Abstract
We have previously reported that, depending on their activation status, mouse γδ T cells can either enhance or inhibit the activity of IL-17+ autoreactive T cells in EAU. In the present study, we showed that γδ T cells in naïve C57BL/6 (B6) mouse do not express the IL-23R, whereas, in immunized mice, it is expressed on more than 50% of γδ T cells. In vitro studies showed that IL-23R expression on γδ T cells is modulated by their state of activation, as weakly activated γδ T cells expressed the IL-23R, but highly activated γδ T cells did not. Functional studies showed that IL-23R+ γδ T cells had the strongest suppressive effect on IL-17+ autoreactive T cells and that this effect was inhibited when the IL-23R was blocked by anti-IL-23R antibody or in the presence of excessive amounts of exogenous IL-23. We conclude that the balance between the enhancing and inhibitory effects of γδ T cells is regulated by their level of IL-23R expression. The expression of variable IL-23R levels allows γδ T cells to have different regulatory effects on adaptive immune responses, conceivably as a result of αβ and γδ T cells competing for IL-23.
Keywords: autoimmunity, EAU, Interleukin-17, IL-23 receptor, Th17, uveitis
Introduction
γδ T cells play a role in the regulation of inflammatory processes associated with infections, tumors, and autoimmunity (1–6). Studies have shown that γδ T cells can either enhance (7–9) or inhibit (2,10–12) an adaptive immune response and that γδ T cell subsets expressing distinct T cell receptors (TCRs) show functional diversity (13–16). Recent studies have shown that the regulatory effect of γδ T cells is not a stable feature, but fluctuates with γδ T cell activation status (17,18). The mechanisms by which γδ T cells enhance or inhibit an adaptive immune response are incompletely understood, and a better understanding of the flexible regulatory effect of γδ T cells should facilitate the development of γδ T cell-targeted immunotherapies for related diseases.
In this study, to define the mechanism by which γδ T cells regulate the autoimmune response, we examined whether the enhancing and inhibitory effects of γδ T cells can be predicted based on the presence of specific biomarkers. By comparing the enhancing or suppressive activities of γδ T cells activated to different extents or by different pathways, we found that the interleukin-23 receptor (IL-23R) was such a marker. Our results showed that IL-23R expression differed between γδ and αβ T cells, that levels of surface-expressed IL-23R were different in γδ T cells activated to different extents, and that Vγ4+ γδ T cells and Vγ1+ γδ T cells differed greatly in IL-23R expression. Functional studies showed that the suppressive effect of γδ T cells was positively correlated with levels of IL-23R expression, both in vitro and in vivo. Manipulation of IL-23R function on γδ T cells using an anti-IL-23 antibody or by the presence of high amounts of exogenous IL-23 reduced γδ T cell suppressive activity. We also showed that γδ T cells express the IL-23R in a biphasic fashion, as partially activated γδ T cells, but not non-activated or highly activated γδ T cells expressed the IL-23R. Weak activation of previously non-activated γδ T cells led to IL-23R expression, whereas exposure to a combination of stimulants resulted in highly activated γδ T cells with no IL-23R expression. We conclude that the enhancing and inhibitory effects of γδ T cells are switched on and off during γδ T cell activation and that the expression of variable IL-23R levels allows γδ T cells to exert different regulatory effects on the adaptive immune response, conceivably by competition between αβ and γδ T cells for IL-23.
Methods
Animals and reagents
Female C57BL/6 (B6) mice were purchased from Jackson Laboratory (Bar Harbor, ME) and were housed and maintained in the animal facilities of the University of Southern California. Institutional approval was obtained and institutional guidelines regarding animal experimentation followed. Recombinant murine IL-23 and IL-12 were purchased from R & D (Minneapolis, MN). Fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated antibodies against mouse IFN-γ, IL-17, αβ T cell receptor (TCR), γδTCR and anti-mouse Vγ1/Vγ4 were purchased from Biolegend (San Diego, CA). PE-anti-IL23R antibody was purchased from R&D Systems, Inc (Minneapolis, MN).
