Abstract
Imagining or simulating future events has been shown to activate the anterior right hippocampus (RHC) more than remembering past events does. One fundamental difference between simulation and memory is that imagining future scenarios requires a more extensive constructive process than remembering past experiences does. Indeed, studies in which this constructive element is reduced or eliminated by “pre-imagining” events in a prior session do not report differential RHC activity during simulation. In this fMRI study, we examined the effects of repeatedly simulating an event on neural activity. During scanning, participants imagined 60 future events; each event was simulated three times. Activation in the RHC showed a significant linear decrease across repetitions, as did other neural regions typically associated with simulation. Importantly, such decreases in activation could not be explained by non-specific linear time-dependent effects, with no reductions in activity evident for the control task across similar time intervals. Moreover, the anterior RHC exhibited significant functional connectivity with the whole-brain network during the first, but not second and third simulations of future events. There was also evidence of a linear increase in activity across repetitions in right ventral precuneus, right posterior cingulate and left anterior prefrontal cortex, which may reflect source recognition and retrieval of internally generated contextual details. Overall, our findings demonstrate that repeatedly imagining future events has a decremental effect on activation of the hippocampus and many other regions engaged by the initial construction of the simulation, possibly reflecting the decreasing novelty of simulations across repetitions, and therefore is an important consideration in the design of future studies examining simulation.
Introduction
Remembering past events and imagining future events recruits a subset of regions of the default network [1], including medial prefrontal and parietal cortices, and the medial temporal lobes (MTL) [2], [3], [4]. Together, these regions have also been described as the ‘core’ network, reflecting the overlapping contributions of these regions to memory and simulation [5], [6]. However, imagining future events has been shown to activate certain regions of this core network, including the anterior right hippocampus (RHC), significantly more than remembering past events [2], [7], [8]. We have argued that while both remembering and imagining employ access to episodic memory details, which form the ‘building blocks’ of events, future simulation also requires the flexible integration of details extracted from various memories into a coherent representation [5], [9]. This process, termed ‘detail recombination’, likely requires additional processing supported by core network regions such as the anterior hippocampus [10].
In contrast, some studies report that remembering past events engages core network regions more than imagining future events [6], [11], [12]. While these findings appear to speak against the idea that, relative to remembering, future simulation requires additional neural resources to support more extensive constructive processes [2], [5], it is notable that the paradigms used in these studies did not require the online construction of imagined events in the scanner. Thus, one way to reconcile these seemingly contradictory findings is to suppose that while the construction of an event draws on hippocampal resources, imagining “pre-constructed” events may not do so to the same degree. We have observed previously that hippocampal activity reduces across the duration of a future simulation trial, with maximal activity evident in the initial moments of event construction [2]. When creating an event ‘from scratch’, the novel binding of details requires more constructive processing than when these details have been linked previously. Indeed, a number of studies have linked robust hippocampal activity with the recombining of familiar elements to form novel associations [13], [14]. Similarly, events that are more demanding to imagine, such as improbable future events, are associated with heightened responses in the anterior RHC [15].
To directly investigate the effects of repetition on simulation-related neural activity, participants repeatedly simulated future events in this study. In particular, we predicted that anterior RHC activity and its connectivity with other structures in the core network would decrease with repetitions, reflecting decreased constructive processing during simulation.
Materials and Methods
Participants
Ethics approval was obtained from the Human Ethics Participants Committee at The University of Auckland, New Zealand. Twenty-five healthy, right-handed adults provided their written consent to participate in this study. All participants were fluent in English, had no history of neurologic or psychiatric conditions or use of psychotropic medications, had no fMRI contraindications (e.g., ferromagnetic implants), and had not participated in our previous fMRI studies on future simulation. Five participants were excluded due to excessive movement or insufficient responses; data from 20 participants (nine males; range, 18–30 years) are presented.
Procedure
We adapted the episodic recombination paradigm [10] to include a repetition-suppression manipulation [16]. The experiment consisted of three phases: A pre-scan session in which memories were recalled, a scan session in which participants imagined future events, and a post-scan interview in which participants were interviewed about the content and features of their imaginings.
Pre-scan session
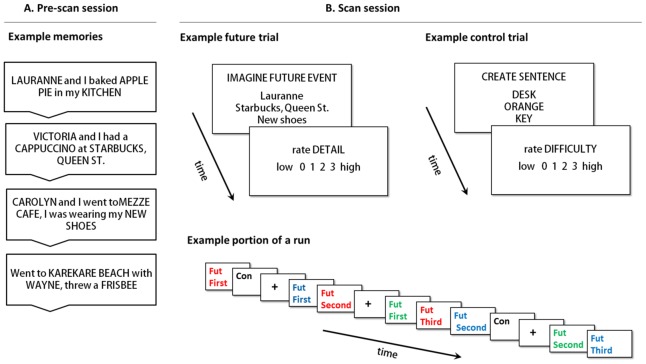
Participants recalled 100 episodic events from the past ten years. For each memory, a person, location and object were identified and described in a few words or less, with the restriction that these details could not be duplicated across events. Details were randomly recombined into new person-location-object sets where all three details came from different memories (see Figure 1).
Figure 1. The future event simulation paradigm.
(A) Pre-scan session: Participants recalled memories and identified a unique person, location and object in each. (B) Scan session: Participants imagined future events containing the three recombined memory details, and subsequently rated these simulations for detail. Participants also completed control trials, during which three common nouns are incorporated in the sentence “X is bigger than Y is bigger than Z”, followed by a difficulty rating. Runs also contained fixation trials. The sequence of trials from a portion of an example run is also provided in the bottom panel of (B): Repetitions of any one future event were separated by a variable number of intervening trials (other future event trials, control trials and fixation trials).
