Abstract
Cytosolic foreign DNA is detected by pattern recognition receptors and mainly induces Type-I IFN production. We found that transfection of different types of DNA into various untreated cells induces Type-III IFN (IFN-lambda1) rather than Type-I IFN, indicating the presence of uncharacterized DNA sensor(s). A pull-down assay using cytosolic proteins identified that Ku70 and Ku80 are the DNA binding proteins. The knockdown studies and the reporter assay revealed that Ku70 is a novel DNA sensor inducing the IFN-lambda1 activation. The functional analysis of IFNL1 promoter revealed that PRDI and ISRE sites are predominantly involved in the DNA-mediated IFNL1 activation. A pull-down assay using nuclear proteins demonstrated that the IFN-lambda1 induction is associated with the activation of IRF-1 and IRF-7. Thus we show for the first time that Ku70 mediates type III IFN induction by DNA.
Introduction
In the activation of innate immune responses triggered by infection with bacterial or viral pathogens, microbe-specific molecular patterns in the pathogens are detected by pattern-recognition receptors (PRR).3 This ligand-receptor interaction triggers the activation of the innate immune system (1–3). Foreign DNA is recognized by membrane-bound PRR and cytoplasmic PRR. Recent studies demonstrate that DNA-dependent activator of IFN-regulatory factor (DAI) (4), absence in melanoma 2 (AIM-2) (5), Leucine-rich repeat flightless-interacting protein 1(LRRFIP1) (6), RNA polymerase III (7) and IFN-γ inducible protein 16 (IFI16) (8) are cytoplasmic PRRs and induce production of Type-I IFN or IL-1β. In this present study, we report that Ku70, a component of a heterodimeric Ku protein which is required for a variety of nuclear processes, including non-homologous end-joining DNA repair, V(D)J recombination, and telomerase maintenance (9, 10), also functions as a cytosolic PRR recognizing DNA and induces the production of IFN-λ1 (a member of Type-III IFN) (11, 12) rather than Type-I IFN. The induction is mediated via the activation of IFN regulatory factor (IRF)-1 and IRF-7.
Materials and Methods
Cell culture, mice and HIV replication assay
HEK293, HEK293T, RD and HeLa cells were obtained from ATCC. Monocyte-derived macrophages (MDM) and dendritic cells (DC) were prepared as previously described (13, 14). HIV replication assay was performed as previously described (13). Female WT C57/B6.129 mice were provided by the National Cancer Institute (NCI)-Frederick. Ku70 deficient mice on a C57/B6.129 background (15) were provided by Dr. Andre Nussenzweig (NCI, Bethesda, MD). All experiments with mice were performed in compliance with the principles and procedures outlined in the National Institutes of Health Guide for the Care and Use of Animals and were approved by NCI-Frederick Animal Care and Use Committee.
Preparation of plasmid DNA and genomic DNA
All plasmids were purified using the Endofree Plasmid Maxi kit (Qiagen). Genomic DNA was extracted from HEK293 cells using QIAamp DNA mini kit (Qiagen).
Transfection
HEK293 cells (100 × 103 cells in 3 mL/well of 6-well plates) were transfected with 1 μg of DNA or 5 nM si-RNA using TransIT-293 (Mirus Bio) or RNAiMAX (Invitrogen), according to the manufacture’s instructions. The siRNA-transfected HEK293 cells were cultured for 48 h followed by DNA transfection for 24 h. Primary monocytes were transfected with siRNA using a Nucleofactor Transfection kit (Lonza) and then differentiated into MDM as described above. DNA transfection into MDM and mouse spleen cells was performed using the Nucleofactor Transfection kit. All si-RNAs were obtained from Ambion (Supplemental Table 1).
Quantitative real-time RT-PCR (qRT-PCR)
The qRT-PCR was performed as previously described (13). All probes were obtained from Applied Biosystems (Supplemental Table 2).
Microarray analysis
Gene expression profiles of DNA-transfected cells were analyzed using the Affimatrix Chip, as previously described (13).
Preparation of cytosolic fraction and nuclear extract
The cytosolic and nuclear proteins were extracted from using a Nuclear Extraction kit (Active Motif).
