequencies, with longer pulse, interpulse, pulse period, and interchirp durations, and shorter chirp durations than G. veletis (Table 1; Figure 2). Note, however, that there was extensive overlap between species in all traits. Both species chirped at similar amplitudes and included 3–4 pulses in their chirps (Table 1; Figure 2F and 2B, respectively), and kept their pulse durations relatively constant through time (Figure 2C).
Figure 2. Signaling diel rhythms in G. pennsylvanicus (solid lines) and G. veletis (dotted lines).
Error bars are standard error of the mean. Horizontal lines at the top of each panel depict the time of day that mating activity is highest (from [32]). Time periods: 1 = 00∶00–03∶59; 2 = 04∶00–07∶59; 3 = 08∶00–11∶59; 4 = 12∶00–15∶59; 5 = 16∶00–19∶59; 6 = 20∶00–23∶59.
Table 1. Interspecific differences in G. pennsylvanicus and G. veletis long distance mate attraction signaling parameters.
| Signaling Trait | Species | Mean ± SD | Range | F | P | DF | R2 adj |
| Signaling Time | G. pennsylvanicus | 12.11±8.05 | 0–34.78 | 59.1074 | <0.0001 | 1,93 | 0.3820 |
| (min/hour) | G. veletis | 5.18±5.56 | 0–19.73 | ||||
| Pulse Duration | G. pennsylvanicus | 18.42±1.55 | 14.61–22.43 | 46.8997 | <0.0001 | 1,89 | 0.3377 |
| (msec) | G. veletis | 16.13±1.35 | 13.64–17.94 | ||||
| Interpulse Duration | G. pennsylvanicus | 39.31±2.54 | 33.99–44.81 | 14.5101 | 0.0003 | 1,89 | 0.1305 |
| (msec) | G. veletis | 36.89±3.38 | 32.81–45.43 | ||||
| Pulse Period | G. pennsylvanicus | 57.74±3.20 | 49.22–66.45 | 37.2571 | <0.0001 | 1,89 | 0.2872 |
| (msec) | G. veletis | 53.02±3.89 | 47.65–63.37 | ||||
| Pulses Per Chirp | G. pennsylvanicus | 3.46±0.35 | 2.53–4.04 | 2.9071 | 0.0917 | 1,89 | 0.0208 |
| (count) | G. veletis | 3.60±0.43 | 2.71–4.67 | ||||
| Chirp Duration | G. pennsylvanicus | 112.02±12.74 | 75.89–132.66 | 23.2040 | <0.0001 | 1,89 | 0.1979 |
| (msec) | G. veletis | 126.14±13.63 | 101.06–151.91 | ||||
| Interchirp Duration | G. pennsylvanicus | 918.27±278.07 | 442.52–1774.00 | 18.9743 | <0.0001 | 1,89 | 0.7996 |
| (msec) | G. veletis | 564.64±137.06 | 371.85–852.20 | ||||
| Amplitude | G. pennsylvanicus | 61.01±7.30 | 41.06–81.72 | 0.2757 | 0.6009 | 1,89 | 0.0081 |
| (dB) | G. veletis | 61.88±7.59 | 47.25–79.03 | ||||
| Carrier Frequency | G. pennsylvanicus | 4.64±0.21 | 4.16–5.11 | 78.6988 | <0.0001 | 1,89 | 0.4633 |
| (kHz) | G. veletis | 5.05±0.20 | 4.63–5.43 |
SD = standard deviation; F = equality of variances test; Our corrected alpha FDRB–Y level of significance is P<0.0177 to account for the 9 models run; DF = degrees of freedom; R2 adj = ratio of variability between group means to the overall sample variability, adjusted for number of explanatory terms.
Individual Differences
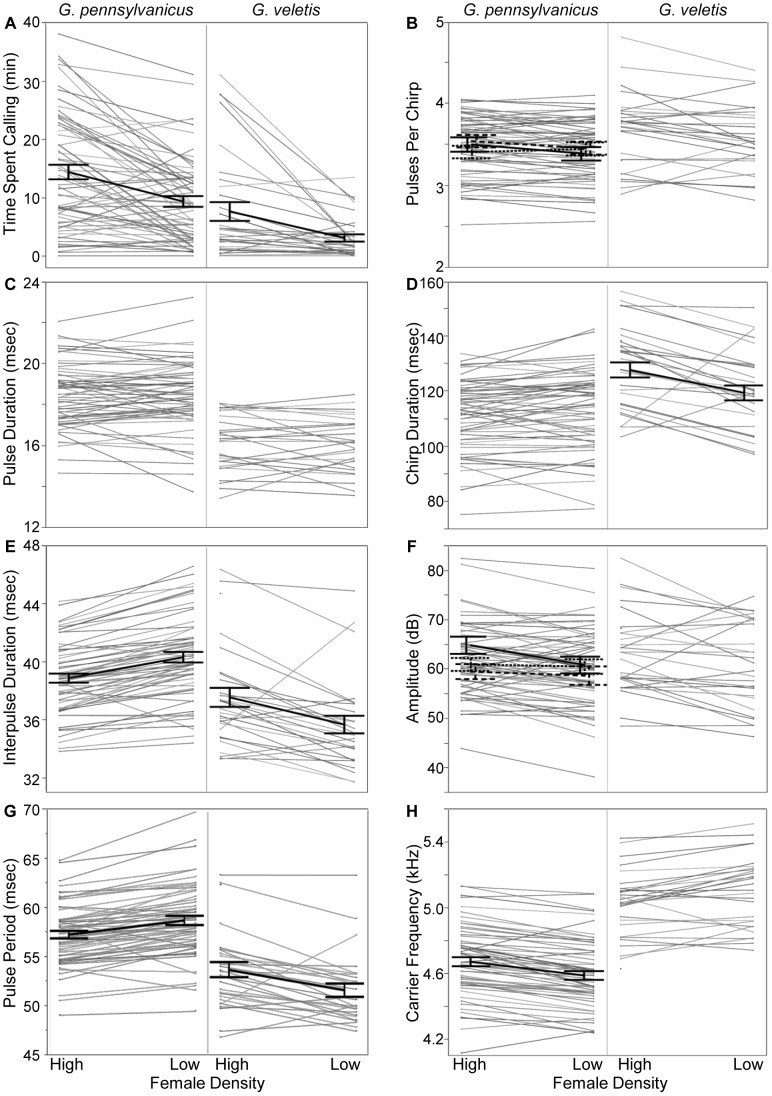
Male crickets used phenotypically plastic signals, generally investing more effort into long distance mate attraction signaling during the time of day that mating activity is highest in the wild. Our models investigating whether individuals exhibit plasticity in their signaling behavior were highly significant, explaining 71–97% of the variation in signaling traits (Tables 2–4). Time period, representing periods of high or low mating activity in the wild, explained 3–22% of the signaling variation; individual identity explained 54–93% (Tables 2–4). During times that mating activity is highest in the wild (22∶00–09∶59; [32]), G. pennsylvanicus males generally signaled more often, with shorter breaks between pulses and shorter pulse periods, more pulses per chirp, louder chirps, and higher carrier frequencies (Figures 1 and 3; Tables 2 and 3). Male G. pennsylvanicus were, however, highly variable in their signaling plasticity, with a few males signaling in a manner that appeared maladaptive. For example, some males signaled less often and with quieter signals when mating activity is typically highest and signaled more often and louder when mating activity is typically lowest. Body size explained some of these differences in phenotypic plasticity. However, contrary to our prediction, average-sized males did not exhibit more phenotypic plasticity in their amplitude or pulses per chirp across mating activity contexts; rather, large and small (based on pronotum area) males did. Large and small males signaled louder and with more pulses per chirp when mating activity is highest in the wild and quieter with fewer pulses per chirp when mating activity is lowest. Residual mass did not explain differences in phenotypic plasticity.
