Early detection of triple-negative (TN) breast cancer is vital, but there are few comprehensive imaging reports. Here we describe mammography, ultrasound, and magnetic resonance imaging findings of TN breast cancers, investigate the specific features of this subtype, and compare the characteristics of TN breast cancers with those of hormone receptor-positive/human epidermal growth factor receptor-negative breast cancers.
Keywords: Breast neoplasms, Triple-negative breast cancer, Mammography, Ultrasound, Magnetic resonance imaging, Ultrasonography, Mammary, Diagnosis
Learning Objectives
Identify the features typical of triple-negative breast cancers on mammography, ultrasound, and magnetic resonance imaging.
Identify this aggressive subtype to accelerate diagnosis and treatment and improve outcomes.
Compare typical imaging features of triple-negative breast cancers with typical imaging features of HR+/HER- breast cancers.
Abstract
Purpose.
Triple-negative (TN) breast cancers have high malignancy potential and are often characterized by early systemic relapse. Early detection is vital, but there are few comprehensive imaging reports. Here we describe mammography, ultrasound, and magnetic resonance imaging (MRI) findings of TN breast cancers, investigate the specific features of this subtype, and compare the characteristics of TN breast cancers with those of hormone receptor (HR)-positive/human epidermal growth factor receptor (HER)-2-negative breast cancers.
Materials and Methods.
From July 2009 to June 2011, mammography and ultrasound findings of 210 patients with pathologically confirmed TN (n = 105) and HR-positive/HER-2-negative breast cancers (n = 105) were retrospectively reviewed from our institutional database. Ultrasound vascularity was notified in 88 cases and elasticity scores were notified in 49 cases overall. Thirty-five patients underwent MRI (22 TN and 13 HR-positive/HER-2-negative). Mammograms, ultrasound, and MRI were reviewed according to the Breast Imaging-Reporting and Data System (BI-RADS) lexicon and classification.
Results.
TN breast cancers were more likely to show round, oval, or lobulated masses with indistinct margins on mammography than HR-positive/HER-2-negative breast cancers. On ultrasound, TN tumors were more likely than HR-positive/HER-2-negative breast cancers to show circumscribed or microlobulated margins and no posterior acoustic features or posterior enhancement-positive. On MRI, TN cancers exhibited suspicious aspects more often than HR-positive/HER-2-negative cancers, often with rim enhancement-positiveHER-2 (84.6% of masses were classified BI-RADS 5).
Conclusion.
This study is the first to describe findings on mammography, ultrasound, and MRI for TN breast cancers with a matched HR-positive/HER-2-negative control group. Several distinctive morphological features of these aggressive tumors are identified that can be used for earlier diagnosis and treatment, and ultimately to improve outcomes.
Implications for Practice:
Our results suggest that there are correlations between underlying phenotypes and distinctive imaging features for estrogen receptor (ER)-negative/progesterone receptor (PR)-negative/human epidermal growth factor receptor (HER)-negative cancers and ER-positive/EP-positive/HER-negative cancers. The findings show that the triple negative (TN) phenotype has a few characteristic radiological findings: an oval or lobulated mass with circumscribed or microlobulated margins and marked hypoechogenicity (p < .001). Some of these masses can be misinterpreted as benign tumors. Breast cancers are a group of diseases with a wide spectrum of clinical, pathological, and molecular characteristics, and these will lead to the development of better-targeted therapies for patients. Breast cancers with a TN phenotype subtype have a poor prognostic outcome; therefore, the detection of the TN phenotype is vital. Radiologists need to know the range of imaging features that occur with different phenotypes and be able to recognize the most aggressive subtypes to speed up pretreatment planning.
Introduction
Recent protein expression profiling in breast cancers has identified the distinctive triple negative (TN) subgroup, defined as breast carcinomas that do not express estrogen receptors, progesterone receptors, or the human epidermal growth factor receptor-2 (HER-2) [1]. They account for 7% to 16% of breast cancers [1–3] and are often associated with young age, high histological grade, suppressed BRCA1 function, and poor prognostic outcomes [3]. Because TN cancers have less specific targets than cancers that overexpress HER-2, endocrine therapy and anti-HER-2 therapy are ineffective in the treatment of TN breast cancers.
The ability to predict the presence of this subtype based on mammography or ultrasound would lead to faster pretreatment planning and improve outcomes, yet there are few reports on the relationship between this tumor subtype and imaging findings. Publications currently available describe a relatively small number of patients with TN cancer, cover only one imaging modality, or do not compare imaging aspects of TN tumors with those of ER-positive/PR-positive/HER-2-negative (hormone receptor [HR]-positive/HER-2-negative) breast cancers [4–11].
The aim of this retrospective study was thus to identify and describe the imaging characteristics of TN breast carcinomas using information obtained from mammography, ultrasound (including Doppler vascularity and elastography when possible), and magnetic resonance imaging (MRI), and to compare the findings with the imaging characteristics of a group of HR-positive/HER-2-negative breast cancers diagnosed over the same period.
Materials and Methods
Patient Selection
We identified 105 consecutive TN breast carcinomas diagnosed at our institution between July 2009 and June 2011 from our prospectively maintained institutional database (TN group). All lobular infiltrating carcinoma in which imaging characteristics can differ across molecular subtypes were excluded. Seventy-three of these patients have already been described in a previous publication [12]. The control group was composed of 105 HR-positive/HER-2-negative breast cancers diagnosed over the same period that were randomly selected from the same database for comparison (RH-positive group).
In the TN group, there were 105 lesions in 101 patients: 97 patients had one lesion, and 4 patients had 2 lesions (1 bilateral). In the HR-positive/HER-2-negative group, there were 105 lesions in 98 patients: 92 patients had 1 lesion, 5 patients had 2 lesions (1 bilateral), and 1 patient had 3 lesions. All analyses are presented per lesion.
The following patient and clinical information was recorded: age, tumor manifestation (palpable mass vs. lesion identified with mammography, sonography, or MRI), and histological type and grade of the tumor. Imaging features were retrieved retrospectively from patient files for all patients and reviewed before analysis. Mammograms and ultrasounds were available for all patients in both groups, and 35 patients additionally underwent MRI (22 in the TN group and 13 in the HR-positive/HER-2-negative group). Initial imaging examination was performed outside our institution on 22 of the lesions (13 TN and 9 HR-positive/HER-2-negative), and these were reviewed internally before analysis.
Institutional review board approval was obtained for these retrospective analyses, and the study was carried out in compliance with the Helsinki Declaration.
Mammography Interpretation
Two standard imaging views (craniocaudal and mediolateral oblique) were used for mammography, with additional views if necessary. Using the American College of Radiology (ACR) Breast Imaging-Reporting and Data System (BI-RADS) lexicon [13], we retrospectively examined breast density (fatty, scattered fibroglandular, heterogeneously dense, or dense) and the presence of lesions. Lesions were described as masses reporting size, shape (oval, round, lobulated, or irregular), and margins (circumscribed, microlobulated, obscured, indistinct, or speculated); microcalcifications reporting size; masses with microcalcifications, asymmetric focal densities reporting size; or architectural distortions reporting size.
