Abstract
Background.
Talactoferrin alfa (talactoferrin), an agent with immune-stimulating properties, has demonstrated safety and preliminary efficacy in clinical trials.
Methods.
Ten patients (five males and five females) with stage IV non-small cell lung cancer (NSCLC) in a single-arm pilot study received orally administered talactoferrin (1.5 g, b.i.d.) for up to 24 weeks. Radiographic and immunologic studies were performed at baseline and at weeks 6 and 12. Circulating immune cells (natural killer cells [NKCs], CD4+, CD8+, and regulatory T cells) and systemic cytokine levels were measured to assess immune response.
Results.
Patients enrolled in the study had received a median of four prior chemotherapy regimens, and all patients were symptomatic. Talactoferrin was well tolerated, with no grade 3 or 4 toxicities. Median time to progression (TTP) and overall survival were 6 weeks and 14.5 weeks, respectively. The four patients with ≥9 weeks TTP had evidence of immunologic activity (three with increased NKC activity).
Conclusions.
The median of four previous chemotherapy regimens, with elevated levels of interleukin (IL) 6 and tumor necrosis factor-alfa in most patients, suggests these patients were poor candidates for immunotherapy.
Discussion
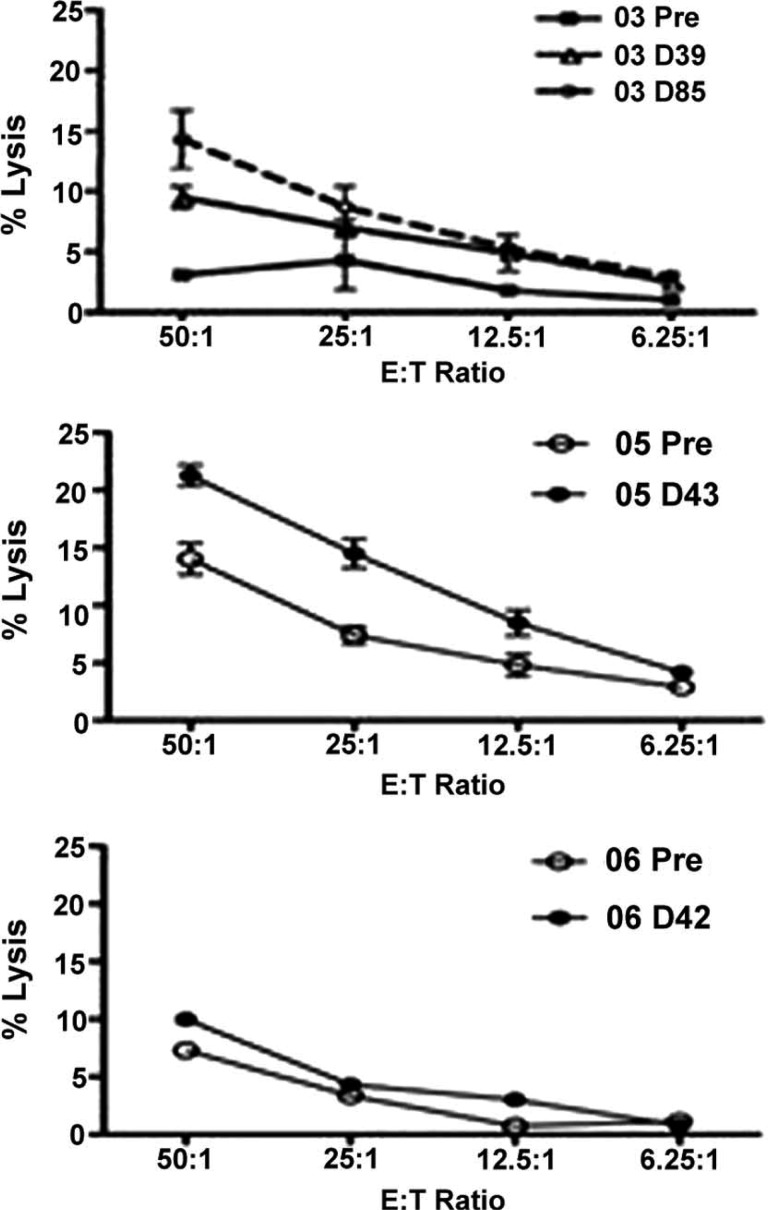
This pilot study of 10 patients evaluated safety and systemic immunologic response with talactoferrin as monotherapy. No grade 3 or grade 4 toxicities were observed. Baseline parameters suggest that the immune systems of enrolled patients were impaired and thus less likely to mount significant immune-mediated responses. In fact, these data suggest that heavily pretreated NSCLC patients with a large disease burden may not be able to generate effective immune responses. In the four patients who remained in the study beyond 6 weeks, there was a suggestion of possible improved innate immunologic activity (NKCs) after treatment with talactoferrin.