Pull-down assay
A pull-down assay was performed using DNA or oligonucleotide-conjugated agarose beads as previously described (16).
Mass spectrometry analysis
Cytosolic proteins bound to beads were analyzed by mass spectrometer (LTQ XP, Thermo Finnigan) as previously described (17).
Western blot
Western blot analysis was performed as previously described (13), using anti-Ku70, anti-DAI and anti-AIM-2 Abs (Abcam), anti-Ku80, anti-IRF-1, anti-NFκB p65, and anti-NFκB p50 Abs (Cell Signaling Technology) or anti-β-actin, anti-IRF-3 and -IRF-7 Abs (Santa Cruz Biotech).
Reporter assay
Luciferase activity was measured using the Dual-Glo luciferase reporter assay system (Promega) and normalized against Renilla luciferase activity following the manufacturer’s protocol.
Statistics
All results are representative of at least three independent experiments. All values are expressed as the mean and s.d. of individual samples. Samples were analyzed using the Student’s t-test.
Results and Discussion
Transfection of various types of DNA induces activation of IFNL1 in different human cells
We have previously reported that IL-27 inhibits replication of HIV-1 and Hepatitis C virus (13, 18). In studies designed to better understand the role of IL-27 in host defense, we constructed an expression vector encoding the human IL-27 gene (pCMV9.IL27) and transiently transfected it into HEK293 cells. As controls, mock and a non-coding empty plasmid (pCMV9) were used. On three days after transfection, the culture supernatants were collected and then studied to determine their ability to inhibit HIV-1 replication in MDM. Surprisingly, anti-HIV activity was seen in both culture supernatants from pCMV9 and pCMV9.IL27 transfected cells (Fig. 1A). In contrast, culture supernatants from mock cells had no significant impact on the antiviral activity, indicating that the transfection of non-coding pCMV9 triggers the induction of anti-HIV mediators in culture supernatants.
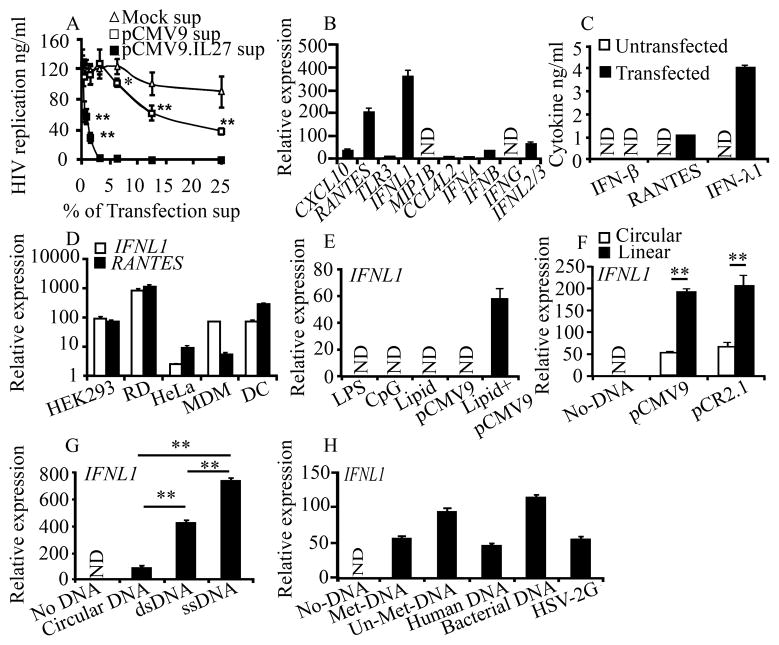
Figure 1. Various types of DNA induce IFNL1 in different human cells.
