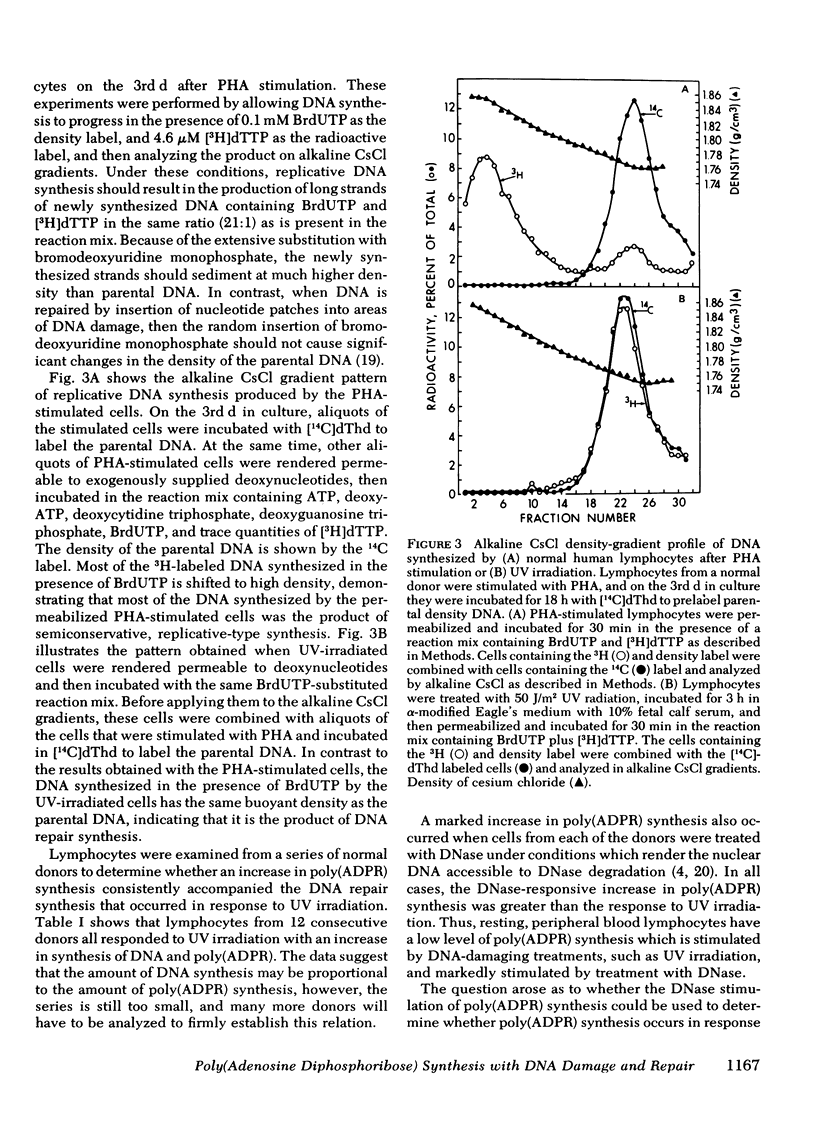
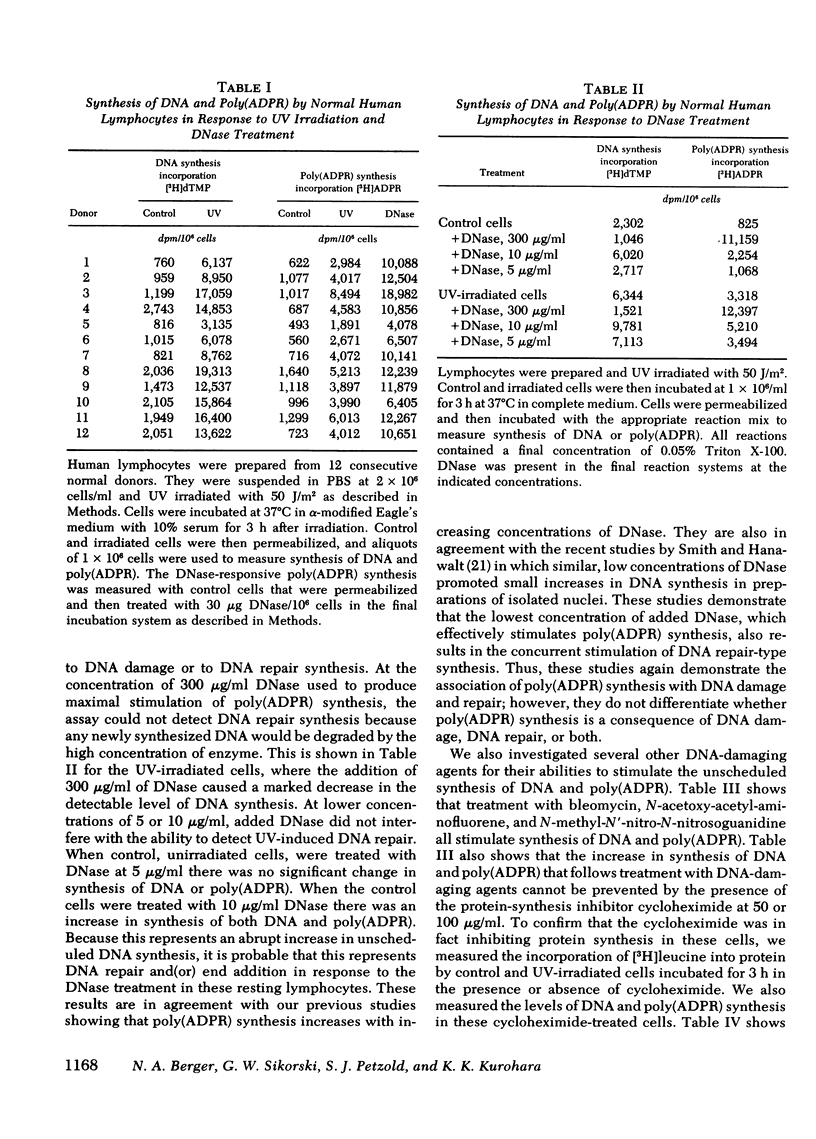
Abstract
A permeable cell technique was used to measure the alterations in synthesis of DNA and poly-(adenosine diphosphoribose) in normal human lymphocytes after treatment of the cells with different types of DNA-damaging agents. The lymphocytes showed an abrupt increase in the unscheduled synthesis of DNA and poly(adenosine diphosphoribose) in response to ultraviolet (UV) irradiation. The increases were apparent within 1 h and reached a maximum between 2 and 4 h after irradiation. The magnitude of the increases in DNA and poly(adenosine diphosphoribose) synthesis was dependent upon the UV dose. Alkaline CsCl gradient studies, with bromodeoxyuridine triphosphate density labeling of DNA, demonstrated that the unscheduled DNA synthesis, which occurred in response to UV irradiation, was actually a result of the repair mode of DNA synthesis. Similar increases in DNA synthesis, and poly(adenosine diphosphoribose) synthesis occurred when lymphocytes were treated with several other DNA-damaging agents, including bleomycin, N-methyl-N′-nitro-N-nitrosoguanidine or N-acetoxyacetyl aminofluorene. Treatment of lymphocytes with DNase, under conditions which allowed degradation of cellular DNA, also resulted in increased synthesis of poly(adenosine diphosphoribose). Cycloheximide did not inhibit the increase in synthesis of DNA or poly(adenosine diphosphoribose) that occurred in response to treatment with the DNA-damaging agents.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger N. A., Adams J. W., Sikorski G. W., Petzold S. J., Shearer W. T. Synthesis of DNA and poly(adenosine diphosphate ribose) in normal and chronic lymphocytic leukemia lymphocytes. J Clin Invest. 1978 Jul;62(1):111–118. doi: 10.1172/JCI109094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger N. A., Erickson W. P., Weber G. Histone inhibition of DNA synthesis in eukaryotic cells permeable to macromolecules. Biochim Biophys Acta. 1976 Sep 20;447(1):65–75. doi: 10.1016/0005-2787(76)90096-4. [DOI] [PubMed] [Google Scholar]
- Berger N. A., Johnson E. S. DNA synthesis in permeabilized mouse L cells. Biochim Biophys Acta. 1976 Feb 18;425(1):1–17. doi: 10.1016/0005-2787(76)90211-2. [DOI] [PubMed] [Google Scholar]
