This study assessed the activity and toxicity of primary carboplatin-based chemoradiotherapy (CarboRT) and compared CarboRT with cisplatin-based chemoradiotherapy (CisRT) in patients with locally advanced cervical cancer and poor general condition. CarboRT was better tolerated than CisRT without compromising tumor response and survival in these patients.
Keywords: Cervical cancer, Carboplatin, Cisplatin, Chemoradiotherapy
Abstract
Purpose.
The aim of this study was to assess the activity and toxicity of primary carboplatin-based chemoradiotherapy (CarboRT) and to compare CarboRT with cisplatin-based chemoradiotherapy (CisRT) in patients with locally advanced cervical cancer and poor general condition.
Patients and Methods.
Fifty-one locally advanced cervical cancer patients with morbidity risks were prospectively enrolled between January 2007 and April 2010. Eligible patients received weekly intravenous CarboRT with carboplatin 100 mg/m2, and a comparison was made with a historical patient group that received weekly CisRT with cisplatin 40 mg/m2.
Results.
Median follow-up was 36 months (range: 4–66 months) in the CarboRT group and 53 months (range: 4–121 months) in the CisRT group. Compared with the historical CisRT group, the CarboRT group showed no statistically significant differences in recurrence (hazard ratio [HR], 1.21; 95% confidence interval [CI], 0.52–2.81) and survival (HR, 1.80; 95% CI, 0.49–6.54). The mean numbers of received cycles of CarboRT and CisRT were 7.5 ± 1.4 and 6.0 ± 1.8, respectively (p < .001). The rates of grade 3–4 toxicity were similar in the two groups.
Conclusions.
CarboRT was better tolerated than CisRT without compromising tumor response and survival in patients with locally advanced cervical cancer and poor general condition.
Implications for Practice:
Cisplatin-based chemoradiotherapy (CisRT) has been regarded as the standard treatment for patients with locally advanced cervical cancer. Cisplatin, however, can cause nephrotoxicity, has highly emetogenic effects, and creates the need for a large amount of hydration. Carboplatin-based chemoradiotherapy was better tolerated than CisRT without compromising tumor response and survival in patients with locally advanced cervical cancer and poor general condition.
Introduction
Cervical cancer is the third most common cancer among females and has the fifth highest mortality among female cancers in Korea [1]. A 2010 National Cancer Institute (NCI) clinical alert recommended that concomitant chemoradiotherapy should be considered instead of radiotherapy alone for the treatment of women with cervical cancer [2]. After the publication of several randomized clinical trials, cisplatin-based chemoradiotherapy (CisRT) is now regarded as the standard treatment for patients with locally advanced cervical cancer [3–6]. An addition of cisplatin to radiotherapy improves survival and raises local control rates up to 80% [3–6]; however, there has been considerable controversy regarding optimal drugs, dosage, timing, and duration of chemotherapy concurrent with radiation [2]. Although weekly CisRT is relatively well tolerated, cisplatin's potential nephrotoxicity, highly emetogenic effects, and creation of need for a large amount of hydration could result in hesitation over its use, particularly in patients with renal dysfunction, such as those with ureteral obstruction in advanced cervical cancer.
Carboplatin is a platinum analog that was introduced in 1981 and has been noted for its reduced toxicity and its equivalent biochemical selectivity and antitumor spectrum relative to cisplatin [7]. Carboplatin is also an effective radiosensitizer both in vivo and in vitro, targeting hypoxic cell populations and potentiating cell killing by radiation [8, 9]. Compared with cisplatin, carboplatin is now used as an effective treatment regimen with an improved toxicity profile in ovarian cancer patients [10]. Despite this evidence, only a few small clinical trials of carboplatin-based chemoradiotherapy (CarboRT) in cervical cancer patients have been reported [11–15]. To our knowledge, there have been no reports comparing CarboRT with CisRT. We initiated this study to evaluate the activity and toxicity of primary CarboRT compared with the activity and toxicity of CisRT in locally advanced cervical cancer patients with morbidity risks.
Materials and Methods
Study Design
We conducted a registry-based study of locally advanced cervical cancer patients with morbidity risks who received CarboRT between January 2007 and April 2010 at a single institution The patients who received CarboRT were recruited prospectively, and comparisons were made to a historical group of patients who received weekly CisRT by using frequency matching (Fig. 1). A regional institutional review board approved the protocol, and informed consent was obtained before treatment.
Figure 1.
