Rectal cancer outcomes vary significantly according to the stage of disease and prognostic factors, including the distance of the tumor from the circumferential resection margin. Accurate staging allows stratification of patients into low-, moderate-, and high-risk disease, which can be used to inform multidisciplinary team decisions regarding the role of neoadjuvant therapy. This article reviews the management of locally advanced rectal cancer, with particular focus on the importance of appropriate patient selection.
Keywords: Rectal cancer, Chemoradiation, Magnetic resonance imaging, Neoadjuvant treatment
Learning Objectives
Explain the use of MRI to stratify patients to undergo different neoadjuvant treatment strategies for locally advanced rectal cancer.
Identify the benefits and risks of currently available neoadjuvant treatment strategies and appraise emerging treatment strategy.
Identify tissue and imaging biomarkers that could predict tumor sensitivity to chemoradiation.
Abstract
Rectal cancer remains a significant problem worldwide. Outcomes vary significantly according to the stage of disease and prognostic factors, including the distance of the tumor from the circumferential resection margin. Accurate staging, including high-resolution magnetic resonance imaging, allows stratification of patients into low-, moderate-, and high-risk disease; this information can be used to inform multidisciplinary team decisions regarding the role of neoadjuvant therapy. Both neoadjuvant short-course radiotherapy and long-course chemoradiation reduce the risk of local recurrence compared with surgery alone, but they have little impact on survival. Although there remains a need to reduce overtreatment of those patients at moderate risk, evaluation of intensified regimens for those with high-risk disease is still required to reduce distant failure rates and improve survival in these patients with an otherwise poor prognosis.
Implications for Practice:
The use of preoperative therapy in rectal cancer should be made in a multidisciplinary team setting based on accurate staging in combination with magnetic resonance imaging findings and patient characteristics. Circumferential resection margin involvement, extent of extramural spread, lymph node burden, extramural venous invasion and low-lying position of primary rectal cancer are all established pathological risk factors and can be identified by MRI preoperatively. This would allow stratification of patients to receive neoadjuvant therapy. Those patients with low risk rectal cancer can undergo surgery alone with favorable long-term outcomes sparing them from radiation-induced long-term toxicities. Those patients with high risk disease will need to be evaluated for intensified preoperative regimens to reduce distant failures and improve survival. Continuous research efforts are essential to identify both tissue and imaging biomarkers to predict efficacy to preoperative therapy which are lacking currently in order to optimize long-term outcome and minimize toxicities.
Introduction
Surgery remains the primary determinant of cure in patients with localized rectal cancer. The surgical approach for rectal cancers is dependent upon the position and stage of the tumor. The development of total mesorectal excision (TME) as a standard surgical technique involves removal of the tumor and all local draining nodes intact [1]. The plane of dissection is formed by the mesorectal fascia, which encloses the fatty mesorectum that envelops the rectum. This fascia forms the circumferential resection margin (CRM), and histological evidence of tumor within 1 mm of the potential CRM strongly predicts local recurrence and poor survival [2]. Magnetic resonance imaging (MRI) has been demonstrated to accurately predict the possibility of achieving a surgically clear CRM [3].
The use of neoadjuvant short-course radiotherapy (SCRT) and long-course chemoradiation (CRT) have both led to improvements in local control rates when compared with surgery alone, preoperative radiotherapy (RT), or postoperative CRT [4–8]. However, they did not consistently translate into an improvement in overall survival (OS) [4–8]. Given the relevance of an involved CRM in predicting outcome, it has become increasingly important that decisions regarding the use of neoadjuvant therapy, made in a multidisciplinary team setting, are based on accurate staging in combination with MRI findings and patient characteristics. This article reviews the management of locally advanced rectal cancer, with particular focus on the importance of appropriate patient selection.
Selection of Patients for Treatment
Many previously established poor prognostic factors are based on resection specimens of rectal cancer. The ability to accurately predict these risk factors preoperatively would allow stratification of patients to receive neoadjuvant therapy. Poor risk factors of importance in rectal cancer include CRM involvement, extent of extramural spread, lymph node burden, extramural venous invasion, and low-lying position of primary rectal cancer.
T3 tumors form a heterogeneous group from tumors that barely extend beyond the lamina muscularis propria to those that extend to or invade the mesorectal fascia. These T3 tumors have been subclassified based on the extent of extramural spread and found to have differing outcomes. Tumors with extramural spread ≤5 mm had a 5-year survival of 83.4% compared with those tumors >5 mm, which had a 5-year OS rate of 54.1% (p < .0001) [9].
Lymph node involvement is one of the most powerful prognostic factors in resectable colorectal cancer. However, as the nodal burden increases, the prognosis also became poorer correspondingly. In pooled analyses of 3,791 patients receiving postoperative therapy for rectal cancer, 5-year OS rates for T3N0, T3N1, and T3N2 were 75%, 60%, and 44%, respectively [10]. In another population-based analysis, the 5-year OS rates were 64%, 52.4%, and 37.5% for N0, N1, and N2 disease, respectively [11].
Extramural venous invasion (EMVI) has been shown to be associated with local recurrence [12] and development of liver metastases [13] in rectal cancer. Histological presence of EMVI has been shown to be associated with significantly worse relapse-free survival [14].
Patients with low-lying tumors requiring abdominoperineal resection (APR) have worse survival rates than patients who could undergo low anterior resection. In a pooled analysis of five (chemo)radiotherapy rectal cancer randomized controlled trials (RCTs), local recurrence, cancer-specific survival and OS rates were all significantly worse in those patients who underwent APR [15].
Tools that could accurately assess the above parameters would be paramount in the decision to offer neoadjuvant therapy. Endorectal ultrasound (EUS) depicts the anatomic layers of the rectal wall with a higher degree of accuracy than computed tomography (CT) and thus enables precise determination of the tumor extent in relation to the different wall layers. Localized cancers involving only the mucosa and submucosa can usually be distinguished from those that penetrate the muscularis propria or extend transmurally into the perirectal fat. However, although EUS allows more accurate characterization of status of perirectal nodes than CT, it is most suitable for evaluating early rectal cancer and is less reliable in assessing more advanced tumors [16–18], especially in relation to the prediction of surgical CRM. Potential disadvantages to EUS include a tendency to understage rather than overstage the primary tumor, interobserver variability, and difficulty in assessing obstructing or highly stenosed lesions.
High-resolution thin-slice (3-mm) MRI allows better differentiation of malignant tissue from the muscularis propria. In the European multicenter prospective Magnetic Resonance Imaging in Rectal Cancer European Equivalence Study (MERCURY) study involving 295 patients, MRI could accurately measure the depth of extramural spread. The primary endpoint of establishing equivalence of MRI and histological assessment of extramural spread to within 0.5 mm was achieved [19]. However, the MERCURY study recruited additional patients for other secondary endpoints. A total of 679 patients consented to the study; complete data on surgery, MRI, and pathology were available for 477 patients. The specificity for predicting clear surgical margins by MRI was 92% [3].
Although the original reports from the MERCURY group focused on the accuracy of predicting histological parameters by high-resolution MRI, outcome data are now available for 386 patients with varying MRI-predicted features. MRI predicted good prognoses for patients with safe mesorectal margins and T2/T3 tumors of <5 mm extramural spread regardless of N stage; for these patients, 5-year disease-free survival (DFS) and OS rates were 85% and 68% with surgery alone [20]. These patients were safely spared from the toxicities of neoadjuvant radiation.
It is unclear what distance the tumor should be from the MRI-predicted margin to be considered a positive CRM that requires preoperative therapy. In the literature, this varies from ≤1 mm to ≤5 mm. The long-term results from MERCURY demonstrated that only those patients with a predicted MRI margin of ≤1 mm had significantly higher rates of local recurrence compared with those with predicted MRI margin of >5 mm. Those patients with margins between >1 and ≤5 mm had similar rates and time to local recurrence compared with those with margins of >5 mm [21].