Ultrasound Interpretation
Most ultrasound examinations were carried out at our institution (Supersonic Imagine Shearwave Aixplorer, France, http://www.supersonicimagine.com, Aix-en-Provence, France) We classified the lesions as masses and non-mass lesions. Non-mass lesions were defined as lesions that showed focal heterogeneity distinct from normal breast parenchyma and the lesion size was specified. Conversely, masses were defined as space-occupying lesions in two orthogonal projections. We used the ACR-BI-RADS lexicon and specified the size, shape (oval, round, lobulated, or irregular), margins (circumscribed, microlobulated, indistinct, angular, spiculated), lesion boundaries (abrupt interface or hyperechoic halo), echogenicity (hypoechoic, hyperechoic, complex), and posterior acoustic features (no change, enhancement, or shadowing). We reanalyzed our cases for echogenicity, which could be analyzed for 93 TN and 83 HR-positive/HER-2-negative cancers. Doppler vascularity was available in 48 TN cases and 40 HR-positive/HER-2-negative cases and quantitative elastography was available for 25 TN cancers and 24 HR-positive/HER-2 cancers.
MRI Interpretation
Breast MRI (1.5 T Philips Electra) was available for 22 patients with TN cancers and 13 patients with HR-positive/HER-2-negative cancers. The imaging protocol consisted of a turbo spin echo T2-weighted non fat-suppressed sequence followed by an axial spin echo T1-weighted precontrast and five serial dynamic postcontrast sets after rapid IV bolus infusion of 0.1 mmol/kg of gadopentetate dimeglumine at a rate of 2 mL/second. Delayed contrast-enhanced images with fat suppression on an axial plane were also obtained. After the dynamic scan was completed, subtraction images were generated. We retrospectively examined images on the Agfa picture archiving and communication system (PACS). The lesion morphology and enhancement kinetic features were defined according to the American College of Radiology–BI-RADS lexicon with final classification. We described lesions as mass and non-mass lesions. For masses, we specified shape (round, oval, lobulated, or irregular), margins (smooth, irregular, speculated), internal enhancement (homogenous, heterogeneous, rim enhancement), kinetic enhancement (visual analyze or kinetic curves after region of interest positioning), and T2 -weighted aspect (hypo or hyper). For non-mass lesions, we described size, distribution, and internal enhancement.
Histopathological Analysis
Histological findings were classified as invasive ductal carcinomas (IDC), ductal carcinoma in situ (DCIS), both (IDC-positive DCIS), or other (for example papillary, tubular, or mucinous carcinomas), and the grade was recorded as low, intermediate, or high. We used the surgical findings from breast-conserving surgery or mastectomy specimens as the reference standard. The cut-off point for ER- and PR-positive expression was 10%. HER-2 status was 0, 1-positive, or 2-positive without amplification in FISH analyses for all TN tumors.
Statistical Analysis
We used χ2's t test or Fisher's exact test for qualitative data and the Mann-Whitney U test or the Student t test for quantitative data. In these exploratory analyses, p < .05 was considered statistically significant. The Bonferroni adjustment was not used to account for multiple testing (approximately 30 factors tested for) because of the study's exploratory and descriptive aims, but it should be used for p values close to 0.05, or more conservatively, p = .001. Descriptive statistics are reported with means and percentages for normally distributed data or medians and ranges for non-normally distributed data. χ2 analysis was used to identify associations between imaging features and groups. All analyses are per lesion. All statistical analyses were performed with the use of the SPSS statistical software (SPSS, Chicago, IL, http://www.spss.com).
Results
Clinicopathological Data
For both groups, 210 mammograms and ultrasounds were available for study and 35 MRI scans were available for study. TN cancers were more often discovered by the patient herself (46.7% vs. 24.8% for HR-positive/HER-2-negative group), whereas HR-positive/HER-2-negative tumors were more often seen on mammography (65.7% vs. 39% for the TN group) (p < .001). Sixty TN tumors (57.1%) and 34 HR-positive/HER-2-negative tumors (32.4%) were palpable and measurable. The median clinical size for tumors palpable at diagnosis was larger for TN tumors than for HR-positive/HER-2-negative tumors: 40 mm (range: 10–150) versus 30 mm (range: 10–70), respectively (p < .001). Patients with TN cancers were generally significantly younger than those with HR-positive/HER-2-negative cancers (54 years vs. 61 years) (p = .02) (Table 1).
Table 1.
Clinicopathological data for TN tumors (n = 105) versus HR+/HER-2- tumors (n = 105) diagnosed from July 2009 through June 2011
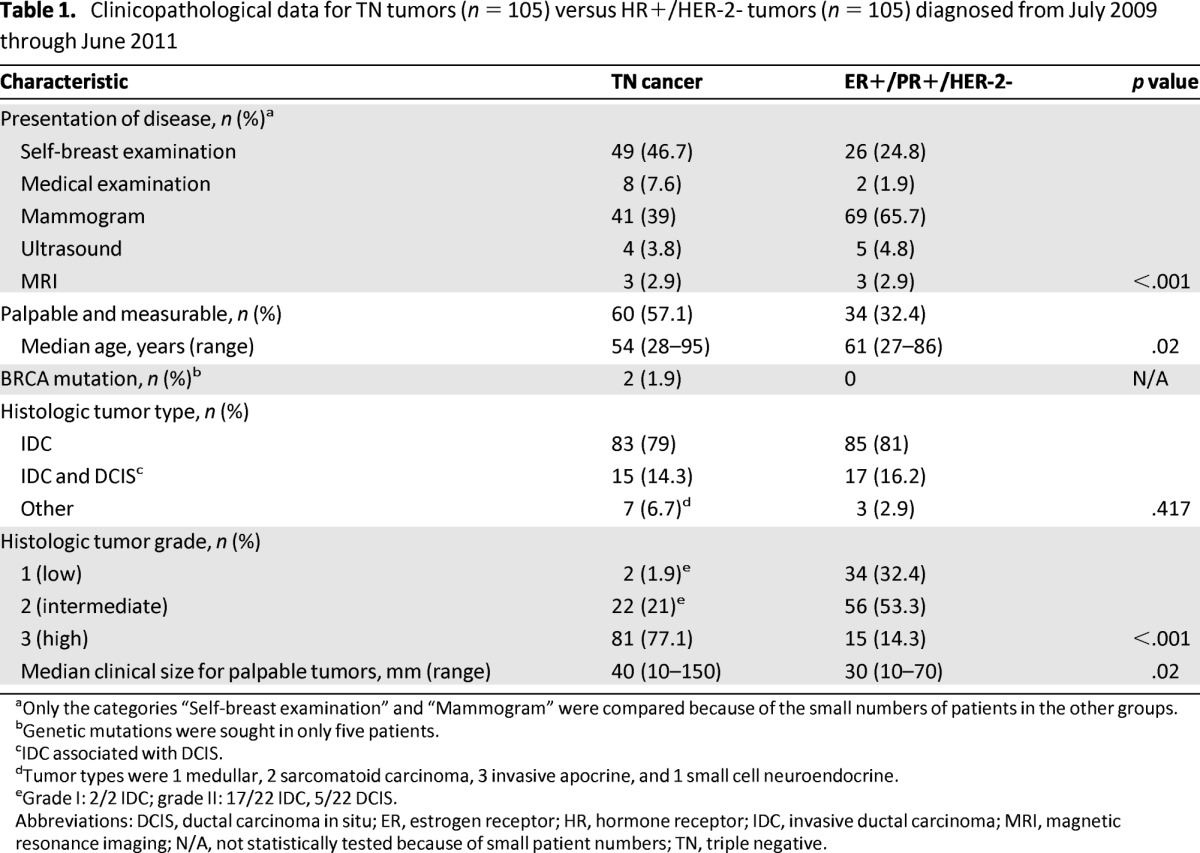
aOnly the categories “Self-breast examination” and “Mammogram” were compared because of the small numbers of patients in the other groups.
bGenetic mutations were sought in only five patients.
cIDC associated with DCIS.
dTumor types were 1 medullar, 2 sarcomatoid carcinoma, 3 invasive apocrine, and 1 small cell neuroendocrine.
eGrade I: 2/2 IDC; grade II: 17/22 IDC, 5/22 DCIS.