Table 2. How signaling effort plasticity is influenced by time of day (subdivided by mating period), residual mass, and body size.
| Species | IndependentVariables | DF | F Ratio | P | R2 adj | IV% |
| G. pennsylvanicus | Mating Activity(MA) | 1,58 | 26.8220 | <0.0001 | 0.8068 | 66.12 |
| Residual Mass(RM) | 2,58 | 0.3436 | 0.7107 | |||
| Body Size(BS) | 2,58 | 1.0531 | 0.3554 | |||
| RM * MA | 2,58 | 1.4248 | 0.2489 | |||
| BS * MA | 2,58 | 1.1146 | 0.3350 | |||
| G. veletis | Mating Activity(MA) | 1,26 | 11.7550 | 0.0020 | 0.7673 | 61.41 |
| Residual Mass(RM) | 2,26 | 0.0556 | 0.9460 | |||
| Body Size (BS) | 2,26 | 1.4155 | 0.2609 | |||
| RM * MA | 2,26 | 0.0250 | 0.9753 | |||
| BS * MA | 2,26 | 0.9030 | 0.4177 |
Repeated measures ANOVA model output investigating plasticity in signaling time across high and low mating activity time periods; MA = Mating Activity; RM = Residual Mass, BS = Body Size; IV% = Variance explained by individual effects in the repeated measures model; FDRB–Y alpha P<0.0120. Note: this legend also applies to Table 3 and 4.
Table 4. How G. veletis fine scale signaling parameter plasticity is influenced by time of day (subdivided by mating period), residual mass, and body size.
| SignalingTraits | IndependentVariables | DF | F Ratio | P | R2 adj | IV % |
| PulseDuration | Mating Activity (MA) | 1,22 | 0.6028 | 0.4457 | 0.8996 | 79.81 |
| Residual Mass (RM) | 2,23 | 2.3163 | 0.1213 | |||
| Body Size (BS) | 2,23 | 2.5329 | 0.1015 | |||
| RM * MA | 2,22 | 0.7563 | 0.4812 | |||
| BS * MA | 2,22 | 2.5092 | 0.1043 | |||
| InterpulseDuration | Mating Activity (MA) | 1,21 | 10.5859 | 0.0037 | 0.8439 | 73.45 |
| Residual Mass (RM) | 2,22 | 0.0534 | 0.9481 | |||
| Body Size (BS) | 2,22 | 1.3417 | 0.2818 | |||
| RM * MA | 2,21 | 0.5118 | 0.6065 | |||
| BS * MA | 2,21 | 0.2086 | 0.8134 | |||
| PulsePeriod | Mating Activity (MA) | 1,21 | 12.0577 | 0.0022 | 0.8845 | 80.79 |
| Residual Mass (RM) | 2,22 | 0.4665 | 0.6332 | |||
| Body Size (BS) | 2,22 | 0.2223 | 0.8024 | |||
| RM * MA | 2,22 | 0.7880 | 0.4674 | |||
| BS * MA | 2,22 | 0.9810 | 0.3911 | |||
| PulsesPer Chirp | Mating Activity (MA) | 1,22 | 2.3425 | 0.1400 | 0.8978 | 80.52 |
| Residual Mass (RM) | 2,23 | 2.3490 | 0.1180 | |||
| Body Size (BS) | 2,23 | 0.5839 | 0.5658 | |||
| RM * MA | 2,22 | 0.3506 | 0.7081 | |||
| BS * MA | 2,22 | 1.2535 | 0.3050 | |||
| ChirpDuration | Mating Activity (MA) | 1,22 | 12.3159 | 0.0019 | 0.8364 | 69.23 |
| Residual Mass (RM) | 2,23 | 1.5760 | 0.2283 | |||
| Body Size (BS) | 2,23 | 0.3790 | 0.6887 | |||
| RM * MA | 2,22 | 0.3121 | 0.7351 | |||
| BS * MA | 2,22 | 2.7546 | 0.0853 | |||
| InterchirpDuration | Mating Activity (MA) | 1,22 | 5.6811 | 0.0261 | 0.7803 | 66.20 |
| Residual Mass (RM) | 2,23 | 0.1097 | 0.8965 | |||
| Body Size (BS) | 2,23 | 0.0798 | 0.9236 | |||
| RM * MA | 2,22 | 1.5068 | 0.2434 | |||
| BS * MA | 2,22 | 1.1061 | 0.3484 | |||
| Amplitude | Mating Activity (MA) | 1,23 | 4.6936 | 0.0411 | 0.8104 | 58.79 |
| Residual Mass (RM) | 2,23 | 3.4634 | 0.0483 | |||
| Body Size (BS) | 2,23 | 4.9375 | 0.0164 | |||
| RM * MA | 2,23 | 0.3832 | 0.6860 | |||
| BS * MA | 2,22 | 0.4849 | 0.6221 | |||
| CarrierFrequency | Mating Activity (MA) | 1,22 | 5.2173 | 0.0326 | 0.7053 | 54.40 |
| Residual Mass (RM) | 2,22 | 1.0355 | 0.3717 | |||
| Body Size (BS) | 2,22 | 0.5955 | 0.5599 | |||
| RM * MA | 2,22 | 0.1494 | 0.8621 | |||
| BS * MA | 2,22 | 0.3335 | 0.7201 |
Figure 3. Signaling plasticity across times of high and low mating activity in nature.