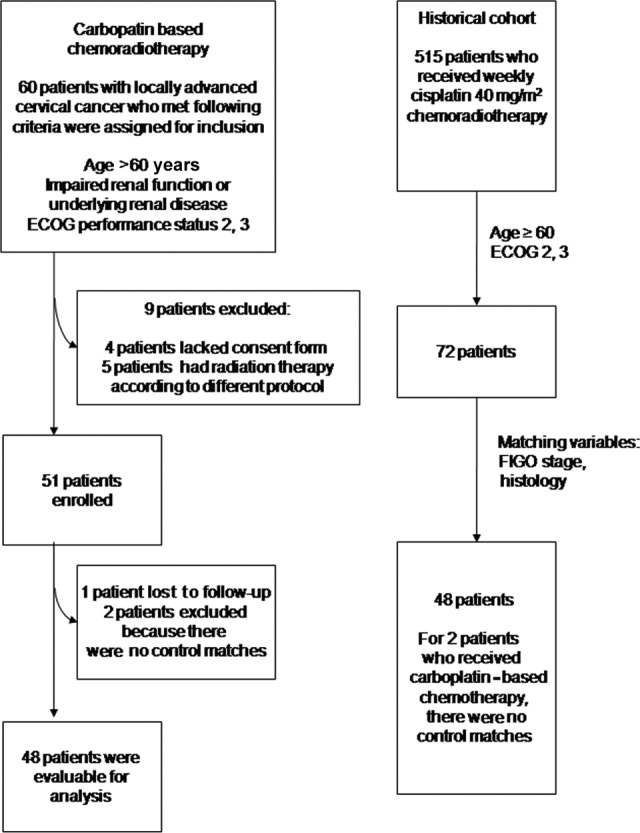
Study design.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; FIGO, International Federation of Gynecology and Obstetrics.
The primary objectives were to evaluate the tumor response rate (RR) and progression-free survival (PFS), and the secondary endpoints were toxicity and overall survival (OS).
Patients
Eligibility criteria included patients who had histologically proven, locally advanced cervical cancer with no prior history of surgery, chemotherapy, or radiotherapy for cervical cancer and who had morbidity risks. Morbidity risks were defined as having one of the following conditions: age greater than 60 years; impaired renal function or underlying renal disease; or poor performance status, which was defined as an Eastern Cooperative Oncology Group (ECOG) performance status of 2 or 3. To be considered eligible for chemotherapy, patients had to have minimum creatinine clearance of 40 mL per minute for CarboRT and 50 mL per minute for CisRT. The rationale for enrollment of patients as outlined was that these conditions are approved by the Korean National Health Insurance for use of carboplatin instead of cisplatin as a chemoradiotherapy regimen. In addition, we speculated that the validity of the toxicity evaluation would be increased in patients with morbidity risks.
At the time of enrollment in the CarboRT group, all patients were required to have adequate bone marrow function, as demonstrated by a white blood cell count ≥3,000 cells per mm3, neutrophil count ≥1,500 cells per mm3, platelet count ≥75,000 cells per mm3, and hemoglobin ≥9.0 g/dL. Other required laboratory criteria included serum bilirubin level ≤2.0 mg/dL, serum aspartate aminotransferase or alanine aminotransferase and alkaline phosphatase levels ≤2 times the upper limit of normal. We excluded patients who had received chemotherapy or radiotherapy within 4 weeks of the beginning of the trial and those who had another malignant disease combined with cervical cancer.
Patients were matched with a similar number of patients selected from 515 patients with locally advanced cervical cancer who received primary CisRT at our institution between January 2002 and February 2009. Patients were matched for age, stage, histologic subtype, and ECOG status (Fig. 1).
Treatment and Follow-up
A previous large randomized phase III study showed that chemoradiotherapy using weekly intravenous (i.v.) carboplatin 100 mg/m2 was effective and had acceptable toxicity [16]. Consequently, in the current study, weekly i.v. carboplatin 100 mg/m2 was administered concurrently with radiotherapy. The CisRT group had received weekly i.v. cisplatin 40 mg/m2 with concurrent radiotherapy. Chemotherapy started at the beginning of radiotherapy. Patients were scheduled to have at least six cycles of chemotherapy, and the duration of chemotherapy could be extended to nine cycles until radiotherapy was finished if the treatment was tolerated and if the patients agreed to the extension.
Radiotherapy involved a combination of external irradiation and high-dose-rate intracavitary irradiation applied using a remote after-loading system with iridium 192 as its source (Gamma-Med II, Mick Radio-Nucelar Instrument, New York, USA). External whole-pelvis irradiation was performed five times per week using a dose of 1.8 Gy per fraction and a midline dose of 27–36 Gy. This was followed by a high-dose-rate intracavitary irradiation with six insertions (twice per week) and fraction doses from 5 Gy to a total dose of 30 Gy at point A. The next week, after completing the intracavitary irradiation, patients were administered a second course of external irradiation with central shielding to a total external beam dose of 45–50.4 Gy [17] in both the CarboRT and CisRT groups. No chemotherapy was given within 48 hours of brachytherapy. Patients with metastatic lymph nodes received a boost of radiation to those nodes.