Abbreviations: DCIS, ductal carcinoma in situ; ER, estrogen receptor; HR, hormone receptor; IDC, invasive ductal carcinoma; MRI, magnetic resonance imaging; N/A, not statistically tested because of small patient numbers; TN, triple negative.
Although mutations were only sought for 5% of the TN group and 20% of the HR-positive/HER-2-negative group (because of familial histories), 2 of the 5 patients with TN cancer who had available genetic mutation data had mutations and none of the 21 women with HR-positive/HER-2-negative cancer had BRCA1 mutations.
No differences were found between the frequency of histological types across groups (p = NS). TN tumors had higher grades in general. Most were grade 3 (77.1%); 21.0% were grade 2, and 1.9% were grade 1. For the HR-positive/HER-2-negative group, 14.3% of tumors were grade 3, 53.3% were grade 2, and 32.4% were grade 1 (p < .001).
Mammography
No differences in breast density were found across groups (Table 2). Most cancers were seen as masses: 57 of 92 (61.9%) for TN and 55 of 95 (57.9%) for the HR-positive/HER-2-negative cancers. TN lesions were more frequently round, oval, or lobulated (67.7%) than those of HR-positive/HER-2-negative cancers (20%), and lesions were more frequently irregular for HR-positive/HER-2-negative tumors (80%) than for TN tumors (32.3%, p < .001). TN tumors more frequently had indistinct margins (61.5%) than tumors in the HR-positive/HER-2-negative group (25%) (p < .001) (Figs. 1A, 2A). On the contrary, margins of masses in HR-positive/HER-2-negative cancers were spiculated in 68.3% (vs. 13.8% in TN tumors). In both groups, masses with microcalcifications, clusters of microcalcifications, asymmetric focal densities, and architectural distortions (other imaging) were relatively rare (Table 2).
Table 2.
Mammography results and findings for breast cancer according to the tumor phenotype (TN, n = 105; HR+/HER-2-, n = 105)
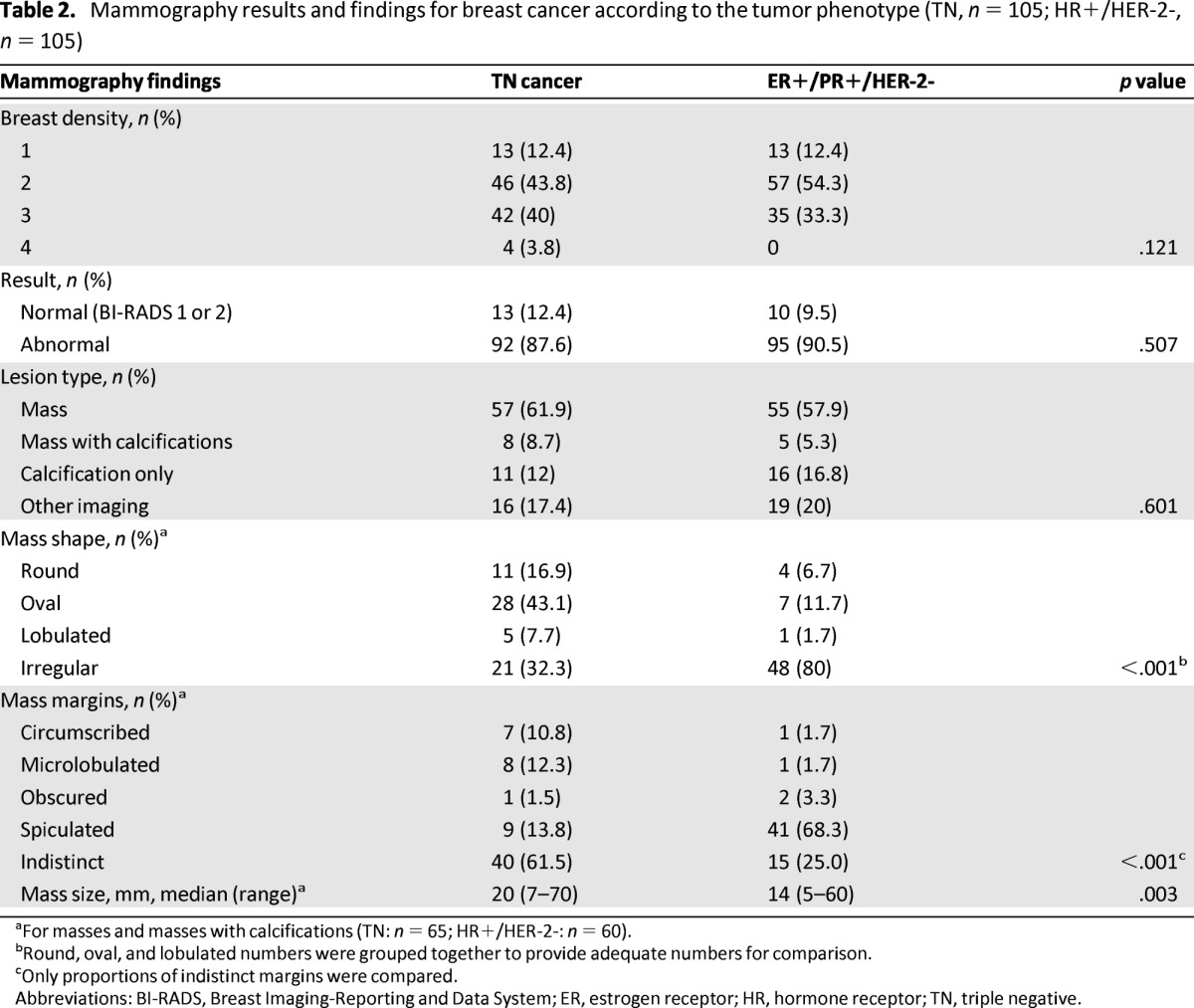
aFor masses and masses with calcifications (TN: n = 65; HR+/HER-2-: n = 60).
bRound, oval, and lobulated numbers were grouped together to provide adequate numbers for comparison.
cOnly proportions of indistinct margins were compared.
Abbreviations: BI-RADS, Breast Imaging-Reporting and Data System; ER, estrogen receptor; HR, hormone receptor; TN, triple negative.
Figure 1.