Mating activity in nature: G. pennsylvanicus high = 22∶00–09∶59, low = 10∶00–21∶59; G. veletis high = 04∶00–15∶59, low = 16∶00–03∶59 ([32]). Each thin gray line represents an individual. Significant time period effects are shown using heavy black lines; significant interactions between mating activity and size are shown with three lines (solid - large, dotted - medium, dashed - small); statistics are shown in Tables 2–4.
Table 3. How G. pennsylvanicus fine scale signaling parameter plasticity is influenced by time of day (subdivided by mating period), residual mass, and body size.
| SignalingTraits | IndependentVariables | DF | F Ratio | P | R2 adj | IV % |
| PulseDuration | Mating Activity (MA) | 1,57 | 0.0124 | 0.9116 | 0.9205 | 84.85 |
| Residual Mass (RM) | 2,57 | 1.3350 | 0.2713 | |||
| Body Size (BS) | 2,57 | 1.6897 | 0.1937 | |||
| RM * MA | 2,57 | 0.7575 | 0.4735 | |||
| BS * MA | 2,57 | 1.3054 | 0.2790 | |||
| InterpulseDuration | Mating Activity (MA) | 1,57 | 95.5756 | <0.0001 | 0.9535 | 90.76 |
| Residual Mass (RM) | 2,57 | 0.4490 | 0.6405 | |||
| Body Size (BS) | 2,57 | 0.8869 | 0.4175 | |||
| RM * MA | 2,57 | 0.5570 | 0.5760 | |||
| BS * MA | 2,57 | 0.6657 | 0.5179 | |||
| PulsePeriod | Mating Activity (MA) | 1,57 | 47.0348 | <0.0001 | 0.9413 | 88.92 |
| Residual Mass (RM) | 2,57 | 0.2271 | 0.7976 | |||
| Body Size (BS) | 2,57 | 0.6112 | 0.5462 | |||
| RM * MA | 2,57 | 0.8750 | 0.4224 | |||
| BS * MA | 2,57 | 0.0027 | 0.9973 | |||
| PulsesPer Chirp | Mating Activity (MA) | 1,57 | 8.9179 | 0.0042 | 0.9614 | 92.85 |
| Residual Mass (RM) | 2,57 | 0.4277 | 0.6541 | |||
| Body Size (BS) | 2,57 | 0.2076 | 0.8132 | |||
| RM * MA | 2,57 | 0.3012 | 0.7411 | |||
| BS * MA | 2,57 | 6.6881 | 0.0025 | |||
| ChirpDuration | Mating Activity (MA) | 1,57 | 4.2342 | 0.0442 | 0.9522 | 91.06 |
| Residual Mass (RM) | 2,57 | 0.8741 | 0.4228 | |||
| Body Size (BS) | 2,57 | 0.4781 | 0.6224 | |||
| RM * MA | 2,57 | 0.7047 | 0.4985 | |||
| BS * MA | 2,57 | 2.6607 | 0.0786 | |||
| InterchirpDuration | Mating Activity (MA) | 1,57 | 0.1573 | 0.6931 | 0.8842 | 79.89 |
| Residual Mass (RM) | 2,57 | 0.0832 | 0.9203 | |||
| Body Size (BS) | 2,57 | 0.9505 | 0.3926 | |||
| RM * MA | 2,57 | 0.6129 | 0.5453 | |||
| BS * MA | 2,57 | 1.7478 | 0.1834 | |||
| Amplitude | Mating Activity (MA) | 1,57 | 13.5078 | 0.0005 | 0.9326 | 87.26 |
| Residual Mass (RM) | 2,57 | 0.4173 | 0.6608 | |||
| Body Size (BS) | 2,57 | 1.3263 | 0.2735 | |||
| RM * MA | 2,57 | 0.6394 | 0.5314 | |||
| BS * MA | 2,57 | 5.5090 | 0.0065 | |||
| CarrierFrequency | Mating Activity (MA) | 1,57 | 85.1932 | <0.0001 | 0.9719 | 93.87 |
| Residual Mass (RM) | 2,57 | 0.6229 | 0.5400 | |||
| Body Size (BS) | 2,57 | 4.4165 | 0.0165 | |||
| RM * MA | 2,57 | 4.1034 | 0.0216 | |||
| BS * MA | 2,57 | 1.7301 | 0.1865 |
Gryllus veletis males also used phenotypically plastic signals and invested more effort into mate signaling during times that mating activity is highest in the wild (04∶00–15∶59; [32]). Males generally signaled more often, with longer breaks between pulses, pulse periods, and chirp durations when mating activity is typically highest in the wild (Figure 3; Tables 2 and 4). Similar to G. pennsylvanicus, G. veletis males were also highly variable in their plasticity, with a few signaling in an apparently maladaptive manner (Figure 3). Some males signaled less often and more quietly during times that mating activity is highest in the wild and more often and louder when mating activity is lowest. Neither body size nor residual mass significantly explained among-male variation in phenotypic plasticity.
Discussion
Given that the density of conspecific rivals and potential mates determines the intensity of sexual selection [33]–[38], male field crickets should be selected to exhibit adaptive phenotypic plasticity in their mate attraction signals that matches the socio-sexual competitive context they experience. Our findings support this idea. Most males signaled plastically, aligning their mate attraction signals to the temporally fluctuating socio-sexual context they faced. Males signaled for mates following distinct diel rhythms that differed across species. Male signaling diel rhythms are synchronized so that they are in phase with female mating activity diel rhythms in the wild. Male G. pennsylvanicus signaled more often (14 versus 9 min/hr), louder (62 vs 60 dB), with elevated carrier frequencies (4.7 vs 4.6 kHz), shorter interpulse durations (38 vs 40 msec), and shorter pulse periods (57 vs 59 msec) during the high versus low mating activity time periods, respectively (high 22∶00–09∶59 vs low 10∶00–21∶59; [32]). This plasticity appears adaptive given that female G. pennsylvanicus preferentially mate with males that signal most often [63] and are more attracted to loud signals played at 5 kHz versus quiet ones played at 4 kHz, and signals with pulse periods falling within the 35–60 msec range [49], [54]. Male G. veletis signaled more often (7 vs 3 min/hr), with longer interpulse durations (37 vs 35 msec), longer pulse periods (53 vs 50 msec) and longer chirp durations (128 vs 118 msec) during the high versus low mating activity time periods (high 04∶00–15∶59 vs low 16∶00–03∶59; [32]). While less is known about female G. veletis’ mating preferences, one study revealed females are attracted to signals with pulse periods that ranged from 40–70 msec [54]. Female G. veletis may be similar to other cricket species and preferentially mate with males that signal most often and with long chirp durations [42], [44], [63]–[66]. If so, plasticity in male G. veletis signaling traits would be adaptive.