A, HIV-infected MDM were cultured with various concentrations (% v/v) of culture supernatants from pCMV9.IL27- or empty pCMV9-transfected HEK293 cells. HIV replication was determined using a p24 antigen capture assay. B, Gene expression in mock- or pCMV9- transfected HEK293 cells was confirmed by qRT-PCR. C, Cytokine concentrations in the transfection supernatants were determined via ELISA. D–H, HEK293, HeLa, RD cells, MDM or DC cells were transfected with pCMV9 (D); HEK293 cells were treated with 100 ngml−1 LPS, 1 μM CpG ODN (InvivoGen), 3 μl of transfection lipid, 1 μg pCMV9 or a combination of the lipid and pCMV9 (E); HEK293 cells were treated with 1 μg supercoil (circular) or linearlized plasmids (pCMV9 and pCR2.1) (F); circular pCR2.1 (circular DNA), linearlized pCR2.1 (dsDNA), single-stranded pCR2.1 DNA (ssDNA) (G); linearized pCR2.1 (Met-DNA), PCR-amplified full-length pCR2.1 (Un-Met DNA), human DNA or bacterial DNA for 24 h, or infected with HSV-2G (MOI=5) for 18h (H), then gene expression was analyzed by qRT-PCR. Gene expression is presented as relative expression units compared with mocked transfection after normalization to GAPDH. Data is shown as the mean ± s.d. Data are mean ± s.d. (n=3), *p<0.05, ** p<0.01. N.D.: Not Detected.
To identify the nature of the anti-HIV mediators associated with the empty vector transfection, we compared patterns of gene expression between untreated and pCMV9-transfected HEK293 cells, using DNA microarray analysis. DNA transfection up-regulated 496 genes and down-regulated 147 genes more than 2-fold compared with the untreated HEK293 control. An annotation analysis using the Database for Annotation, Visualization and Integrated Discovery (DAVID) bioinformatic tool (19) illustrated that the transfection led to an upregulation of genes associated with viral infection and immune responses including some known anti-HIV proteins, IFN-λ1 (20) and RANTES (21) (Supplemental Table 3). A qRT-PCR assay confirmed that pCMV9 transfection induced high levels of IFNL1 and RANTES mRNA with lower levels of IFNA, IFNB, and IFNL2/3 mRNA (Fig. 1B). Quantitation of cytokine concentration using ELISA indicated that pCMV9 transfection significantly produced both IFN-λ1 and RANTES; however, the induction of IFN-β was below the level of detection (<25pg/ml) (Fig. 1C).
To characterize the DNA-mediated IFNL1 activation, cell-type specificity in the gene activation was analyzed. The activation of IFNL1 and RANTES was detected not only in HEK293, but also in RD, HeLa, MDM and DC (Fig. 1D). It has been reported that DNA transfection induces RANTES (4); however, the induction of IFN-λ1 has not been reported yet. Thus, we mainly focused on the activation of IFNL1. To evaluate whether endogenous TLR4 or TLR9 is involved in IFNL1 activation, HEK293 cells were treated with LPS (TLR4 ligand) or CpG motif oligodinucleotides (TLR9 ligand), and then the gene activation was analyzed. As a positive control, MDM were treated with LPS or CpG for 6 or 24 h. Even though LPS and CpG induced IFNB mRNA within 24 h in MDM (data not shown), neither reagent had any impact on the activation of IFNL1 in HEK293 cells (Fig. 1E). Expression of TLR7, DAI, AIM-2 and LRRFIP1 mRNA was not detected after 38 cycle qRT-PCR. Western blot illustrated that neither DAI nor AIM-2 was detected (data not shown), and transfection of siRNA RNA polymerase III (POLR3F) suppressed the expression of POLR3F mRNA by 50%, the siRNA, however, had no impact on IFNL1 activation (Supplemental Fig 2); thus we concluded that none of those DNA sensors is involved in the activation of IFNL1 in HEK293 cells. To characterize the DNA-mediated IFNL1 activation, DNA length-, dose-, incubation time-, sequence- and structure dependency were assessed. The IFNL1 mRNA was induced in a DNA size-dependent (>500 bp) and dose-dependent (> 250 ng/ml) (Supplemental Fig. 1A, 1B). The kinetic experiment illustrated that the gene activation could be detected within 6 h of transfection and that activation persisted for more than 48 h (Supplemental Fig. 1C). IFNL1 mRNA was induced by both supercoil or linearized forms of pCMV9 (6.4 Kbp) and pCR2.1 (3.9 Kbp) plasmids, and the activation was significantly enhanced by the linearized plasmids (Fig. 1F). In addition, transfection of ss-pCR2.1, PCR-amplified pCR2.1 (Un-Met-DNA), human genomic DNA (~500 bp), bacterial DNA and infection of DNA virus (HSV-2G) (Fig 1G, H) also induced IFNL1 activation. Taken together, these data indicated that an uncharacterized DNA sensor recognizes DNA without any restriction in structure or sequence, and induces activation of IFNL1. The sensor may preferentially recognize long linearized DNA. Since DNA fragment of Human DNA induced IFNL1 gene activation, apoptotic cells may also induce the gene activation.