Scan session
Approximately one week later (M = 8.05 days, SD = 1.73 days), participants completed the scanning session. Prior to entering the scanner, a practice session was completed to familiarize the participants with the tasks and to allow time for questions. The scanning session consisted of a structural scan (10 mins) and five 12-min functional runs (60 mins). Sixty recombined detail sets were presented during magnetic resonance imaging (MRI); each was shown three times across the scanning session. During the First presentation (8s), the instruction “imagine future” was provided and participants imagined a novel event incorporating the three details that might occur in the next 5 years. Once participants had an event in mind, they made a button-press and continued imagining until a four-point rating scale for imagined detail (0 = vague; 3 = vivid) was presented (4s). The Second and Third presentation of each set occurred within the same run after an average interval of 93.57 seconds (SD = 26.00s). The duration was pseudo-randomized to avoid regularity, and included one to three future trials and a variable number of null and control trials. Participants were re-presented with an already viewed detail set and instructed to “re-imagine” the original simulation. Participants were encouraged to allow the previously constructed event come to mind, but to refrain from radically changing the simulation (such as progressing the event in time). It was considered acceptable if details in the event became ‘clearer’ over presentations (i.e., better or more easily visualized) as long as the event itself was not changed in any major way (e.g., a change in location, a change in people present, etc.) Participants also completed 60 trials of a size judgement task (adapted from [10]). This control task was designed to include similar elements to the future tasks, namely the presentation of the task instruction and three stimuli words on the screen, mental imagery and the formation of an integrated representation. In this task, participants were presented with a set of three nouns taken from Clark and Paivio’s extended norms [17], and were required to visualize the stimuli and incorporate them into a sentence of the form “X is bigger than Y is bigger than Z”, thus performing a relative size judgment task. Nouns were all rated highly familiar (M = 5.69), imageable (M = 5.55) and concrete (M = 6.94) [17]. Participants then rated the size judgment task for difficulty (4s) on a four-point scale (0 = not difficult; 3 = extremely difficult), included to control for the act of making a rating in the future task. One fifth of total scan time comprised jittered fixation-cross trials (4–16s) interspersed through the five runs (each 720s), as determined using Optseq2 [18].
Post-scan session
Immediately following scanning, participants described each event that was imagined in the MRI, estimated the date of future occurrence, rated event novelty relative to previous thoughts and experiences (0 = novel; 3 = identical) and the consistency of the simulation across repetitions (0 = different; 3 = identical; note that consistency referred to event content rather than clarity of the representation, as described above).
MRI Acquisition
Anatomical data were acquired on a Siemens 1.5T Avanto MRI scanner using an MP-RAGE sequence. Functional scans (25 coronal-oblique interleaved 5 mm slices) were collected perpendicular to the long axis of the hippocampus with a T2*-weighted EPI sequence (TR = 2000 ms, TE = 23 ms, FOV = 200 mm, flip angle = 90o). Stimuli were projected onto a screen reflected into a mirror within the head coil. E-Prime software (Psychology Software Tools Inc.) was used to present stimuli and collect responses made on a 4-button MR-compatible button box.
MRI Preprocessing
Imaging data were preprocessed and analyzed using SPM8 (Wellcome Trust Centre for Neuroimaging, London). Standard preprocessing included slice-timing correction, rigid-body motion correction and unwarping, spatial normalization to the Montreal Neurological Institute (MNI) template (using normalization parameters derived during segmentation; resampled at 2 mm3), spatial smoothing (8 mm full-width half-maximum Gaussian kernel), and high-pass filtering (128 s cut-off). One participant’s data contained slight movement artefacts (<6 mm) in 4.7% of TRs. ArtRepair software (http://web.mit.edu/swg/software.htm) was used to repair slice artifacts in raw functional images before preprocessing, and volume artifacts after realignment but before estimation. Each event was modeled by SPM’s canonical hemodynamic response function, applied at stimulus onset.
fMRI Contrast Analyses
Fixed-effects subject-level models consisted of four regressors of interest: First, Second, and Third future conditions, Control condition. Two regressors of no interest (excluded trials; ratings phase) were also modelled. The future condition regressors included trials for which a reaction time (RT) was collected on all three repetitions (94.65% of all trials). We also used additional RT criteria to exclude trials on which accidental button presses were made (3.6% of all trials). For the First condition, we excluded trials where RT was less than 2 seconds, in line with previous research indicating that it takes participants approximately 2 seconds to read a screen with instructions and cue words on the first presentation [2]. For repeated imaginings, we excluded any trials where RT was more than two standard deviations lower than the mean RT for that condition and therefore trials faster than 374 ms for Second and 15 ms for Third were excluded. Trials for which simulations did not comply with task instructions were also excluded, according to the following criteria: simulations rated (at post-scan) as “identical” to previous thoughts/experiences (0.9% of all trials); simulations rated as “different” with respect to consistency over presentations (0.3% of all trials). Given that for some trials multiple exclusionary criteria applied, in all 91.42% of the original trials were entered into the analyses.
A random-effects flexible factorial model with two factors, condition and subject, was computed using contrast images for conditions (relative to the implicit baseline) from the fixed-effects models. Following an omnibus F test to assess the effect of condition, we examined the regions engaged during future simulation relative to the control task with the contrast Future(First, Second, Third)>Control. We also computed two contrasts testing for linear trends: First>Second>Third (c = [1 0–1]) and Third>Second>First (c = [−1 0 1]). Note that these contrast weights are recommended for testing linear trends over three conditions [19]. We tested for non-linear effects using two quadratic contrasts (c = [−1 2 −1] and [1 −2 1]).
We computed additional analyses to determine whether any changes in signal across repetitions were the result of non-specific linear time-dependent effects, that is, increases or decreases in signal unrelated to the repetition manipulation that occur across the time window between the First and Third future event trials. While we had controlled for low frequency signal drift across the duration of the entire run with a high-pass filter (128 s cut-off), we computed a new analysis to control for any non-specific linear time-dependent effects occurring across the time interval separating the first and third future trials (M = 93.57 s, SD = 26.00 s). In order to model the change in signal over this time window, we divided our control trials into pairs separated by a time interval similar to that between the First and Third future trials (M = 89.31 s, SD = 48.29 s; these intervals were not significantly different from those for the future trials, t38 = .49, p = .63). We re-ran the fixed-effects and random-effects flexible factorial models to include the time1 and time2 Control conditions. A repetition × condition interaction analysis was computed to identify regions with significant repetition effects (increases or decreases) for the Future but not the Control conditions.
To investigate whether decreasing reaction times were related to changes in neural activity across repetitions, we entered reaction times as a parametric modulation regressor in the fixed-effects model, producing contrast images for each condition of interest that were independent of the effect of reaction time. These contrast images were entered into the random-effects flexible factorial model and we re-computed our First>Second>Third and Third> Second>First contrasts, controlling for reaction time. We also used these fixed-effects models to run a random-effects parametric modulation analysis to identify regions in which neural activity correlated with reaction time. A contrast image of the parametric modulation effect from each participant’s fixed effects model was entered into a random-effects one-sample t-test to identify regions where activity was significantly correlated with reaction times at the group level.
A correction for multiple comparisons was applied to all contrasts (pFWE<.05). Peak MNI coordinates were transformed into Talairach space for localization using a stereotactic atlas [20]. All coordinates are reported in MNI space. For descriptive purposes, percent signal change data were extracted from 2 mm spheres centred on peak voxels. Masks were created in MarsBaR [21] and the REX toolbox (http://web.mit.edu/swg/software.htm) was used to extract and rescale beta values to percent signal change.