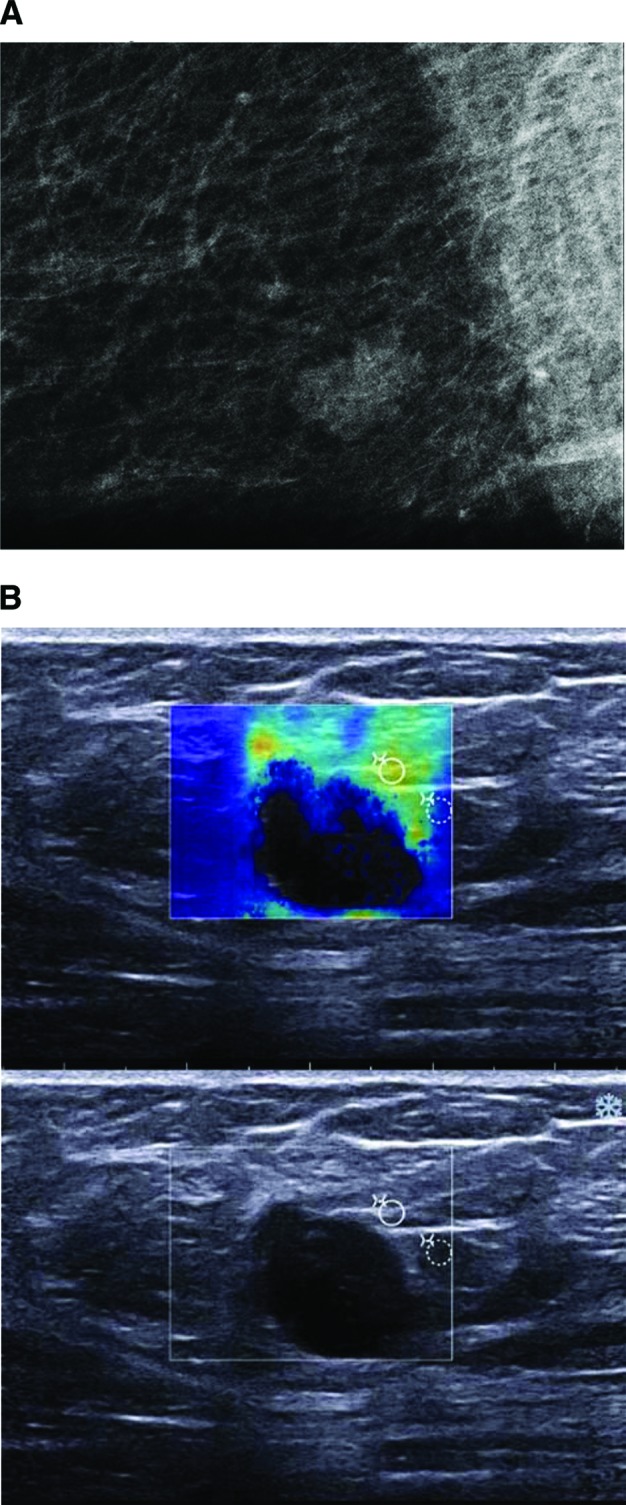
Imaging studies of a 66-year-old woman with a diagnosis of triple-negative ductal invasive carcinoma. (A): Mammogram shows a microlobulated-shaped mass with mostly circumscribed margins. (B): Ultrasound image shows an oval-shaped mass with microlobulated margins, marked hypogenicity, abrupt interface, and posterior acoustic enhancement. The periphery of the lesion was hard on elastography.
Figure 2.
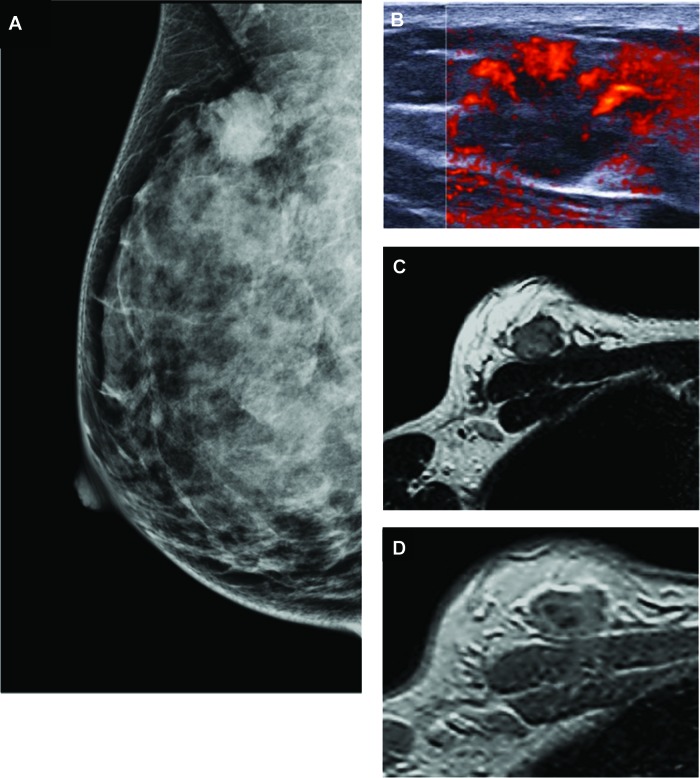
Imaging studies of a 32-year-old woman with a palpable lump in her right breast corresponding with triple-negative ductal invasive carcinoma. (A): Mammogram (mediolateral oblique view) shows an oval-shaped mass with indistinct margins. (B): Sonogram shows an oval-shaped mass with circumscribed margins, hypogenicity, abrupt interface, posterior acoustic enhancement, and diffusely increased vascularity. (C): Axial T2-weighted magnetic resonance imaging (MRI) shows hyperintense oval mass with smooth margins. (D): Axial T1-weighted early phase dynamic contrast-enhanced MRI shows mass with rim enhancement.
Only one cancer (a TN cancer) was classified BI-RADS 3. A comparison of the different types of abnormal mammograms revealed that fewer TN lesions than HR-positive/HER-2-negative lesions were classified BI-RADS 5 (34.1% vs. 51.6%, respectively) and more TN tumors than HR-positive/HER-2-negative tumors were classified BI-RADS 4 (65.9% vs. 48.4%, respectively) (p = .016).
Ultrasound
Ultrasound scans were available for all lesions. Masses were present on the same percentage of TN and HR-positive/HER-2-negative lesion images (94% vs. 93%). TN cancers more frequently had a round or oval shape (65.6% vs. 23.3% for HR-positive/HER-2-negative lesions) and HR-positive/HER-2-negative tumors more frequently had an irregular shape (76.7% vs. 34.4% TN), (p < .001). TN tumors had circumscribed or microlobulated margins more often than the HR-positive/HER-2-negative group (47.3% vs. 17.5%, respectively), and HR-positive/HER-2-negative tumors more often had indistinct, angular, spiculated margins (82.6% vs. 52.7% for TN) (p < .001) (Table 3).
Table 3.
