The success of scalp cooling in preventing or reducing chemotherapy-induced alopecia (CIA) is highly variable. In a review of the literature, this study found that the factors influencing the effectiveness of scalp cooling to prevent CIA in patients with cancer include chemotherapy type and dose, as well as the degree and duration of cooling.
Keywords: Chemotherapy, Scalp cooling, Side effects, Alopecia
Learning Objectives
Compare the effectiveness of scalp cooling with different chemotherapy regimens.
Describe techniques that may improve effectiveness of scalp cooling.
Abstract
Introduction.
The success of scalp cooling in preventing or reducing chemotherapy-induced alopecia (CIA) is highly variable between patients and chemotherapy regimens. The outcome of hair preservation is often unpredictable and depends on various factors.
Methods.
We performed a structured search of literature published from 1970 to February 2012 for articles that reported on factors influencing the effectiveness of scalp cooling to prevent CIA in patients with cancer.
Results.
The literature search identified 192 reports, of which 32 studies were considered relevant. Randomized studies on scalp cooling are scarce and there is little information on the determinants of the result. The effectiveness of scalp cooling for hair preservation depends on dose and type of chemotherapy, with less favorable results at higher doses. Temperature seems to be an important determinant. Various studies suggest that a subcutaneous scalp temperature less than 22°C is required for hair preservation.
Conclusions.
The effectiveness of scalp cooling for hair preservation varies by chemotherapy type and dose, and probably by the degree and duration of cooling.
Implications for Practice:
Despite the continuous development of cytotoxics and new targeted therapies, chemotherapy-induced alopecia (CIA) remains a major problem. The ongoing underestimation by medical professionals of the high impact of CIA for patients and their relatives has resolved in minimal efforts to prevent CIA. The literature is mainly restricted to patient series evaluating the effectiveness. Future research should focus on determinants of the result, in particular, scalp cooling temperature and time. Reviews on scalp cooling clearly show that scalp cooling is an effective method to prevent CIA. Scalp cooling should therefore be available in every hospital and health care professionals should offer the possibility of scalp cooling to all eligible patients.
Introduction
Chemotherapy-induced alopecia (CIA), although reversible, is one of the most distressing side effects for patients undergoing chemotherapy. CIA has psychosocial implications and may affect body image and acceptance of treatment [1–5]. For some patients, CIA is a reason to refuse chemotherapy, and up to 8% of patients may choose less effective chemotherapy regimens that do not cause hair loss [6, 7].
In healthy individuals, scalp hair follicles show a pattern of cyclic activity. The hair growth (anagen) phase involves the growth of a hair from a hair follicle, lasting for 3–7 years. During the transitional (catagen) phase, the hair follicle atrophies and migrates upward to a resting level in the skin. During the resting (telogen) phase, the hair does not grow but stays attached to the hair follicle. The telogen phase ends when the old hair is shed and a new hair is regenerated in the anagen phase. In the adult scalp, approximately 90% of the follicles are in the growth phase [8, 9].
Chemotherapy acts on rapidly growing cells, including hair follicles. Chemotherapy-induced shedding of hairs usually occurs 7–14 days after infusion [8, 10]. The incidence and severity of alopecia depends both on the type (Table 1) and dose of chemotherapy [6, 9, 11–13]. Apart from hair loss from the scalp, patients may also lose their eyebrows, eyelashes, and pubic hair after several cycles of chemotherapy. Although alopecia is a reversible side effect, permanent alopecia has been reported incidentally after high-dose chemotherapy [10].
Table 1.
Cytotoxic drugs that cause chemotherapy-induced alopecia
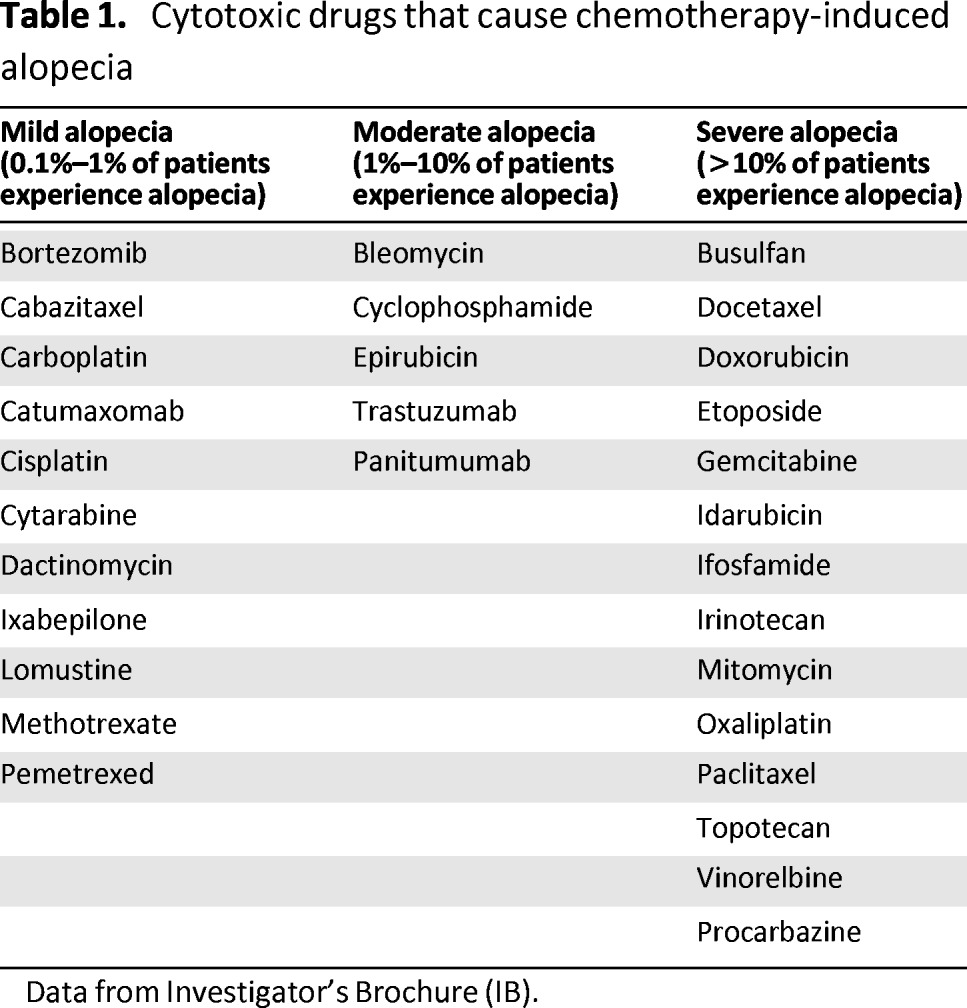
Data from Investigator's Brochure (IB).