We also computed a laterality index for hippocampal activity, using the Laterality Index (LI) toolbox [22] and an anatomical AAL atlas mask of the bilateral hippocampus from the WFU PickAtlas Tool (http://fmri.wfubmc.edu/software/PickAtlas). The LI for the Future>Control and First>Second>Third contrast images was determined using a bootstrap analysis that determines the laterality of activity using a sum of voxel values at different thresholds. LI values range from −1 (extreme right) to +1 (extreme left).
Functional Connectivity Analyses
We used partial least squares (PLS), a covariance-based multivariate technique [23], [24], to examine whether the connectivity of the anterior RHC with other brain regions also differs according to repetition. A seed analysis was computed using maximal signal extracted from the peak RHC voxel in contrast of Future>Control (see Results); this contrast was used for voxel selection so as not to bias activity to show decreasing connectivity across repetitions. Correlations between activity in this seed voxel and all other voxels were computed for each condition (across an 18s trial window) across participants. The resulting correlation maps were stacked and analyzed with singular value decomposition. We utilized a non-rotated version of PLS, specifying two a priori contrasts: (1) stronger RHC connectivity during First relative to Second and Third; and (2) stronger RHC connectivity during First and Second relative to Third. For each contrast, a latent variable was produced, comprising a singular value (indicating the amount of covariance for which the LV accounts), a linear contrast between the seeds and the conditions (coding for the effect depicted by voxels), and a singular image of voxel weights or “saliences” (akin to a component loadings in principle components analysis) that are proportional to the covariance of activity with the linear contrast.
The significance of each LV was determined using permutation testing in which each participant’s data were randomly reassigned to experimental conditions and the PLS analysis recomputed to obtain a new singular value for each reordering. This permutation procedure was done 500 times, and thus significance reflects the number of times the singular value from the permuted data exceed the original singular value (p≤.05). Because whole-brain patterns are assessed in one analytic step, corrections for multiple comparisons are not required. The reliability of voxel saliences was determined using bootstrap estimation of the SE: participants were randomly resampled with replacement, the PLS analysis was rerun and new saliences were determined. After 300 iterations, the SE of the salience was computed. Clusters of five or more voxels in which bootstrap ratios were greater than ±5 (p<.0001) were considered reliable.
Results
Behavioral Results
Behavioral data are presented in Table 1. A repeated-measures ANOVA showed that RTs significantly decreased over repetitions F (1.15,21.92) = 120.85, p<.001, with differences between all future conditions (pBonferroni<.002). A Friedman test showed that detail ratings increased across repetitions (χ2 (2) = 33.60, p<.001), and Wilcoxon Signed-Rank Tests confirmed that detail ratings differed between all future conditions (Z scores <−3.06; p-values ≤.002). The average estimated date of future simulated events was 1.84 years from the present. Simulations were rated as having minimal similarity to previous thoughts and past experiences, and highly consistent over repetitions.
Table 1. Mean reaction times, detail ratings, and post-scan ratings of future events.
| Measure | Mean scores (SD) according to condition | ||
| First | Second | Third | |
| Reaction time (s) | 4.37 (.92) | 2.80 (1.21) | 2.55 (1.27) |
| Detail of simulation† | 1.39 (.38) | 2.04 (.29) | 2.26 (.29) |
| Mean scores (SD) | |||
| Temporal distance of event (years) | 1.84 (.73) | ||
| Similarity of event to previous experiences† | 0.36 (.19) | ||
| Similarity of event to previous thoughts† | 0.11 (.12) | ||
| Consistency of event across repetitions† | 2.75 (.17) | ||
Note: †Participant ratings made using a four-point rating scale, ranging from 0 (low) to 3 (high).
fMRI Contrast Results
All contrast results presented here were computed within the random-effects flexible factorial model. In order to identify the regions associated with the construction of future simulations, we computed a contrast of Future>Control. This contrast replicated previous findings of simulation-related activity in medial prefrontal and parietal cortex, lateral temporal cortex and MTL (a cluster which extended into the RHC, xyz 32 −16 −18; see Table 2).
Table 2. Regions evident in Future>Control contrast analysis.
| Brain Region | MNI co-ordinates | Z-score | ||
| x | y | z | ||
| Future>Control* | ||||
| L Posterior Cingulate Gyrus/Cingulate Gyrus (BA 31)† | −6 | −62 | 24 | Infinite |
| L Medial Frontal Gyrus (BA 10/11)† | −2 | 58 | −6 | Infinite |
| R Middle Temporal Gyrus (BA 21) | 58 | −6 | −18 | 7.75 |
| L Middle Temporal Gyrus (BA 39) | −42 | −70 | 30 | 7.59 |
| R Parahippocampal Gyrus (BA 36) | 24 | −38 | −10 | 7.08 |
| L Superior Frontal Gyrus (BA 9) | −18 | 34 | 38 | 6.92 |
| R Supramarginal Gyrus (BA 40) | 52 | −53 | 22 | 6.89 |
| L Inferior Temporal Gyrus | −62 | −10 | −20 | 6.66 |
| R Middle Frontal Gyrus | 20 | 34 | 44 | 6.47 |
| L Parahippocampal Gyrus | −24 | −40 | −8 | 5.61 |
Note: All activations evident at a height threshold of pFWE <.05; for brevity, only those clusters with more than 100 voxels are reported. Only the maximal peak voxel of each cluster is reported. BA = Brodmann area; L = left; R = right; †Cluster extends bilaterally. *All regions in future>control contrast were also evident in an F-test assessing the main effect of condition.
To further explore whether activation of these simulation-related regions were modulated by repetition, we ran a set of linear and quadratic contrasts. The contrast of First>Second>Third revealed a profile of decreasing activity across repetitions in MTL regions, including a cluster in the anterior RHC (that extended into the right amygdala, xyz 16 −6 −18 and parahippocampal gyrus, 34 −28 −18), the left amygdala, bilateral inferior frontal gyri, and left medial prefrontal and posterior cingulate cortices (Table 3, Figure 2A). LI results confirmed that hippocampal activity during both this contrast and the Future>Control contrast was strongly right-lateralized (LIs: Future>Control, −0.71; First>Second>Third, −0.51). To further explore the nature of this linear decrease in RHC activity across repetitions, we computed whole-brain contrasts First>Second and Second>Third. Both contrasts revealed activation of the same regions of the RHC activity (xyz 32 −16 −18), albeit at a lower threshold (puncorrected <.001) than when the entire linear trend was assessed, but still in line with the finding that the right hippocampus exhibits a linear rather than non-linear decrease over repetitions.