Ultrasonography results and findings for breast cancer according to the tumor phenotype: TN (n = 105) or ER+/PR+/HER- (n = 105)
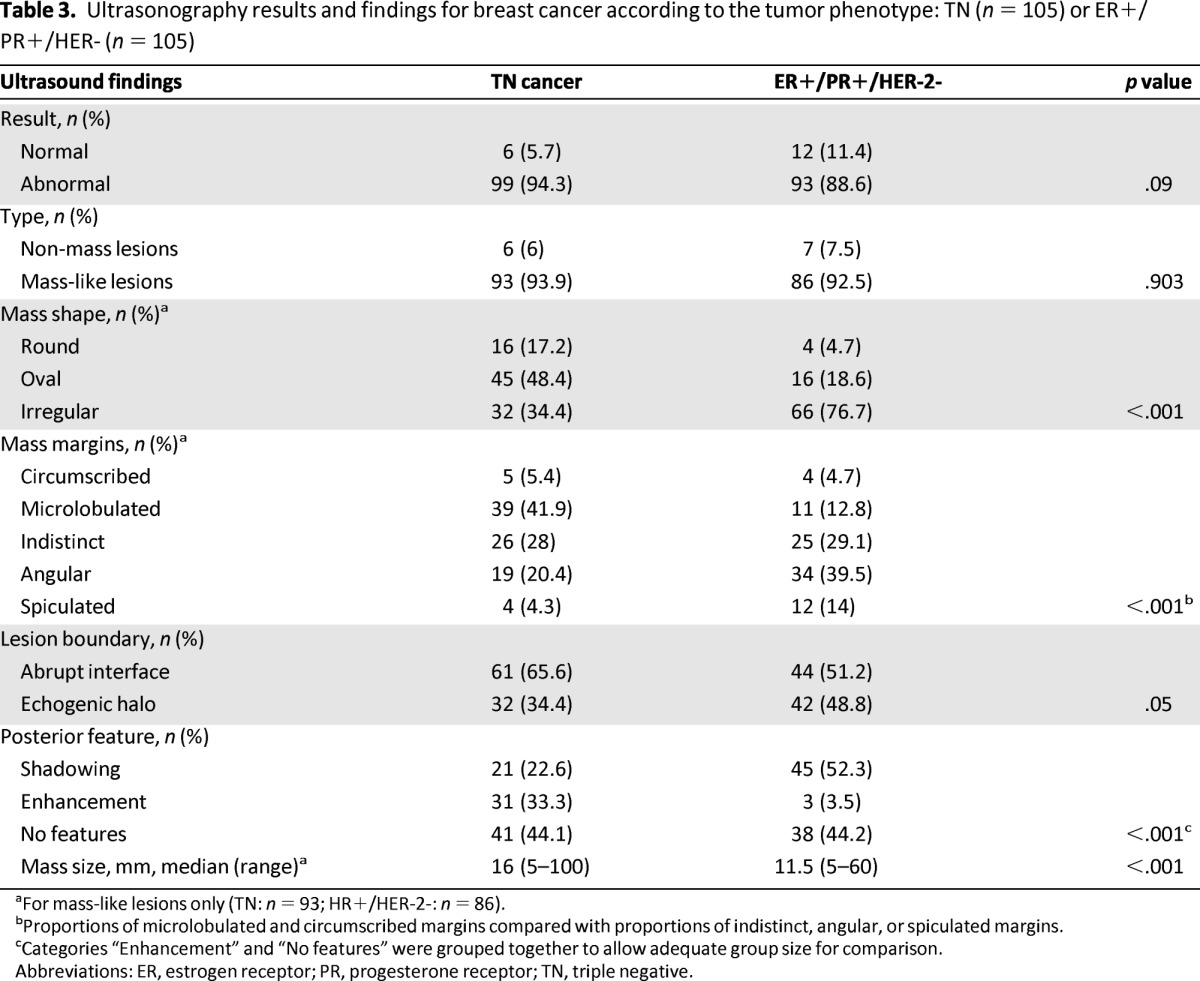
aFor mass-like lesions only (TN: n = 93; HR+/HER-2-: n = 86).
bProportions of microlobulated and circumscribed margins compared with proportions of indistinct, angular, or spiculated margins.
cCategories “Enhancement” and “No features” were grouped together to allow adequate group size for comparison.
Abbreviations: ER, estrogen receptor; PR, progesterone receptor; TN, triple negative.
There was borderline statistical significance for differences in lesion boundaries, with TN cancers more frequently having an abrupt interface (65.6%) than HR-positive/HER-2-negative cancers (51.2%) and HR-positive/HER-2-negative cancers having echogenic halos more frequently than TN cancers (48.8% vs. 34.4%) (p = .05) (Table 3). Ultrasound showed no posterior acoustic features and no posterior enhancement in 72 of 93 TN lesions (77.4% vs. 47.7% for HR-positive/HER-2-negative lesions) (p < .001). Posterior shadowing was present in 45 out of 86 HR-positive/HER-2-negative tumors (52.3% vs. 22.6% TN) (p < .001) (Table 3). TN cancers were significantly more often markedly hypoechoic (46.2%) than HR-positive/HER-2-negative cancers (10.8%) (p < .001) (Table 4).
Table 4.
Pattern on ultrasound for masses according the tumor phenotype: TN (n = 93); ER+/PR+/HER- (n = 83)
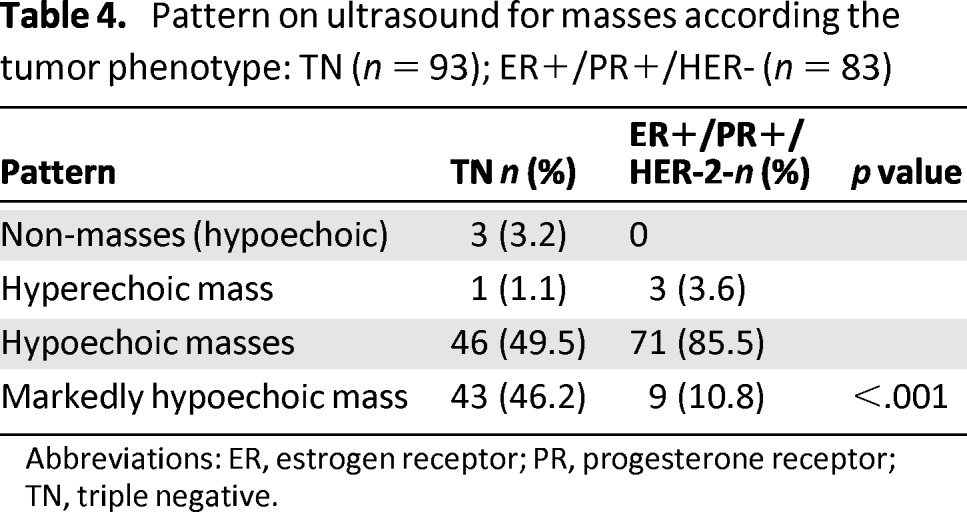
Abbreviations: ER, estrogen receptor; PR, progesterone receptor; TN, triple negative.
Doppler color imaging was available for 48 of 100 TN cancers and for 40 of 90 HR-positive/HER-2-negative cancers and was positive in 38 TN (79%) and 33 HER-positive/HER-negative tumors (83%) (Figs. 1B, 2B). For 25 TN tumors and 24 HR-positive/HER-2-negative tumors, we could use the quantitative elastography technique of Shear Wave Elastography (SWE). There was one false negative among the TN group (10-mm cancer initially classified BI-RADS 3). The small number of patients and the variability of the data (kPa measures were inside the lesion for some tumors and in the periphery of the lesion for others) ruled out the investigation of this parameter.