Furthermore, the clinical significance of pelvic side wall lymph nodes is currently unclear. Although pelvic side wall dissection is practiced in some parts of the world such as Japan, surgery is less commonly carried out in patients in Western countries, partly because of the technical challenge with the larger habitus and pelvic shape of the Western population. MERCURY study also found that 11.7% of patients had baseline pelvic side wall involvement; these patients had poorer DFS rates [22]. Pelvic side wall lymph node involvement was associated with other poor prognostic factors, such as presence of EMVI, MRI-defined mesorectal nodal involvement, and pathological T staging. However, preoperative radiotherapy appeared to eliminate the poor prognostic significance of pelvic side wall involvement [22].
Despite the reproducibility demonstrated in the MERCURY study, the high degree of staging accuracy has not consistently been replicated by others, possibly because of the technical aspects of imaging and image interpretation, which are critical to the success of this approach; in addition, there is variable acceptance of the technique worldwide. In a recent study, images from 168 consecutive pelvic MRIs of patients with rectal cancer were evaluated by radiologists at five imaging centers, by two expert reviewers, and by a resident [23]. The authors demonstrated that measurements of extramural tumor spread are more reproducible among different observers than predicting the anticipated CRM. Using 1 mm as a cutoff is more reproducible than 5 mm; as discussed before, only tumors ≤1 mm from the CRM have prognostic significance for local recurrence [21]. Using a 5-mm cutoff for MRI-predicted margins might potentially overtreat large numbers of patients. Indeed, from the MERCURY data, 89 of 216 additional patients (an additional 41%) would have been treated to prevent seven potential local recurrences.
In a recent systematic review and meta-analysis [24], 21 studies were included to assess the diagnostic accuracy of MRI compared with the histological gold standard. The specificity of MRI in assessing the CRM involvement was significantly higher than T and N staging, although there were no significantly differences in MRI sensitivity in all three categories. The diagnostic performance for MRI was significantly better in assessing CRM involvement compared with N staging, but it was not different than T staging.
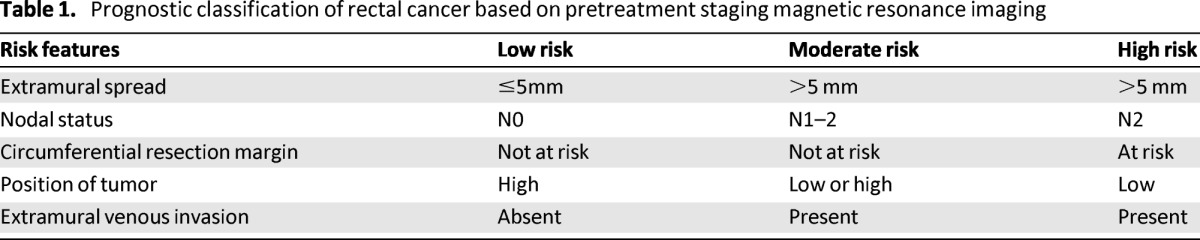
EUS and high-resolution MRI are both acceptable radiographic methods to determine preoperative local tumor stage, although MRI is now standard practice in the U.K. and many European countries. On the basis of MRI staging, patients can be categorized into three prognostic groups according to stage, the predicted relationship of the tumor to the CRM, lymph node status, the degree of extramural spread, and the presence of extramural venous invasion enabling patient selection for preoperative treatment (Table 1).
Table 1.
Prognostic classification of rectal cancer based on pretreatment staging magnetic resonance imaging
Management of Low-Risk Disease
With early stage low-risk rectal cancer (T1-T2N0), surgery alone is associated with 5-year survival rates greater than 90%. Therefore, neoadjuvant treatment is generally reserved for more locally advanced, moderate- to high-risk operable patients (T3–4, N0–2). Transanal excision is possible in some patients with early stage rectal cancer. The degree of submucosal (SM) invasion influences the risk of nodal metastases, increasing from 0%–3% for SM1 to 8%–10% for SM2 and 23%–25% for SM3 [25]. In practice, transanal resection is usually only offered to those with T1 SM1/SM2 tumors that are well to moderately differentiated with no clinical or radiological evidence of lymphadenopathy [26]. Those with T1 SM3 or T2 tumors are at higher risk of local nodal involvement with subsequent higher rates of local recurrence when compared with traditional surgical approaches [27, 28]. Accurate staging and patient selection are essential in this setting. Patients who are understaged and subsequently found to have high-risk features after local excision require consideration of further resection. For those with low-risk disease (>T1 SM2 N0), radical resection with TME should be considered.
Multimodality management is associated with an increase in acute and late toxicity. Moreover, despite a reduction in local recurrence rates, this has not consistently been demonstrated to improve survival. Although acute side effects and surgical complications are only slightly increased following pelvic radiotherapy, long-term toxicity can be more problematic.
As discussed before, for T3 rectal cancer with extramural spread <5 mm without nodal or extramural venous involvement, preoperative treatment may also be spared. However, many patients in this category would have been receiving preoperative or postoperative (chemo)radiation. The long-term side effects of pelvic RT need to be carefully balanced against the possibly marginal benefit over surgery alone.
Moderate-Risk Disease
Historically, patients with moderate-risk disease would be subjects of RCTs to assess pre- and postoperative therapy in locally advanced rectal cancer in conjunction with patients with CRM-threatened rectal cancer. The use of both neoadjuvant short-course RT (25 Gy in five fractions) and long-course chemoradiotherapy (CRT 45–54 Gy in 25–30 fractions with concomitant fluropyrimidine-based chemotherapy) have both shown benefits in local control, but OS has not been improved apart from the Swedish Cancer trial [5], perhaps due to lack of impact on reducing distant recurrences.
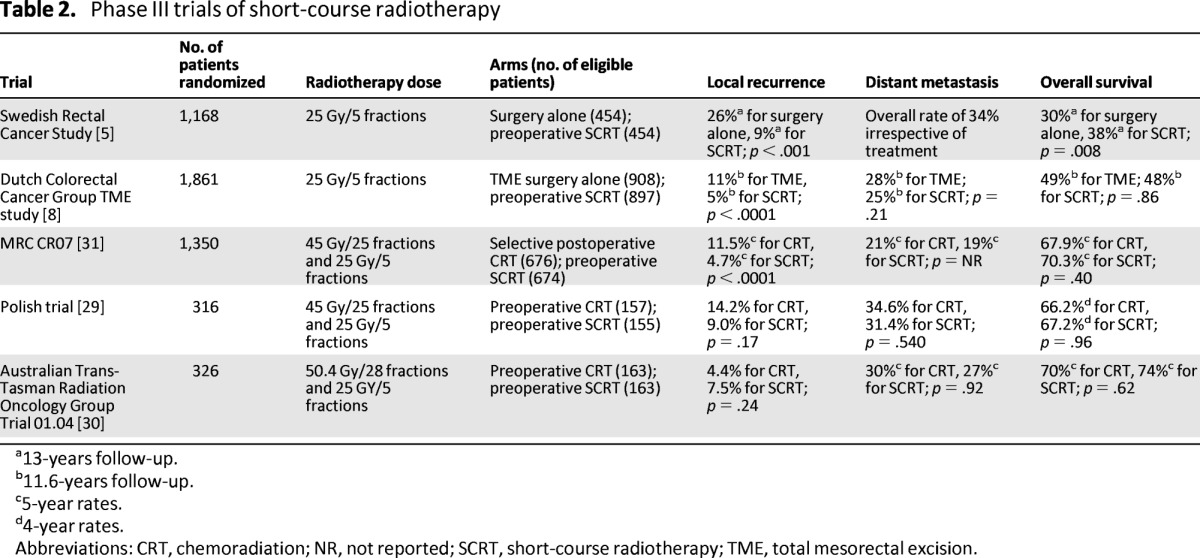
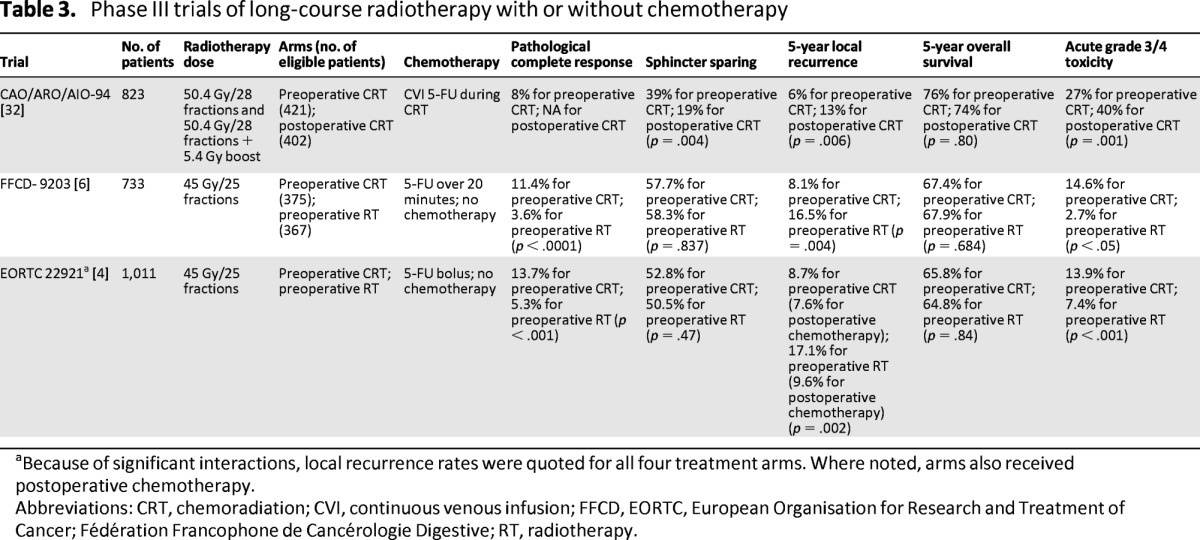
Tables 2 [5, 8, 29–31] and 3 [4, 6, 32] show the recent RCTs in short-course RT and long-course CRT, respectively. However, multimodality management is associated with an increase in acute and late toxicity. Moreover, despite a reduction in local recurrence rates, this has not consistently been demonstrated to improve survival [4–8]. Although acute side effects and surgical complications are only slightly increased following pelvic radiotherapy, long-term toxicity can be more problematic. Increased rates of fecal incontinence, bowel obstruction, and sexual dysfunction have all been reported [33–36]. Importantly, a second cancer may in fact compromise long-term survival; this could widen further with longer term follow-up [8, 37]. In the Dutch TME study, patients who received RT had a 14% second cancer rate versus 9% in the nonirradiated group after a median follow-up of 12 years [8]. The long-term sequelae of pelvic radiotherapy must be therefore considered when making treatment decisions. Subsequently, there remains significant variation in practice for patients with moderate risk disease.