Since the 1970s, scalp cooling has been used to reduce and prevent CIA [14]. Scalp cooling reduces skin temperature, thereby affecting the exposure and metabolism of cytotoxic agents in the hair follicles. However, the effectiveness of scalp cooling in preventing alopecia is highly variable and unpredictable. We therefore wanted to explore possible reasons why scalp cooling works in one patient but fails in another.
Materials and Methods
We designed a search strategy to identify relevant literature that described the use of scalp cooling in preventing CIA in patients with cancer. We performed our search on February 24, 2012, in the electronic databases PubMed, Embase, and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) for literature published from 1970 through February 24, 2012, linking the subject search headings with text word and MESH terms.
A combination of the following search terms were used: (“chemotherapy” [all fields] OR “antineoplastic protocols” [Mesh] OR “antineoplastic agents” [Mesh] OR “neoplasms/drug therapy” [Mesh Terms] OR “chemotherapy-induced” [all fields]) AND (“hair loss” [all fields] OR “alopecia” [Mesh] OR “alopecia” [all fields] OR “alopecia/chemically induced” [Mesh]) AND (“scalp cooling” [all fields] OR “scalp hypothermia” OR “cold cap” [all fields] OR “hypothermia, induced” [Mesh]) AND (“scalp” [Mesh] OR “scalp” [all fields]). We did not restrict the search strategy to a particular type of study design.
Articles were selected if they assessed any possible factors affecting the effectiveness of scalp cooling in preventing alopecia after chemotherapy. Only full-text articles in English and Dutch were considered. We also did a manual search for any relevant references used in the articles found. Papers that described scalp cooling as a safety issue or that focused solely on impact or tolerance were excluded.
Results
The initial search resulted in a total of 192 citations (76 in PubMed, 92 in Embase, and 24 in CINAHL). After the duplicates were removed, 102 citations remained; 70 were discarded based on title or abstract because they did not meet the inclusion criteria. The 32 citations that were considered relevant were included in this review (Table 2). A manual search of references in the relevant articles did not yield any additional studies. The majority of the articles (20 of 32) were published between 1980 and 2000. Since 2010, only three articles were published on possible factors influencing the effectiveness of scalp cooling in the prevention of CIA. Excluding one large multicenter observational study of 1,411 patients [15], the median number of reported patients was 35 (range: 9–180).
Table 2.
Determinants of chemotherapy-induced alopecia
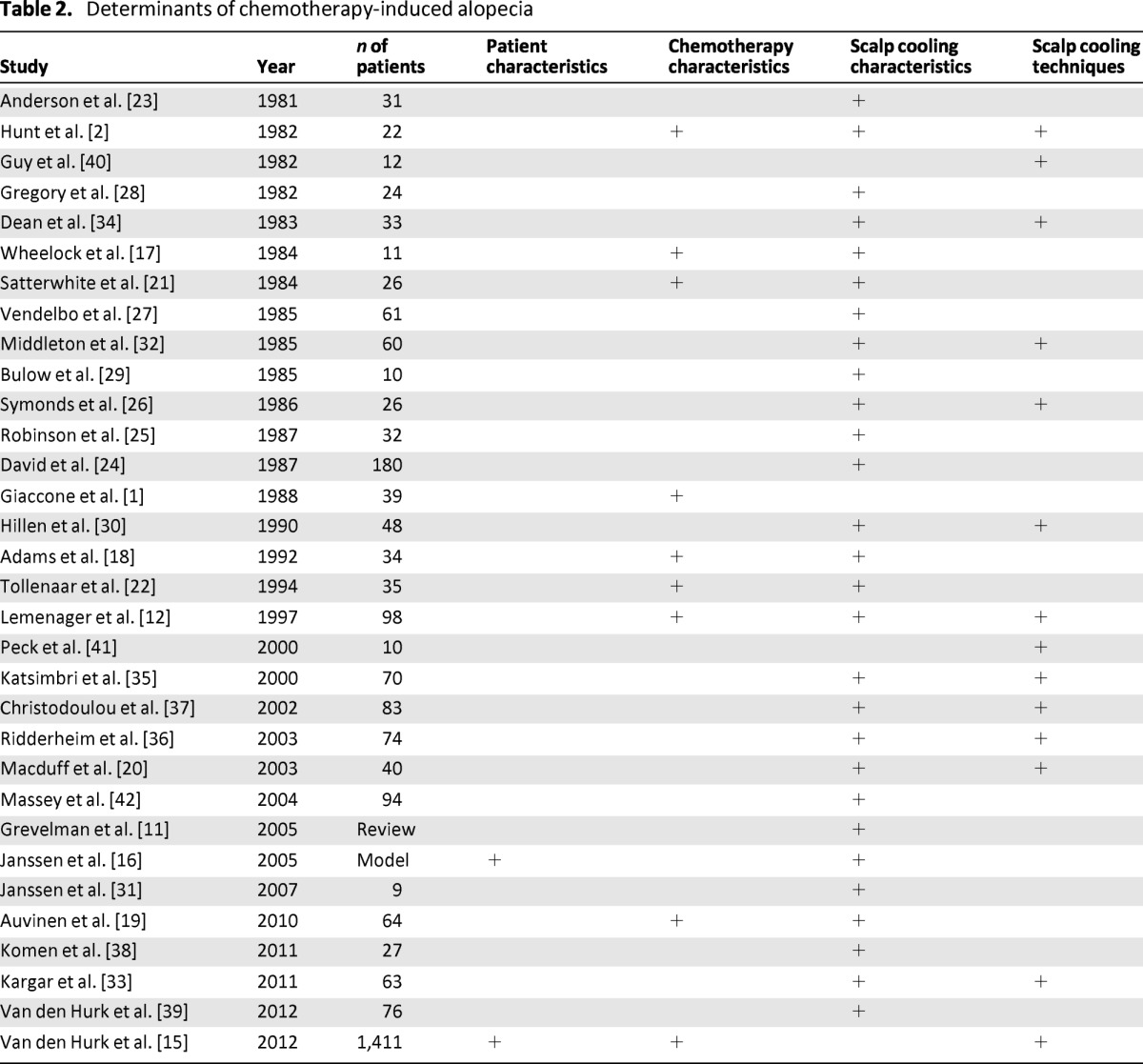