Evaluation of Response and Toxicity
During chemoradiotherapy, patients were monitored weekly on the day prior to chemotherapy with complete physical examination and laboratory tests, including complete blood cell count, relevant blood chemistry, and 24-hour urine creatinine clearance. Patients that had received at least two cycles of chemotherapy were evaluated to determine treatment efficacy. At the follow-up visit 2 months after radiotherapy, the response to treatment was assessed using the Response Evaluation Criteria in Solid Tumors [18]. Acute toxicities were defined as those occurring between the start of treatment and 90 days after its completion and were graded according to the NCI Common Toxicity Criteria version 3.0. Depending on the severity and duration of toxicity, the ongoing treatment schedule was delayed or stopped.
Follow-up after the completion of chemoradiotherapy consisted of visits every 3 months for the first 2 years, then every 6 months for the following 3 years, and annually thereafter for both the CarboRT and CisRT groups. Physical examination and cervical cytology were performed at each follow-up visit. Imaging studies, including abdominopelvic computed tomography (CT), magnetic resonance imaging (MRI), or positron emission tomography/CT (PET/CT), were obtained every 6 months for the first 2 years and annually thereafter. PFS was defined as the interval between the date that treatment began and the date of documented disease progression or death from any cause. OS was defined as the interval between the date treatment began and the date of death from any cause. If a patient was lost to follow-up, that patient was censored as of the date of last contact. Two group comparisons were carried out using a nonparametric Wilcoxon two-group test (SPSS version 18.0; IBM, New York, USA). The Kaplan-Meier method was used to calculate estimates for PFS and OS. The log-rank test was used to compare PFS and OS between the CarboRT and CisRT groups. Cox regression was used to calculate the hazard ratios (HRs) between these two groups for PFS and OS.
Results
Patient Characteristics
Between January 2007 and April 2010, 60 patients were eligible for the study. We excluded four patients who lacked a consent form, and five patients had radiotherapy according to a different protocol. Consequently, 51 patients were enrolled in the CarboRT group. Patients who received CarboRT were matched with 48 patients who were selected from 515 patients who previously received CisRT at our institution. Table 1 outlines the patient characteristics. The mean tumor diameter was 45.2 ± 16.4 mm in the CarboRT group and 40.8 ± 20.2 mm in the CisRT group (p = .24). Three patients in the CarboRT group (5.9%) and four patients in the CisRT group (8.3%) had adenocarcinoma. There were no statistically significant differences in mean age, mean body mass index, distribution of ECOG score, stage, or lymph node metastasis status between the two groups. Lymph node metastasis status was evaluated before treatment by an imaging study, such as MRI, CT, or PET/CT, and pelvic or para-aortic lymph node metastasis was noted in 22 patients (43.1%) in the CarboRT group and in 27 patients (56.2%) in the CisRT group. Isolated para-aortic lymph node metastasis was not found during this study.
Table 1.
Patient characteristics
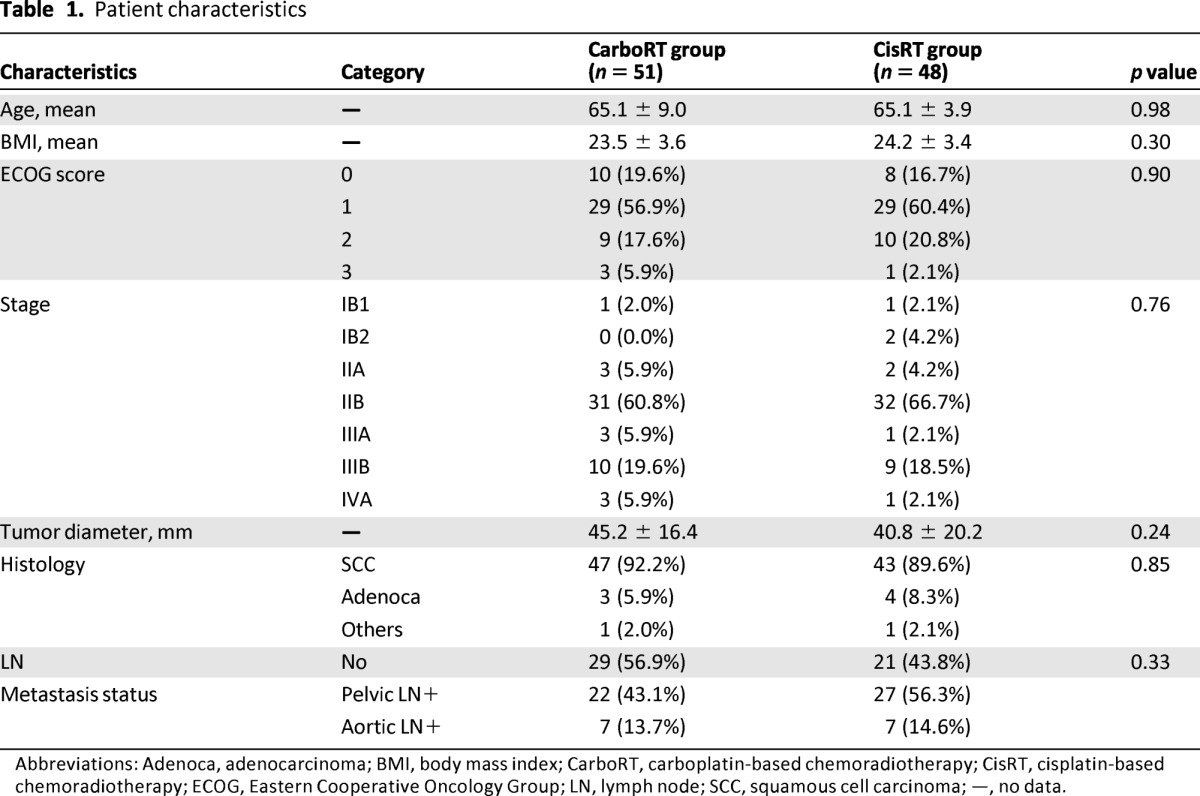
Abbreviations: Adenoca, adenocarcinoma; BMI, body mass index; CarboRT, carboplatin-based chemoradiotherapy; CisRT, cisplatin-based chemoradiotherapy; ECOG, Eastern Cooperative Oncology Group; LN, lymph node; SCC, squamous cell carcinoma; —, no data.