Species-level differences in temporal signaling rhythms also appear adaptive. The mating seasons of G. pennsylvanicus and G. veletis overlap spatially and temporally. Since G. pennsylvanicus and G. veletis both breed in grassy fields and rock crevices (SMB, SJH, IRT, LPF), it is likely that their habitats overlap in mid-summer. Gryllus pennsylvanicus and G. veletis differ in the time of day when peak calling and mating activity occurs, with G. pennsylvanicus peaking at night and G. veletis peaking in the morning. Given the fine-scale structure of most mating signal parameters overlap extensively across the two species, these temporal differences may act as a behavioral barrier to reproductively isolate the two species.
While we conclude that males are changing their signaling throughout the day in response to female mating activity, it is also possible that females are instead mating more during these times of day because males are signaling more. The causal relationships between male signaling and female mating activities should be explored by measuring female receptivity to mating throughout the day while controlling for male signaling behavior.
Condition Dependency?
Sexually selected traits should act as phenotypically plastic gauges of an individual’s condition because only individuals in top condition should be able to bear the costs of advertising with the most exaggerated traits. Predictions differ about how condition influences plasticity. Bretman et al. [29] predicted a concave relationship between condition and plasticity, where males in high and low condition exhibit minimal plasticity, whereas males in intermediate condition exhibit high plasticity. They theorized that intermediate condition males should have the stores available to amplify their signals when environmental conditions are best, but not enough stores to constantly maintain amplified signals. Conversely, Jacot et al. [37] predicted a negative linear relationship between condition and plasticity. They suggested males in low condition should exhibit high phenotypic plasticity because they only have sufficient resources available to invest when the net benefits are highest. These differences in predictions likely results from how the authors define low condition males, but regardless, our results do not support either of them. Although variation in body size (pronotum area) explained some of the variation in signaling plasticity in G. pennsylvanicus, the largest individuals exhibited the highest adaptive plasticity, not the intermediate-sized individuals (as predicted by Bretman et al. [29]) or smallest individuals (as hypothesized by Jacot et al. [37]). Body size is partially indicative of resource availability during development [66], but is also a heritable trait in many field cricket species [67]–[70]. Larger individuals may have superior genotypes, making larger males capable of both growing larger during development and exhibiting more adaptive signaling plasticity during adulthood, a hypothesis that requires testing.
Residual mass did not explain variation in signaling plasticity. We assumed that males with low residual mass were in worse condition than males with average or high residual mass. However, some low residual mass males may be better at acquiring resources and therefore more willing to risk investing energy into signaling, because it can be easily replaced. If so, this would have confounded our results for how residual mass influenced plasticity. Our ad libitum feeding regime following field capture could also have confounded our results for how residual mass influences plasticity. For these reasons and many others [57], [71]–[74], residual mass may not be a strong measure of condition in crickets [75], [76].
Hill [23] recommends quantifying condition using physiological, cellular, and biochemical processes. Supporting Hill’s [23] assertion, our recent research suggests that differences in carbohydrate metabolism may more accurately denote condition in chirping crickets [29]. Metabolic power for signaling comes from work caused by the thoracic muscles closing the plectrum against the file during the production of a sound pulse [77]. Energetic signaling costs are dependent on the total number of pulses produced and on the average number of teeth struck during the production of a pulse [78]–[80]. While the energetic costs of chirping are not very high, reaching only 1–4× resting metabolic rate [78]–[85], the costs may be high enough for some males to not be able to alter the quality or quantity of their signals. Future research should examine whether plastic signaling is tied to the ability to metabolize carbohydrates.
Temperature-Induced Plasticity?
Our fine scale signaling plasticity results were unlikely to result from minor changes in ambient temperature. We controlled for temperature by conducting our study in a climate-controlled greenhouse. However, while the temperature remained close to 28°C throughout our experiment, it occasionally climbed as high as 30°C during the day and fell as low as 26°C at night. Increasing temperature decreases interpulse, chirp and interchirp durations [78], [86]–[91]. The slight temperature fluctuations experienced in the greenhouse are unlikely to explain the signaling plasticity because we observed that interpulse, chirp, and interchirp durations increased throughout the morning hours, as the greenhouse warmed with the morning sun.
Maladaptive Behavior?
A handful of males behaved in a seemingly maladaptive manner, consistently producing unattractive signals or signaling most during the time of day that mating activity is lowest in the wild. Why do some males signal maladaptively? While G. pennsylvanicus mating activity is highest at night and around sunrise (22∶00–09∶59), some mating (12%) occurred during the rest of the day (10∶00–21∶59; [32]). Similarly, while G. veletis mating activity is highest in the early morning and throughout the day (04∶00–15∶59), some mating (19%) occurred during evening hours (16∶00–03∶59; [32]). Males that signal more when mating activity is lowest may be less aggressive or less able to compete with males during peak mating hours, and may instead signal during times of lower competition. Variation in signaling plasticity could also stem from age, fighting experience, or mating experience differences among males. We captured males as adults in the field, so age and social experience are unknown. Male signaling changes with age (G. pennsylvanicus: [92]; G. veletis: [93]) and so males may also alter signaling plasticity with age/experience.
Male variation in signaling plasticity could also stem from female behavioral plasticity in the wild. Wild female G. pennsylvanicus and G. veletis are likely to exhibit variation in the types of signals to which they are most attracted. Whereas female field crickets are generally most responsive to specific signaling parameters, some females exhibit narrow mating preferences while other females exhibit broad preferences [54]. Female age and mating history may play a role, because older and mated females are typically much choosier than younger and virgin females [54]. Virgin females are more likely to mate and mate more quickly than previously-mated females [94]. Differences in choosiness could result from physiological differences, changes in hormonal levels altering auditory receptiveness, and/or lower residual reproductive value [95]. Female social experience could also shape differences in preference. The perceived attractiveness of previously encountered males influences pre- and post-copulatory mate choice decisions with subsequent males [96]. Given that females in the wild may exhibit mate preference plasticity, the seemingly maladaptive mate signaling strategies of males may in fact not be maladaptive.
Regardless of the underlying causes of some individuals signaling more attractively or more often during the non-peak mating hours, this signaling may be risky. Signaling during non-peak mating hours may increase the potential of interbreeding (G. pennsylvanicus with G. veletis). Given hybridization is lethal [43], signaling more attractively or more often during the non-peak mating hours may be maladaptive.