Ku70 is a novel cytosolic DNA sensor and positively regulates the activation of IFNL1
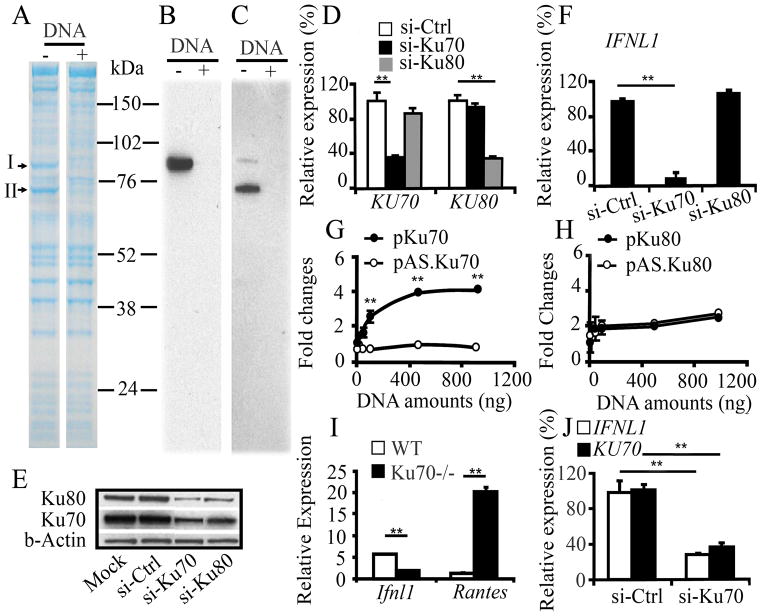
To identify the potential cytoplasmic DNA sensor present in HEK293 cells, a proteomic approach using immobilized DNA beads and mass spectrometric analysis was performed. Cytosolic fractions from untreated HEK293 cells were incubated with the DNA beads conjugated with PCR-amplified full-length pCR2.1, and then proteins bound on the beads were separated on SDS-PAGE, followed by Coomassie blue staining (Supplemental Fig. 3). To determine DNA-specific binding proteins on the gel, 10 x fold excess amounts of pCR2.1 (DNA competitor) were mixed with the cytosol fraction prior to incubation with the beads. The addition of the competitor DNA reproducibly led to the disappearance of protein bands at m.w. 80 kDa (Band I) and 70 kDa (Band II) (Fig. 2A). The two bands were analyzed by mass spectrometer. Database searching revealed that Band I is Ku80 and Band II is Ku70, respectively (Supplemental Fig. 4). Both Ku80 and Ku70 were confirmed by Western blot (Fig. 2B, C). To determine the roles of Ku70 and Ku80 in the activation of IFNL1, siRNA-Ku70 (si-Ku70) or siRNA-Ku80 (si-Ku80) were applied in HEK293 and MDM. Transfection with si-Ku70 into HEK293 cells led to a 70% reduction in KU70 mRNA and no change in KU80 mRNA compared to siRNA-Ctrl (si-Ctrl) transfected cells, while transfection with si-Ku80 led to a 75% decrease in KU80 mRNA and no change in KU70 mRNA (Fig. 2D). Western blot using cytosol fraction from the siRNA-transfected cells resulted in si-Ku70 decreasing Ku70 protein by 54% (Fig. 2E). Consistent with other reports illustrating that each subunit of the Ku protein stabilizes the other (22), the si-Ku70 transfections also decreased the protein level of Ku80 by 65% (Fig. 2E). In MDM, si-Ku70 transfection, but not si-Ku80 down regulated the expression of KU70 mRNA by 40% compared to si-Ctrl transfected cell (Supplemental Fig 5A). The transfection of si-Ku70, but not si-Ku80 significantly decreased the DNA-mediated IFNL1 activation in HEK293 cells and MDM (Fig. 2F, Supplemental Fig. 5B). HSV-2G- and the ssDNA-mediated IFNL1 activation was also significantly suppressed by si-Ku70 (Fig. 2J, Supplemental Fig. 6), indicating that only Ku70 is the positive regulator of IFNL1 activation. To further delineate the roles of Ku70 and Ku80, FLAG-tagged Ku70 (pKu70) or HA-tagged Ku80 (pKu80) expression vectors were transfected and IFNL1 promoter activation was analyzed using a reporter assay. As a control, expression vector encoding FLAG-tagged anti-sense Ku70 (pAS.Ku70) or HA-tagged Ku80 (pAS.Ku80) was applied. Transfection of pKu70 and pKu80 over-expressed Ku70 and Ku80 proteins, respectively (Supplemental Fig. 7). Over-expression of Ku70, but not Ku80 increased IFNL1 promoter activity in a dose-dependent manner (Fig. 2G, 2H). To precisely determine the role of Ku70, DNA was transfected into Ku70−/− mice spleen cells. DNA transfection induced transcripts of Ifnl1 in WT but not in Ku70−/−, while the transfection enhanced the expression of rantes (Fig. 2I). Taken together, Ku70 is a novel cytosolic DNA sensor protein and positively regulates IFNL1 activation by invaded cytosolic DNA. Since si-Ku70 inhibited the expression of RATNES mRNA in HEK293 cells (Supplemental Fig. 8), Ku70 may differentially regulate RANTES gene in between human and mouse.
Figure 2. Ku70 is a cytosolic DNA sensor positively regulating IFNL1 activation.
A, Cytosol proteins from untreated HEK293 cells were incubated with DNA-conjugated beads in the absence or presence of DNA competitor (Supplemental Fig. 3). Proteins bound to the beads were separated on SDS–PAGE under reducing conditions, followed by Coomassie blue staining. B,C, Western blot analysis using anti-Ku70 (B) or anti-Ku80 (C) antibody demonstrated intended proteins. Due to a cross reactivity in the anti-Ku70 antibody, it detected Ku70 as well as Ku80. D–E, HEK293 cells were transfected with si-Ctrl, si-Ku70 or si-Ku80, and the expression level of KU70 or KU80 mRNA (D) and protein (E) was analyzed by qRT-PCR and Western blot, respectively. The expression level of mRNA was compared with that in the cells transfected with si-Ctrl. Relative amounts of Ku70 and Ku80 protein levels were densitometrically analyzed using the NIH image, and normalized against β-actin. F, HEK293 cells were transfected with si-Ctrl, si-Ku70 or si-Ku80 followed by DNA transfection. Expression levels of IFNL1, KU70 and KU80 mRNA were determined by qRT-PCR. The level of mRNA was compared with that in the cells transfected with si-Ctrl. G–H, HEK293T cells were co-transfected with 100 ng full-length IFNL1–luciferase reporter plasmid and 10 ng Renilla luciferase plasmid with pKu70, pAS.Ku70, pKu80 or pAS.Ku80 for 24 h, then stimulated for 18 h by transfection with 500 ng of pCR2.1. I, Spleen cells from WT or KO Ku70−/− mice were transfected with linearized pCR2.1 using Nucleofactor Transfection kit, and then expression level of mRNA was analyzed by qRT-PCR. J, si-Ctrl or si-Ku70-transfected HEK293 cells were infected with HSV-2G, and then gene expression was analyzed. Data are shown as the mean ± s.d. (n = 3). **p<0.01.
