Abstract
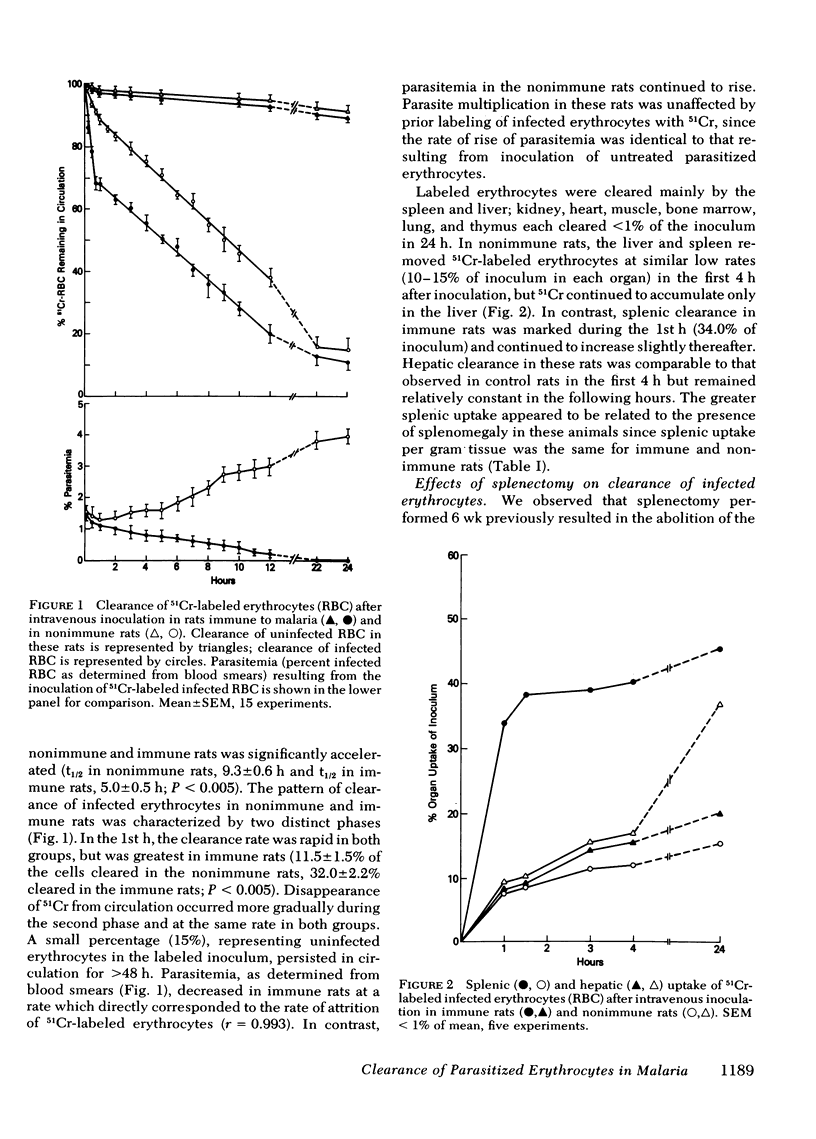
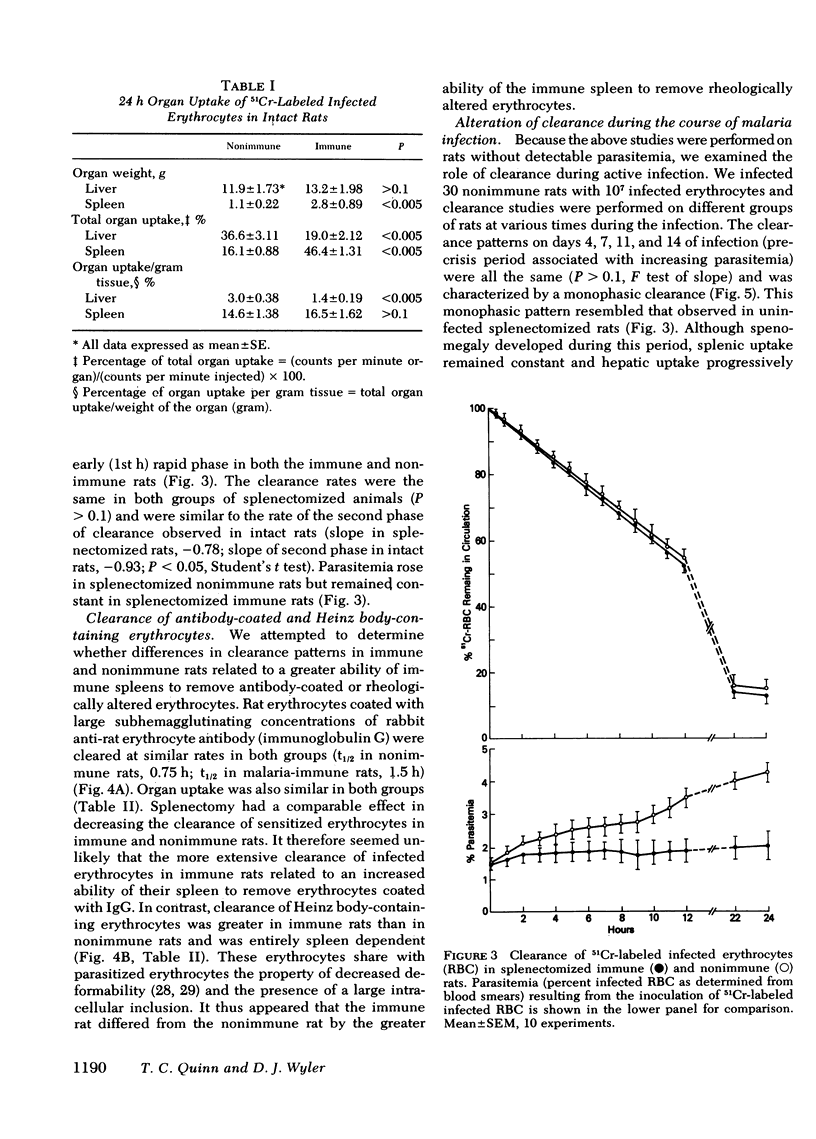
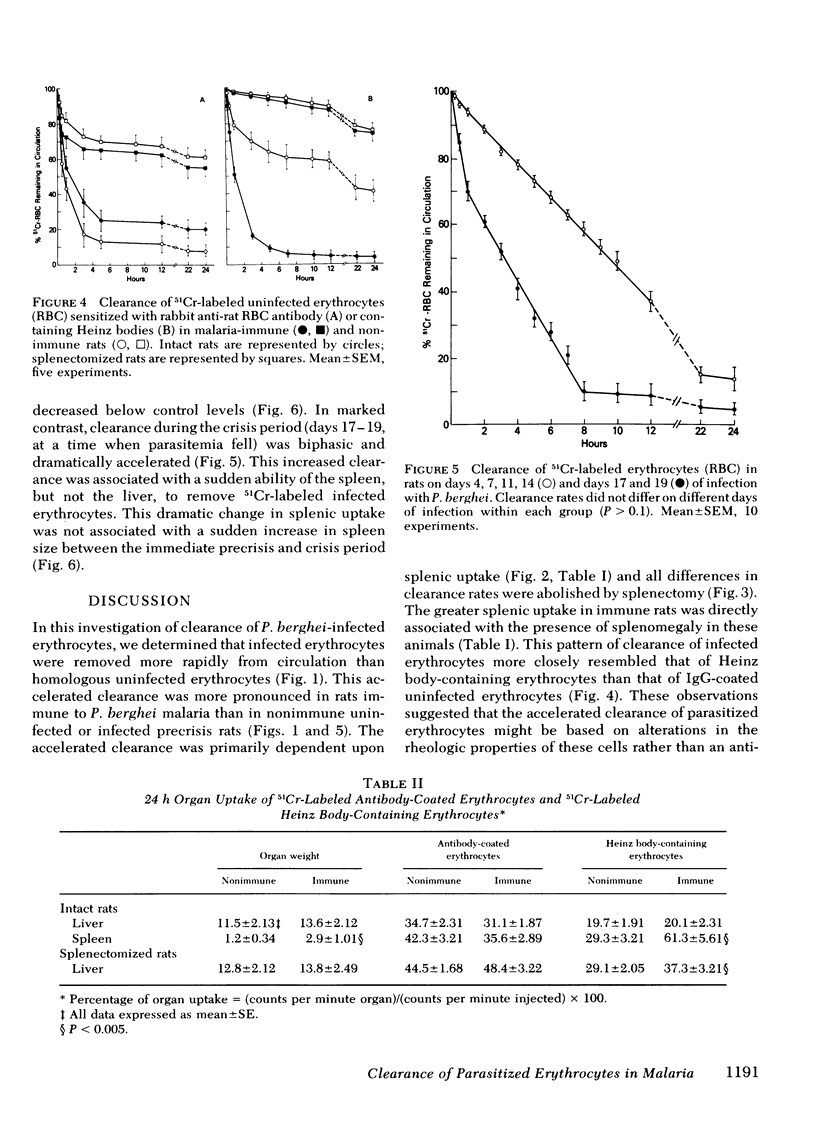
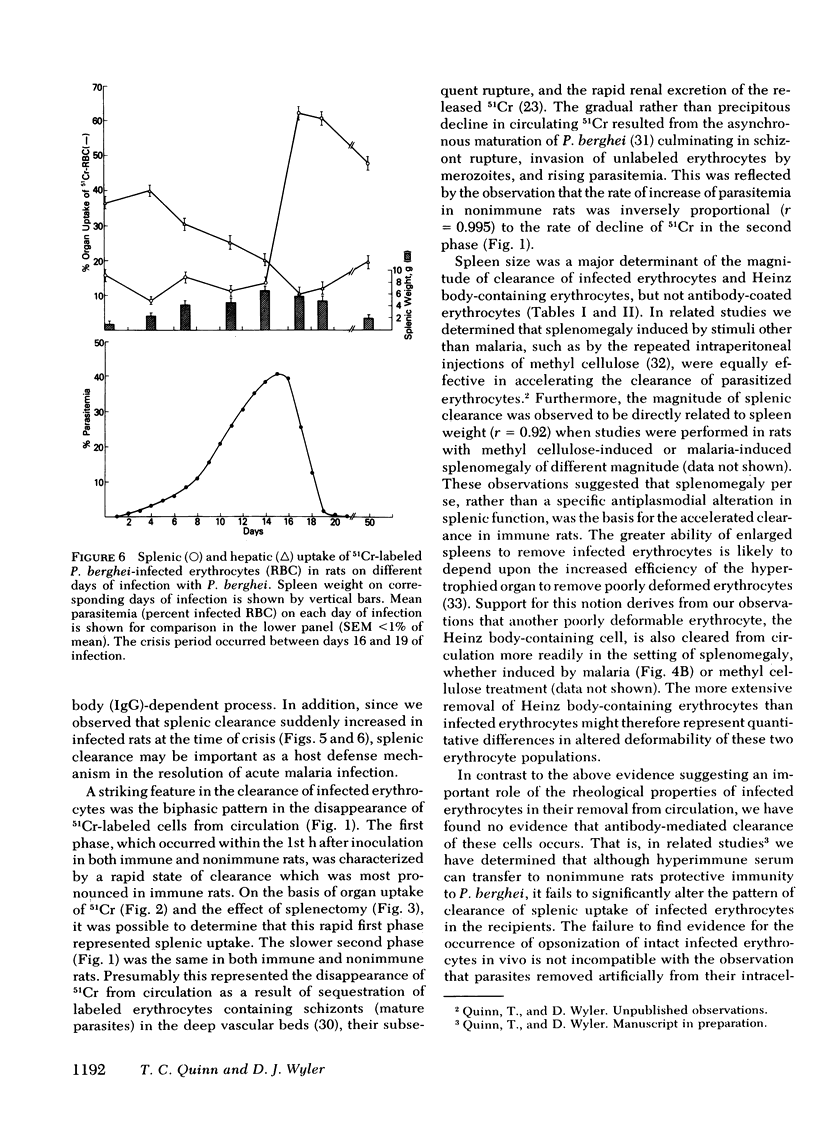
Little is known about host defense mechanisms responsible for protective immunity in malaria. The intravascular location of the infection suggested that removal of parasitized erythrocytes by reticuloendothelial organs might be important. To study this possibility, we examined the clearance of 51Crlabeled Plasmodium berghei-infected erythrocytes in rats. Infected erythrocytes were removed more rapidly from circulation than homologous uninfected erythrocytes. The rate of clearance of infected cells during the 1st hour after inoculation was approximately three times greater in rats rendered immune by prior infection than in control rats. This accelerated clearance resulted from greater splenic uptake in immune rats and appeared to correlate with spleen size. Since the clearance pattern of infected erythrocytes more closely resembled the clearance of Heinz body-containing uninfected erythrocytes than of antibody-coated (immunoglobulin G) uninfected erythrocytes, rheologic alterations of parasitized erythrocytes might be a more important determinant of clearance than an antibody-dependent process. During the phase of malaria infection in which increasing parasitemia is observed, organ uptake of infected erythrocytes did not increase despite splenic and hepatic enlargement. However during the spontaneous onset of resolution of malaria infection characterized by decreasing parasitemia, a marked enhancement of splenic clearance was noted. These observations suggest that sudden alteration in splenic clearance of parasitized erythrocytes might be important in the resolution of acute malaria.