Conclusions and Future Directions
Phenotypic plasticity can be adaptive when phenotypes align with environmental changes. In crickets, environmental changes result in rhythmic fluctuations in the density of sexually active individuals (summarized in Table S1). Given that density strongly influences the intensity of sexual selection [33]–[38], we asked whether crickets exhibit plasticity in their signaling behavior that aligns with these rhythmic fluctuations in the socio-sexual environment. Our findings suggest that most crickets exhibit phenotypically plastic mate attraction signals that match the temporally fluctuating socio-sexual context they experience. Our findings also suggest cricket signaling plasticity may be adaptive. Our correlational study exploring variation in plasticity is the first step towards determining whether crickets exhibit genotype by environment interactions in their signaling behaviors (e.g., [58]–[60]). Future research should (1) quantify the signaling plasticity of crickets reared in the laboratory to allow us to control for mating experience, condition, and age effects [97, submitted] and (2) examine whether plastic signaling is tied to the ability to metabolize carbohydrates. If signaling plasticity of crickets reared in the laboratory is similar to wild crickets, then future research should also (3) quantify the underlying genetic basis of signaling plasticity.
Supporting Information
Literature review of how cricket and parasitoid density change over the course of a day. Studies with different results are presented in separate rows, and studies with the same results are presented in the same row. A dash (−) indicates the species was not observed during that time period. a French Polynesia; b Australia; c Hawaii;.
(DOCX)
Repeated measures ANOVA using repeated measures general linear mixed models (GLMM) utilizing the restricted maximum likelihood (REML) approach to investigate whether signaling behavior changed over the course of a day. Individual was classified as a random effect, while time was classified as a fixed effect using six time periods: Time: 1 = 00∶00–03∶59; 2 = 04∶00–07∶59; 3 = 08∶00–11∶59; 4 = 12∶00–15∶59; 5 = 16∶00–19∶59; 6 = 20∶00–23∶59. Separate models were run for G. pennsylvanicus and G. veletis and for each of the 9 signaling parameters. Our corrected alpha FDRB-Y level of significance is P<0.0143 to account for the 18 models run. Our models were generally highly significant explaining 55–88% of the variation in signaling traits: time of day explained 3–15% of the signaling variation, while individual identity explained 40–85%.
(DOCX)
Acknowledgments
We thank G.R. Kolluru for stimulating statistical and theoretical discussions during the preparation of this manuscript and for helpful comments on an earlier version of this manuscript. We also thank the Biological Sciences Department at California Polytechnic State University in San Luis Obispo for hosting S.M.B. during the preparation of this manuscript. We thank J. Dawson for the use of his equipment, J. Fitzsimmons, A. Mikhail and S. Klaus for assistance in field collections, and C. Grant for assisting in both the field collections and the laboratory recordings.
Funding Statement
Funding was provided by a Natural Science and Engineering Research Council of Canada (NSERC) Canada Graduate Scholarship to LPF, an Ontario Graduate Scholarship (OGS) to SJF, an NSERC Undergraduate Research Scholarships to IRT and SJH, and a Philanthropic Education Organization (PEO) Scholar Award to LPF. SMB received funding support from an NSERC Discovery Grant, the Canadian Foundation for Innovation (CFI), the Ontario Research Fund (ORF), and Carleton University (NSERC: http://www.nserc-crsng.gc.ca/, OGS: https://osap.gov.on.ca/OSAPPortal/en/A-ZListofAid/TCONT003465.html, PEO: http://www.peointernational.org/, CFI: http://www.innovation.ca/, ORF: http://www.mri.gov.on.ca/english/programs/ResearchFund.asp, Carleton: http://www.carleton.ca). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. West-Eberhard M (1989) Phenotypic plasticity and the origins of diversity. Ann Rev Ecol Syst 20: 249–278. [Google Scholar]
- 2. Scheiner S (1993) Genetics and evolution of phenotypic plasticity. Ann Rev Ecol Syst 24: 35–68. [Google Scholar]
- 3. Kelly SA, Panhuis T, Stoehr A (2012) Phenotypic plasticity: molecular mechanisms and adaptive significance. Comp Physiol 2: 1417–1439 doi:10.1002/cphy.c110008 [DOI] [PubMed] [Google Scholar]
- 4.Levins R (1968) Evolution in changing environments: Some theoritcal explorations. Princeton, New Jersey: Princeton University Press.
- 5. Moran N (1992) The evolutionary maintenance of alternative phenotypes. Am Nat 139: 971–989. [Google Scholar]
- 6.Schlichting C, Pigliucci M (1998) Phenotypic evolution: a reaction norm perspective. Sunderland, MA: Sinauer Associates Inc.
- 7. Snell-Rood EC (2012) Selective processes in development: implications for the costs and benefits of phenotypic plasticity. Integr Comp BIol 52: 31–42 doi:10.1093/icb/ics067 [DOI] [PubMed] [Google Scholar]
- 8. Van Tienderen P (1991) Evolution of generalists and specialist in spatially heterogeneous environments. Evolution 45: 1317–1331. [DOI] [PubMed] [Google Scholar]
- 9. Dewitt TJ, Sih A, Wilson DS (1998) Costs and limits of phenotypic plasticity. Trends Ecol Evol 13: 77–81 doi:10.1016/S0169-5347(97)01274-3 [DOI] [PubMed] [Google Scholar]
- 10. Pigliucci M (2005) Evolution of phenotypic plasticity: where are we going now? Trends Ecol Evol 20: 481–486 doi:10.1016/j.tree.2005.06.001 [DOI] [PubMed] [Google Scholar]
- 11. Van Kleunen M, Fischer M (2005) Constraints on the evolution of adaptive phenotypic plasticity in plants. New Phytol 166: 49–60 doi:10.1111/j.1469-8137.2004.01296.x [DOI] [PubMed] [Google Scholar]
- 12. Valladares F, Gianoli E, Gómez JM (2007) Ecological limits to plant phenotypic plasticity. New Phytol 176: 749–763 doi:10.1111/j.1469-8137.2007.02275.x [DOI] [PubMed] [Google Scholar]
- 13. Ghalambor CK, McKay JK, Carroll SP, Reznick DN (2007) Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct Ecol 21: 394–407 doi:10.1111/j.1365-2435.2007.01283.x [Google Scholar]
- 14. Auld JR, Agrawal AA, Relyea RA (2010) Re-evaluating the costs and limits of adaptive phenotypic plasticity. Proc R Soc B 277: 503–511 doi:10.1098/rspb.2009.1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Komers PE (1997) Behavioural plasticity in variable environments. Can J Zool 75: 161–169 doi:10.1139/z97-023 [Google Scholar]
- 16. Ryan M, Keddy-Hector A (1992) Directional patterns of female mate choice and the role of sensory biases. Am Nat 139: S4–S35. [Google Scholar]
- 17. Price TD (1998) Sexual selection and natural selection in bird speciation. Phil Trans R Soc B 353: 251–260 doi:10.1098/rstb.1998.0207 [Google Scholar]
- 18. Price TD (2006) Phenotypic plasticity, sexual selection and the evolution of colour patterns. J Exp Biol 209: 2368–2376 doi:10.1242/jeb.02183 [DOI] [PubMed] [Google Scholar]
- 19. Zahavi A (1975) Mate selection: a selection for a handicap. J Theor Biol 53: 205–214. [DOI] [PubMed] [Google Scholar]
- 20. Nur N, Hasson O (1984) Phenotypic plasticity and the handicap principle. J Theor Biol 110: 275–297. [Google Scholar]
- 21. Grafen A (1990) Biological signals as handicaps. J Theor Biol 144: 517–546. [DOI] [PubMed] [Google Scholar]
- 22. Qvarnström A, Price TD (2001) Maternal effects, paternal effects and sexual selection. Trends Ecol Evol 16: 95–100. [DOI] [PubMed] [Google Scholar]
- 23. Hill GE (2011) Condition-dependent traits as signals of the functionality of vital cellular processes. Ecol Lett 14: 625–634 doi:10.1111/j.1461-0248.2011.01622.x [DOI] [PubMed] [Google Scholar]
- 24. Piersma T, Drent J (2003) Phenotypic flexibility and the evolution of organismal design. Trends Ecol Evol 18: 228–233 doi:10.1016/S0169-5347(03)00036-3 [Google Scholar]
- 25.Ghalambor CK, Angeloni L, Carroll SP (2010) Behavior as phenotypic plasticity. In: Westneat D, Fox C, editors. Evolutionary Behavioural Ecology. Oxford: Oxford University Press. 90–107.