The effectiveness of scalp cooling in patients with cancer depends on many factors, including patient characteristics, chemotherapy characteristics, and the procedure used for scalp cooling (Table 2). Only one study reported on a relationship between patient characteristics and scalp cooling effectiveness. In nine articles, various chemotherapy schedules were tested. In 31 articles, technical aspects of scalp cooling were described.
Patient Characteristics
In a large multicenter observational study in The Netherlands, Van den Hurk et al. [15] concluded that scalp cooling was more effective at younger age, in male patients, and in patients with a Caucasian type of hair. In a computer model study, Janssen et al. [16] found that the thickness of the hair layer correlated with the scalp skin temperature during scalp cooling. This may explain the lower effectiveness of scalp cooling in patients with black African hair, who have a thick layer of hair that acts as an insulating layer between the cooling cap and the scalp.
Chemotherapy Characteristics
The incidence and severity of CIA in patients using scalp cooling depends on the type and dose of chemotherapy [1, 2, 12, 15, 17–22]. Only three studies randomized patients to chemotherapy either with or without scalp cooling [1, 20, 21]. In those studies, minimal or no hair loss was seen in patients who received scalp cooling, in contrast to almost 100% alopecia for patients in the control groups.
Of note, scalp cooling failed to prevent alopecia in most patients who were treated with the combination of docetaxel, adriamycin, and cyclophosphamide (TAC) chemotherapy for early breast cancer.
Because of the small number of randomized studies, the results on the effectiveness of scalp cooling have to be compared with historical series with identical chemotherapy regimens. An ongoing Dutch observational study is collecting data on the effectiveness of scalp cooling for various types and doses of chemotherapy regimens (Table 3) [15]. For anthracycline-containing regimens, a higher dose of anthracycline was correlated with a worse outcome of scalp cooling [15, 17]. With 5-fluorouracil-epirubicin-cyclophosphamide (FEC) chemotherapy, 33% of the patients treated with epirubicin at a dose of 100 mg/m2 did not require a head cover versus 52% of the patients treated with epirubicin at a dose of 90 mg/m2. Likewise, 59% of the patients treated with docetaxel at a dose of 100 mg/m2 did not require a head cover versus 79% of the patients treated at a dose of 75 mg/m2 (Table 3) [15]. Of note, scalp cooling failed to prevent alopecia in most patients who were treated with the combination of docetaxel, adriamycin, and cyclophosphamide (TAC) chemotherapy for early breast cancer.
Table 3.