Tumor Responses
Fifty patients in the CarboRT group and 48 patients in the CisRT group were evaluated for tumor response (Table 2). One patient withdrew from the CarboRT group and did not have response assessment. Complete RRs were 50.0% in the CarboRT group and 62.5% in the CisRT group. There were no differences in the overall RRs between the CarboRT and CisRT groups (90.0% and 87.5%, respectively; p = .31).
Table 2.
Tumor responses
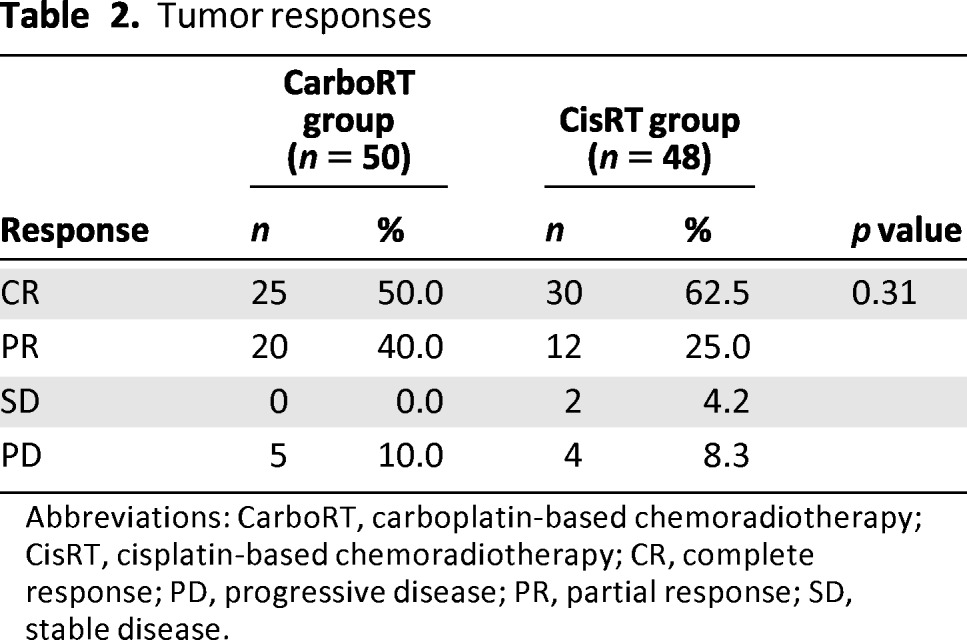
Abbreviations: CarboRT, carboplatin-based chemoradiotherapy; CisRT, cisplatin-based chemoradiotherapy; CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
PFS and OS
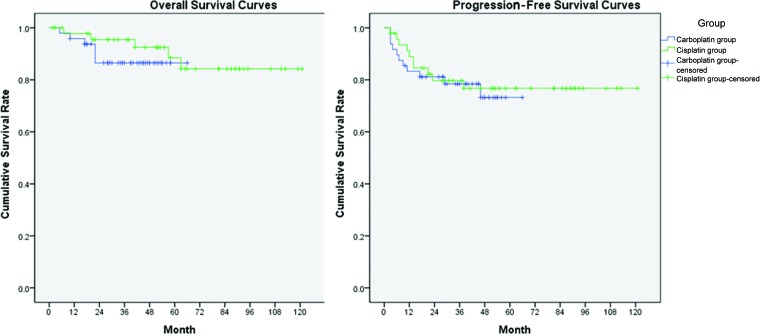
The median follow-up duration was 36 months (range: 4–66 months) in the CarboRT group and 54 months (range: 4–121 months), significantly longer, in the CisRT group (p < .01). There were no significant differences in PFS or OS, neither of which reached the median survival time (Fig. 2). Five patients in the CarboRT group and four patients in the CisRT group had disease progression during the first 2 months after treatment completion. Compared with the historical CisRT group, the CarboRT group showed no statistically significant difference in recurrence (HR, 1.21; 95% confidence interval [CI], 0.52–2.81) or survival (HR, 1.80; 95% CI, 0.49–6.54). Among the patients who experienced recurrence, two had adenocarcinoma and four had lymph node metastasis in the CarboRT group and two patients had adenocarcinoma and all four patients had lymph node metastasis in the CisRT group.