Table 2.
Phase III trials of short-course radiotherapy
a13-years follow-up.
b11.6-years follow-up.
c5-year rates.
d4-year rates.
Abbreviations: CRT, chemoradiation; NR, not reported; SCRT, short-course radiotherapy; TME, total mesorectal excision.
Table 3.
Phase III trials of long-course radiotherapy with or without chemotherapy
aBecause of significant interactions, local recurrence rates were quoted for all four treatment arms. Where noted, arms also received postoperative chemotherapy.
Abbreviations: CRT, chemoradiation; CVI, continuous venous infusion; FFCD, EORTC, European Organisation for Research and Treatment of Cancer; Fédération Francophone de Cancérologie Digestive; RT, radiotherapy.
As shown in Table 2, preoperative short-course radiotherapy improved only OS in the Swedish Rectal Cancer Study when surgery was not optimal; the subsequent Dutch study and the more recent Medical Research Council (MRC) CR07 trial have yet to demonstrate an OS advantage.
Although the use of long-course CRT for patients with high-risk disease is widely accepted as standard practice (Table 3), its use in patients with moderate-risk disease is more variable. The Chirurgische Arbeitsgemeinschaft für Onkologie/Arbeitsgemeinschaft Radiologische Onkologie/Arbeitsgemeinschaft Internistische Onkologie (CAO/ARO/AIO-94) German Rectal Study Group clearly demonstrated that preoperative CRT was superior to postoperative CRT. Patients with T3 or T4 node positive rectal cancer were randomized to receive pre- or postoperative CRT with concurrent infused 5-FU. Local recurrence rates were significantly reduced in the preoperative arm; however, there was no difference in DFS or OS rates [32]. The majority of patients had T3 disease, although the T stage was unknown in 24% of patients and subgroup analysis of benefit by stage was not performed. The improvement in local recurrence with the addition of chemotherapy persisted after 11 years of follow-up (7.1% vs. 10.1% in the pre- and postoperative arms, respectively; p = .048). There remained no significant difference in OS, DFS, or cumulative incidence of distant metastases [7]. Interestingly, the median time to local recurrence was only 18.7 months in the postoperative CRT arm, whereas it was 30.7 months for those after preoperative CRT. Notably, seven local recurrences occurred beyond 5 years of follow-up—five in the preoperative arm and two in the postoperative arm—which suggested that preoperative CRT delayed but did not eliminate local recurrences. No such discrepancy was found in distant metastases between the pre-and postoperative CRT arms, with 7.6% of distant metastases occurring beyond 5 years of follow-up.
Although all three RCTs that established the benefit of preoperative CRT used 5-FU in conjunction with radiotherapy [4, 6, 32], capecitabine has been readily incorporated in CRT in routine clinical practice. Two RCTs comparing capecitabine with 5-FU-based CRT have reported their results. The smaller German study showed that OS was noninferior in the capecitabine arm compared with the fluorouracil group [38]. Interestingly, there was a trend toward improved survival in the capecitabine arm, perhaps because of fewer distant metastases in the capecitabine group. However, there was a significant improvement in DFS in favor of capecitabine. The second U.S. study also compared capecitabine with 5-FU [39]. No significant difference was seen with the pathological complete response (pCR) rate between capecitabine and 5-FU with or without oxaliplatin. The survival outcomes have not yet been reported.
A number of trials have compared the relative benefits of SCRT and CRT. The Medical Research Council (MRC) CR07 trial compared the use of SCRT plus surgery with surgery and selective postoperative CRT reserved for patients with an involved CRM [31]. There was a reduction of 61% in the relative risk of local recurrence for patients receiving preoperative radiotherapy with an absolute difference at 3 years of 6.2%. However, interpretation of this trial is complicated by the relatively small number of patients having selective postoperative CRT (<10%; n = 68) in comparison to those completing preoperative SCRT (n = 624). Although TME surgery was encouraged, it was not mandated. In addition, although surgeons considered TME complete in 93% of patients, histological review confirmed that in fact only 52% of patients had a “good” (mesorectal) excision [31]. However, independent assessment of CRM positivity and the plane of surgery within the CR07 trial demonstrated that the benefit from SCRT radiotherapy was independent of the extent of excision [40]. Although these results strengthen the argument for SCRT, the trial cannot confidently be used as a direct comparison of preoperative SCRT versus postoperative CRT in the post-TME era when <10% of trial patients indeed received postoperative radiation.
Neoadjuvant chemotherapy has demonstrated high radiological response rates in phase II trials. This raises the possibility that CRT could be omitted in a selected group of patients without affecting outcome, thus potentially avoiding radiotherapy-associated toxicity.
A Polish trial compared sphincter preservation rates following SCRT and TME or CRT and TME. The study found that despite significant downsizing and increased pCR rates with CRT, there was no increase in sphincter preservation rate when compared with SCRT [41]. After 4 years of follow-up, there were no differences in local failure, DFS, or OS rates in the SCRT and CRT groups, respectively [29]. Local recurrence rates in the Polish trial were higher in both arms than in other randomized studies using TME. The authors postulated that TME was a relatively new technique at the time of the study, which may have affected the quality of the TME. In addition, there was no pathological control of surgery quality.
A more recently published Australasian trial also compared SCRT with preoperative CRT [30]. Once again, no statistically significant differences were seen in local recurrence, distant metastasis, and survival, although the trend in reduction of local recurrence was in favor of long-course CRT.