Overview of scalp cooling results in The Netherlands, 2006–2009 [14]
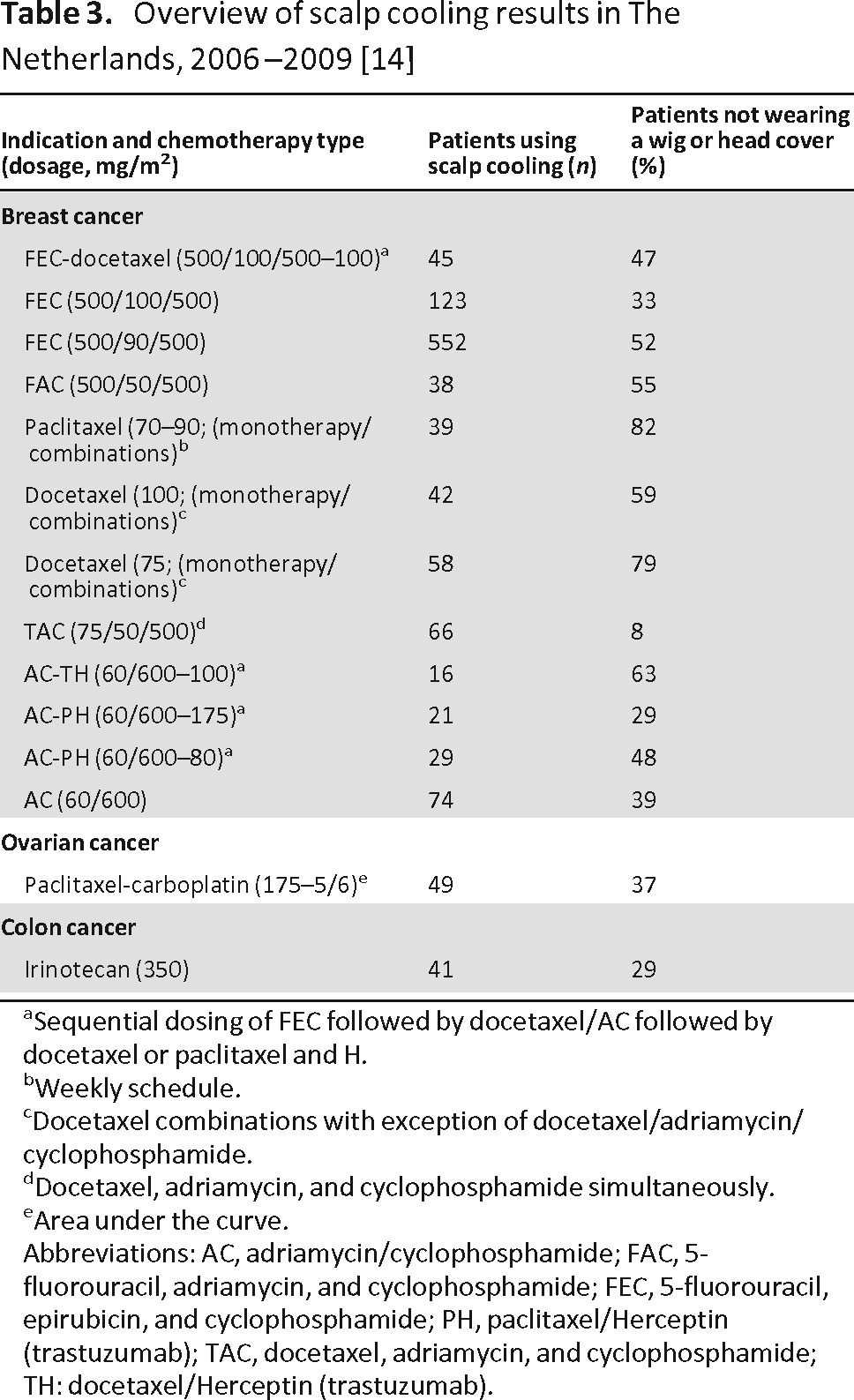
aSequential dosing of FEC followed by docetaxel/AC followed by docetaxel or paclitaxel and H.
bWeekly schedule.
cDocetaxel combinations with exception of docetaxel/adriamycin/cyclophosphamide.
dDocetaxel, adriamycin, and cyclophosphamide simultaneously.
eArea under the curve.
Abbreviations: AC, adriamycin/cyclophosphamide; FAC, 5-fluorouracil, adriamycin, and cyclophosphamide; FEC, 5-fluorouracil, epirubicin, and cyclophosphamide; PH, paclitaxel/Herceptin (trastuzumab); TAC, docetaxel, adriamycin, and cyclophosphamide; TH: docetaxel/Herceptin (trastuzumab).
Liver Function
According to several studies, scalp cooling does not prevent CIA in most patients with biochemical evidence of abnormal liver function [2, 23–27]. This may be predicted on the basis of pharmacokinetics of chemotherapy that is metabolized by the liver. For these drugs, an impaired liver function is associated with higher and more prolonged plasma concentrations [23]. In contrast, Grevelman et al. [11] concluded that impaired liver function seemed to be related to less benefit from scalp cooling in only 6 out of 13 studies [11]. In these studies, patients were treated with doxorubicin or epirubicin.
Scalp Cooling Characteristics
Temperature
It is evident that optimal fitting of the cold cap is an important factor for success. Bald areas may be seen where the cap did not fit properly (Fig. 1). Contact between the cold cap and the scalp skin is important for lowering the skin temperature [16]. In 1982, Gregory et al. [28] found a relationship between the degree of decrease in scalp skin temperature and the protective effect of scalp cooling against hair loss in patients treated with doxorubicin. They concluded that the subcutaneous scalp skin temperature should be reduced to less than 22°C to prevent CIA, corresponding to an epicutaneous scalp temperature of less than 19°C to prevent CIA [29].
Figure 1.
Bald areas are seen where the cold cap did not fit properly.
Hillen et al. [30] attributed the success of their air-cooling methods in part to achieving epicutaneous scalp temperatures below 15°C. Bülow reported that it was impossible to obtain a subcutaneous scalp temperatures less than 28°C in 2 out of 10 healthy volunteers, which implies that some persons consistently respond to scalp cooling with only a minor reduction in subcutaneous temperature; this conclusion is in agreement with the findings of Gregory et al. and Janssen et al. [28, 29, 31]. As these studies used different and obsolete scalp cooling techniques and report different cutoff levels of scalp skin temperature, a study on scalp temperature using the modern Paxman (Paxman Coolers, Huddersfield, UK, http://www.paxman-coolers.co.uk) system is presently being conducted at our center.
