Abstract
We have studied the fate of inert phagocytized particles in rabbit neutrophils. Neutrophils release significant quantities of preingested oil emulsion. Roughly 50% of an ingested load is released in 40 min at 37 degrees C. By electron microscopy the process of release appears to be by exocytosis: particles appear extruded through a network of processes often accompanied by membranous vesicles. Exocytosis is temperature and glucose dependent but unlike phagocytosis does not require divalent cations. From Coulter counter measurements virtually the entire cell population appears to undergo the phagocytosis-exocytosis sequence. Neutrophils undergoing exocytosis remain intact as determined by direct counts, electron microscopy, and absence of lactate dehydrogenase release. Moreover, by sequentially feeding differently labeled particles, it is shown that the processes of phagocytosis and exocytosis can occur concurrently. Indeed, it is found that ingestion accelerates release. The implications of these phenomena for membrane recycling, lysosomal enzyme release, and the killing of microorganisms are briefly discussed.
Full text
PDF

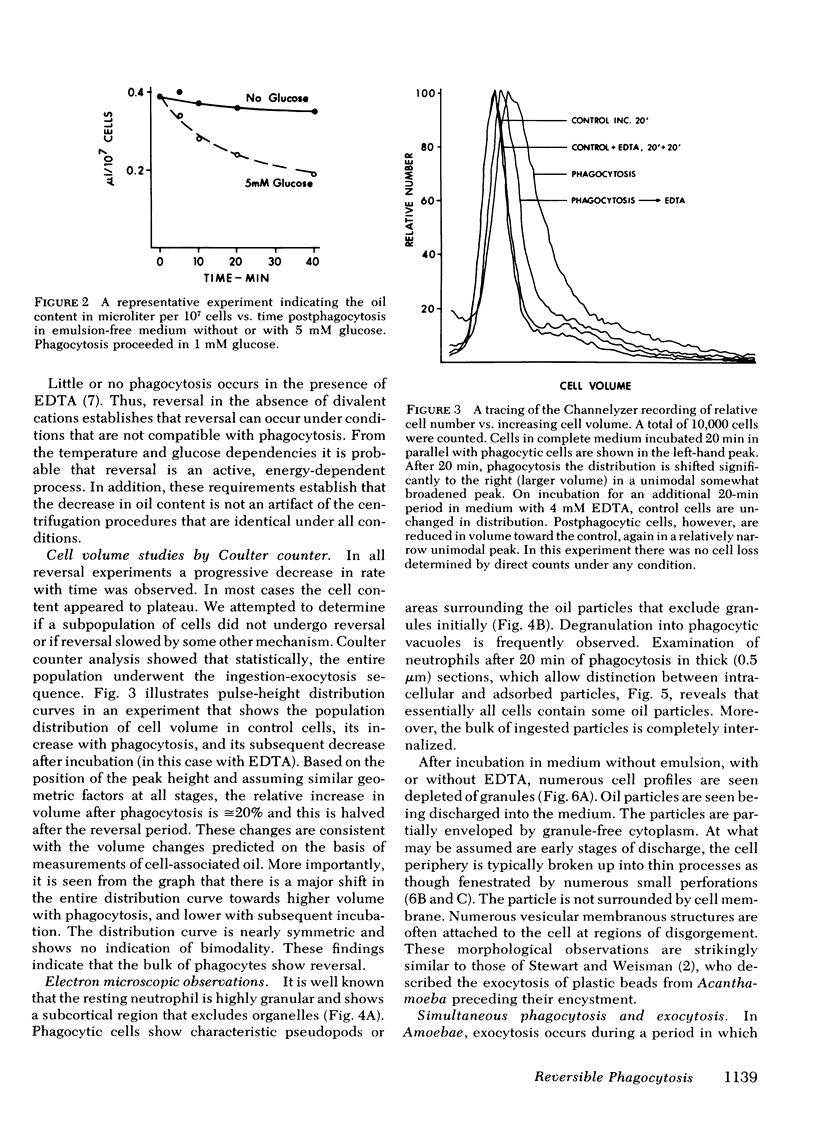




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berlin R. D., Fera J. P. Changes in membrane microviscosity associated with phagocytosis: effects of colchicine. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1072–1076. doi: 10.1073/pnas.74.3.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
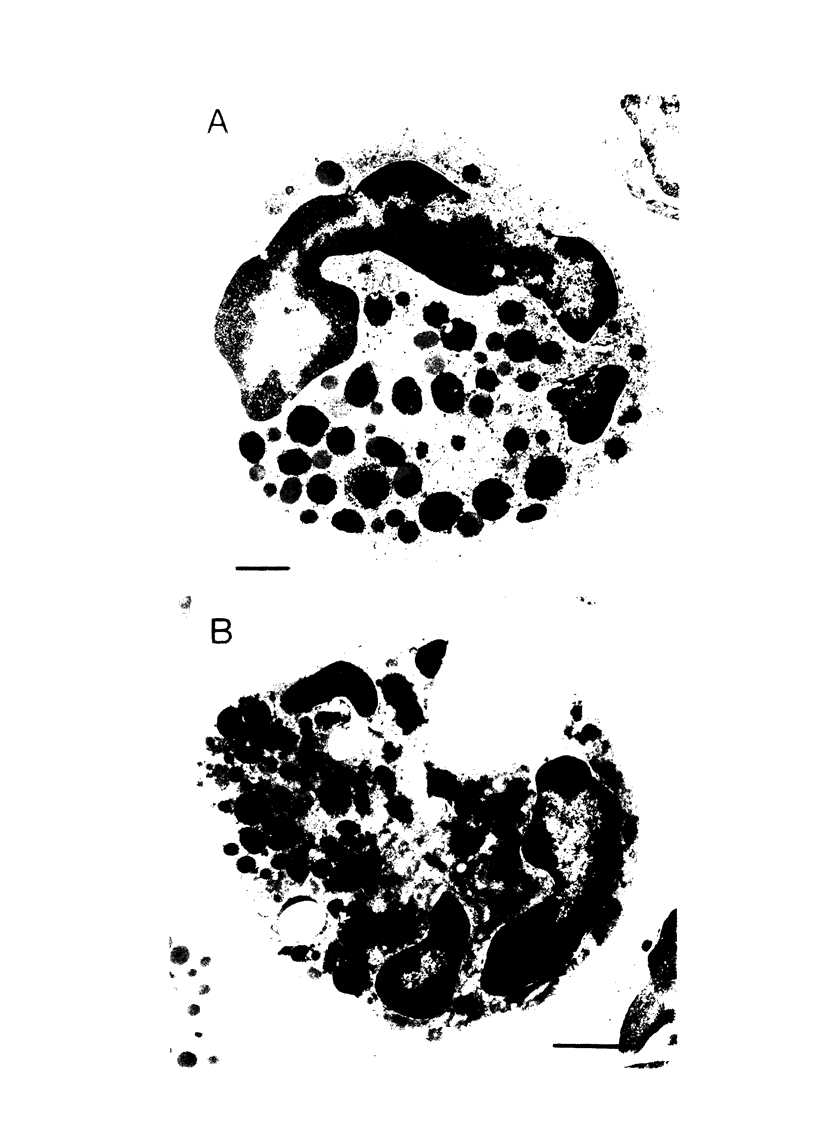
- Hawkins R. A., Berlin R. D. Purine transport in polymorphonuclear leukocytes. Biochim Biophys Acta. 1969 Mar 11;173(2):324–337. doi: 10.1016/0005-2736(69)90115-1. [DOI] [PubMed] [Google Scholar]