Figure 2.
Progression-free survival curves and overall survival curves. Compared with the historical cisplatin group, the carboplatin group showed no statistically significant difference in terms of recurrence (hazard ratio [HR], 1.21; 95% confidence interval [CI], 0.52–2.81; p = .66) and survival (HR, 1.80; 95% CI, 0.49–6.54; p = .38).
Abbreviations: CarboRT, carboplatin-based chemoradiotherapy; CisRT, cisplatin-based chemoradiotherapy.
In the CarboRT group, the 3-year PFS rate was 78% and the 3-year OS rate was 88%. In the CisRT group, the 3-year PFS rate was 80% and the 3-year OS rate was 94%. In the CarboRT group, nine patients experienced treatment failure, three locally (pelvic side wall or pelvic lymph node) and six at extrapelvic sites. In the CisRT group, 10 patients experienced treatment failure, 2 locally and 8 at extrapelvic sites.
Compliance and Toxicity Profiles
A total of 379 cycles (at 4–9 cycles per patient) of CarboRT and 290 cycles (at 3–9 cycles per patient) of CisRT were administered (Table 3). The mean number of administered cycles was 7.5 ± 1.4 for CarboRT and 6.0 ± 1.8 for CisRT (p < .001; Table 3). Five patients dropped out before the sixth cycle of chemotherapy in the CarboRT group, and 10 patients dropped out before the sixth cycle of chemotherapy in the CisRT group (p = .16; Table 3). Six patients in the CisRT group received only three cycles of chemotherapy during radiation.
Table 3.
Number of cycles administered
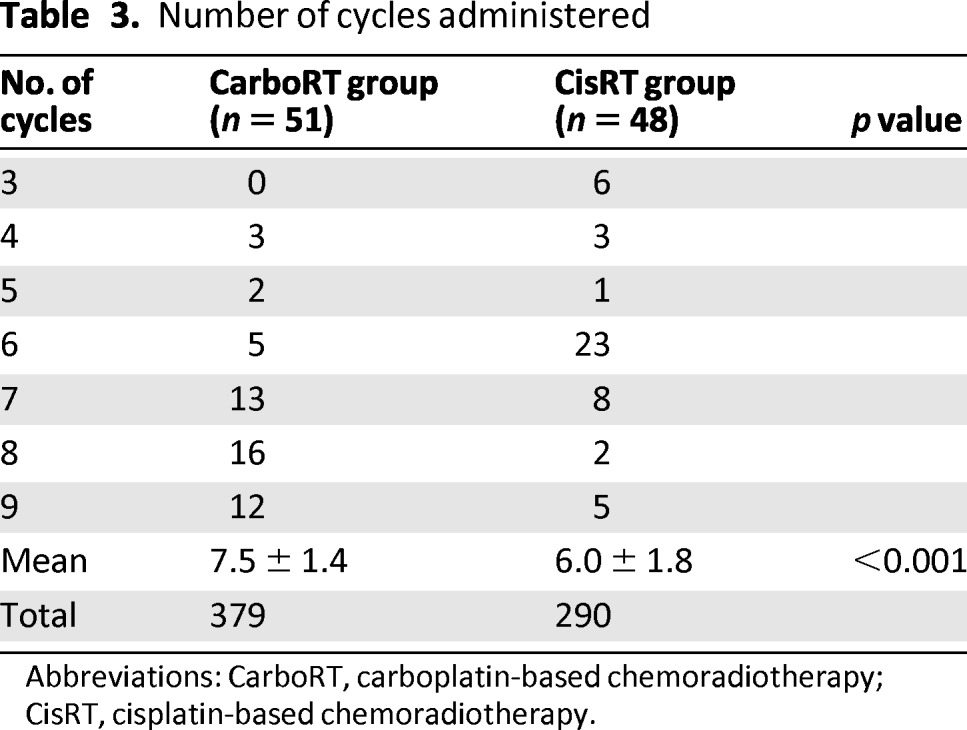
Abbreviations: CarboRT, carboplatin-based chemoradiotherapy; CisRT, cisplatin-based chemoradiotherapy.