Janssen et al. demonstrated that wetting the hair increased the conductivity of the hair layer, resulting in a further decrease in scalp skin temperature [16]. However, there are no randomized studies regarding the influence of wetting on scalp skin temperature and scalp cooling success rates. Wetting the hair may also increase the burden for the patient.
Perfusion
To gain more insight into the effect of cooling, Janssen et al. studied the relationship between skin temperature and skin perfusion during a cooling experiment in nine healthy subjects [16]. During scalp cooling, relative perfusion of the scalp skin gradually dropped down to 28%. A plateau in perfusion was reached after prolonged cooling to lower skin temperatures. This reduction in perfusion was in line with the findings of Bülow et al. [29] and Hillen et al. [30], who found that blood flow during scalp cooling was reduced to 25% of the basal value. Bülow et al. also stated that blood flow was not reduced any further when the subcutaneous scalp temperature was less than 30°C [29].
Scalp Cooling Time
The duration of scalp cooling might influence the hair protective effect of scalp cooling. In most studies, the precooling time (time between the start of scalp cooling and the start of intravenous infusion of chemotherapy) ranged from 5 to 30 minutes [12, 17–23, 25–27, 30, 32–37]. At the Medical Centre Alkmaar, we measured serial scalp skin temperatures during scalp cooling in healthy subjects and patients with cancer to determine the optimal preinfusion cooling time. In 27 individuals treated with scalp cooling using the Paxman PSC1 system, scalp temperature reached a constant level of approximately 18°C after 45 minutes. These preliminary data suggest that as no further reduction in temperature occurred, a preinfusion cooling time of 45 minutes seems optimal when the cap has not been precooled [38].
Although the preinfusion cooling time is well-known, there is much uncertainty about the postinfusion cooling time. Theoretically, the cooling period after infusion of chemotherapy should be related to pharmacokinetics of exposure to the cytostatic agent and its active metabolites [11, 22]. However, research on postinfusion cooling time is very scarce. In daily practice, postinfusion cooling times range from 15 minutes to 4 hours [12, 17–23, 25–28, 30, 32, 34–37]. A study comparing the effect of a shorter postinfusion time in patients treated with docetaxel (90 vs. 45 minutes) showed no difference in results on hair preservation (95% vs. 79% of patients did not need head covering) [39]. Therefore, a new docetaxel study has started in which patients are randomized between 45 or 20 minutes of postinfusion cooling time. In contrast, for patients with breast cancer who are treated with adjuvant FEC chemotherapy for which scalp cooling is less effective (about 50% with no head covering), it is investigated whether prolonging the postinfusion cooling time to 150 minutes is favorable over 90 minutes.
Scalp Cooling Techniques
Several techniques have been used to induce hypothermia: chilled air, bags with crushed ice, frozen Cryogel packs or packs with an endothermic cooling reaction, special caps with Cryogel and an insulation layer, and caps connected to a cooling device using air or fluid as a medium and equipped with a thermostat [2, 12, 18, 20, 26, 30, 32–37, 40, 41]. Few studies compared the effectiveness of different methods of scalp cooling [30, 32, 34]. Dean et al. compared a Kold Kap (American Kaye Laboratory, San Diego, CA) device with ice packs for 62 patients treated with doxorubicin [34]; they found that 63% of patients using Kold Kaps and 56% of patients using ice packs did not require wigs.
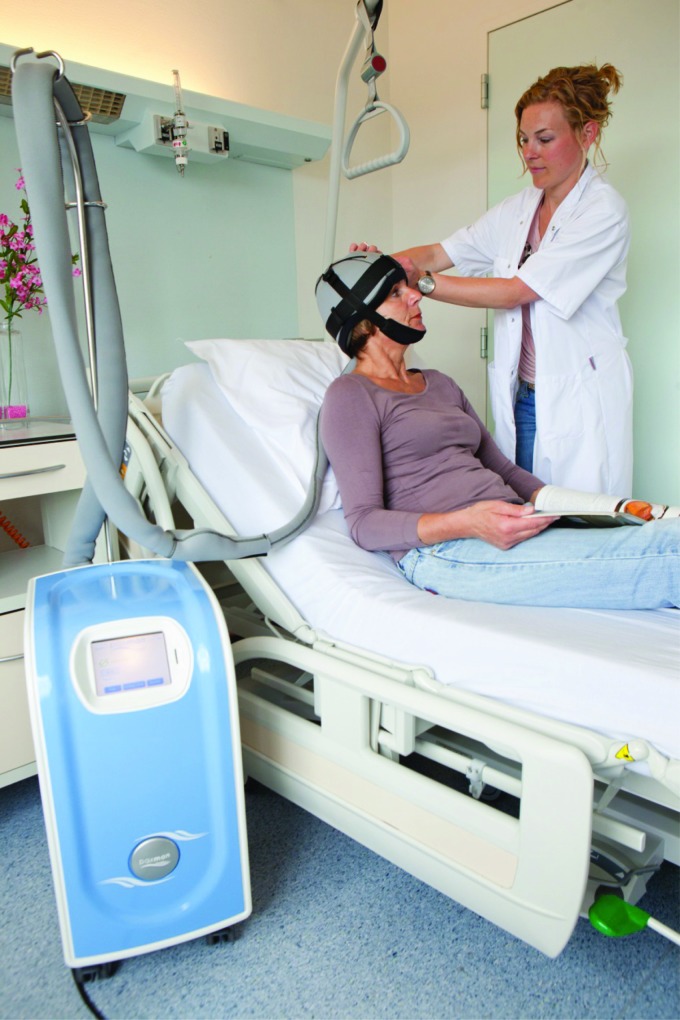
Although cooling devices that use air or fluid as a medium and are equipped with a thermostat (Fig. 2) provide more constant cooling that can be maintained for longer, there is no conclusive evidence that permanently cooled caps result in better hair preservation. Studies comparing skin temperatures and skin perfusion as obtained with various methods of scalp cooling are lacking. Although bags with ice as well as special caps are both well tolerated, caps are lighter in weight and easier to apply, which might offer a comfort advantage.