IRF-1 and IRF-7 are associated with DNA-mediated IFN-λ1 activation
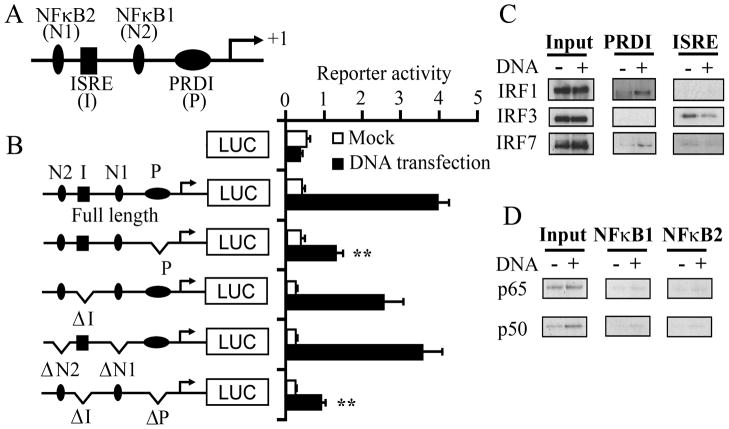
To investigate mechanism of the DNA-mediated IFNL1 activation, we constructed mutants on the IFNL1 promoter region lacking PRDI, ISRE, NFκB1, NFκB2 or different combinations (Fig. 3A, B). The construct lacking PRDI or ISRE domain predominantly reduced the Ku70-mediated IFNL1 promoter activation, while the construct lacking NFκB sites were able to induce the Ku70-mediated IFNL1 promoter activation (Fig. 3B), indicating that the PRDI and ISRE binding sites play key roles in the DNA-mediated IFNL1 activation. The pull-down assay using oligonucleotide (PRDI, ISRE, or NFκB element)-conjugated beads with nuclear extract from mock- or pCR2.1-transfected cells, followed by Western blotting, demonstrated that DNA transfection significantly induced the binding activity of IRF-1 to the PRDI element, and a subtle increase of the IRF-7 binding activity to the same element rather than IRF-3 (Fig. 3C). Analysis of the activation profile of NFκB indicated that DNA transfection increased only p65 and p50 binding activity (Supplemental Fig. 9). The pull-down assay illustrated that p65 and p50 bound to the NFκB1 and NFκB2 sites are at low, but detectable level (Fig. 3D). Taken together, these results indicated that both PRDI and ISRE sites are involved in the DNA-mediated IFNL1 activation. IRF-1 and IRF-7 play key roles in DNA-dependent IFNL1 activation.
Figure 3. PRDI and ISRE elements of the IFNL1 promoter are import for the DNA-mediated IFNL1 activation and IFNL1 activation is associated with the activation of IRF1 and 7.
A–B, Schematic representation of the IFNL1 promoter region and different mutant constructs on the IFNL1 promoter region. This diagram does not indicate the exact position of the elements. HEK293T cells were transfected with a series of variants of IFN-λ1–luciferase reporter and pTK-Ren for 24 h, and then stimulated with transfection of pCR2.1; the luciferase activities were normalized with Renilla activities and data are presented as fold inductions from promoter activity from basal promoter activation without pCR2.1 transfection. Data are shown as the mean ± s.d. (n = 3). **p<0.01. C–D, Nuclear extracts from mock- or DNA-transfected HEK293 cells were allowed to bind to oligonucleotides (PRDI or ISRE or NFκB elements from the IFNL1 promoter) conjugated to beads. Proteins bound to the beads were separated on SDS-PAGE, followed by Western blot analysis with specific antibodies.
It is known that the signaling pathway and biological activity of IFN-λ1 are the same as those of IFN-β; however, anti-proliferative activity by IFN-λ1 is lower than that by IFN-β, therefore, the selective induction of IFN-λ1 appears to be less cytotoxicity (23). The Ku70-mediated IFNL1 activation required a longer size of DNA (> 500bp DNA). Ku protein bounds to multiple sites along linear DNA on dsDNA or ssDNA (24, 25), thus, unlike IFI16 (8), binding of multiple molecules of Ku70 on DNA may need to induce the gene activation. As previously reported (9), Ku70 and Ku80 protein expressed in cytosol fraction of all cell types tested (Supplemental Fig.10A). A comparative analysis demonstrated no correlation between Ku70 expression and IFNL1 activation (Supplemental Fig. 10B), indicating that although Ku70 plays a key role to activate IFNL1 via activated IRF-1 and -7, some other factor(s) may be involved in the activation of IRFs (supplemental Fig 11). Further study needs to precisely determine the mechanism by which Ku70 induces IFN-λ1 induction sensing ds and ssDNA, and the physiological relevance in the selective induction of IFN-λ1 as innate immune response.