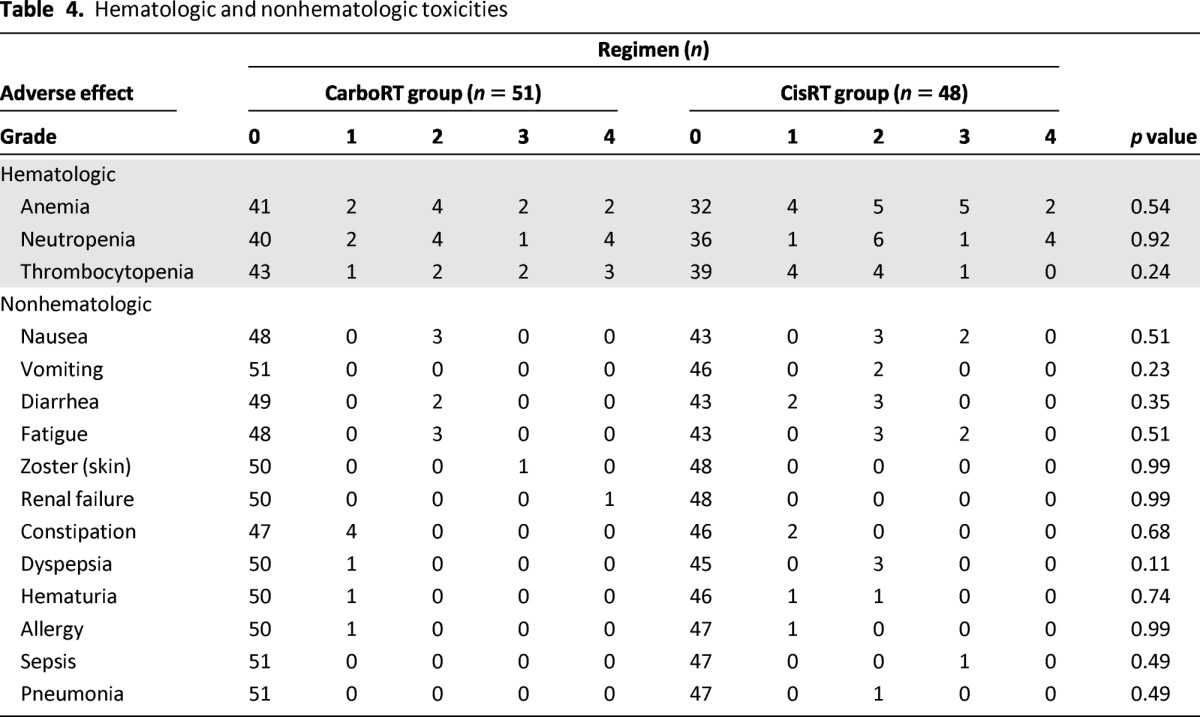
There were no treatment-related deaths. The major toxicity observed for both regimens was bone marrow suppression (Table 4). Thrombocytopenia tended to occur more in the CarboRT group; nausea, vomiting, and fatigue tended to occur more in the CisRT group. There were no differences between the groups in the incidence of hematologic and nonhematologic toxicities. One patient withdrew from the trial because of progressive renal dysfunction after receiving four cycles of CarboRT. The patient had diabetes mellitus and suffered from chronic renal dysfunction with creatinine clearance of 41.2 mL per minute when she enrolled in this trial.
Table 4.
Hematologic and nonhematologic toxicities
Discussion
In this study, we found that CarboRT and CisRT had similar outcomes in terms of toxicity and efficacy, and CarboRT was better tolerated than CisRT. These results are promising when we consider that the patients enrolled in this study had morbidity risks, a poor prognostic factor in cervical cancer. To the best of our knowledge, this study was the first to compare primary weekly CarboRT and CisRT in the treatment of locally advanced cervical cancer.
Only four studies have been published that used concurrent weekly CarboRT. Corn and colleagues evaluated weekly CarboRT at a dose of 60 mg/m2 and showed a complete RR of 43%; however, the study included only seven patients [11]. Duenas-Gonzalez and colleagues tested four different weekly CarboRT dosages (100, 116, 133, and 150 mg/m2) in 24 patients and reported an RR of 75% and a recommended a dose of 133 mg/m2 [13]. Higgins and colleagues evaluated 31 patients treated with CarboRT at an area under the curve of 2, which corresponds to 60–90 mg/m2, and showed an RR of 90% [14]. Last, Veerasarn and colleagues reported the largest randomized phase III study comparing weekly CarboRT 100 mg/m2 and CarboRT plus tegafur-uracil 225 mg/m2 per day orally for 5 days a week in 469 locally advanced cervical cancer patients [16]. They reported a complete RR of 72% with acceptable toxicity in the CarboRT group. Tegafur-uracil did not increase the efficacy of the treatment.