Figure 2.
Cooling device equipped with a thermostat.
Side Effects and Contraindications for Scalp Cooling
Scalp cooling is generally well-tolerated [2, 3, 11, 42]. Results obtained from patients appear to indicate high levels of comfort and acceptability with evidence of only minor and reversible side effects [1]. The most often reported side effects of scalp cooling include headaches, complaints of coldness and/or uncomfortable sensations, and claustrophobia [11, 20, 42]. Scalp cooling is contraindicated for patients with cold sensitivity, cold agglutinin disease, cryoglobulinemia, cryofibrinogenemia, and post-traumatic cold dystrophy [11]. Scalp metastases have rarely been reported in the literature, but caution regarding their development has been a limitation for the broad-scale application of scalp cooling during chemotherapy [6, 43]. Theoretically, tumor cells that have seeded in the scalp might not receive adequate chemotherapy during hypothermia, thus allowing them to grow at a later date [6].
Scalp metastases have rarely been reported in the literature, but caution regarding their development has been a limitation for the broad-scale application of scalp cooling during chemotherapy. Theoretically, tumor cells that have seeded in the scalp might not receive adequate chemotherapy during hypothermia, thus allowing them to grow at a later date.
Discussion
Although the incidence and severity of alopecia as a side effect of chemotherapy depends on the type and dose of chemotherapy [6, 9, 11–13], the outcome of hair preservation by scalp cooling is often unpredictable and varies between patients. The effectiveness of scalp cooling to prevent CIA depends on various factors such as patient characteristics, type and dose of chemotherapy, and scalp cooling technique. Currently, some hospitals in Europe, Canada, and Japan use scalp cooling in daily practice. In the United States, a feasibility study regarding scalp cooling for patients with cancer has recently started. A small number of randomized studies on the effectiveness and safety of scalp cooling in CIA exist. Few studies have investigated which patient characteristics could be of influence and which method of scalp cooling is the most effective. In this review, we found that scalp cooling results are better with certain chemotherapy types (taxanes). The results of scalp cooling are less favorable at higher doses of chemotherapy. Skin temperature seems to play an important role; however, currently there is no evidence for a cutoff point under which alopecia can be prevented by scalp cooling. Some studies suggest that a subcutaneous scalp temperature less than 22°C is required for hair preservation; however, these studies used different and obsolete scalp cooling techniques.
Ideally, the use of scalp cooling should be personalized for each patient. If the threshold level of scalp temperature is a critical issue, the timing and technique of scalp cooling should be adapted to individual measurements of skin temperature. To advise patients on an individual basis regarding scalp cooling for prevention of CIA, factors such as optimal temperature and postinfusion cooling time should be investigated further.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Author Contributions
Conception/Design: Manon M.C. Komen, Carolien H. Smorenburg, Corina J.G. van Den Hurk, Johan W.R. Nortier
Provision of study materials or patients: Manon M.C. Komen, Carolien H. Smorenburg, Corina J.G. van Den Hurk, Johan W.R. Nortier
Collection and/or assembly of data: Manon M.C. Komen
Data analysis and interpretation: Manon M.C. Komen, Carolien H. Smorenburg, Corina J.G. van Den Hurk, Johan W.R. Nortier
Manuscript writing: Manon M.C. Komen, Carolien H. Smorenburg, Corina J.G. van Den Hurk, Johan W.R. Nortier
Final approval of manuscript: Manon M.C. Komen, Carolien H. Smorenburg, Corina J.G. van Den Hurk, Johan W.R. Nortier
Disclosures
The authors indicated no financial relationships.