Tumor downstaging is not typically seen following SCRT because tumor regression is integrally related to the time interval between treatment and surgery; patients receiving SCRT usually proceed to surgery 7–10 days after completion of radiotherapy. Although there is preliminary data to suggest that SCRT followed by a delay of 6–8 weeks may allow tumor downstaging and be a useful alternative to CRT, this has yet to be validated in a clinical trial setting for patients with advanced disease but comorbidities precluding a longer treatment schedule.
A small Polish study randomized 154 patients to have 5 × 5 Gy preoperative SCRT followed by surgery either 7–10 days or 4–5 weeks after RT [42]. With a minimum follow-up of about 4 years, 5-year survival rates were 63% and 73% for the immediate and delayed surgery groups, respectively (p = .24). The longer time interval between RT and surgery resulted in greater downstaging rate (44.2% vs. 13%), resulting in significantly better survival in this subgroup of patients who experienced downstaging. However, no improvements in other surgical outcomes, such as the rates of sphincter-saving procedures and curative resections, were observed.
The ongoing Stockholm III trial is randomizing patients to either SCRT with immediate (1–10 days) or delayed (4–7 weeks) surgery. An interim analysis performed after 303 patients were recruited demonstrated acceptable compliance. However, the trial has been slow to recruit and final results are still awaited. At present, patients with bulky tumors who require downstaging prior to surgery should be offered CRT.
Neoadjuvant chemotherapy has demonstrated high radiological response rates in phase II trials [43, 44]. This raises the possibility that CRT could be omitted in a selected group of patients without affecting outcome, thus potentially avoiding radiotherapy-associated toxicity. In a single-arm phase II study, selected patients with stage II or III disease (excluding those with T4 or bulky disease) were reassessed following six cycles of neoadjuvant folinic acid, fluorouracil, and oxaliplatin (FOLFOX) and bevacziumab. Patients with a partial or complete response proceeded directly to surgery, with selective postoperative CRT used in those with a positive CRM. In those with stable or progressive disease after chemotherapy, CRT was given. Early results demonstrated that all 31 patients responded to treatment and did not require CRT. Treatment was well-tolerated and resulted in a pCR rate of 27% with no local recurrence observed yet. Longer term follow-up is required to assess the impact on local control, PFS, and OS rates [45].
In another Spanish study Grupo Español Multidisciplinar en Cáncer Digestivo (GEMCAD 0801), patients with moderate-risk disease received neoadjuvant chemotherapy with capecitabine and oxaliplatin (CAPOX) and bevacizumab [46]. Patients without documented disease progression would undergo surgery directly without any RT. Early results showed that 88% of patients had radiological response, resulting in 100% R0 resection and 15% pCR rates. However, both studies are currently in abstract form and the full peer-reviewed publications have not been published. Nevertheless, the first study conducted in the U.S. has led to an U.S. Cooperative Group phase II–III study North Central Cancer Treatment Group (NCCTG-N1048). In this study, 1,060 patients will be randomized to neoadjuvant FOLFOX followed by low anterior resection with TME if ≥20% tumor regression is observed. In those patients with <20% tumor regression after chemotherapy, CRT will be given before surgery. The control group will receive neoadjuvant CRT alone followed by low anterior resection with TME. The primary endpoint for the phase II portion will be pelvic R0 resection rate, whereas disease-free survival will be the primary outcome measure for the phase III trial.
In the planned U.K. phase II BACCHUS (NCT01650428) study, patients with moderate-risk rectal cancers (tumors >4 cm from anal verge with a nonthreatened CRM) will be randomized to receive six cycles of bevacizumab with either FOLFOX or folinic acid, 5-fluorouracil, oxaliplatin, and irinotecan (FOLFOXIRI) followed by TME surgery. In another U.K. phase II trial, COPERNICUS (NCT01263171), investigators aim to assess the role of neoadjuvant chemotherapy followed by SCRT for those with moderate-risk disease.
High-Risk Disease
By definition, patients with high-risk disease have a potentially involved or threatened CRM and are at increased risk of both local and distant recurrence with an associated poorer prognosis. Long-course CRT with a radiation dose of 45–54 Gy and concomitant fluoropyrimidine chemotherapy is now widely accepted as standard practice. However, despite improvements in local recurrence rates from 40% to <10% with the addition of neoadjuvant long-course CRT, distant recurrence remains a significant problem, with 5-year distant metastasis rates of about 35% [4, 32]. Intensification of CRT schedules in an attempt to improve outcomes, with either radiation dose escalation or the use of more effective radiation sensitizers, has been evaluated in a number of clinical trials.
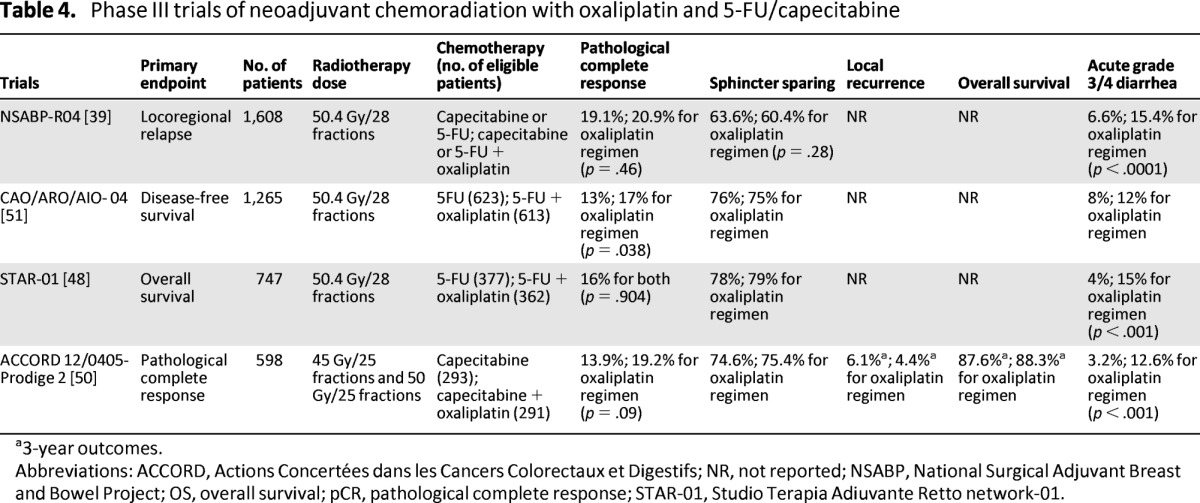
Although preclinical studies found oxaliplatin to be a potent radiosensitizing agent [47] and single-arm noncomparative phase II studies showed pCR rates of 20%–25% with adding oxaliplatin to fluoropyrmidine-based CRT, improvements in efficacy have not been demonstrated in several phase III studies. Table 4 shows the four phase III trials that have evaluated the addition of oxaliplatin to fluoropyrimidine-based CRT [39, 48–51]. Only the German study showed an improvement in pCR in an unplanned exploratory analysis [51]. The other three studies reported no improvements in pCR, as well as increased grade 3/4 toxicities, especially diarrhea [39, 48, 49]. Thus far, only the ACCORD study reported 3-year survival outcomes, but no significant differences have been found between the two treatment arms [50].
Table 4.
Phase III trials of neoadjuvant chemoradiation with oxaliplatin and 5-FU/capecitabine
a3-year outcomes.
Abbreviations: ACCORD, Actions Concertées dans les Cancers Colorectaux et Digestifs; NR, not reported; NSABP, National Surgical Adjuvant Breast and Bowel Project; OS, overall survival; pCR, pathological complete response; STAR-01, Studio Terapia Adiuvante Retto network-01.