In summary, the present study has demonstrated a role for Ku70 protein in the innate immune responses to foreign DNA through induction of IFNL1 activation. The finding that an endogenously expressed cytosolic protein can immediately trigger IFN-λ1, but not IFN-β production in response to exogenous DNA describes a new pathway of host defense to viral infection and DNA vaccination.
Supplementary Material
Acknowledgments
We thank D. Perlegas, Q. Chen, B. Fullmer, X. Zhen, G. Degray, M. Bosche and R. Anderson for technical assistance; L. Feigenbaum and A. Nussenzweig for providing the Ku70 knockout mice; M. Grau and R. Dewar for critical reading; A. Troxell-Gussio for assistance with supplying materials.
Footnotes
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This research was supported [in part] by the National Institute of Allergy and Infectious Disease.
Abbreviations used in this paper: PRR, pattern-recognition receptors; DAI, DNA-dependent activator of interferon (IFN)-regulatory factor; AIM-2, Absence in melanoma 2; LRRFIP1, Leucine-rich repeat flightless-interacting protein 1; IFI16, IFN-γ inducible protein 16; HEK293, human embryonic kidney cell line 293; RD, human rhabdomyosarcoma cell line; HeLa, cervical cancer cell line; IRF, IFN regulatory factor; siRNA, small interfering RNA; si-Ctrl, siRNA-control; siKu70, siRNA-Ku70; si-Ku80, siRNA-Ku80, MDM, monocytes-derived macrophages; DC, dendritic cells; AS, anti-sense.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- 2.Pichlmair A, Reis ESC. Innate recognition of viruses. Immunity. 2007;27:370–383. doi: 10.1016/j.immuni.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Ranjan P, Bowzard JB, Schwerzmann JW, Jeisy-Scott V, Fujita T, Sambhara S. Cytoplasmic nucleic acid sensors in antiviral immunity. Trends Mol Med. 2009;15:359–68. doi: 10.1016/j.molmed.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, Ban T, Lu Y, Miyagishi M, Kodama T, Honda K, Ohba Y, Taniguchi T. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 5.Bird L. Innate immunity: Ready, AIM, fire! Nature reviews. 10:287. doi: 10.1038/nri2771. [DOI] [PubMed] [Google Scholar]
- 6.Yang P, An H, Liu X, Wen M, Zheng Y, Rui Y, Cao X. The cytosolic nucleic acid sensor LRRFIP1 mediates the production of type I interferon via a beta-catenin-dependent pathway. Nat Immunol. 11:487–494. doi: 10.1038/ni.1876. [DOI] [PubMed] [Google Scholar]
- 7.Chiu YH, Macmillan JB, Chen ZJ. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell. 2009;138:576–591. doi: 10.1016/j.cell.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, Sharma S, Sirois CM, Jin T, Latz E, Xiao TS, Fitzgerald KA, Paludan SR, Bowie AG. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11:997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker JR, Corpina RA, Goldberg J. Structure of the Ku heterodimer bound to DNA and its implications for double-strand break repair. Nature. 2001;412:607–614. doi: 10.1038/35088000. [DOI] [PubMed] [Google Scholar]
- 10.Lieber MR, Ma Y, Pannicke U, Schwarz K. Mechanism and regulation of human non-homologous DNA end-joining. Nat Rev Mol Cell Biol. 2003;4:712–720. doi: 10.1038/nrm1202. [DOI] [PubMed] [Google Scholar]
- 11.Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, Langer JA, Sheikh F, Dickensheets H, Donnelly RP. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol. 2003;4:69–77. doi: 10.1038/ni875. [DOI] [PubMed] [Google Scholar]