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALGER N. E. Distribution of schizonts of Plasmodium berghel in tissues of rats, mice and hamsters. J Protozool. 1963 Feb;10:6–10. doi: 10.1111/j.1550-7408.1963.tb01633.x. [DOI] [PubMed] [Google Scholar]
- Allison A. C., Clark I. A. Specific and non-specific immunity to haemoprotozoa. Am J Trop Med Hyg. 1977 Nov;26(6 Pt 2):216–222. doi: 10.4269/ajtmh.1977.26.216. [DOI] [PubMed] [Google Scholar]
- Cohen S., Butcher G. A., Crandall R. B. Action of malarial antibody in vitro. Nature. 1969 Jul 26;223(5204):368–371. doi: 10.1038/223368a0. [DOI] [PubMed] [Google Scholar]
- DEUTSCH H. F., MORTON J. I. Dissociation of human serum macroglobulins. Science. 1957 Mar 29;125(3248):600–601. doi: 10.1126/science.125.3248.600. [DOI] [PubMed] [Google Scholar]
- EBAUGH F. G., Jr, EMERSON C. P., ROSS J. F. The use of radioactive chromium 51 as an erythrocyte tagging agent for the determination or red cell survival in vivo. J Clin Invest. 1953 Dec;32(12):1260–1276. doi: 10.1172/JCI102855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnham P. C. The role of the spleen in protozoal infections with special reference to splenectomy. Acta Trop. 1970;27(1):1–14. [PubMed] [Google Scholar]
- George J. N., Stokes E. F., Wicker D. J., Conrad M. E. Studies of the mechanism of hemolysis in experimental malaria. Mil Med. 1966 Sep;131(9 Suppl):1217–1224. [PubMed] [Google Scholar]