A fifth RCT Pan-European Trials in Adjuvant Colon Cancer (PETTAC 6) randomized patients to capecitabine and radiation with or without oxaliplatin; results are not yet available. It is unlikely that results from PETTAC 6 will lead to the widespread incorporation of oxaliplatin to fluoropyrimidine-based CRT given that three large phase III trials of nearly 3,000 patients have failed to demonstrate improvement in pCR. The survival outcomes of these trials are important to determine the role of oxaliplatin-based CRT, but a significant survival effect is unlikely to be observed. Whether this is a consequence of the increased toxicity demonstrated with oxaliplatin, resulting in reduced-dose intensity of the fluropyrimidine or radiation, or whether oxaliplatin is simply not an effective radiosensitizer remains unclear.
A number of phase II trials have demonstrated that irinotecan-based CRT is well-tolerated, with resultant pCR rates of 15%–28%. However, there has been some concern over the rate of surgical complications with the addition of irinotecan to CRT schedules. Rates of grade 3/4 diarrhea in these studies ranged from 11%–32%. The limited data available on surgical morbidity after neoadjuvant CRT with irinotecan suggest an increase in the rate of anastamotic leakage, which clearly needs further evaluation in prospective clinical trials before these regimens can be adopted. The U.K. ARISTOTLE trial of concurrent irinotecan and capecitabine versus capecitabine-based CRT is currently recruiting.
Further intensification of CRT with the addition of targeted agents, including the antiangiogenic antibody bevacizumab and antiepidermal growth factor receptor inhibitors cetuximab and panitumumab, have also been evaluated. Bevacizumab, a monoclonal antibody to vascular endothelial growth factor, has been incorporated into neoadjuvant CRT with variable toxicity and pCR rates (0%–36%) [52–55]. The data are limited by the relatively small numbers of patients in these studies; therefore, conclusions regarding the benefits of the addition of bevacizumab cannot be made at this time.
Incorporation of the anti-epidermal growth factor receptor (EGFR) monoclonal antibodies cetuximab and panitumumab into management of rectal cancer has been evaluated in multiple phase I and II studies based on their proven efficacy in KRAS wild-type patients in the advanced setting [56, 57]. Cetuximab has recognized radiosensitization properties in head and neck cancers. However, to date, phase I and II studies of cetuximab-based CRT in unselected patients with rectal cancer have not shown any improvement in clinical outcomes. A recent pooled analysis of unselected cetuximab-based CRT studies demonstrated pCR rates ranging 0%–20%. The overall pooled pCR for cetuximab-based CRT was 9.1% (29 of 316 patients) [58].
Radiation doses to the pelvis have classically been restricted by concerns over potential small bowel toxicity, sphincter preservation, and the risks of surgical morbidity, including anastomotic dehiscence [59]. Early dose-escalation studies evaluating doses ≥50 Gy in rectal cancer have demonstrated respectable pCR rates. In the French ACCORD12/0405 PRODIGE 2 study, apart from incorporating oxaliplatin to capecitabine RT, the dose of RT was also increased from 45 Gy in the control arm to 50 Gy in the experimental arm [49]. No significant difference was observed in the CAPOX 50 arm; thus, it is unclear whether increasing RT dose would improve survival outcome [50].
Alternative methods of radiotherapy delivery to increase dose to the primary tumor while minimizing dose to the surrounding normal tissue include contact radiotherapy and endorectal brachytherapy. The use of endorectal high-dose rate (HDR) brachytherapy allows a higher dose to be delivered to the primary tumor. With use of a combined approach, doses up to 100 Gy have been delivered without significantly affecting toxicity [60]. In a small French RCT, 88 patients were randomly allocated to be treated with external beam RT (EBRT; 39 Gy in 13 fractions) or the same EBRT plus endocavitary contact radiotherapy boost (85 Gy in 3 fractions). The 10-year rate of permanent colostomy was halved in the contact RT group compared with EBRT alone. A phase III trial of 50.4 Gy in 28 fractions with concomitant uftoral and leucovorin with or without an HDR endorectal boost (10 Gy in two 5-Gy fractions) in patients with high-risk disease demonstrated no difference in pCR. However, the R0 resection rate was significantly increased following the endorectal boost (90% vs. 99%; p = .03) [61].
More recently, intensity-modulated radiotherapy (IMRT) using multiple radiation fields to create highly conformal dose distributions has been evaluated in an attempt to minimize the doses to adjacent critical pelvic structures. This approach has been evaluated in small clinical studies and in a retrospective review, but it has yet to be confirmed in larger studies [62]. IMRT with concurrent capecitabine and oxaliplatin was demonstrated to be feasible in the phase II Radiation Therapy Oncology Group (RTOG) 0822 study [63].
With an optimized dose of radiation and fluropyrimidine, which both serve to maximize local tumor response, there currently appears to be little room for further improvement with additional radiosensitizing agents. However, there remains a need to improve outcomes for patients with high-risk rectal cancer, suggesting that alternative strategies are required to tackle the challenge of reducing distant metastases.
The rationale for neoadjuvant chemotherapy includes downstaging of the primary tumor, reduction of distant recurrence by early initiation of systemic treatment, and early identification of those with aggressive biology who are unlikely to respond. The time from diagnosis to initiation of systemic chemotherapy following SCRT is 6–10 weeks (up to 16–19 weeks following CRT). Given that systemic relapse is responsible for the majority of deaths in patients with rectal cancer, there is a strong clinical rationale for upfront chemotherapy, particularly in patients who are likely to require chemotherapy as part of their treatment strategy.
A number of phase II trials have evaluated the addition of neoadjuvant chemotherapy to the treatment paradigm of localized rectal cancer with encouraging results. A single-arm phase II trial (EXPERT; [Oxaliplatin (Eloxatin) Capecitabine (Xeloda) and preoperative radiotherapy for patients with locally advanced and inoperable rectal cancer]) evaluated neoadjuvant CAPOX followed by CRT and TME in 105 patients with MRI-defined poor-prognosis rectal cancer [43]. Patients received four cycles of CAPOX followed by CRT (54 Gy in 32 fractions) with capecitabine, TME, and 12 weeks of postoperative adjuvant capecitabine. Radiological response rates were 74% after neoadjuvant chemotherapy and 89% after CRT, with a pCR of 20%. The 5-year PFS and OS rates were 64% and 75%, respectively, despite the poor-risk population [43]. These encouraging results were replicated in a recent study from Denmark [64] where two cycles of CAPOX were given before CRT in 84 patients. A pathological CR rate of 25% was observed, with 5-year DFS and OS rates of 63% and 67%, respectively.
A subsequent phase II European multicenter trial (EXPERT-C) evaluated neoadjuvant chemotherapy with the addition of cetuximab to a similar treatment protocol as was used in EXPERT trial [44]. Although the study did not meet the primary endpoint of improving complete response, the addition of cetuximab resulted in a 20% improvement in radiological response to the neoadjuvant chemotherapy. This significant improvement was maintained after CRT. Progression-free survival was similar in both arms; however, there was a significant OS benefit at 3 years in the KRAS wild-type patients receiving cetuximab. These results indicate the potential benefit of systemic treatment prior to local therapy in patients with high-risk rectal cancer and warrants further investigation in patients who would otherwise receive chemotherapy as a component of their postoperative treatment. Indeed, a phase III study has commenced in France, in which 460 patients with at least clinical T3 rectal cancer with risk of local recurrence will be randomized to standard capecitabine-based CRT alone or modified 5-FU/leucovorin/irinotecan/oxaliplatin (FOLFIRINOX) followed by capecitabine-based CRT. Both groups would then undergo TME surgery and further postoperative adjuvant chemotherapy with either FOLFOX or capecitabine depending on TNM and the center's choice.
Biomarkers To Predict Efficacy
Relatively few robust data have elucidated the molecular mechanism of chemoradiosensitivity or resistance. Both high-throughput (whole genome analysis) and low-throughput (single or multibiomarker analyses) investigations have been performed. A comprehensive systematic review of single biomarkers identified several promising predictive biomarkers including EGFR, thymidylate synthase, and p21 [65]. In brief, the main studies evaluating single/multiple biomarkers focused on DNA mutation in the RAS-MAPK pathway, single nucleotide polymorphisms, and various single or multiple immunohistochemical markers. For whole genomic analysis, several gene signatures have been identified, which were differentially expressed between responders and nonresponders to preoperative CRT. These studies have recently been reviewed by Grade et al. [66].