One HR-positive/HER-2-negative and four TN cancers were classified BI-RADS 3. When we compared BI-RADS 4 and 5 classifications, there were more BI-RADS 5 classifications in HR-positive/HER-2-negative tumors (58.7%) than in TN lesions (32.6%) (p < .001). For the TN group, 18 BI-RADS 4 tumors were classified BI-RADS 5 when using elastography. In the HR-positive/HER-2-negative group, 6 tumors appeared more suspect with elastography.
Dynamic Contrast-Enhanced MRI Findings
MRI was available for 22 patients with TN cancer (21.4%) and 13 patients with HR-positive/HER-2-negative cancer (12.4%). All cancers were detected on MRI in the two subgroups and showed significant abnormal contrast enhancement. For the TN cohort, 17 of 22 cancers (77%) appeared as masses and 4 of 22 (18%) appeared as non-mass lesions. For the HR-positive/HER-2-negative group, 12 of 13 cancers (92.3%) appeared as masses and 1 of 13 (7.7%) appeared as non-mass lesions (limited statistical testing because of small sample size).
MRI characteristics for tumors with mass-like contrast enhancement (n = 17 TN and 12 HER-positive/HER-negative) are shown in Table 5. Statistical testing was not performed because of the small number of patients in each group. All HR-positive/HER-2-negative mass-like lesions showed low intratumoral signal intensity on T2-weighted images, whereas approximately half of TN lesions showed high signal intensity and half showed low signal intensity (Fig 2C). Among the TN cancers with masses, the most common internal enhancement pattern was rim enhancement (10/17; 58.8%), which was much more frequent in TN tumors than in the HR-positive/HER-2-negative group (1/12: 8.3%) (Fig. 2D). For non-mass TN lesions, MRI showed 1 of 4 with focal zone enhancement, 1 of 4 with regional enhancement, and 2 of 4 lesions with ductal enhancement. The internal enhancement was homogenous (2/4), heterogeneous (1/4) or micronodular (1/4). The principal imaging differences between TN and HER-positive/HER-negative tumors are summarized in supplemental online Table 1.
Table 5.
MRI findings in TN (n = 17) and HR+ (n = 12) cancers showing mass-like contrast enhancement
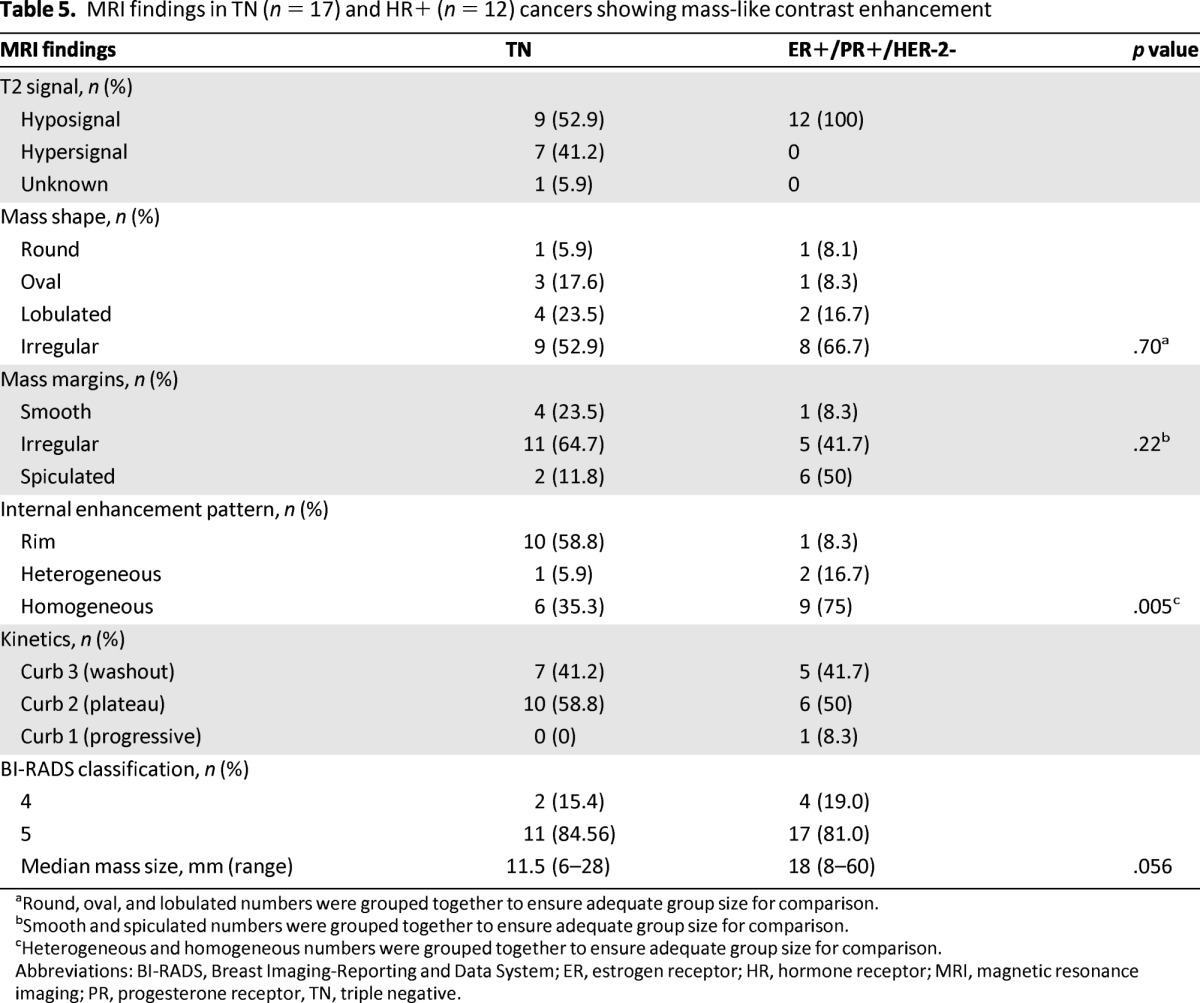
aRound, oval, and lobulated numbers were grouped together to ensure adequate group size for comparison.
bSmooth and spiculated numbers were grouped together to ensure adequate group size for comparison.
cHeterogeneous and homogeneous numbers were grouped together to ensure adequate group size for comparison.
Abbreviations: BI-RADS, Breast Imaging-Reporting and Data System; ER, estrogen receptor; HR, hormone receptor; MRI, magnetic resonance imaging; PR, progesterone receptor, TN, triple negative.