- Hamburger J., Kreier J. P. Antibody-mediated elimination of malaria parasites (plasmodium berghei) in vivo. Infect Immun. 1975 Aug;12(2):339–345. doi: 10.1128/iai.12.2.339-345.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber H., Polley M. J., Linscott W. D., Fudenberg H. H., Müller-Eberhard H. J. Human monocytes: distinct receptor sites for the third component of complement and for immunoglobulin G. Science. 1968 Dec 13;162(3859):1281–1283. doi: 10.1126/science.162.3859.1281. [DOI] [PubMed] [Google Scholar]
- JANDL J. H., JONES A. R., CASTLE W. B. The destruction of red cells by antibodies in man. I. Observations of the sequestration and lysis of red cells altered by immune mechanisms. J Clin Invest. 1957 Oct;36(10):1428–1459. doi: 10.1172/JCI103542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANDL J. H., KAPLAN M. E. The destruction of red cells by antibodies in man. III. Quantitative factors influencing the patterns of hemolysis in vivo. J Clin Invest. 1960 Jul;39:1145–1156. doi: 10.1172/JCI104129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKIN C. R., ROWLEY D. The role of opsonins in the clearance of living and inert particles by cells of the reticuloendothelial system. J Exp Med. 1961 Sep 1;114:363–374. doi: 10.1084/jem.114.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchen A. G., Di Luzio N. R. Influence of Plasmodium berghei infections on phagocytic and humoral recognition factor activity. J Reticuloendothel Soc. 1971 Mar;9(3):237–247. [PubMed] [Google Scholar]