Table 3. Regions evident in repetition contrast analyses.
| Brain Region | MNI co-ordinates | Z-score | ||
| x | y | z | ||
| First>Second>Third | ||||
| L Medial Frontal Gyrus (BA 10) | −4 | 58 | −6 | 7.76 |
| L Caudate (Head)* | −6 | 12 | −6 | 7.06 |
| L Inferior Frontal Gyrus (BA 47) | −38 | 30 | −16 | 6.42 |
| R Inferior Frontal Gyrus (BA 47) | 30 | 32 | −12 | 6.41 |
| R Hippocampus§ | 32 | −16 | −18 | 6.32 |
| L Middle Temporal Gyrus (BA 39) | −42 | −70 | 30 | 6.32 |
| L Posterior Cingulate/Retrosplenial Cortex (BA 31)∫ | −4 | −56 | 22 | 6.31 |
| R Superior Temporal Gyrus*† | 40 | 18 | −30 | 6.27 |
| L Middle Temporal Gyrus (BA 21)* | −60 | −10 | −16 | 6.01 |
| L Medial Frontal Gyrus (BA 10)† | −12 | 64 | 12 | 5.97 |
| L Amygdala | −18 | −6 | −14 | 5.61 |
| R Middle Frontal Gyrus (BA 9) | 40 | 14 | 28 | 5.57 |
| L Medial Frontal Gyrus (BA 8)*§ | −14 | 34 | 46 | 5.47 |
| Third>Second>First | ||||
| R Inferior Frontal Gyrus (BA 44)¥ | 58 | 14 | 10 | 7.39 |
| L Superior Frontal Gyrus (BA 10) | −30 | 54 | 20 | 6.98 |
| R Inferior Parietal Lobule (BA 40)¥ | 56 | −44 | 38 | 6.85 |
| R Precuneus (BA 7) | 14 | −66 | 36 | 6.62 |
| L Cingulate Gyrus (BA 23) | −2 | −26 | 28 | 6.09 |
| R Middle Frontal Gyrus (BA 10)† | 44 | 50 | 8 | 6.02 |
Note: All activations evident at a height threshold of PFWE <.05; for brevity, only those clusters with more than 100 voxels are reported. Only the maximal peak voxel of each cluster is reported. BA = Brodmann area; L = left; R = right; ¥Cluster also exhibits a quadratic effect. †Cluster not evident in the interaction analysis (controlling for non-specific linear time-dependent effects). *Cluster not evident when controlling for reaction time. § ∫Peak voxel for cluster is shifted to an adjacent voxel when controlling for time-dependent (§) and reaction time (∫) effects.
Figure 2. Results from contrast analyses.
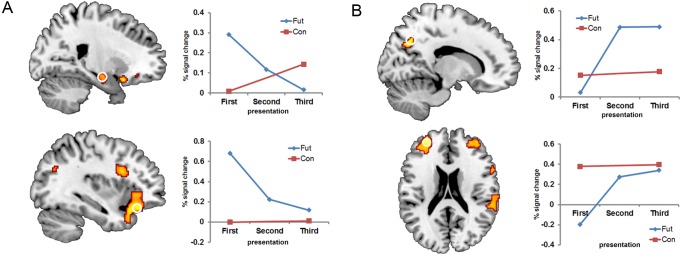
(A) Regions from the interaction analysis in which fMRI signal decreased across repetitions for the future condition only: right hippocampus (xyz 22 −10 −14, top panel) and left inferior frontal gyrus (−36 28 −16, bottom panel), and associated percent signal change data for future and control conditions (First = First Future and time1 Control conditions; Second = Second Future; Third = Third Future and time2 Control conditions). These regions were also evident in the contrast of First>Second>Third (see Table 3). (B) Regions from the interaction analysis in which fMRI signal increased across repetitions for the future condition only: right precuneus (14 −64 38, top panel) and left anterior prefrontal cortex (−30 52 20, bottom panel) with associated percent signal change data. These regions were also evident in the contrast of Third>Second>First (see Table 3). Activity is shown at puncorrected <.0001 overlaid on a standard anatomical template; all peak activations survived a corrected threshold of pFWE <.05; see Table 3. Note that error bars are not included as these plots are for descriptive purposes only [54].
The opposite linear contrast, Third>Second>First, demonstrated that activity in left anterior and right ventrolateral prefrontal cortex, right inferior parietal lobule, right posterior cingulate gyrus and right ventral precuneus increased with repetition (Table 3, Figure 2B). Importantly, no MTL region showed increasing activation across repetitions. Although some regions are evident in both linear contrasts (First>Second>Third and Third>Second>First), such as the left posterior cingulate, right inferior and middle frontal gyri, it is important to note that different subregions of these neural structures exhibit opposite linear effects, as indicated by the different Brodmann area labels in Table 3; there was in fact no overlap between the statistical maps resulting from these two linear contrasts.
To test for possible nonlinear effects, we computed two quadratic contrasts over the First, Second and Third conditions. Neither contrast revealed any activation in the RHC (neither at pFWE<.05 nor at a more lenient puncorrected <.001 threshold), indicating the repetition effect in the RHC was predominantly linear in nature. In fact, none of the regions exhibiting a linear decrease over repetitions were evident in the quadratic contrasts. On the other hand, two regions exhibiting a Third>Second>First effect also exhibited a quadratic effect (see annotations in Table 3), with a steep increase in activity between First and Second which then plateaued. Specifically, activity in the right inferior frontal gyrus (xyz 58 14 10; see Figure 2B) and right inferior parietal lobule (−58 −46 36) followed this pattern. Note that although a quadratic effect is apparent for the right precuneus in Figure 2B, this effect just failed to reach significance (pFWE = .06).
To ensure that these changes in signal across repetitions were not the result of non-specific linear time-dependent effects, we computed an additional analysis to control for any non-specific linear time-dependent effects occurring across the time interval separating the first and third future trials. In a random-effects flexible factorial model that included the First, Second and Third future conditions as well as time1 and time2 Control conditions (created by pairing control trials separated by a time interval similar to that between the First and Third future trials), we computed a repetition × condition interaction analysis to identify regions with significant repetition effects (increases or decreases) for the Future but not the Control conditions. Importantly, we found that the majority of regions reported in our original First>Second>Third Future contrast were again evident in this repetition × condition interaction (although some peak voxels were at slightly different locations in the same cluster; see annotations in Table 3), including the left medial prefrontal cortex, bilateral inferior frontal gyrus, left middle temporal gyrus, anterior RHC and bilateral amygdala (pFWE <.05). In all these regions, the repetition-related decreases in signal were only evident in the future condition, confirming these effects are not influenced by non-specific linear time-dependent effects (Figure 2A). The only regions in which activation decreases were no longer evident in this interaction analysis were right parahippocampal gyrus, right superior temporal gyrus, and right middle frontal gyrus (see Table 3). Moreover, only one of the regions exhibiting repetition-related increases in the original Third>Second>First contrast, right middle frontal gyrus, was no longer significant in the interaction analysis (see Table 3). For all other regions in the original Third>Second>First contrast, increases in signal over repetitions were only present for the future condition. Overall, these results confirm that the majority of repetition effects we report cannot be explained by non-specific linear time effects.