- 26. Gross M (1996) Alternative reproductive strategies and tactics: diversity within sexes. Trends Ecol Evol 11: 92–98 doi:10.1016/0169-5347(96)81050-0 [DOI] [PubMed] [Google Scholar]
- 27. Buzatto BA, Tomkins JL, Simmons LW (2012) Maternal effects on male weaponry: female dung beetles produce major sons with longer horns when they perceive higher population density. BMC Evol Biol 12: 118 doi:10.1186/1471-2148-12-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lyons C, Barnard CJ (2006) A learned response to sperm competition in the field cricket, Gryllus bimaculatus (de Geer). Anim Behav 72: 673–680 doi:10.1016/j.anbehav.2005.12.006 [Google Scholar]
- 29. Bretman A, Gage MJG, Chapman T (2011) Quick-change artists: male plastic behavioural responses to rivals. Trends Ecol Evol 26: 467–473 doi:10.1016/j.tree.2011.05.002 [DOI] [PubMed] [Google Scholar]
- 30. Alexander RD (1961) Aggressiveness, territoriality, and sexual behavior in field crickets (Orthoptera: Gryllidae). Behaviour 17: 130–223. [Google Scholar]
- 31. Bertram SM, Orozco SX, Bellani R (2004) Temporal shifts in conspicuousness: mate attraction displays of the Texas field cricket, Gryllus texensis . Ethology 110: 963–975 doi:10.1111/j.1439-0310.2004.01031.x [Google Scholar]
- 32. French BW, Cade WH (1987) The timing of calling, movement, and mating in the field crickets Gryllus veletis, G. pennsylvanicus, and G. integer . Behav Ecol Sociobiol 21: 157–162 doi:10.1007/BF00303205 [Google Scholar]
- 33. Kokko H, Monaghan P, Letters E (2001) Predicting the direction of sexual selection. Ecol Lett 4: 159–165 doi:10.1046/j.1461-0248.2001.00212.x [Google Scholar]
- 34. Davidson AJ, Menaker M (2003) Birds of a feather clock together – sometimes: social synchronization of circadian rhythms. Curr Opin Neurobiol 13: 765–769 doi:10.1016/j.conb.2003.10.011 [DOI] [PubMed] [Google Scholar]
- 35. Helm B, Piersma T, Van der Jeugd H (2006) Sociable schedules: interplay between avian seasonal and social behaviour. Anim Behav 72: 245–262 doi:10.1016/j.anbehav.2005.12.007 [Google Scholar]
- 36. Kokko H, Rankin DJ (2006) Lonely hearts or sex in the city? Density-dependent effects in mating systems. Phil Trans R Soc B 361: 319–334 doi:10.1098/rstb.2005.1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jacot A, Scheuber H, Holzer B, Otti O, Brinkhof MWG (2008) Diel variation in a dynamic sexual display and its association with female mate-searching behaviour. Proc R Soc B 275: 579–585 doi:10.1098/rspb.2007.1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roth T, Sprau P, Schmidt R, Naguib M, Amrhein V (2009) Sex-specific timing of mate searching and territory prospecting in the nightingale: nocturnal life of females. Proc R Soc B 276: 2045–2050 doi:10.1098/rspb.2008.1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berrigan D, Scheiner S (2004) Modeling the evolution of phenotypic plasticity. In: DeWitt T, Scheiner S, editors. Phenotypic plasticity: Functional and conceptual approaches. New York: Oxford University Press. 82–97.
- 40. Garland T, Kelly SA (2006) Phenotypic plasticity and experimental evolution. J Exp BIol 209: 2344–2361 doi:10.1242/jeb.02244 [DOI] [PubMed] [Google Scholar]
- 41. Kasumovic MM, Brooks RC (2011) It’s all who you know: the evolution of socially cued anticipatory plasticity as a mating strategy. Q Rev Biol 86: 181–197 doi:10.1086/661119 [DOI] [PubMed] [Google Scholar]
- 42. Holzer B, Jacot A, Brinkhof MWG (2003) Condition-dependent signaling affects male sexual attractiveness in field crickets, Gryllus campestris . Behav Ecol 14: 353–359 doi:10.1093/beheco/14.3.353 [Google Scholar]
- 43.Bigelow R (1960) Interspecific hybrids and speciation in the genus Acheta (Orthoptera, Gryllidae). Can J Zool 38 509–524.