One example of such biomarker analysis would be the evaluation of PI3 kinase mutation in rectal cancer. In a subset of patients (n = 240) enrolled in the Dutch TME study, PIK3CA mutation was found in 7.9% of nonirradiated rectal cancer cases [67]. Tumors with PIK3CA mutations had a significantly higher risk of local recurrence (5-year risk of local recurrence: 27.8% vs. 9.4%; p = .006) compared with those without PIK3CA mutation. Interestingly, there were no differences in distant metastases between the PIK3CA mutant and wild-type patients. In particular, those with an E545K mutation had an aggressive clinical course, with 81% of patients with this type of mutation developing a relapse. However, in the 30 tumor samples out of all 32 patients who developed local recurrence in the irradiated arms, preoperative RT had 3 times greater benefits in the PIK3CA mutants compared with those who were wild type [68]. Thus, PIK3CA mutants might be a group that would require preoperative RT.
There are several major drawbacks of the published studies. For instance, most studies tend to use tissues from retrospectively assembled cohorts without prospectively collected outcomes. A variety of endpoints were used to predict positive successful outcome. Many studies used pathological responses such as pCR or tumor regression grades, whereas others used survival outcomes such as DFS or OS. The definition of “response” and “resistance” differed among published studies. Often, the studies had small sample sizes, which greatly limited the statistical power to detect smaller but clinically relevant differences. This was especially true when multiple biomarkers were examined—even more so with gene expression profiling, for which the multidimensionality of data posed further problems in their interpretation.
Recent technological advances in biomarker discovery and validation bring much excitement as well as challenges. Whole exome sequencing using next-generation sequencing (NGS) will allow rare mutations to be identified. The sensitivity of detecting small quantities of known DNA mutations will be improved by digital polymerase chain reaction technology. However, there are further logistic challenges in future prospective studies in rectal cancer. Firstly, current CRT regimens result in pCR rates of 15%–20%. Therefore, in approximately one fifth of patients, the only tissue available for such biomarker studies would be from the original diagnostic biopsy, which often is of relatively minute amount of available material. Resection specimens after CRT would not have viable tumors in these cases; however, it is usually this particular cohort of patients who achieved pCR that would constitute the main group of interest for chemoradiosensitivity. Secondly, patients often participate in multiple clinical trials throughout their cancer journey. Each individual trial may request archival tissues, which means that the tissues soon would be exhausted for further testing. Thus, it is vital to perform these biomarker studies in real time rather than waiting for the entire clinical trial to finish recruitment. Thirdly, many multicenter clinical trials are now part of an international collaboration. Each country and each institution may have individual regulations/guidelines with regard to storage and release of tissues. Retrieval of tissues from other countries would potentially be problematic.
Aside from tissue-based biomarkers, imaging-based biomarkers have also been examined. Recently, the MERCURY group published data suggesting that an MRI-assessed tumor regression grade (mrTRG) based on the degree of low signal intensity appearances of fibrosis can predict for improved outcomes following CRT in patients with rectal cancer. In this study, the 5-year survival rate for patients with poor mrTRG was 27% compared with 72% for those with good mrTRG (p = .001); the DFS rates were 31% versus 64% (p = .007), respectively [69].
Conclusions
Although accurate staging and the use of high-resolution MRI have allowed stratification of patients with locally advanced rectal cancers, current multimodal neoadjuvant treatment has failed to significantly improve OS. For patients with low-risk disease, surgery alone remains an appropriate option. For patients with moderate-risk disease, neoadjuvant SCRT or CRT are accepted as standard of care. However, further investigation is needed to determine if patients with moderate-risk disease may be considered for neoadjuvant chemotherapy alone, followed by surgery, which would avoid unnecessary RT-associated toxicity. Conversely, patients with high-risk disease remain in need of intensified treatment to improve long-term outcomes; the role of neoadjuvant chemotherapy in addition to CRT warrants further evaluation in these patients. These hypotheses clearly require evaluation in clinical trial settings, ideally incorporating modern imaging techniques and molecular biomarkers to allow accurate prediction and assessment of response.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgments
This study was funded by the National Institute for Health Research Biomedical Research Centre and the Peter Stebbings Memorial Charity.
Author Contributions
Conception/Design: Alice Dewdney, David Cunningham, Ian Chau
Data analysis and interpretation: Alice Dewdney, David Cunningham, Ian Chau
Manuscript writing: Alice Dewdney, David Cunningham, Ian Chau
Final approval of manuscript: Alice Dewdney, David Cunningham, Ian Chau
Disclosures
David Cunningham: Amgen, Merck (RF); The Royal Marsden NHS Foundation Trust (E). The other authors indicated no financial relationships.
Section editors: Richard Goldberg: Amgen, Bayer, Genentech, Genomic Health, Lilly, Sanofi (C/A); Amgen, Bayer, Genentech, Myriad, Sanofi, Enzon (RF); Patrick Johnston: Almac Diagnostics (E), Chugai Pharmaceuticals, Pfizer, Sanofi, Roche (H), AstraZeneca, Amgen (RF); Almac Diagnostics, Fusion Antibodies (O); Peter O'Dwyer: Tetralogic Pharmaceuticals, PrECOG, AstraZeneca, Sanofi, Topotarget (C/A); Pfizer, Bristol-Myers Squibb, Methylgene, Novartis, Genentech, Ardea, Exelixis, FibroGen, AstraZeneca, Incyte, ArQule, GlaxoSmithKline (RF); Genentech, Bristol-Myers Squibb, Pfizer (H); Tetralogic Pharmaceuticals (O)
Reviewer “A”: UpToDate, Inc. (H)
Reviewer “B”: Roche (H)
C/A: Consulting/advisory relationship; RF: Research funding; E: Employment; H: Honoraria received; OI: Ownership interests; IP: Intellectual property rights/inventor/patent holder; SAB: scientific advisory board
Reference
- 1.Heald RJ, Moran BJ, Ryall RD, et al. Rectal cancer: The Basingstoke experience of total mesorectal excision, 1978–1997. Arch Surg. 1998;133:894–899. doi: 10.1001/archsurg.133.8.894. [DOI] [PubMed] [Google Scholar]
- 2.Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol. 2008;26:303–312. doi: 10.1200/JCO.2007.12.7027. [DOI] [PubMed] [Google Scholar]
- 3.MERCURY Study Group. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: Prospective observational study. BMJ. 2006;333:779. doi: 10.1136/bmj.38937.646400.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–1123. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 5.Folkesson J, Birgisson H, Pahlman L, et al. Swedish Rectal Cancer Trial: Long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol. 2005;23:5644–5650. doi: 10.1200/JCO.2005.08.144. [DOI] [PubMed] [Google Scholar]
- 6.Gerard JP, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3–4 rectal cancers: Results of FFCD 9203. J Clin Oncol. 2006;24:4620–4625. doi: 10.1200/JCO.2006.06.7629. [DOI] [PubMed] [Google Scholar]
- 7.Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926–1933. doi: 10.1200/JCO.2011.40.1836. [DOI] [PubMed] [Google Scholar]