Additionally, as we found a significant decrease in reaction time over repetitions, we wanted to ensure that this decrease in activation in several core regions was not due simply to a decrease in task difficulty. To examine this possibility, we entered reaction times as a parametric modulation regressor, which allowed us to compute the First>Second>Third and Third>Second>First contrasts while controlling for reaction time. Importantly, many of the same regions, including the MTL regions remained activated for the contrast of First>Second>Third even when controlling for reaction time. However, some regions were no longer active at a corrected threshold (pFWE <.05): left caudate, right superior temporal gyrus, and left middle temporal gyrus; see annotations in Table 3. The same regions were activated for the Third>Second>First contrast when controlling for reaction time.
We also ran a random-effects parametric modulation analysis to identify regions in which neural activity correlated with reaction time. However, there was very little activation correlated with reaction time, with no voxels surviving a corrected threshold of pFWE <.05. Even at a very lenient threshold of puncorrected <.05, the prefrontal clusters that did emerge were not in regions comprising the core network. Based on these additional analyses, we believe that difficulty, as indexed by reaction time, cannot explain the repetition effects evident in the current study.
Functional Connectivity Results
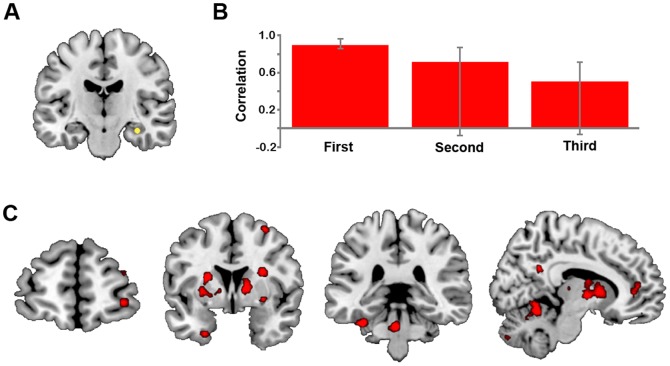
We used a seed PLS analysis to examine statistically whether the strength of connectivity of the anterior RHC (xyz 32 −16 −18; see Figure 3A) with other brain regions also differs according to repetition. Two a priori contrasts were tested: (1) RHC connectivity during First but not Second and Third; and (2) RHC connectivity during First and Second but not Third. This analysis determined that only the first contrast, testing for stronger RHC connectivity during First relative to Second and Third, was significant (p = .05), explaining 42.35% of the covariance. This LV indicated that during the First simulation, the RHC was strongly connected with a distributed pattern of activity that included many regions associated with simulation, including bilateral medial prefrontal cortex, left MTL regions (parahippocampal and perirhinal cortices) and lingual/fusiform gyrus, and right inferior frontal gyrus, thalamus, and precuneus (see Figure 3C and Table 4). Not only was RHC connectivity reduced during the Second and Third conditions relative to First, but the RHC was not reliably connected to this network during these two repetition conditions, see Figure 3B. Many of the regions exhibiting functional connectivity with the RHC during the first simulation overlapped with, or were adjacent to, regions exhibiting a linear decrease in activity across repetitions (e.g., medial prefrontal cortex, precuneus, putamen), but many regions identified in the two analyses were also distinct.
Figure 3. Results from functional connectivity analyses.
(A) Location of the RHC seed (xyz 32 −16 −18). (B) Average brain scores indicating the strength of correlation between activity in the seed region and the associated whole brain network, with 95% confidence intervals for the First, Second and Third presentations (p = .05). (C) Regions which were significantly connected with the RHC seed during First, but not during Second and Third during TR 5, including: right frontopolar cortex (leftmost image), putamen, cerebellum and lingual gyrus (middle images), posterior cingulate cortex, thalamus and right medial frontal gyrus (rightmost image). These data are thresholded using a BSR of 5, which corresponds to p<.0001.
Table 4. Regions showing significant functional connectivity with the right hippocampal seed region.
| Brain Region | MNI co-ordinates | BSR | ||
| x | y | z | ||
| L Cerebellum | −6 | −74 | −30 | 15.89 |
| R Thalamus | 12 | −4 | 6 | 12.52 |
| R Cerebellum | 8 | −52 | −14 | 12.09 |
| R Inferior Frontal Gyrus (BA 47) | 20 | 14 | −16 | 11.52 |
| R Medial Frontal Gyrus (BA 10) | 20 | 42 | 12 | 11.28 |
| L Parahippocampal Gyrus (BA 36) | −36 | −32 | −28 | 11.01 |
| L Cerebellum† | −4 | −34 | −32 | 10.68 |
| L Putamen | −24 | 0 | 18 | 10.61 |
| R Precuneus (BA 31) | 8 | −52 | 30 | 10.04 |
| L Lingual Gyrus (BA 18) | −22 | −76 | 0 | 8.87 |
| R Frontopolar Cortex (BA 10) | 32 | 58 | −4 | 8.33 |
| R Middle Frontal Gyrus (BA 6) | 30 | 4 | 62 | 8.16 |
| L Perirhinal Cortex (BA 36) | −26 | 2 | −38 | 8.10 |
| R Thalamus | 4 | −22 | 6 | 7.92 |
| R Middle Frontal Gyrus (BA 46) | 50 | 32 | 28 | 7.78 |
| R Superior Frontal Gyrus (BA 9) | 18 | 54 | 30 | 7.51 |
| L Cerebellum† | −4 | −70 | −42 | 7.29 |
| L Middle Frontal Gyrus (BA 9) | −34 | 32 | 32 | 7.01 |
| L Lingual/Fusiform Gyrus (BA 18/19) | −18 | −82 | −20 | 6.98 |
| R Putamen | 26 | 16 | 2 | 6.93 |
| L Medial Frontal Gyrus (BA 9) | −20 | 34 | 32 | 6.89 |
during first but not second and third presentation.
Note: Only clusters evident during peak time point (TR 5) with a bootstrap ratio greater than ±5 (roughly equivalent to a p-value of <.0001) and with a minimum extent of 20 voxels are shown here. BA = Brodmann area; BSR = Bootstrap ratio; L = left; R = right. †Cluster extends bilaterally.
Discussion
This study extends previous findings of anterior RHC activation during future simulation by demonstrating that activity in this region is stronger during the initial construction of a future event than during repetitions. Right-lateralized activity in the anterior hippocampus was evident during the first simulation trial, and then decreased significantly across repeated imaginings of the same event. The functional connectivity of the anterior RHC with other regions of the core network, including medial and inferior frontal gyrus, was evident during the first, but not the second and third, simulation condition. These findings have important implications with respect to interpreting hippocampal activity (or lack thereof) during simulation: when interpreting such neural effects, it is important to consider whether or not the paradigm requires the active construction of imagined events or reimagining of pre-constructed events during the scanning session. Thus, differential activity for past relative to future events in some previous studies may reflect the influence of the ‘pre-imagination’ sessions in these studies [6], [11], [12].