- Berger N. A., Kaichi A. S., Steward P. G., Klevecz R. R., Forrest G. L., Gross S. D. Synthesis of poly(adenosine diphosphate ribose) in synchronized Chinese hamster cells. Exp Cell Res. 1978 Nov;117(1):127–135. doi: 10.1016/0014-4827(78)90435-4. [DOI] [PubMed] [Google Scholar]
- Berger N. A., Petzold S. J., Johnson E. S. High molecular weight DNA intermediates synthesized by permeabilized L cells. Biochim Biophys Acta. 1977 Sep 6;478(1):44–58. doi: 10.1016/0005-2787(77)90242-8. [DOI] [PubMed] [Google Scholar]
- Berger N. A., Weber G., Kaichi A. S. Characterization and comparison of poly(adenosine dephosphoribose) synthesis and DNA synthesis in nucleotide-permeable cells. Biochim Biophys Acta. 1978 Jun 22;519(1):87–104. doi: 10.1016/0005-2787(78)90064-3. [DOI] [PubMed] [Google Scholar]
- Berger N. A., Weber G., Kaichi A. S., Petzold S. J. Relation of poly(adenosine diphosphoribose) synthesis to DNA synthesis and cell growth. Biochim Biophys Acta. 1978 Jun 22;519(1):105–117. doi: 10.1016/0005-2787(78)90065-5. [DOI] [PubMed] [Google Scholar]
- Davies M. I., Shall S., Skidmore C. J. Poly(adenosine diphosphate ribose) polymerase and deoxyribonucleic acid damage [proceedings]. Biochem Soc Trans. 1977;5(4):949–950. doi: 10.1042/bst0050949. [DOI] [PubMed] [Google Scholar]
- Hahn G. M. Radiation and chemically induced potentially lethal lesions in noncycling mammalian cells: recovery analysis in terms of x-ray- and ultraviolet-like-systems. Radiat Res. 1975 Dec;64(3):533–545. [PubMed] [Google Scholar]
- Hayaishi O., Ueda K. Poly(ADP-ribose) and ADP-ribosylation of proteins. Annu Rev Biochem. 1977;46:95–116. doi: 10.1146/annurev.bi.46.070177.000523. [DOI] [PubMed] [Google Scholar]
- Masker W. E., Hanawalt P. C. Ultraviolet-stimulated DNA synthesis in toluenzied Escherichia coli deficient in DNA polymerase I. Proc Natl Acad Sci U S A. 1973 Jan;70(1):129–133. doi: 10.1073/pnas.70.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn J., Skinner A., Kornfeld S. The rapid induction by phytohemagglutinin of increased alpha-aminoisobutyric acid uptake by lymphocytes. J Clin Invest. 1971 Apr;50(4):818–826. doi: 10.1172/JCI106553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. G. Stimulation of nuclear poly (adenosine diphosphate-ribose) polymerase activity from HeLa cells by endonucleases. Biochim Biophys Acta. 1975 Jun 16;395(2):191–200. doi: 10.1016/0005-2787(75)90158-6. [DOI] [PubMed] [Google Scholar]