Discussion
Although constituting only a relatively small proportion of breast cancers, TN tumors are aggressive in nature and responsible for a large proportion of breast cancer deaths. Nearly all (98%) of the TN cancers in our retrospective single-center study were of intermediate or high grades, which is similar to previous reports [3, 7]. TN cancers were larger in size and affected younger women more often than the control group of HR-positive/HER-2-negative tumors. In addition, we found that nearly half of the patients with TN cancers (46.7%) had previously presented to a primary care practitioner with a breast lump, compared with less than a quarter of the women with HR-positive/HER-2-negative tumors. This difference supports previous reports that, compared with other phenotypes, TN tumors are more likely to be detected clinically by the patient or physician than radiographically by mammography or ultrasound [14]. Early detection of these tumors is important, and it should be noted that more than 12% of TN tumors were occult on mammography and 6% were occult on ultrasound. This is in agreement with the report by Dogan et al. of 9% of TN tumors that were occult on mammography and 6% occult on sonography [6].
Mammography
Our results support previous reports showing that most TN and HR-positive/HER-2-negative cancers are seen as masses in differences in the frequency of negative mammograms or architectural distortions. For tumors presenting as masses, we found striking differences in characteristics between TN cancers and HR-positive/HER-2-negative breast cancers. In our study, 67.7% of TN cancers were seen as round, oval, or lobulated masses, compared with 70% [10] to 75% [11] in previously reports. We found circumscribed margins in the TN lesions less often than was described in other studies [10.8% vs. 24% [4] to 43% [11]. In our study, circumscribed margins occurred in similar rates in TN and HR-positive/HER-2-negative tumors. Of TN tumors, 61.5% showed indistinct margins. This is a much higher rate than HR-positive/HER-2-negative tumors and higher than other reports, which range from 32% [4] to 45% [11]. This difference could be the result of variability among clinicians' interpretations of findings, or differences in patients' ages in previous reports and in our study (e.g., patients in the study by Yang et al. had a median age of 32 years for example) [11]. The characteristic of round-shaped masses reflects an aggressive, rapidly proliferating tumor [15] with pushing margins [10] without any stromal reaction.
TN cancers reportedly have associated microcalcifications less frequently than other phenotypes [7, 10, 11]. It should be noted that among the seven non-ductal TN tumors, two invasive apocrine cancers appeared as spiculated masses with microcalcifications. Ko et al. suggested that TN cancers have a more rapid pattern of carcinogenesis that leads directly to invasive cancer, with no major in situ component or precancerous stage [7]. In our series, we found 12% of TN cancers revealed by a cluster of microcalcifications, which is higher than the rate generally reported in the literature (0%–9%) [7, 10, 11]. However, this may be explained by a smaller proportion of patients in our study with BRCA mutations.
In our study, mammograms were normal for 12.4% of the TN tumors and 9.5% of the HR-positive/HER-2-negative tumors. Using the same equipment we used, Ko et al. reported no occult TN tumors and 2%–4% occult tumors in the two control groups [7]. A negative or normal mammogram can be explained by the presence of dense breast tissue and by the rapid progression of these types of tumors that is not accompanied by architectural distortion.
Sonography
Masses were observed on ultrasound in most of tumors for both TN and HR-positive/HER-2-negative phenotypes, in agreement with a previous report by Au-Yong et al. [16]. The major differences observed between TN and HR-positive/HER-2-negative tumors on sonography were that TN tumors had higher rates of round or oval shapes, circumscribed or microlobulated margins, and no posterior acoustic features or posterior enhancement than HR-positive/HER-2-negative tumors. Echogenicity was also more markedly hypoechoic for TN tumors. These distinctive features should indicate the presence of a TN tumor, in contrast to irregular shapes; indistinct, angular, or spiculated margins; and echogenic halos that are more indicative of HR-positive/HER-2-negative tumors. The rate of circumscribed margins in our study is somewhat lower than that reported in the literature [5.4% vs. 27% to 57% [7, 10]] and the occurrence of microlobulated margins was somewhat higher in our study (41.9% vs. 9% to 33%). This difference could be the result of variations in how observers use the BI-RADS classification, with Ko et al. using the term “irregular shape” for masses that we would instead have described as “oval with microlobulated margins” [7]. Differences may also be the result of the ultrasound equipment used. Some authors recognize that their results may have been different using more modern equipment [16]. Some of the TN cancers might have also been misinterpreted as benign, similar to other subtypes of high-grade tumors and familiar breast cancers [6, 17]. Our results highlight the need to correctly identify microlobulated margins, which are poorly described in the literature, but which may help characterize a malignant tumor.
We did not find a significant difference between the presentation of lesion boundaries on TN or HR-positive/HER-2-negative tumors, compared with Ko et al., who reported an abrupt interface in 84% of TN cancers compared with 64% in ER-positive/PR-negative/HER-negative tumors and an echogenic halo in 36% of ER-positive/PR-negative/HER-negative tumors [7]. The halo is thought to represent the appearance of a stromal reaction, which is poor or absent in TN tumors. Ultrasound technology may aid in its detection.
The presence of tumor vascularity on color Doppler has been reported only once previously, by Kojima and Tsunoda [4], who reported 90% vascularity, which is somewhat higher than the rate found in our study (38% for TN cancers and 36.7% for HR-positive/HER-2-negative cancers). SWE allows measurement of the propagation speed of SWs throughout tissues to locally quantify the stiffness of tissue in kilopascals per second and improves the specificity of breast ultrasound. We are not aware of any study about quantitative elastography specifically for TN cancers.
MRI
In agreement with Youk et al. [18], we identified several distinctive features of TN tumors on MRI, including irregular margins; a higher rate of round, oval, or lobulated shapes (as in sonography or mammography); high intratumoral signal intensity; and the presence of rim enhancement. Rim enhancement, generally reported as relatively infrequent across all tumors and as having high positive predictive value for malignancy [19], was much more common in TN tumors (10/17) than in HR-positive/HER-2-negative tumors (1/12) in our series. HR-positive/HER-2-negative tumors in contrast showed more homogenous enhancement. We found that morphological criteria on MRI were more suspicious compared with those of mammography and ultrasound: the most frequent findings on MRI were a round or an oval contrast-enhanced mass with irregular or spiculated margins and rim enhancement, which is similar to the findings of Dogan et al. [6].
This single-center retrospective study has some limitations. First, we do not mention the sensitivity and specificity of the different imaging modalities because the majority of patients were symptomatic at presentation, and the reviewing radiologists were not blinded to the patient information or lesion pathology. Second, the small size of patients with color Doppler studies and elastography limits the statistical significance of the data obtained. Further evaluation of the diagnostic efficacy of elastography, MRI, and functional imaging is warranted. In addition, the small number of patients for whom genetic mutations were tested should be noted. Only those with familial histories are recommended for genetic testing in our institute. Our rate of 2% of BRCA1 mutations may underestimate real rates. In recent reports specifically investigating the prevalence of BRCA mutations in an unselected population of women with TN breast cancer, BRCA1 mutations were found in 6.5% [19–21].