All patients enrolled in this study were symptomatic, and four had a relatively poor ECOG performance status of 2, the most powerful prognostic factor in advanced NSCLC. But perhaps more important than having end-stage NSCLC, these patients had received multiple prior chemotherapy regimens. Six of the 10 patients enrolled had four or more previous chemotherapy regimens (4 of those 6 had received six previous regimens). A previous analysis of patients treated with an immune-stimulating agent (therapeutic cancer vaccine) demonstrated that immunologic response was inversely related to the number of previous chemotherapy regimens [1]. In this regard, it is interesting to note that the two patients (patient 5 and patient 6) with the fewest number of prior anticancer regimens were among the four who remained in the trial the longest and had increased or stable NKC function.
Figure 1.
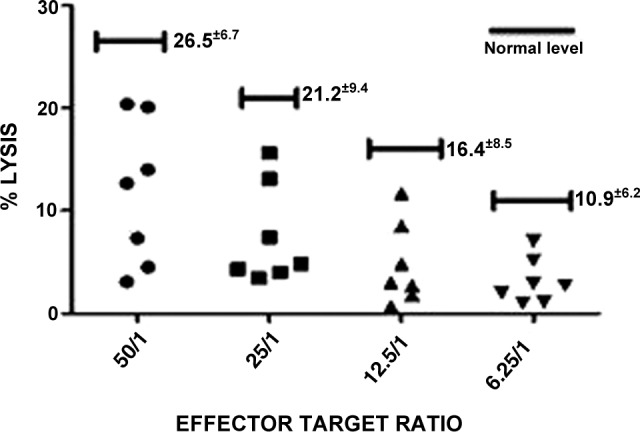
Baseline natural killer cell function is below normal in the patients enrolled in this study.
Figure 2.
Changes in natural killer function after treatment with talactoferrin for patients 03, 05, and 06.
It is also important to note that survival after study enrollment was only 14.5 weeks, suggesting that these patients were in the final months of their disease course and had greater tumor quantities. It has been demonstrated that tumor burden is proportionally related to production of immunosuppressive cytokines such as transforming growth factor-β and IL-10 [2, 3]. From this perspective, the lack of immunologic responses leading to improved clinical outcomes could potentially be the result of an immunologic monotherapy administered too late in the disease course.
Results of a phase III trial with talactoferrin in NSCLC showed no improvement in overall survival in the second-line setting [4]. Limiting therapy to patients who have received fewer chemotherapy regimens and who have better performance status may improve the likelihood of increased immunologic response and clinical benefit.
Supplementary Material
Footnotes
ClinicalTrials.gov Identifier: NCT00923741 Sponsor(s): National Cancer Institute
Principal Investigator: James L. Gulley IRB Approved: Yes
Disclosures
Author disclosures available online.
Reference
- 1.von Mehren M, Arlen P, Tsang KY, et al. Pilot study of a dual gene recombinant avipox vaccine containing both carcinoembryonic antigen (CEA) and B7.1 transgenes in patients with recurrent CEA-expressing adenocarcinomas. Clin Cancer Res. 2000;6:2219–2228. [PubMed] [Google Scholar]
- 2.Teicher BA. Transforming growth factor-beta and the immune response to malignant disease. Clin Cancer Res. 2007;13:6247–6251. doi: 10.1158/1078-0432.CCR-07-1654. [DOI] [PubMed] [Google Scholar]
- 3.Muller AJ, Prendergast GC. Indoleamine 2,3-dioxygenase in immune suppression and cancer. Curr Cancer Drug Targets. 2007;7:31–40. doi: 10.2174/156800907780006896. [DOI] [PubMed] [Google Scholar]
- 4.Agennix Reports Results of FORTIS-M Phase III Trial with Talactoferrin Alfa in Non-Small Cell Lung Cancer. 2012. [Accessed April 5, 2013]. Available at http://agennix.com/index.php?option=com_content&view=article&id=227%3Aagennix-reports-results-of-fortis-m-phase-iii-trial-with-talactoferrin-alfa-in-non-small-cell-lung-cancer.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.