- Mortelmans K., Friedberg E. C., Slor H., Thomas G., Cleaver J. E. Defective thymine dimer excision by cell-free extracts of xeroderma pigmentosum cells. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2757–2761. doi: 10.1073/pnas.73.8.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter R. B., Cleaver J. E. Repair replication, unscheduled DNA synthesis, and the repair of mammalian DNA. Radiat Res. 1969 Mar;37(3):451–466. [PubMed] [Google Scholar]
- Regan J. D., Setlow R. B. Two forms of repair in the DNA of human cells damaged by chemical carcinogens and mutagens. Cancer Res. 1974 Dec;34(12):3318–3325. [PubMed] [Google Scholar]
- Smith C. A., Hanawalt P. C. Phage T4 endonuclease V stimulates DNA repair replication in isolated nuclei from ultraviolet-irradiated human cells, including xeroderma pigmentosum fibroblasts. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2598–2602. doi: 10.1073/pnas.75.6.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smulson M. E., Schein P., Mullins D. W., Jr, Sudhakar S. A putative role for nicotinamide adenine dinucleotide-promoted nuclear protein modification in the antitumor activity of N-methyl-N-nitrosourea. Cancer Res. 1977 Sep;37(9):3006–3012. [PubMed] [Google Scholar]
- Suzuki H., Nagai K., Akutsu E., Yamaki H., Tanaka N. On the mechanism of action of bleomycin. Strand scission of DNA caused by bleomycin and its binding to DNA in vitro. J Antibiot (Tokyo) 1970 Oct;23(10):473–480. [PubMed] [Google Scholar]
- Ueda K., Reeder R. H., Honjo T., Nishizuka Y., Hayaishi O. Poly adenosine diphosphate ribose synthesis associated with chromatin. Biochem Biophys Res Commun. 1968 May 10;31(3):379–385. doi: 10.1016/0006-291x(68)90486-5. [DOI] [PubMed] [Google Scholar]
- YOFFEY J. M., WINTER G. C., OSMOND D. G., MEEK E. S. MORPHOLOGICAL STUDIES IN THE CULTURE OF HUMAN LEUCOCYTES WITH PHYTOHAEMAGGLUTININ. Br J Haematol. 1965 Jul;11:488–497. doi: 10.1111/j.1365-2141.1965.tb06613.x. [DOI] [PubMed] [Google Scholar]