- Klausner M. A., Hirsch L. J., Leblond P. F., Chamberlain J. K., Klemperer M. R., Segel G. B. Contrasting splenic mechanisms in the blood clearance of red blood cells and colloidal particles. Blood. 1975 Dec;46(6):965–976. [PubMed] [Google Scholar]
- Loose L. D., di Luzio N. R. A temporal relationship between reticuloendothelial system phagocytic alterations and antibody responses in mice infected with Plasmodium berghei (NYU-2 strain). Am J Trop Med Hyg. 1976 Mar;25(2):221–228. doi: 10.4269/ajtmh.1976.25.221. [DOI] [PubMed] [Google Scholar]
- Lubin A., Desforges J. F. Effect of Heinz bodies on red cell deformability. Blood. 1972 May;39(5):658–665. [PubMed] [Google Scholar]
- Lucia H. L., Nussenzweig R. S. Plasmodium chabaudi and Plasmodium vinckei: phagocytic activity of mouse reticuloendothelial system. Exp Parasitol. 1969 Aug;25(1):319–323. doi: 10.1016/0014-4894(69)90077-0. [DOI] [PubMed] [Google Scholar]
- Miller L. H., Aikawa M., Dvorak J. A. Malaria (Plasmodium knowlesi) merozoites: immunity and the surface coat. J Immunol. 1975 Apr;114(4):1237–1242. [PubMed] [Google Scholar]
- Miller L. H., Usami S., Chien S. Alteration in the rheologic properties of Plasmodium knowlesi--infected red cells. A possible mechanism for capillary obstruction. J Clin Invest. 1971 Jul;50(7):1451–1455. doi: 10.1172/JCI106629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PALMER J. G., EICHWALD E. J., CARTWRIGHT G. E., WINTROBE M. M. The experimental production of splenomegaly, anemia and leukopenia in Albino rats. Blood. 1953 Jan;8(1):72–80. [PubMed] [Google Scholar]