The overall RRs for CarboRT and CisRT in this study were 90.0% and 87.5%, respectively, and the complete RRs for CarboRT and CisRT were 50% and 62.5%, respectively. CarboRT and CisRT had a similar tumor RR in locally advanced cervical cancer patients with morbidity risks; however, the complete RRs in this study seem to be relatively low compared with previous studies [13, 14, 16]. We speculated about some reasons for the low complete RRs in our study. First, the optimal timing for the evaluation of response after radiation or chemoradiotherapy has not been fully investigated. We evaluated the tumor response at 2 months after treatment, whereas most other researchers evaluated the response at the 3-month follow-up. If we consider that regression may continue for several months after completion of radiotherapy, we might have observed a higher complete RR if we had evaluated the tumor response at 3 months after treatment. Second, lesions that look like residual disease at the site of the primary cervical cancer on imaging studies after chemoradiotherapy are often nonviable “scars” unless disease progression occurs. We performed radical hysterectomy in two patients who had a partial response on imaging study after CarboRT; however, no residual disease was found on pathologic examination.
Survival outcomes were also encouraging, as disease recurrence was noted in 10 patients in each group. PFS and OS of the CarboRT group were not inferior to those of the CisRT group. The PFS rate for CisRT at the median follow-up of 53 months after treatment was 79% in this study, and these survival data for CisRT are consistent with other studies [3–6]. In addition, our results showed that the CarboRT and CisRT groups had similar recurrence patterns. Only 3 of 10 recurrences in the CarboRT group and 2 of 10 recurrences in the CisRT group resulted in a pelvic failure pattern, and the others were recurrences at distant sites.
A major strength of our study is that the patient population had morbidity risks; therefore, the results of the analysis of toxicity and compliance may be informative. The most common toxicity of chemoradiotherapy in both groups was hematologic. As expected with carboplatin, thrombocytopenia was more common in the CarboRT group, although the difference was not statistically significant (p = .24). Specifically, grade 3–4 thrombocytopenia was noted in five patients (9.8%); this rate is higher than those reported in previous studies (range: 0%–6.4%) [11–14, 16]. We speculated that the rate of thrombocytopenia was relatively higher because our patients had morbidity risks. For other, nonhematologic, grade 3–4 toxicities, grade 3 nausea and fatigue were more common in the CisRT group, although there was not a statistically significant difference between groups.
This study has some limitations because of the small sample size and the retrospectively selected historical CisRT group. Duration of follow-up in the CisRT group was much longer, and the difference in the number of cycles received could result from treatment periods that did not coincide. In the CisRT group, some adverse effects might have been omitted, antiemetic treatments were not uniformly administered, some patients did not undergo pretreatment PET/CT, and patients who received CisRT did not have the opportunity to sign the consent form. To prove equivalence between the CarboRT and CisRT groups with a margin of 0.1 cumulative survival, 209 events should be observed in each group. Randomized controlled multicenter studies are warranted to overcome these limitations.
It is important to note that the mean number of completed cycles per patient for the CarboRT group was higher than that for the CisRT group (7.5 cycles vs. 6.0 cycles; p < .001). We usually recommend that patients have six to nine cycles of chemotherapy during radiation treatment because our radiotherapy protocol is different from that recommended by the American Brachytherapy Society (ABS) guidelines [19]. The ABS recommends starting brachytherapy following completion of external beam irradiation; however, brachytherapy is performed in the middle of external beam radiation at our institution. Based on our radiation treatment protocol, we recommended that patients have extended treatment to nine cycles of chemotherapy in both regimens and found that CarboRT was better tolerated.
A recent meta-analysis reported that no evidence of the superiority of certain chemotherapy doses, regimens, or scheduling was seen when comparing various kinds of chemoradiotherapy for cervical cancer [20]. High emetogenic effects, potential nephrotoxity, low compliance, and the need for a large amount of hydration with cisplatin make it desirable to explore more practical drugs for use in chemoradiotherapy.
Conclusion
CarboRT and CisRT showed similar tumor response and survival, and CarboRT was better tolerated. Based on the results of this trial, CarboRT appears to be a viable alternative to CisRT for the treatment of cervical cancer, especially in patients with morbidity risks. The results from this study underscore the need for a randomized controlled trial comparing CarboRT and CisRT in patients with cervical cancer.
Acknowledgments
This study was supported by grants from the National Research Foundation of Korea Grant funded by the Korean Government (2011-0010800).
Author Contributions
Conception/Design: Eun Ji Nam, Sunghoon Kim, Sang Wun Kim
Provision of study material or patients: Eun Ji Nam, Maria Lee, Ga Won Yim, Sunghoon Kim, Sang Wun Kim, Jae Wook Kim, Young Tae Kim
Collection and/or assembly of data: Eun Ji Nam, Maria Lee, Jae Wook Kim, Young Tae Kim
Data analysis and interpretation: Eun Ji Nam, Maria Lee, Jae Hoon Kim, Young Tae Kim
Conception/Design: Eun Ji Nam, Sunghoon Kim, Sang Wun Kim, Young Tae Kim
Manuscript writing: Eun Ji Nam, Young Tae Kim
Final approval of manuscript: Eun Ji Nam, Young Tae Kim
Disclosures
The authors indicated no financial relationships.