Section editors: Eduardo Bruera: None; Russell K. Portenoy: Grupo Ferrer, Purdue Pharma (C/A); Allergan, Boston Scientific, Covidien Mallinckrodt Inc., Endo Pharmaceuticals, Medtronic, Otsuka Pharma, ProStrakan, Purdue Pharma, Salix, St. Jude Medical (RF)
Reviewer “A”: None
Reviewer “B”: None
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Reference
- 1.Giaccone G, Di Giulio F, Morandini MP, et al. Scalp hypothermia in the prevention of doxorubicin-induced hair loss. Cancer Nurs. 1988;11:170–173. [PubMed] [Google Scholar]
- 2.Hunt JM, Anderson JE, Smith IE. Scalp hypothermia to prevent adriamycin-induced hair loss. Cancer Nurs. 1982;5:25–31. [PubMed] [Google Scholar]
- 3.Mols F, van den Hurk CJ, Vingerhoets AJ, et al. Scalp cooling to prevent chemotherapy-induced hair loss: Practical and clinical considerations. Support Care Cancer. 2009;17:181–189. doi: 10.1007/s00520-008-0475-4. [DOI] [PubMed] [Google Scholar]
- 4.Rosman S. Cancer and stigma: Experience of patients with chemotherapy-induced alopecia. Patient Educ Couns. 2004;52:333–339. doi: 10.1016/S0738-3991(03)00040-5. [DOI] [PubMed] [Google Scholar]
- 5.Van den Hurk CJ, Mols F, Vingerhoets AJ, et al. Impact of alopecia and scalp cooling on the well-being of breast cancer patients. Psychooncology. 2010;19:701–709. doi: 10.1002/pon.1615. [DOI] [PubMed] [Google Scholar]
- 6.Batchelor D. Hair and cancer chemotherapy: Consequences and nursing care—A literature study. Eur J Cancer Care (Engl) 2001;10:147–163. doi: 10.1046/j.1365-2354.2001.00272.x. [DOI] [PubMed] [Google Scholar]
- 7.Hesketh PJ, Batchelor D, Golant M, et al. Chemotherapy-induced alopecia: Psychosocial impact and therapeutic approaches. Support Care Cancer. 2004;12:543–549. doi: 10.1007/s00520-003-0562-5. [DOI] [PubMed] [Google Scholar]
- 8.Botchkarev VA, Komarova EA, Siebenhaar F, et al. p53 is essential for chemotherapy-induced hair loss. Cancer Res. 2000;60:5002–5006. [PubMed] [Google Scholar]
- 9.Trueb RM. Chemotherapy-induced hair loss. Skin Therapy Lett. 2010;15:5–7. [PubMed] [Google Scholar]
- 10.Trueb RM. Chemotherapy-induced alopecia. Semin Cutan Med Surg. 2009;28:11–14. doi: 10.1016/j.sder.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Grevelman EG, Breed WP. Prevention of chemotherapy-induced hair loss by scalp cooling. Ann Oncol. 2005;16:352–358. doi: 10.1093/annonc/mdi088. [DOI] [PubMed] [Google Scholar]
- 12.Lemenager M, Lecomte S, Bonneterre ME, et al. Effectiveness of cold cap in the prevention of docetaxel-induced alopecia. Eur J Cancer. 1997;33:297–300. doi: 10.1016/s0959-8049(96)00374-7. [DOI] [PubMed] [Google Scholar]
- 13.Munstedt K, Manthey N, Sachsse S, et al. Changes in self-concept and body image during alopecia induced cancer chemotherapy. Support Care Cancer. 1997;5:139–143. doi: 10.1007/BF01262572. [DOI] [PubMed] [Google Scholar]
- 14.Breed W, van den Hurk CJ, Peerbooms M. Presentation, impact and prevention of chemotherapy induced hair loss: Scalp cooling potentials and limitations. Dermatology. 2011;6:109–125. [Google Scholar]
- 15.Van den Hurk CJ, Peerbooms M, van de Poll-Franse LV, et al. Scalp cooling for hair preservation and associated characteristics in 1411 chemotherapy patients: Results of the Dutch Scalp Cooling Registry. Acta Oncol. 2012;51:497–504. doi: 10.3109/0284186X.2012.658966. [DOI] [PubMed] [Google Scholar]
- 16.Janssen FE, van Leeuwen GM, van Steenhoven AA. Modelling of temperature and perfusion during scalp cooling. Phys Med Biol. 2005;50:4065–4073. doi: 10.1088/0031-9155/50/17/010. [DOI] [PubMed] [Google Scholar]
- 17.Wheelock JB, Myers MB, Krebs HB, et al. Ineffectiveness of scalp hypothermia in the prevention of alopecia in patients treated with doxorubicin and cisplatin combinations. Cancer Treat Rep. 1984;68:1387–1388. [PubMed] [Google Scholar]
- 18.Adams L, Lawson N, Maxted KJ, et al. The prevention of hair loss from chemotherapy by the use of cold-air scalp-cooling. Eur J Cancer Care (Engl) 1992;1:16–18. [Google Scholar]
- 19.Auvinen PK, Mahonen UA, Soininen KM, et al. The effectiveness of a scalp cooling cap in preventing chemotherapy-induced alopecia. Tumori. 2010;96:271–275. doi: 10.1177/030089161009600214. [DOI] [PubMed] [Google Scholar]
- 20.Macduff C, Mackenzie T, Hutcheon A, et al. The effectiveness of scalp cooling in preventing alopecia for patients receiving epirubicin and docetaxel. Eur J Cancer Care (Engl) 2003;12:154–161. doi: 10.1046/j.1365-2354.2003.00382.x. [DOI] [PubMed] [Google Scholar]
- 21.Satterwhite B, Zimm S. The use of scalp hypothermia in the prevention of doxorubicin-induced hair loss. Cancer. 1984;54:34–37. doi: 10.1002/1097-0142(19840701)54:1<34::aid-cncr2820540109>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 22.Tollenaar RA, Liefers GJ, Repelaer van Driel OJ, et al. Scalp cooling has no place in the prevention of alopecia in adjuvant chemotherapy for breast cancer. Eur J Cancer. 1994;30A:1448–1453. doi: 10.1016/0959-8049(94)00280-i. [DOI] [PubMed] [Google Scholar]