- 44. Wagner WE (1996) Convergent song preferences between female field crickets and acoustically orienting parasitoid flies. Behav Ecol 7: 279–285 doi:10.1093/beheco/7.3.279 [Google Scholar]
- 45. Hedwig B, Poulet J (2004) Complex auditory behaviour emerges from simple reactive steering. Nature 430: 781–785 doi:10.1038/nature02723.1 [DOI] [PubMed] [Google Scholar]
- 46. Popov A, Shuvalov V (1977) Phonotactic behavior of crickets. J Comp Physiol A 126: 111–126. [Google Scholar]
- 47. Simmons LW, Ritchie MGGM (1996) Symmetry in the songs of crickets. Proc R Soc B 263: 1305–1311 doi:10.1098/rspb.1996.0191 [Google Scholar]
- 48. Scheuber H, Jacot A, Brinkhof MWG (2003) The effect of past condition on a multicomponent sexual signal. Proc R Soc B 270: 1779–1784 doi:10.1098/rspb.2003.2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jeffery J, Navia B, Atkins G, Stout J (2005) Selective processing of calling songs by auditory interneurons in the female cricket, Gryllus pennsylvanicus: possible roles in behavior. J Exp Zool 303: 377–392 doi:10.1002/jez.a.176 [DOI] [PubMed] [Google Scholar]
- 50. Popov A, Shuvalov V (1974) The spectrum, intensity, and direction of the calling song of the cricket Gryllus campestris under natural conditions. J Evol Biochem Physiol 10: 61–68. [PubMed] [Google Scholar]
- 51. Wagner WE, Murray A, Cade WH (1995) Phenotypic variation in the mating preferences of female field crickets, Gryllus integer . Anim Behav 49: 1269–1281 doi:10.1006/anbe.1995.0159 [Google Scholar]
- 52. Thorson J, Weber T, Huber F (1982) Auditory behavior of the cricket II. Simplicity of calling song recognition in Gryllus, and anamalous phototaxis at abnormal carrier frequencies. J Comp Physiol A 146: 361–378. [Google Scholar]
- 53. Doherty J, Huber F (1983) Temperature effects on acoustic communication in the cricket Gryllus bimaculatus DeGeer. Verh Dtsch Zool Ges 188: 188. [Google Scholar]
- 54. Stout J, Navia B, Jeffery J, Samuel L, Hartwig L, et al. (2010) Plasticity of the phonotactic selectiveness of four species of chirping crickets (Gryllidae): Implications for call recognition. Physiol Entomol 35: 99–116 doi:10.1111/j.1365-3032.2009.00713.x [Google Scholar]
- 55. Simmons LW (1988) The calling song of the field cricket, Gryllus bimaculatus (De Geer): constraints on transmission and its role in intermale competition and female choice. Anim Behav 36: 380–394. [Google Scholar]
- 56. Hedrick AV (1986) Female preferences for male calling bout duration in a field cricket. Behav Ecol and Sociobiol 19: 73–77. [Google Scholar]
- 57. Tomkins JL, Radwan J, Kotiaho JS, Tregenza T (2004) Genic capture and resolving the lek paradox. Trends Ecol Evol 19: 323–328 doi:10.1016/j.tree.2004.03.029 [DOI] [PubMed] [Google Scholar]
- 58. Endler J (1978) A predator’s view of animal color patterns. Evol Biol 11: 319–364. [Google Scholar]
- 59. Endler J (1980) Natural selection on color patterns in Poecilia reticulata. . Evolution 34: 76–91. [DOI] [PubMed] [Google Scholar]
- 60. Millar N, Reznick DN, Kinnison M, Hendry A (2006) Disentangling the selective factors that act on male colour in wild guppies. Oikos 1: 1–12 doi:10.1111/j.0030-1299.2006.14038.x [Google Scholar]
- 61. Whattam EM, Bertram SM (2011) Effects of juvenile and adult condition on long-distance call components in the Jamaican field cricket, Gryllus assimilis . Anim Behav 81: 135–144 doi:10.1016/j.anbehav.2010.09.024 [Google Scholar]
- 62. Benjamini Y, Yekutieli D (2001) The control of the false discovery rate in multiple testing under dependency. Ann Stat 29: 1165–1188 doi:10.1214/aos/1013699998 [Google Scholar]
- 63. Judge KA, Ting JJ, Gwynne DT (2008) Condition dependence of male life span and calling effort in a field cricket. Evolution 62: 868–878 doi:10.1111/j.1558-5646.2008.00318.x [DOI] [PubMed] [Google Scholar]
- 64. Cade WH, Cade ESE (1992) Male mating success, calling and searching behaviour at high and low densities in the field cricket, Gryllus integer . Anim Behav 43: 49–56 doi:10.1016/S0003-3472(05)80070-3 [Google Scholar]
- 65. Crnokrak P, Roff DA (1995) Fitness differences associated with calling behaviour in the two wing morphs of male sand crickets, Gryllus firmus . Anim Behav 50: 1475–1481 doi:10.1016/0003-3472(95)80004-2 [Google Scholar]
- 66. Hunt J, Brooks RC, Jennions MD, Smith MJ, Bentsen CL, et al. (2004) High-quality male field crickets invest heavily in sexual display but die young. Nature 432: 1024–1027 doi:10.1038/nature03084 [DOI] [PubMed] [Google Scholar]
- 67. Mousseau TA, Roff D (1989) Adaptation to seasonality in a cricket: patterns of phenotypic and genotypic variation in body size and diapause expression along a cline in season length. Evolution 43: 1483–1496. [DOI] [PubMed] [Google Scholar]
- 68. Fedorka KM, Mousseau TA (2004) Female mating bias results in conflicting sex-specific offspring fitness. Nature 429: 65–67 doi:10.1038/nature02492 [DOI] [PubMed] [Google Scholar]
- 69. Del Castillo R (2005) The quantitative genetic basis of female and male body size and their implications on the evolution of body size dimorphism in the house cricket Acheta domesticus (Gryllidae). Genet Mol Biol 28: 843–848 doi:10.1590/S1415 [Google Scholar]
- 70. Simmons LW (1987) Female choice contributes to offspring fitness in the field cricket, Gryllus bimaculatus (De Geer). Behav Ecol Sociobiol 21: 313–321. [Google Scholar]
- 71. Gould SJ (1975) Allometry in primates, with emphasis on scaling and the evolution of the brain. Contrib Primatol 5: 244–292. [PubMed] [Google Scholar]
- 72. Kotiaho JS, Marshall SD, Barrow JH, Jakob EM, Uetz GW (1999) Estimating fitness: comparison of body condition indices revisited. Oikos 87: 399–402. [Google Scholar]
- 73. García-Berthou E (2001) On the misuse of residuals in ecology: testing regression residuals vs. the analysis of covariance. J Anim Ecol 70: 708–711 doi:10.1046/j.1365-2656.2001.00524.x [Google Scholar]