- 8.van Gijn W, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575–582. doi: 10.1016/S1470-2045(11)70097-3. [DOI] [PubMed] [Google Scholar]
- 9.Merkel S, Mansmann U, Siassi M, et al. The prognostic inhomogeneity in pT3 rectal carcinomas. Int J Colorectal Dis. 2001;16:298–304. doi: 10.1007/s003840100309. [DOI] [PubMed] [Google Scholar]
- 10.Gunderson LL, Sargent DJ, Tepper JE, et al. Impact of T and N stage and treatment on survival and relapse in adjuvant rectal cancer: A pooled analysis. J Clin Oncol. 2004;22:1785–1796. doi: 10.1200/JCO.2004.08.173. [DOI] [PubMed] [Google Scholar]
- 11.Gunderson LL, Jessup JM, Sargent DJ, et al. Revised tumor and node categorization for rectal cancer based on surveillance, epidemiology, and end results and rectal pooled analysis outcomes. J Clin Oncol. 2010;28:256–263. doi: 10.1200/JCO.2009.23.9194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dresen RC, Peters EE, Rutten HJ, et al. Local recurrence in rectal cancer can be predicted by histopathological factors. Eur J Surg Oncol. 2009;35:1071–1077. doi: 10.1016/j.ejso.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Ouchi K, Sugawara T, Ono H, et al. Histologic features and clinical significance of venous invasion in colorectal carcinoma with hepatic metastasis. Cancer. 1996;78:2313–2317. doi: 10.1002/(sici)1097-0142(19961201)78:11<2313::aid-cncr7>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 14.Smith NJ, Barbachano Y, Norman AR, et al. Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer. Br J Surg. 2008;95:229–236. doi: 10.1002/bjs.5917. [DOI] [PubMed] [Google Scholar]
- 15.den DM, Putter H, Collette L, et al. The abdominoperineal resection itself is associated with an adverse outcome: The European experience based on a pooled analysis of five European randomised clinical trials on rectal cancer. Eur J Cancer. 2009;45:1175–1183. doi: 10.1016/j.ejca.2008.11.039. [DOI] [PubMed] [Google Scholar]
- 16.Puli SR, Bechtold ML, Reddy JB, et al. Can endoscopic ultrasound predict early rectal cancers that can be resected endoscopically? A meta-analysis and systematic review. Dig Dis Sci. 2010;55:1221–1229. doi: 10.1007/s10620-009-0862-9. [DOI] [PubMed] [Google Scholar]
- 17.Puli SR, Reddy JB, Bechtold ML, et al. Accuracy of endoscopic ultrasound to diagnose nodal invasion by rectal cancers: A meta-analysis and systematic review. Ann Surg Oncol. 2009;16:1255–1265. doi: 10.1245/s10434-009-0337-4. [DOI] [PubMed] [Google Scholar]
- 18.Puli SR, Bechtold ML, Reddy JB, et al. How good is endoscopic ultrasound in differentiating various T stages of rectal cancer? Meta-analysis and systematic review. Ann Surg Oncol. 2009;16:254–265. doi: 10.1245/s10434-008-0231-5. [DOI] [PubMed] [Google Scholar]
- 19.Extramural depth of tumor invasion at thin-section MR in patients with rectal cancer: Results of the MERCURY Study. Radiology. 2007;243:132–139. doi: 10.1148/radiol.2431051825. [DOI] [PubMed] [Google Scholar]
- 20.Taylor FG, Quirke P, Heald RJ, et al. Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II, and III rectal cancer best managed by surgery alone: A prospective, multicenter, European study. Ann Surg. 2011;253:711–719. doi: 10.1097/SLA.0b013e31820b8d52. [DOI] [PubMed] [Google Scholar]
- 21.Taylor FG, Quirke P, Heald RJ, et al. One millimetre is the safe cut-off for magnetic resonance imaging prediction of surgical margin status in rectal cancer. Br J Surg. 2011;98:872–879. doi: 10.1002/bjs.7458. [DOI] [PubMed] [Google Scholar]
- 22.Shihab OC, Taylor F, Bees N, et al. Relevance of magnetic resonance imaging-detected pelvic sidewall lymph node involvement in rectal cancer. Br J Surg. 2011;98:1798–1804. doi: 10.1002/bjs.7662. [DOI] [PubMed] [Google Scholar]
- 23.Pedersen BG, Moran B, Brown G, et al. Reproducibility of depth of extramural tumor spread and distance to circumferential resection margin at rectal MRI: Enhancement of clinical guidelines for neoadjuvant therapy. AJR Am J Roentgenol. 2011;197:1360–1366. doi: 10.2214/AJR.11.6508. [DOI] [PubMed] [Google Scholar]
- 24.Al-Sukhni E, Milot L, Fruitman M, et al. Diagnostic accuracy of MRI for assessment of T category, lymph node metastases, and circumferential resection margin involvement in patients with rectal cancer: A systematic review and meta-analysis. Ann Surg Oncol. 2012;19:2212–2223. doi: 10.1245/s10434-011-2210-5. [DOI] [PubMed] [Google Scholar]
- 25.Kikuchi R, Takano M, Takagi K, et al. Management of early invasive colorectal cancer. Risk of recurrence and clinical guidelines. Dis Colon Rectum. 1995;38:1286–1295. doi: 10.1007/BF02049154. [DOI] [PubMed] [Google Scholar]
- 26.Baxter NN, Garcia-Aguilar J. Organ preservation for rectal cancer. J Clin Oncol. 2007;25:1014–1020. doi: 10.1200/JCO.2006.09.7840. [DOI] [PubMed] [Google Scholar]
- 27.Peng J, Chen W, Venook AP, et al. Long-term outcome of early-stage rectal cancer undergoing standard resection and local excision. Clin Colorectal Cancer. 2011;10:37–41. doi: 10.3816/CCC.2011.n.005. [DOI] [PubMed] [Google Scholar]
- 28.Wu ZY, Zhao G, Chen Z, et al. Oncological outcomes of transanal local excision for high risk T(1) rectal cancers. World J Gastrointest Oncol. 2012;4:84–88. doi: 10.4251/wjgo.v4.i4.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg. 2006;93:1215–1223. doi: 10.1002/bjs.5506. [DOI] [PubMed] [Google Scholar]
- 30.Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group Trial 01.04. J Clin Oncol. 2012;30:3827–3833. doi: 10.1200/JCO.2012.42.9597. [DOI] [PubMed] [Google Scholar]
- 31.Sebag-Montefiore D, Stephens RJ, Steele R, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): A multicentre, randomised trial. Lancet. 2009;373:811–820. doi: 10.1016/S0140-6736(09)60484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 33.Braendengen M, Tveit KM, Bruheim K, et al. Late patient-reported toxicity after preoperative radiotherapy or chemoradiotherapy in nonresectable rectal cancer: Results from a randomized Phase III study. Int J Radiat Oncol Biol Phys. 2011;81:1017–1024. doi: 10.1016/j.ijrobp.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Marijnen CA, Van de Velde CJ, Putter H, et al. Impact of short-term preoperative radiotherapy on health-related quality of life and sexual functioning in primary rectal cancer: Report of a multicenter randomized trial. J Clin Oncol. 2005;23:1847–1858. doi: 10.1200/JCO.2005.05.256. [DOI] [PubMed] [Google Scholar]
- 35.Peeters KC, Van de Velde CJ, Leer JW, et al. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: Increased bowel dysfunction in irradiated patients—A Dutch colorectal cancer group study. J Clin Oncol. 2005;23:6199–6206. doi: 10.1200/JCO.2005.14.779. [DOI] [PubMed] [Google Scholar]
- 36.Stephens RJ, Thompson LC, Quirke P, et al. Impact of short-course preoperative radiotherapy for rectal cancer on patients' quality of life: Data from the Medical Research Council CR07/National Cancer Institute of Canada Clinical Trials Group C016 randomized clinical trial. J Clin Oncol. 2010;28:4233–4239. doi: 10.1200/JCO.2009.26.5264. [DOI] [PubMed] [Google Scholar]
- 37.Birgisson H, Pahlman L, Gunnarsson U, et al. Occurrence of second cancers in patients treated with radiotherapy for rectal cancer. J Clin Oncol. 2005;23:6126–6131. doi: 10.1200/JCO.2005.02.543. [DOI] [PubMed] [Google Scholar]
- 38.Hofheinz RD, Wenz F, Post S, et al. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: A randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol. 2012;13:579–588. doi: 10.1016/S1470-2045(12)70116-X. [DOI] [PubMed] [Google Scholar]
- 39.Roh MS, Yothers GA, O'Connell MJ, et al. The impact of capecitabine and oxaliplatin in the preoperative multimodality treatment in patients with carcinoma of the rectum: NSABP R-04. J Clin Oncol. 2011;29:3503. [Google Scholar]