Hippocampal Responses to Repetition in Episodic Simulation
Our analyses revealed the presence of repetition-suppression effects in the anterior RHC that were not due to decreases in reaction time or drifts in signal across the repetition interval. The observation that hippocampal activity decreased across repetitions is broadly consistent with other reports that the hippocampus is not consistently active during episodic simulation and may even exhibit a phasic profile of activity [25]. Rather, episodic simulation is likely to be more of a dynamic process, placing varying demands on the hippocampus throughout the generation of a scenario, particularly during the initial phases of simulation, as our results suggest.
One explanation for our results is that RHC activity during the first simulation condition is a novelty response, and that this activity decreases gradually across repetitions with the reducing novelty of simulations across repetitions. Previous work has shown that associative novelty triggers robust hippocampal activation [13], [14], [26], [27]. This idea is also in line with findings from Weiler et al. [15] that the construction of low probability (and more novel) future events invokes increased RHC activation relative to more probable future events. While it might be expected that novelty effects should result in a more steep decline where RHC activity decreases rapidly after initial construction, in line with the idea of novelty detection [28], activity in this region showed a linear decrease with no evidence of a quadratic component. This more linear decrease might suggest that other processes supported by RHC are required less and less, such as binding processes [29]. It is also notable that the functional connectivity of the RHC did follow a marked step-wise pattern: during the first simulation, the RHC was strongly connected with other core network regions, including the left MTL, right inferior frontal gyrus and precuneus, and by the second simulation this connectivity was no longer evident.
Another possibility is that these decreases in activation and connectivity reflect the increasing ease of event construction with repetition. This idea is in line with previous research demonstrating that pre-imagining hypothetical future events increases the fluency of the imagined event, leading to increased plausibility with repetition [30], [31], [32]. It is also possible that reducing demands on detail recombination may have resulted in lowered activity in the hippocampus [10], [14]. One problem with this suggestion is that detail ratings increased across repetitions, and to the extent that increased detail ratings reflect increased constructive processing, it might be expected that hippocampal activity should show a corresponding increase, which it does not. It is also important to note that encoding processes may have also diminished across repetitions. However, unlike other variants of this paradigm [33] that allow a distinction between successful encoding and construction by comparing remembering and forgetting of simulations on a later recall test, we cannot distinguish encoding and constructive processes in this study due to the lack of forgotten trials after three repetitions.
Although it is not possible in this study to distinguish definitively between neural responses to novelty and constructive processes, the finding from the parametric modulation analysis that reaction time did not correlate with activity in medial temporal regions provides some evidence against the interpretation of these repetition effects in terms of increased ease of constructive processes. However, it was somewhat surprising that no regions associated with simulation exhibited correlations with reaction time, which might be expected if reaction time is closely associated with changes in the ease or difficulty of construction processes. While this observation raises some concerns about the use of reaction time as a proxy for difficulty of future event construction, it is noted that some regions were no longer significant in the First>Second>Third analysis once we controlled for reaction time. But nonetheless, while the observed repetition effects cannot be attributed to reaction time effects, they may not necessarily be independent of difficulty. It might also be that some neural regions, such as the hippocampus, respond differently to task difficulty and possibly in ways not adequately captured by reaction time or by a parametric modulation analysis. For example, Summerfield et al. [25] reported that constructing scenes with an increasing number of elements (an increasing difficulty as indicated by ratings) resulted in an overall increase in RHC activation. However, this increase was not linear but phasic. Moreover, the lack of reaction time effects in regions exhibiting repetition effects could also be taken as reflecting processes on a time scale not associated with reaction time, such as novelty effects. Although more fine-grained research is needed to draw strong conclusions, novelty effects may be an important mechanism underlying at least some of the repetition effects reported here. Thus, it remains an important challenge for future research to develop a manipulation or paradigm than can distinguish between novelty and construction of autobiographical future events.
Another important consideration is whether these novelty effects are tied to future simulations specifically, or whether they would be evident for any form of episodic simulation, such as simulations of past or atemporal events [34]. Recent studies on counterfactual episodic simulation, where individuals simulate alternative outcomes to past events, raise the possibility that this effect is not restricted to future simulations, but would extend to other forms of episodic simulation. For instance, De Brigard et al. [35] have shown that episodic memory and episodic counterfactual simulation rely, to a large degree, on the same common pattern of brain activity that is associated with episodic future simulation [2].
Our results suggest that repetition-related decreases in RHC activation and connectivity may occur even when there are some changes to the event representation across repetitions; for related results, see [36]. Although participants rated their repeated simulations as highly consistent (although these ratings may have been somewhat inflated due to the delay between the simulation and post-scan rating phases), there was an incremental increase in detail ratings. We suggest that consistency ratings reflected the maintenance of the gist or core components of the event representation across repetitions, and the small changes in overall event clarity or vividness as indexed by detail ratings were not sufficient to disrupt repetition suppression effects. This point may have important implications for studies using any form of pre-generation of future events.
Core Network Region Responses to Repetition in Episodic Simulation
Decreases in activity over repetitions were evident in other MTL regions including bilateral amygdala, and were unrelated to reaction time or signal drifts. While amygdala activity is not always evident in future simulation, it has been documented in the two other studies using the recombination paradigm [10], [33]. The randomized recombinations of episodic details probably resulted in a number of uncommon scenarios, and indeed the simulations generated in this paradigm are particularly novel, with low ratings of similarity to previous experiences and thoughts relative to other reports (e.g., [8]). This finding is consistent with studies implicating the bilateral amygdala in the processing of unusual stimuli [37], [38] and novelty detection [28], [39].
A number of extra-MTL regions thought to play an important role in event construction also exhibited a reduction of activity across repetitions that could not be accounted for by reaction time or signal drift. Such regions included the bilateral inferior frontal gyri, an area of prefrontal cortex that is thought to play a role in the generative aspects of future simulation, including semantic generation of event schemas [2], [40]. Interestingly, neither Botzung et al. [11] nor D’Argembeau et al. [12] reported inferior frontal activity, possibly indicative of diminished generative processes following a pre-imagination session. Moreover, our functional connectivity results indicated that during the initial construction, the RHC was functionally connected to the right inferior frontal gyrus, and also to left MTL regions, namely the perirhinal and parahippocampal cortex. The connectivity between MTL regions is consistent with the predictions of the binding of items and context (BIC) model [41], [42], [43], which posits that in order to construct complete representations of episodes, the hippocampus binds item information represented in perirhinal cortex with context information represented in parahippocampal gyrus. It is also possible that connectivity with the fusiform gyrus reflected the integration of person (face) information into the scenario [44]. Together, this connectivity pattern is consistent with the demands of the recombination paradigm, which requires integration of objects and people into particular contexts, and thus suggests this pattern of connectivity may reflect, at least to some extent, the construction demands of the task. This is also broadly consistent with scene construction theory, which poses that event construction involves the binding of multimodal elements into a spatially coherent scene [45], [46].