- KAISER H. K., WOOD W. B., Jr Studies on the pathogensis of fever. IX. The production of endogenous pyrogen by polymorphonuclear leucocytes. J Exp Med. 1962 Jan 1;115:27–36. doi: 10.1084/jem.115.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson K. E., Dahlgren C., Stendahl O., Sundqvist T. Characteristics of the phagocytic process assessed by Coulter Counter. Acta Pathol Microbiol Scand C. 1977 Jun;85(3):215–221. doi: 10.1111/j.1699-0463.1977.tb03633.x. [DOI] [PubMed] [Google Scholar]
- Silverstein S. C., Steinman R. M., Cohn Z. A. Endocytosis. Annu Rev Biochem. 1977;46:669–722. doi: 10.1146/annurev.bi.46.070177.003321. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Brodie S. E., Cohn Z. A. Membrane flow during pinocytosis. A stereologic analysis. J Cell Biol. 1976 Mar;68(3):665–687. doi: 10.1083/jcb.68.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J. R., Weisman R. A. Exocytosis of latex beads during the encystment of Acanthamoeba. J Cell Biol. 1972 Jan;52(1):117–130. doi: 10.1083/jcb.52.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P., Mason R. J., Hartwig J., Vaughan M. Quantitative studies of phagocytosis by polymorphonuclear leukocytes: use of emulsions to measure the initial rate of phagocytosis. J Clin Invest. 1972 Mar;51(3):615–624. doi: 10.1172/JCI106851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P., Pollard T. D., Mason R. J., Vaughan M. Isolation and properties of phagocytic vesicles from polymorphonuclear leukocytes. J Clin Invest. 1971 Aug;50(8):1745–1747. doi: 10.1172/JCI106664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsan M. F., Berlin R. D. Effect of phagocytosis on membrane transport of nonelectrolytes. J Exp Med. 1971 Oct 1;134(4):1016–1035. doi: 10.1084/jem.134.4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]