- 23.Anderson JE, Hunt JM, Smith IE. Prevention of doxorubicin-induced alopecia by scalp cooling in patients with advanced breast cancer. Br Med J (Clin Res Ed) 1981;282:423–424. doi: 10.1136/bmj.282.6262.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.David J, Speechley V. Scalp cooling to prevent alopecia. Nurs Times. 1987;83:36–37. [PubMed] [Google Scholar]
- 25.Robinson MH, Jones AC, Durrant KD. Effectiveness of scalp cooling in reducing alopecia caused by epirubicin treatment of advanced breast cancer. Cancer Treat Rep. 1987;71:913–914. [PubMed] [Google Scholar]
- 26.Symonds RP, McCormick CV, Maxted KJ. Adriamycin alopecia prevented by cold air scalp cooling. Am J Clin Oncol. 1986;9:454–457. doi: 10.1097/00000421-198610000-00019. [DOI] [PubMed] [Google Scholar]
- 27.Vendelbo JL. Scalp hypothermia in the prevention of chemotherapy-induced alopecia. Acta Radiol Oncol. 1985;24:113–116. doi: 10.3109/02841868509134372. [DOI] [PubMed] [Google Scholar]
- 28.Gregory RP, Cooke T, Middleton J, et al. Prevention of doxorubicin-induced alopedia by scalp hypothermia: Relation to degree of cooling. Br Med J (Clin Res Ed) 1982;284:1674. doi: 10.1136/bmj.284.6330.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bulow J, Friberg L, Gaardsting O, et al. Frontal subcutaneous blood flow, and epi- and subcutaneous temperatures during scalp cooling in normal man. Scand J Clin Lab Invest. 1985;45:505–508. doi: 10.3109/00365518509155250. [DOI] [PubMed] [Google Scholar]
- 30.Hillen HF, Breed WP, Botman CJ. Scalp cooling by cold air for the prevention of chemotherapy-induced alopecia. Neth J Med. 1990;37:231–235. [PubMed] [Google Scholar]
- 31.Janssen FP, Rajan V, Steenbergen W, et al. The relationship between local scalp skin temperature and cutaneous perfusion during scalp cooling. Physiol Meas. 2007;28:829–839. doi: 10.1088/0967-3334/28/8/006. [DOI] [PubMed] [Google Scholar]
- 32.Middleton J, Franks D, Buchanan RB, et al. Failure of scalp hypothermia to prevent hair loss when cyclophosphamide is added to doxorubicin and vincristine. Cancer Treat Rep. 1985;69:373–375. [PubMed] [Google Scholar]
- 33.Kargar M, Sarvestani RS, Khojasteh HN, et al. Efficacy of penguin cap as scalp cooling system for prevention of alopecia in patients undergoing chemotherapy. J Adv Nurs. 2011;67:2473–2477. doi: 10.1111/j.1365-2648.2011.05668.x. [DOI] [PubMed] [Google Scholar]
- 34.Dean JC, Griffith KS, Cetas TC, et al. Scalp hypothermia: A comparison of ice packs and the Kold Kap in the prevention of doxorubicin-induced alopecia. J Clin Oncol. 1983;1:33–37. doi: 10.1200/JCO.1983.1.1.33. [DOI] [PubMed] [Google Scholar]
- 35.Katsimbri P, Bamias A, Pavlidis N. Prevention of chemotherapy-induced alopecia using an effective scalp cooling system. Eur J Cancer. 2000;36:766–771. doi: 10.1016/s0959-8049(00)00012-5. [DOI] [PubMed] [Google Scholar]
- 36.Ridderheim M, Bjurberg M, Gustavsson A. Scalp hypothermia to prevent chemotherapy-induced alopecia is effective and safe: A pilot study of a new digitized scalp-cooling system used in 74 patients. Support Care Cancer. 2003;11:371–377. doi: 10.1007/s00520-003-0451-y. [DOI] [PubMed] [Google Scholar]
- 37.Christodoulou C, Klouvas G, Efstathiou E, et al. Effectiveness of the MSC cold cap system in the prevention of chemotherapy-induced alopecia. Oncology. 2002;62:97–102. doi: 10.1159/000048253. [DOI] [PubMed] [Google Scholar]
- 38.Komen MMC, Smorenburg CH, Breed WPM, et al. Optimal pre-infusion cooling time in patients treated with chemotherapy and scalp cooling. Eur J Cancer. 2011;47:S320. [Google Scholar]
- 39.Van den Hurk CJ, Breed WP, Nortier JW. Short post-infusion scalp cooling time in the prevention of docetaxel-induced alopecia. Support Care Cancer. 2012;20:3255–3260. doi: 10.1007/s00520-012-1465-0. [DOI] [PubMed] [Google Scholar]
- 40.Guy R, Shah S, Parker H, et al. Scalp cooling by thermocirculator. Lancet. 1982;1:937–938. doi: 10.1016/s0140-6736(82)91936-5. [DOI] [PubMed] [Google Scholar]
- 41.Peck HJ, Mitchell H, Stewart AL. Evaluating the efficacy of scalp cooling using the Penguin cold cap system to reduce alopecia in patients undergoing chemotherapy for breast cancer. Eur J Oncol Nurs. 2000;4:246–248. doi: 10.1054/ejon.2000.0094. [DOI] [PubMed] [Google Scholar]
- 42.Massey CS. A multicentre study to determine the efficacy and patient acceptability of the Paxman Scalp Cooler to prevent hair loss in patients receiving chemotherapy. Eur J Oncol Nurs. 2004;8:121–130. doi: 10.1016/j.ejon.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 43.Coukell AJ, Faulds D. Epirubicin. An updated review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in the management of breast cancer. Drugs. 1997;53:453–482. doi: 10.2165/00003495-199753030-00008. [DOI] [PubMed] [Google Scholar]