Repetition Enhancements in Core Network Regions
A distinct set of prefrontal and parietal regions showed the opposite profile, with activity increasing across repetitions (i.e., ‘repetition enhancement’), including left anterior and right ventrolateral prefrontal cortex, right inferior parietal lobule, right posterior cingulate gyrus and right ventral precuneus. Numerous studies report repetition enhancements in posterior cingulate and ventral precuneus [47], [48], which are thought to reflect source recognition and retrieval [49], [50]. Moreover, left anterior prefrontal cortex (BA 10) has been associated with context retrieval [51], [52], in particular the retrieval of details that have been internally generated [53]. Thus, in the current study, these increases may be a result of retrieving the contextual details comprising previously imagined events, ensuring the consistency of the simulated event across repetitions. Even so, such retrieval processes did not influence levels of MTL activity which steadily decreased across repetitions.
Summary
Our results demonstrate that the anterior RHC plays an important role in the functional network supporting the initial construction of imagined future events, but that RHC activation and connectivity decreases over repetitions. This finding is important for understanding some incongruent findings reported in the literature concerning hippocampal activation during simulation by confirming that pre-imagining future events has a significant effect on both the activation of the RHC and the broader network supporting the future event simulation. This experiment also provides further support for the idea that generating novel future events particularly enhances the activation of the RHC. Whether such novelty effects are evident for all forms of simulated events (such as counterfactual and atemporal events) remains a question for future research.
Acknowledgments
We thank R.P. Roberts and V.C. Martin for assistance.
Funding Statement
This work was supported by a Royal Society of NZ Marsden Grant UOA0810 to DRA and a National Institute of Mental Health grant MH060941 to DLS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Andrews-Hanna JR (2012) The Brain’s Default Network and Its Adaptive Role in Internal Mentation. Neuroscientist 18: 251–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Addis DR, Wong AT, Schacter DL (2007) Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia 45: 1363–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Okuda J, Fujii T, Ohtake H, Tsukiura T, Tanji K, et al. (2003) Thinking of the future and past: the roles of the frontal pole and the medial temporal lobes. NeuroImage 19: 1369–1380. [DOI] [PubMed] [Google Scholar]
- 4. Szpunar KK, Watson JM, McDermott KB (2007) Neural substrates of envisioning the future. Proceedings of the National Academy of Sciences of the United States of America 104: 642–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schacter DL, Addis DR (2007) The cognitive neuroscience of constructive memory: Remembering the past and imagining the future. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences 362: 773–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hassabis D, Kumaran D, Maguire EA (2007) Using imagination to understand the neural basis of episodic memory. Journal of Neuroscience 27: 14365–14374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weiler JA, Suchan B, Daum I (2010) When the future becomes the past: Differences in brain activation patterns for episodic memory and episodic future thinking. Behavioural Brain Research 212: 196–203. [DOI] [PubMed] [Google Scholar]
- 8. Addis DR, Cheng T, Roberts RP, Schacter DL (2011) Hippocampal contributions to the episodic simulation of specific and general future events. Hippocampus 21: 1045–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Addis DR, Schacter DL (2012) The hippocampus and imagining the future: where do we stand? Frontiers in Human Neuroscience 5: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Addis DR, Pan L, Vu M, Laiser N, Schacter DL (2009) Constructive episodic simulation of the future and the past: Distinct subsystems of a core brain network mediate imagining and remembering. Neuropsychologia 47: 2222–2238. [DOI] [PubMed] [Google Scholar]
- 11. Botzung A, Denkova E, Manning L (2008) Experiencing past and future personal events: functional neuroimaging evidence on the neural bases of mental time travel. Brain and Cognition 66: 202–212. [DOI] [PubMed] [Google Scholar]
- 12. D’Argembeau A, Xue G, Lu ZL, Van der Linden M, Bechara A (2008) Neural correlates of envisioning emotional events in the near and far future. NeuroImage 40: 398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Köhler S, Danckert S, Gati JS, Menon RS (2005) Novelty responses to relational and non-relational information in the hippocampus and the parahippocampal region: A comparison based on event-related fMRI. Hippocampus 15: 763–774. [DOI] [PubMed] [Google Scholar]
- 14. Preston AR, Shrager Y, Dudukovic NM, Gabrieli JDE (2004) Hippocampal contribution to the novel use of relational information in declarative memory. Hippocampus 14: 148–152. [DOI] [PubMed] [Google Scholar]
- 15. Weiler JA, Suchan B, Daum I (2010) Foreseeing the Future: Occurrence Probability of Imagined Future Events Modulates Hippocampal Activation. Hippocampus 20: 685–690. [DOI] [PubMed] [Google Scholar]
- 16. Wagner AD, Maril A, Schacter DL (2000) Interactions between forms of memory: When priming hinders new episodic learning. Journal of Cognitive Neuroscience 12: 52–60. [DOI] [PubMed] [Google Scholar]
- 17. Clark JM, Paivio A (2004) Extensions of the Paivio, Yuille, and Madigan (1968) norms. Behavior Research Methods, Instruments and Computers 36: 371–383. [DOI] [PubMed] [Google Scholar]
- 18. Dale AM (1999) Optimal experimental design for event-related fMRI. Human Brain Mapping 8: 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mumford JA (2008) Using contrasts to test trends: precautions. Available: http://mumford.fmripower.org/lin_cont_illus.pdf. Accessed 2013 Jun 29.
- 20.Talairach J, Tournoux P (1988) Co-Planar Stereotactic Atlas of the Human Brain. New York: Thieme.
- 21.Brett M, Anton J, Valabregue R, Poline J (2002) Region of interest analysis using an SPM toolbox. 8th International Conference on Functional Mapping of the Human Brain. Sendai, Japan.