- 40.Quirke P, Steele R, Monson J, et al. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: A prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet. 2009;373:821–828. doi: 10.1016/S0140-6736(09)60485-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Sphincter preservation following preoperative radiotherapy for rectal cancer: Report of a randomised trial comparing short-term radiotherapy vs. conventionally fractionated radiochemotherapy. Radiother Oncol. 2004;72:15–24. doi: 10.1016/j.radonc.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 42.Pach R, Kulig J, Richter P, et al. Randomized clinical trial on preoperative radiotherapy 25 Gy in rectal cancer–treatment results at 5-year follow-up. Langenbecks Arch Surg. 2012;397:801–807. doi: 10.1007/s00423-011-0890-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chua YJ, Barbachano Y, Cunningham D, et al. Neoadjuvant capecitabine and oxaliplatin before chemoradiotherapy and total mesorectal excision in MRI-defined poor-risk rectal cancer: A phase 2 trial. Lancet Oncol. 2010;11:241–248. doi: 10.1016/S1470-2045(09)70381-X. [DOI] [PubMed] [Google Scholar]
- 44.Dewdney A, Cunningham D, Tabernero J, et al. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C) J Clin Oncol. 2012;30:1620–1627. doi: 10.1200/JCO.2011.39.6036. [DOI] [PubMed] [Google Scholar]
- 45.Schrag D, Weiser MR, Goodman KA, et al. Neoadjuvant FOLFOX-bev, without radiation, for locally advanced rectal cancer. J Clin Oncol. 2010;28:3511. [Google Scholar]
- 46.Fernandez-Martos C, Estevan R, Salud A, et al. Neoadjuvant capecitabine, oxliplatin, and bevacizumab (CAPOX-B) in intermediate-risk rectal cancer (RC) patients defined by magnetic resonance (MR): GEMCAD 0801 trial. J Clin Oncol. 2012;30:3586. [Google Scholar]
- 47.Cividalli A, Ceciarelli F, Livdi E, et al. Radiosensitization by oxaliplatin in a mouse adenocarcinoma: Influence of treatment schedule. Int J Radiat Oncol Biol Phys. 2002;52:1092–1098. doi: 10.1016/s0360-3016(01)02792-4. [DOI] [PubMed] [Google Scholar]
- 48.Aschele C, Cionini L, Lonardi S, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: Pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011;29:2773–2780. doi: 10.1200/JCO.2010.34.4911. [DOI] [PubMed] [Google Scholar]
- 49.Gerard JP, Azria D, Gourgou-Bourgade S, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: Results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol. 2010;28:1638–1644. doi: 10.1200/JCO.2009.25.8376. [DOI] [PubMed] [Google Scholar]
- 50.Gerard JP, Azria D, Gourgou-Bourgade S, et al. Clinical outcome of the ACCORD 12/0405 PRODIGE 2 randomized trial in rectal cancer. J Clin Oncol. 2012;30:4558–4565. doi: 10.1200/JCO.2012.42.8771. [DOI] [PubMed] [Google Scholar]
- 51.Rodel C, Liersch T, Becker H, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: Initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol. 2012;13:679–687. doi: 10.1016/S1470-2045(12)70187-0. [DOI] [PubMed] [Google Scholar]
- 52.Crane CH, Eng C, Feig BW, et al. Phase II trial of neoadjuvant bevacizumab, capecitabine, and radiotherapy for locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2010;76:824–830. doi: 10.1016/j.ijrobp.2009.02.037. [DOI] [PubMed] [Google Scholar]
- 53.Nogue M, Salud A, Vicente P, et al. Addition of bevacizumab to XELOX induction therapy plus concomitant capecitabine-based chemoradiotherapy in magnetic resonance imaging-defined poor-prognosis locally advanced rectal cancer: The AVACROSS study. The Oncologist. 2011;16:614–620. doi: 10.1634/theoncologist.2010-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Willett CG, Boucher Y, Duda DG, et al. Surrogate markers for antiangiogenic therapy and dose-limiting toxicities for bevacizumab with radiation and chemotherapy: Continued experience of a phase I trial in rectal cancer patients. J Clin Oncol. 2005;23:8136–8139. doi: 10.1200/JCO.2005.02.5635. [DOI] [PubMed] [Google Scholar]
- 55.Willett CG, Duda DG, di TE, et al. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: A multidisciplinary phase II study. J Clin Oncol. 2009;27:3020–3026. doi: 10.1200/JCO.2008.21.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bokemeyer C, Bondarenko I, Hartmann JT, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: The OPUS study. Ann Oncol. 2011;22:1535–1546. doi: 10.1093/annonc/mdq632. [DOI] [PubMed] [Google Scholar]
- 57.Van Cutsem E, Kohne CH, Lang I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: Updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011–2019. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- 58.Glynne-Jones R, Mawdsley S, Harrison M. Cetuximab and chemoradiation for rectal cancer—Is the water getting muddy? Acta Oncol. 2010;49:278–286. doi: 10.3109/02841860903536010. [DOI] [PubMed] [Google Scholar]
- 59.Cummings BJ. Is there a limit to dose escalation for rectal cancer? Clin Oncol (R Coll Radiol) 2007;19:730–737. doi: 10.1016/j.clon.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 60.Ortholan C, Romestaing P, Chapet O, et al. Correlation in rectal cancer between clinical tumor response after neoadjuvant radiotherapy and sphincter or organ preservation: 10-year results of the Lyon R 96–02 randomized trial. Int J Radiat Oncol Biol Phys. 2012;83:e165–e171. doi: 10.1016/j.ijrobp.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 61.Jakobsen AKM, Appelt AL, Lindebjerg J, et al. The dose-effect relationship in preoperative chemoradiation of locally advanced rectal cancer: Preliminary results of a phase III trial. J Clin Oncol. 2011;29:3512. [Google Scholar]
- 62.Samuelian JM, Callister MD, Ashman JB, et al. Reduced acute bowel toxicity in patients treated with intensity-modulated radiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2012;82:1981–1987. doi: 10.1016/j.ijrobp.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 63.Garofalo M, Moughan J, Hong T, et al. RTOG 0822: A phase II study of preoperative (PREOP) chemoradiotherapy (CRT) utilizing IMRT in combination with capecitabine (C) and oxaliplatin (O) for patients with locally advanced rectal cancer. Int J Rad Oncol Biol Phys. 2011;81:S3–S4. [Google Scholar]
- 64.Schou JV, Larsen FO, Rasch L, et al. Induction chemotherapy with capecitabine and oxaliplatin followed by chemoradiotherapy before total mesorectal excision in patients with locally advanced rectal cancer. Ann Oncol. 2012;23:2627–2633. doi: 10.1093/annonc/mds056. [DOI] [PubMed] [Google Scholar]
- 65.Kuremsky JG, Tepper JE, McLeod HL. Biomarkers for response to neoadjuvant chemoradiation for rectal cancer. Int J Radiat Oncol Biol Phys. 2009;74:673–688. doi: 10.1016/j.ijrobp.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 66.Grade M, Wolff HA, Gaedcke J, et al. The molecular basis of chemoradiosensitivity in rectal cancer: implications for personalized therapies. Langenbecks Arch Surg. 2012;397:543–555. doi: 10.1007/s00423-012-0929-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.He Y, Van't Veer LJ, Mikolajewska-Hanclich I, et al. PIK3CA mutations predict local recurrences in rectal cancer patients. Clin Cancer Res. 2009;15:6956–6962. doi: 10.1158/1078-0432.CCR-09-1165. [DOI] [PubMed] [Google Scholar]
- 68.He Y, Van't Veer LJ, Lopez-Yurda M, et al. Do rectal cancer patients with PIK3CA mutations benefit from preoperative radiotherapy with regard to local recurrences? Clin Cancer Res. 2010;16:6179. doi: 10.1158/1078-0432.CCR-10-1437. [DOI] [PubMed] [Google Scholar]
- 69.Patel UB, Taylor F, Blomqvist L, et al. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol. 2011;29:3753–3760. doi: 10.1200/JCO.2011.34.9068. [DOI] [PubMed] [Google Scholar]