Conclusion
The presence of triple negative breast cancer is suggested by noncalcified mammography masses that are seen as a markedly hypoechoic masses with microlobulated margins across combined imaging modalities. The radiologist must be aware that this subtype of cancer can mimic lesions with benign morphologies. Improved recognition and detection on mammography and ultrasound imaging should add to a better understanding of the biological behavior of the disease entity and should lead to rapid pretreatment planning.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Author Contributions
Conception/Design: Martine Boisserie-Lacroix, Gaetan MacGrogan, Marc Debled, Gabrielle Hurtevent Labrot
Provision of study material or patients: Martine Boisserie-Lacroix, Gaetan MacGrogan, Marc Debled, Stéphane Ferron, Maryam Asad-Syed, Gabrielle Hurtevent Labrot
Collection and/or assembly of data: Martine Boisserie-Lacroix, Stéphane Ferron, Maryam Asad-Syed, Véronique Brouste
Data analysis and interpretation: Martine Boisserie-Lacroix, Pippa McKelvie Sebileau, Simone Mathoulin-Pélissier, Véronique Brouste
Manuscript writing: Martine Boisserie-Lacroix, Gaetan MacGrogan, Marc Debled, Stéphane Ferron, Maryam Asad-Syed, Pippa McKelvie Sebileau, Simone Mathoulin-Pélissier, Véronique Brouste, Gabrielle Hurtevent Labrot
Final approval of manuscript: Martine Boisserie-Lacroix, Gaetan MacGrogan, Marc Debled, Stéphane Ferron, Maryam Asad-Syed, Pippa McKelvie Sebileau, Simone Mathoulin-Pélissier, Véronique Brouste, Gabrielle Hurtevent Labrot
Disclosures
The authors indicated no financial relationships.
Section editors: Gabriel Hortobágyi: Antigen Express, Galena Biopharma, Novartis, Rockpointe (C/A); Novartis (RF); Taivex (O); founder and member of the board of directors for Citizen's Oncology Foundation; Kathleen Pritchard: Novartis, Roche, AstraZeneca, Pfizer, Boehringer-Ingelheim, GlaxoSmithKline, Sanofi, Ortho-Biotech, Amgen, and Bristol-Myers Squibb (C/A); (H)
Reviewer “A”: None
Reviewer “B”: None
Reviewer “C”: None
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Reference
- 1.Billar JA, Dueck AC, Stucky CC, et al. Triple-negative breast cancers: Unique clinical presentations and outcomes. Ann Surg Oncol. 2010;17(suppl 3):384–390. doi: 10.1245/s10434-010-1260-4. [DOI] [PubMed] [Google Scholar]
- 2.Shah SP, Roth A, Goya R, et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. 2012;486:395–399. doi: 10.1038/nature10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitman GJ, Albarracin CT, Gonzalez-Angulo AM. Triple-negative breast cancer: What the radiologist needs to know. Semin Roentgenol. 2011;46:26–39. doi: 10.1053/j.ro.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Kojima Y, Tsunoda H. Mammography and ultrasound features of triple-negative breast cancer. Breast Cancer. 2011;18:146–151. doi: 10.1007/s12282-010-0223-8. [DOI] [PubMed] [Google Scholar]
- 5.Chen JH, Agrawal G, Feig B, et al. Triple-negative breast cancer: MRI features in 29 patients. Ann Oncol. 2007;18:2042–2043. doi: 10.1093/annonc/mdm504. [DOI] [PubMed] [Google Scholar]
- 6.Dogan BE, Gonzalez-Angulo AM, Gilcrease M, et al. Multimodality imaging of triple receptor-negative tumors with mammography, ultrasound, and MRI. AJR Am J Roentgenol. 2010;194:1160–1166. doi: 10.2214/AJR.09.2355. [DOI] [PubMed] [Google Scholar]
- 7.Ko ES, Lee BH, Kim HA, et al. Triple-negative breast cancer: Correlation between imaging and pathological findings. Eur Radiol. 2010;20:1111–1117. doi: 10.1007/s00330-009-1656-3. [DOI] [PubMed] [Google Scholar]
- 8.Li SP, Padhani AR, Taylor NJ, et al. Vascular characterisation of triple negative breast carcinomas using dynamic MRI. Eur Radiol. 2011;21:1364–1373. doi: 10.1007/s00330-011-2061-2. [DOI] [PubMed] [Google Scholar]
- 9.Uematsu T, Kasami M, Yuen S. Triple-negative breast cancer: Correlation between MR imaging and pathologic findings. Radiology. 2009;250:638–647. doi: 10.1148/radiol.2503081054. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Ikeda DM, Narasimhan B, et al. Estrogen receptor-negative invasive breast cancer: Imaging features of tumors with and without human epidermal growth factor receptor type 2 overexpression. Radiology. 2008;246:367–375. doi: 10.1148/radiol.2462070169. [DOI] [PubMed] [Google Scholar]
- 11.Yang WT, Dryden M, Broglio K, et al. Mammographic features of triple receptor-negative primary breast cancers in young premenopausal women. Breast Cancer Res Treat. 2008;111:405–410. doi: 10.1007/s10549-007-9810-6. [DOI] [PubMed] [Google Scholar]
- 12.Boisserie-Lacroix M, MacGrogan G, Debled M, et al. Radiological features of triple-negative breast cancers (73 cases) Diagn Interv Imaging. 2012;93:183–190. doi: 10.1016/j.diii.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda DM, Hylton NM, Kuhl CK, et al. BI-RADS: Magnetic Resonance Imaging. In: D'Orsi CJ, Mendelson EB, Ikeda DM, et al., editors. Breast Imaging Reporting and Data System: ACR BI-RADS—Breast Imaging Atlas. 1st ed. Reston, VA: American College of Radiology; 2003. [Google Scholar]
- 14.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 15.Shin HJ, Kim HH, Huh MO, et al. Correlation between mammographic and sonographic findings and prognostic factors in patients with node-negative invasive breast cancer. Br J Radiol. 2011;84:19–30. doi: 10.1259/bjr/92960562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Au-Yong IT, Evans AJ, Taneja S, et al. Sonographic correlations with the new molecular classification of invasive breast cancer. Eur Radiol. 2009;19:2342–2348. doi: 10.1007/s00330-009-1418-2. [DOI] [PubMed] [Google Scholar]
- 17.Schrading S, Kuhl CK. Mammographic, US, and MR imaging phenotypes of familial breast cancer. Radiology. 2008;246:58–70. doi: 10.1148/radiol.2461062173. [DOI] [PubMed] [Google Scholar]
- 18.Youk JH, Son EJ, Chung J, et al. Triple-negative invasive breast cancer on dynamic contrast-enhanced and diffusion-weighted MR imaging: Comparison with other breast cancer subtypes. Eur Radiol. 2012;22:1724–1734. doi: 10.1007/s00330-012-2425-2. [DOI] [PubMed] [Google Scholar]
- 19.Schnall MD, Blume J, Bluemke DA, et al. Diagnostic architectural and dynamic features at breast MR imaging: Multicenter study. Radiology. 2006;238:42–53. doi: 10.1148/radiol.2381042117. [DOI] [PubMed] [Google Scholar]
- 20.Jinguji M, Kajiya Y, Kamimura K, et al. Rim enhancement of breast cancers on contrast-enhanced MR imaging: Relationship with prognostic factors. Breast Cancer. 2006;13:64–73. doi: 10.2325/jbcs.13.64. [DOI] [PubMed] [Google Scholar]
- 21.Hartman AR, Kaldate RR, Sailer LM, et al. Prevalence of BRCA mutations in an unselected population of triple-negative breast cancer. Cancer. 2012;118:2787–2795. doi: 10.1002/cncr.26576. [DOI] [PubMed] [Google Scholar]