- 22. Wilke M, Lidzba K (2007) LI-tool: A new toolbox to assess lateralization in functional MR-data. Journal of Neuroscience Methods 163: 128–136. [DOI] [PubMed] [Google Scholar]
- 23. McIntosh AR, Bookstein FL, Haxby JV, Grady CL (1996) Spatial pattern analysis of functional brain images using partial least squares. Neuroimage 3: 143–157. [DOI] [PubMed] [Google Scholar]
- 24. Lobaugh NJ, West R, McIntosh AR (2001) Spatiotemporal analysis of experimental differences in event-related potential data with partial least squares. Psychophysiology 38: 517–530. [DOI] [PubMed] [Google Scholar]
- 25. Summerfield JJ, Hassabis D, Maguire EA (2010) Differential engagement of brain regions within a ‘core’ network during scene construction. Neuropsychologia 48: 1501–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Knight RT (1996) Contribution of human hippocampal region to novelty detection. Nature 383: 256–259. [DOI] [PubMed] [Google Scholar]
- 27. Nyberg L (2005) Any novelty in hippocampal formation and memory? Current Opinion in Neurology 18: 424–428. [DOI] [PubMed] [Google Scholar]
- 28. Balderston NL, Schultz DH, Helmstetter FJ (2011) The human amygdala plays a stimulus specific role in the detection of novelty. NeuroImage 55: 1889–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Svoboda E, Levine B (2009) The Effects of Rehearsal on the Functional Neuroanatomy of Episodic Autobiographical and Semantic Remembering: A Functional Magnetic Resonance Imaging Study. Journal of Neuroscience 29: 3073–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carroll JS (1978) The effect of imagining an event on expectations for the event: An interpretation in terms of the availability heuristic. Journal of Experimental Social Psychology 14: 88–96. [Google Scholar]
- 31. Sherman SJ, Cialdini RB, Schwartzman DF, Reynolds KD (1985) Imagining can heighten or lower the perceived likelihood of contracting a disease: The mediating effect of ease of imagery. Personality and Social Psychology Bulletin 11: 118–127. [Google Scholar]
- 32. Szpunar KK, Schacter DL (2013) Get real: Effects of repeated simulation and emotion on the percieved plausibility of future interpersonal experiences. Journal of Experimental Psychology: General 142(2): 323–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Martin VC, Schacter DL, Corballis MC, Addis DR (2011) A role for the hippocampus in encoding simulations of future events. Proceedings of the National Academy of Sciences of the United States of America 108: 13858–13863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schacter DL, Addis DR, Hassabis D, Martin VC, Spreng RN, et al. (2012) The future of memory: Remembering, imagining, and the brain. Neuron 76: 677–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Brigard F, Addis DR, Ford JH, Schacter DL, Giovanello KS (2013) Remembering what could have happened: Neural correlates of episodic counterfactual thinking. Neuropsychologia. In press. doi:10.1016/j.neuropsychologia.2013.01.015. [DOI] [PMC free article] [PubMed]
- 36.Szpunar KK, St Jacques PL, Robbins CA, Wig GS, Schacter DL (2013) Repetition-related reductions in neural activity reveal component processes of mental simulation. Social, Cognitive, and Affective Neuroscience. doi: 10.1093/scan/nst035. [DOI] [PMC free article] [PubMed]
- 37. Hamann SB, Ely TD, Hoffman JM, Kilts CD (2002) Ecstasy and agony: Activation of the human amygdala in positive and negative emotion. Psychological Science 13: 135–141. [DOI] [PubMed] [Google Scholar]
- 38. Rotshtein P, Malach R, Hadar U, Graif M, Hendler T (2001) Feeling or Features: Different Sensitivity to Emotion in High-Order Visual Cortex and Amygdala. Neuron 32: 747–757. [DOI] [PubMed] [Google Scholar]
- 39. Blackford JU, Buckholtz JW, Avery SN, Zald DH (2010) A unique role for the amygdala in novelty detection. NeuroImage 50: 1188–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Conway MA, Pleydell-Pearce CW, Whitecross SE, Sharpe H (2003) Neurophysiological correlates of memory for experienced and imagined events. Neuropsychologia 41: 334–340. [DOI] [PubMed] [Google Scholar]
- 41. Eichenbaum H, Yonelinas AR, Ranganath C (2007) The medial temporal lobe and recognition memory. Annual Review of Neuroscience 0: 123–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Diana RA, Yonelinas AP, Ranganath C (2007) Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends in Cognitive Sciences 11: 379–386. [DOI] [PubMed] [Google Scholar]
- 43. Ranganath C (2010) A unified framework for the functional organization of the medial temporal lobes and the phenomenology of episodic memory. Hippocampus 20: 1263–1290. [DOI] [PubMed] [Google Scholar]
- 44. Kanwisher N, McDermott J, Chun MM (1997) The fusiform face area: a module in human extrastriate cortex specialized for face perception. The Journal of Neuroscience 17: 4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hassabis D, Maguire EA (2007) Deconstructing episodic memory with construction. Trends in Cognitive Sciences 11: 299–306. [DOI] [PubMed] [Google Scholar]
- 46. Hassabis D, Maguire EA (2009) The construction system of the brain. Philosophical Transactions of the Royal Society B-Biological Sciences 364: 1263–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dobbins IG, Foley H, Schacter DL, Wagner AD (2002) Executive control during episodic retrieval: multiple prefrontal processes subserve source memory. Neuron 35: 989–996. [DOI] [PubMed] [Google Scholar]
- 48. Hasson U, Nusbaum HC, Small SL (2006) Repetition suppression for spoken sentences and the effect of task demands. Journal of Cognitive Neuroscience 18: 2013–2029. [DOI] [PubMed] [Google Scholar]
- 49. Dobbins IG, Rice HJ, Wagner AD, Schacter DL (2003) Memory orientation and success: separable neurocognitive components underlying episodic recognition. Neuropsychologia 41: 318–333. [DOI] [PubMed] [Google Scholar]
- 50. Lundstrom BN, Petersson KM, Andersson J, Johansson M, Fransson P, et al. (2003) Isolating the retrieval of imagined pictures during episodic memory: activation of the left precuneus and left prefrontal cortex. Neuroimage 20: 1934–1943. [DOI] [PubMed] [Google Scholar]
- 51. Rugg MD, Fletcher PC, Chua PML, Dolan RJ (1999) The role of the prefrontal cortex in recognition memory and memory for source: An fMRI study. NeuroImage 10: 520–529. [DOI] [PubMed] [Google Scholar]
- 52. Ranganath C, Johnson MK, D’Esposito M (2000) Left anterior prefrontal activation increases with demands to recall specific perceptual information. Journal of Neuroscience 20: RC108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Simons JS, Owen AM, Fletcher PC, Burgess PW (2005) Anterior prefrontal cortex and the recollection of contextual information. Neuropsychologia 43: 1774–1783. [DOI] [PubMed] [Google Scholar]
- 54.Vul E, Kanwisher N (2009) Begging the question: The non-independence error in fMRI data analysis. In: Hanson S, Bunzl M, editors. Foundations and Philosophy for Neuroimaging. 71–91.