- 12.Sheppard P, Kindsvogel W, Xu W, Henderson K, Schlutsmeyer S, Whitmore TE, Kuestner R, Garrigues U, Birks C, Roraback J, Ostrander C, Dong D, Shin J, Presnell S, Fox B, Haldeman B, Cooper E, Taft D, Gilbert T, Grant FJ, Tackett M, Krivan W, McKnight G, Clegg C, Foster D, Klucher KM. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol. 2003;4:63–68. doi: 10.1038/ni873. [DOI] [PubMed] [Google Scholar]
- 13.Fakruddin JM, Lempicki RA, Gorelick RJ, Yang J, Adelsberger JW, Garcia-Pineres AJ, Pinto LA, Lane HC, Imamichi T. Noninfectious papilloma virus-like particles inhibit HIV-1 replication: implications for immune control of HIV-1 infection by IL-27. Blood. 2007;109:1841–1849. doi: 10.1182/blood-2006-02-001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang D, Chen Q, Stoll S, Chen X, Howard OM, Oppenheim JJ. Differential regulation of responsiveness to fMLP and C5a upon dendritic cell maturation: correlation with receptor expression. J Immunol. 2000;165:2694–2702. doi: 10.4049/jimmunol.165.5.2694. [DOI] [PubMed] [Google Scholar]
- 15.Ouyang H, Nussenzweig A, Kurimasa A, Soares VC, Li X, Cordon-Cardo C, Li W, Cheong N, Nussenzweig M, Iliakis G, Chen DJ, Li GC. Ku70 is required for DNA repair but not for T cell antigen receptor gene recombination In vivo. J Exp Med. 1997;186:921–929. doi: 10.1084/jem.186.6.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osterlund PI, Pietila TE, Veckman V, Kotenko SV, Julkunen I. IFN regulatory factor family members differentially regulate the expression of type III IFN (IFN-lambda) genes. J Immunol. 2007;179:3434–3442. doi: 10.4049/jimmunol.179.6.3434. [DOI] [PubMed] [Google Scholar]
- 17.Ilani T, Khanna C, Zhou M, Veenstra TD, Bretscher A. Immune synapse formation requires ZAP-70 recruitment by ezrin and CD43 removal by moesin. J Cell Biol. 2007;179:733–746. doi: 10.1083/jcb.200707199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frank AC, Zhang X, Katsounas A, Bharucha JP, Kottilil S, Imamichi T. Interleukin-27, an anti-HIV-1 cytokine, inhibits replication of hepatitis C virus. J Interferon Cytokine Res. 30:427–431. doi: 10.1089/jir.2009.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 20.Hou W, Wang X, Ye L, Zhou L, Yang ZO, Riedel E, Ho WZ. Lambda interferon inhibits human immunodeficiency virus type 1 infection of macrophages. J Virol. 2009;83:3834–3842. doi: 10.1128/JVI.01773-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simmons G, Clapham PR, Picard L, Offord RE, Rosenkilde MM, Schwartz TW, Buser R, Wells TN, Proudfoot AE. Potent inhibition of HIV-1 infectivity in macrophages and lymphocytes by a novel CCR5 antagonist. Science. 1997;276:276–279. doi: 10.1126/science.276.5310.276. [DOI] [PubMed] [Google Scholar]
- 22.Smider V, Rathmell WK, Lieber MR, Chu G. Restoration of X-ray resistance and V(D)J recombination in mutant cells by Ku cDNA. Science. 1994;266:288–291. doi: 10.1126/science.7939667. [DOI] [PubMed] [Google Scholar]
- 23.Steen HC, Gamero AM. Interferon-lambda as a potential therapeutic agent in cancer treatment. J Interferon Cytokine Res. 2010;30:597–602. doi: 10.1089/jir.2010.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blier PR, Griffith AJ, Craft J, Hardin JA. Binding of Ku protein to DNA. Measurement of affinity for ends and demonstration of binding to nicks. J Biol Chem. 1993;268:7594–7601. [PubMed] [Google Scholar]
- 25.Torrance H, Giffin W, Rodda DJ, Pope L, Haché RJ. Sequence-specific binding of Ku autoantigen to single-stranded DNA. J Biol Chem. 1998;273:20810–20819. doi: 10.1074/jbc.273.33.20810. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.