- ROWLEY D. The role of opsonins in non-specific immunity. J Exp Med. 1960 Jan 1;111:137–144. doi: 10.1084/jem.111.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkind R. A. Destruction of injured red cells in vivo. Am J Med. 1966 Nov;41(5):711–723. doi: 10.1016/0002-9343(66)90032-5. [DOI] [PubMed] [Google Scholar]
- SPIEGELBERG H. L., MIESCHER P. A., BENACERRAF B. STUDIES ON THE ROLE OF COMPLEMENT IN THE IMMUNE CLEARANCE OF ESCHERICHIA COLI AND RAT ERYTHROCYTES BY THE RETICULOENDOTHELIAL SYSTEM IN MICE. J Immunol. 1963 May;90:751–759. [PubMed] [Google Scholar]
- Saba T. M. Physiology and physiopathology of the reticuloendothelial system. Arch Intern Med. 1970 Dec;126(6):1031–1052. [PubMed] [Google Scholar]
- Schulkind M. L., Ellis E. F., Smith R. T. Effect of antibody upon clearance of I-125-labelled pneumococci by the spleen and liver. Pediatr Res. 1967 May;1(3):178–184. doi: 10.1203/00006450-196705000-00004. [DOI] [PubMed] [Google Scholar]
- Sheagren J. N., Tobie J. E., Fox L. M., Wolff S. M. Reticuloendothelial system phagocytic function in naturally acquired human malaria. J Lab Clin Med. 1970 Mar;75(3):481–487. [PubMed] [Google Scholar]
- Weed R. I. The importance of erythrocyte deformability. Am J Med. 1970 Aug;49(2):147–150. doi: 10.1016/s0002-9343(70)80069-9. [DOI] [PubMed] [Google Scholar]
- Ziessman H. A. Lung uptake of 99mTc-sulfur colloid in falciparum malaria: case report. J Nucl Med. 1976 Sep;17(9):794–796. [PubMed] [Google Scholar]