Reference
- 1.Kim YT. Current status of cervical cancer and HPV infection in Korea. J Gynecol Oncol. 2009;20:1–7. doi: 10.3802/jgo.2009.20.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chemoradiotherapy for Cervical Cancer Meta-analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: Individual patient data meta-analysis. Cochrane Database Syst Rev. 2010:CD008285. doi: 10.1002/14651858.CD008285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144–1153. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 4.Keys HM, Bundy BN, Stehman FB, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340:1154–1161. doi: 10.1056/NEJM199904153401503. [DOI] [PubMed] [Google Scholar]
- 5.Morris M, Eifel PJ, Lu J, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–1143. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 6.Whitney CW, Sause W, Bundy BN, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: A Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol. 1999;17:1339–1348. doi: 10.1200/JCO.1999.17.5.1339. [DOI] [PubMed] [Google Scholar]
- 7.Muggia FM. Overview of carboplatin: Replacing, complementing, and extending the therapeutic horizons of cisplatin. Semin Oncol. 1989;16:7–13. [PubMed] [Google Scholar]
- 8.Schwachofer JH, Crooijmans RP, Hoogenhout J, et al. Effectiveness in inhibition of recovery of cell survival by cisplatin and carboplatin: Influence of treatment sequence. Int J Radiat Oncol Biol Phys. 1991;20:1235–1241. doi: 10.1016/0360-3016(91)90233-t. [DOI] [PubMed] [Google Scholar]
- 9.Pekkola-Heino K, Kulmala J, Grenman R. Carboplatin-radiation interaction in squamous cell carcinoma cell lines. Arch Otolaryngol Head Neck Surg. 1992;118:1312–1315. doi: 10.1001/archotol.1992.01880120038007. [DOI] [PubMed] [Google Scholar]
- 10.Ozols RF, Bundy BN, Greer BE, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: A Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 11.Corn BW, Hernandez E, Anderson L, et al. Phase I/II study of concomitant irradiation and carboplatin for locally advanced carcinoma of the uterine cervix: An interim report. Am J Clin Oncol. 1996;19:317–321. doi: 10.1097/00000421-199606000-00023. [DOI] [PubMed] [Google Scholar]
- 12.Dubay RA, Rose PG, O'Malley DM, et al. Evaluation of concurrent and adjuvant carboplatin with radiation therapy for locally advanced cervical cancer. Gynecol Oncol. 2004;94:121–124. doi: 10.1016/j.ygyno.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 13.Duenas-Gonzalez A, Cetina L, Sanchez B, et al. A phase I study of carboplatin concurrent with radiation in FIGO stage IIIB cervix uteri carcinoma. Int J Radiat Oncol Biol Phys. 2003;56:1361–1365. doi: 10.1016/s0360-3016(03)00347-x. [DOI] [PubMed] [Google Scholar]
- 14.Higgins RV, Naumann WR, Hall JB, et al. Concurrent carboplatin with pelvic radiation therapy in the primary treatment of cervix cancer. Gynecol Oncol. 2003;89:499–503. doi: 10.1016/s0090-8258(03)00151-3. [DOI] [PubMed] [Google Scholar]
- 15.Micheletti E, La Face B, Bianchi E, et al. Continuous infusion of carboplatin during conventional radiotherapy treatment in advanced squamous carcinoma of the cervix uteri IIB-IIIB (UICC) A phase I/II and pharmacokinetic study Am J Clin Oncol. 1997;20:613–620. doi: 10.1097/00000421-199712000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Veerasarn V, Lorvidhaya V, Kamnerdsupaphon P, et al. A randomized phase III trial of concurrent chemoradiotherapy in locally advanced cervical cancer: Preliminary results. Gynecol Oncol. 2007;104:15–23. doi: 10.1016/j.ygyno.2006.06.045. [DOI] [PubMed] [Google Scholar]
- 17.Kim YB, Cho JH, Keum KC, et al. Concurrent chemoradiotherapy followed by adjuvant chemotherapy in uterine cervical cancer patients with high-risk factors. Gynecol Oncol. 2007;104:58–63. doi: 10.1016/j.ygyno.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 19.Nag S, Chao C, Erickson B, et al. The American Brachytherapy Society recommendations for low-dose-rate brachytherapy for carcinoma of the cervix. Int J Radiat Oncol Biol Phys. 2002;52:33–48. doi: 10.1016/s0360-3016(01)01755-2. [DOI] [PubMed] [Google Scholar]
- 20.Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol. 2008;26:5802–5812. doi: 10.1200/JCO.2008.16.4368. [DOI] [PMC free article] [PubMed] [Google Scholar]