- 74. Tomkins JL, Simmons LW (2002) Measuring relative investment: a case study of testes investment in species with alternative male reproductive tactics. Anim Behav 63: 1009–1016 doi:10.1006/anbe.2001.1994 [Google Scholar]
- 75. Bertram SM, Fitzsimmons LP, McAuley EM, Rundle HD, Gorelick R (2011) Phenotypic covariance structure and its divergence for acoustic mate attraction signals among four cricket species. Ecol Evol 1: 1–15 doi:10.1002/ece3.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gray DA, Eckhardt G (2001) Is cricket courtship song condition dependent? Anim Behav 62: 871–877 doi:10.1006/anbe.2001.1825 [Google Scholar]
- 77. Pfau H, Koch UT (1994) The functional morphology of singing in the cricket. J Exp Biol 195: 147–167. [DOI] [PubMed] [Google Scholar]
- 78. Prestwich KN, Walker TJ (1981) Energetics of singing in crickets: effect of temperature in three trilling species (Orthoptera: Gryllidae). J Comp Physiol B 143: 199–212. [Google Scholar]
- 79. Hoback WW, Wagner WE (1997) The energetic cost of calling in the variable field cricket, Gryllus lineaticeps . Physiol Entomol 22: 286–290 doi:10.1111/j.1365-3032.1997.tb01170.x [Google Scholar]
- 80. Prestwich KN, O’Sullivan K (2005) Simultaneous measurement of metabolic and acoustic power and the efficiency of sound production in two mole cricket species (Orthoptera: Gryllotalpidae). J Exp Biol 208: 1495–1512 doi:10.1242/jeb.01550 [DOI] [PubMed] [Google Scholar]
- 81. Kavanagh MW (1987) The efficiency of sound production in two cricket species, Gryllotalpa australis and Teleogryllus commodus (Orthoptera: Grylloidea). J Exp Biol 119: 107–119. [Google Scholar]
- 82. Bailey WJ, Withers PC, Endersby M, Gaull K (1993) The energetic costs of calling in the bushcricket Requena verticalis (Orthoptera: Tettigoniidae: Listroscelidinae). J Exp Biol 178: 21–37. [Google Scholar]
- 83. Prestwich KN (1994) The energetics of acoustic signaling in Anurans and Insects. Am Zool 34: 625–643 doi:10.1093/icb/34.6.625 [Google Scholar]
- 84. Hack MA (1998) The energetics of male mating strategies in field crickets (Orthoptera: Gryllinae: Gryllidae). J Insect Behav 11: 853–867 doi:10.1023/A:1020864111073 [Google Scholar]
- 85. Prestwich KN (2007) Measuring the efficiency of sound production. Physiol Biochem Zool 80: 157–165 doi:10.1086/509238 [DOI] [PubMed] [Google Scholar]
- 86. Walker TJ (1957) Specificity in the response of female tree crickets (Orthoptera, Gryllidae, Oecanthinae) to calling songs of the males. Ann Entomol Soc Am 50: 626–636. [Google Scholar]
- 87. Walker TJ (1962) Factors responsible for intraspecific variation in the calling songs of crickets. Evolution 16: 407–428. [Google Scholar]
- 88. Walker TJ (1962) The taxonomy and calling songs of United States tree crickets (Orthoptera: Gryllidae: Oecaiithinae). I. The genus Neoxabea and the niveus and varicornis groups of the genus. Ann Entomol Soc Am 55: 303–323. [Google Scholar]
- 89. Walker TJ (1975) Effects of temperature on rates in poikilotherm nervous systems: evidence from the calling songs of meadow katydids (Orthoptera: Tettigoniidae: Orchelimum) and reanalysis of published data. J Comp Physiol A 101: 57–69. [Google Scholar]
- 90. Doherty J (1985) Trade-off phenomena in calling song recognition and phonotaxis in the cricket, Gryllus bimaculatus (Orthoptera, Gryllidae). J Comp Physiol A 156: 787–801. [Google Scholar]
- 91. Martin SD, Gray DA, Cade WH (2000) Fine-scale temperature effects on cricket calling song. Can J Zool 78: 706–712 doi:10.1139/cjz-78-5-706 [Google Scholar]
- 92. Judge KA (2011) Do male field crickets, Gryllus pennsylvanicus, signal their age? Anim Behav 81: 185–194 doi:10.1016/j.anbehav.2010.09.032 [Google Scholar]
- 93. Fitzsimmons LP, Bertram SM (2011) The calling songs of male spring field crickets (Gryllus veletis) change as males age. Behaviour 148: 1045–1065 doi:10.1163/000579511X588812 [Google Scholar]
- 94. Judge KA, Tran K-C, Gwynne DT (2010) The relative effects of mating status and age on the mating behaviour of female field crickets. Can J Zool 88: 219–223 doi:10.1139/Z09-139 [Google Scholar]
- 95. Gray DA (1999) Intrinsic factors affecting female choice in house crickets: time cost, female age, nutritional condition, body size, and size-relative reproductive investment. J Insect Behav 12: 691–700 doi:10.1023/A:1020983821436 [Google Scholar]
- 96. Rebar D, Zuk M, Bailey NW (2011) Mating experience in field crickets modifies pre- and postcopulatory female choice in parallel. Behav Ecol 22: 303–309 doi:10.1093/beheco/arq195 [Google Scholar]
- 97.Bertram SM, Thomson IR, Harrison SH, Ferguson GL, Fitzsimmons LP (submitted) Variation in mate signaling and size: comparison of wild and laboratory-reared males of two field cricket species. PeerJ (#2013:05: 536:0:0:REVIEW): Under review (Submitted May 29, 2013).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Literature review of how cricket and parasitoid density change over the course of a day. Studies with different results are presented in separate rows, and studies with the same results are presented in the same row. A dash (−) indicates the species was not observed during that time period. a French Polynesia; b Australia; c Hawaii;.
(DOCX)
Repeated measures ANOVA using repeated measures general linear mixed models (GLMM) utilizing the restricted maximum likelihood (REML) approach to investigate whether signaling behavior changed over the course of a day. Individual was classified as a random effect, while time was classified as a fixed effect using six time periods: Time: 1 = 00∶00–03∶59; 2 = 04∶00–07∶59; 3 = 08∶00–11∶59; 4 = 12∶00–15∶59; 5 = 16∶00–19∶59; 6 = 20∶00–23∶59. Separate models were run for G. pennsylvanicus and G. veletis and for each of the 9 signaling parameters. Our corrected alpha FDRB-Y level of significance is P<0.0143 to account for the 18 models run. Our models were generally highly significant explaining 55–88% of the variation in signaling traits: time of day explained 3–15% of the signaling variation, while individual identity explained 40–85%.
(DOCX)