Immunization procedure and in vitro stimulation of in vivo primed T cells
B6 mice were immunized subcutaneously over 6 spots at the tail base and on the flank with 200 μl of emulsion containing 150 μg of the uveitogenic peptide IRBP1–20 [amino acids 1-20 of human interphotoreceptor retinoid-binding protein (IRBP; Sigma, St. Louis, MO)] emulsified in complete Freund’s adjuvant (CFA; Difco, Detroit, MI). Concurrently, 200 ng of pertussis toxin (PTX) (Sigma, St. Louis, MO) was injected intraperitoneally. At day 13 post-immunization, T cells were isolated from lymph node cells and spleen cells by passage through a nylon wool column, then 1 x 107 cells in 2 ml of RPMI 1640 medium (Cellgro, VA, USA) containing 10% fetal calf serum were added to each well of a 6-well plate (Costar) and stimulated for 48 h with 10 μg/ml of IRBP1–20 in the presence of 1 x 107 irradiated syngeneic spleen cells as antigen-presenting cells (APCs) in the presence of either IL-12 (Th1 polarized) or IL-23 (Th17-polarized) (10 ng/ml), then activated T cell blasts were separated by Ficoll gradient centrifugation and cultured for another 72 h in the same medium used for stimulation without the peptide.
γδ T cell preparation
γδ T cells were purified from IRBP1–20 immunized B6 mice (5,19,20). Nylon wool-enriched splenic T cells from immunized mice were incubated for 10 min at 4°C with FITC-conjugated anti-mouse γδ TCR or αβ TCR antibody, then for 15 min at 4°C with anti-FITC Microbeads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). The cells were then separated into bound and non-bound on an autoMACSTM separator column (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). The purity of the isolated cell, as determined by flow cytometric analysis PE-conjugated antibodies against αβ or γδ T cells was >95%. Resting cells were harvested from this isolate after culture in cytokine-free medium for 5-7 days, when they showed down-regulation of CD69 expression. Activated γδ T cells were prepared by incubating the resting γδ T cells with anti-γδTCR (GL3) and anti-CD28 antibodies (2 μg/ml) for 2 days.
Assessment of in vitro effect of IL-23+ and IL-23R− γδ T cells
Responder αβ T cells (1 x 106/well) were co-cultured with IRBP1–20 (10 μg/ml) and antigen presenting cells (APCs) (irradiated spleen cells) in a 24-well plate, with or without addition of 2% (2 x 104/well) of IL-23+ or IL-23R− γδ T cells. The numbers of antigen-specific T cells expressing IL-17 or IFN-γ determined by intracellular staining followed by FACS analysis after 5 days of stimulation.
Testing the in vivo effect of injected γδ T cells
TCR-δ−/− mice were randomly grouped and left untreated or injected intraperitoneally with IL-23R+ or IL-23R− γδ T cells (5 x 105), prepared as described above, then all the mice were immunized with a pathogenic dose of IRBP1–20. On day 13 post-immunization, CD4+αβTCR+ T cells were separated by magnetic sorting from lymph node and spleen cells and stimulated with 10 μg/ml of immunizing peptide in the presence of irradiated syngeneic spleen cells. The activated T cell blasts were then separated by Ficoll gradient centrifugation and cultured in polarizing culture medium for 3 days, then cytoplasmic expression of IFN-γ and IL-17 by the responder T cells was determined and cytokine levels in the culture supernatants assessed.
Cytokine assays
Enriched T cells (3 x 104 cells/well) from the draining lymph nodes and spleens were cultured at 37° C for 48 h in 96-well microtiter plates with irradiated syngeneic spleen APCs (1 x 105) in the presence of IRBP1–20, then a fraction of the culture supernatant was assayed for IL-17 using ELISA kits (R & D).
Intracellular staining and FACS analysis
For intracellular staining, T cells (2 x 105 in 100 μl of PBS) were incubated for 4 h with 50 ng/ml of PMA, 1 μg/ml of ionomycin, and 1 μg/ml of brefeldin A (Sigma-Aldrich), then were washed, fixed, permeabilized overnight with Cytofix/Cytoperm buffer (eBioscience), intracellularly stained with antibodies against IFN-γ and IL-17, and analyzed on a FACScalibur flow cytometer.
Statistical analysis
Experiments were repeated at least twice, usually three or more times. Experimental groups were typically composed of four mice. The figures show data from a representative experiment. Differences between the values for different groups were examined using the two-tailed t test.
Results
Biphasic expression of the IL-23R by γδ T cells
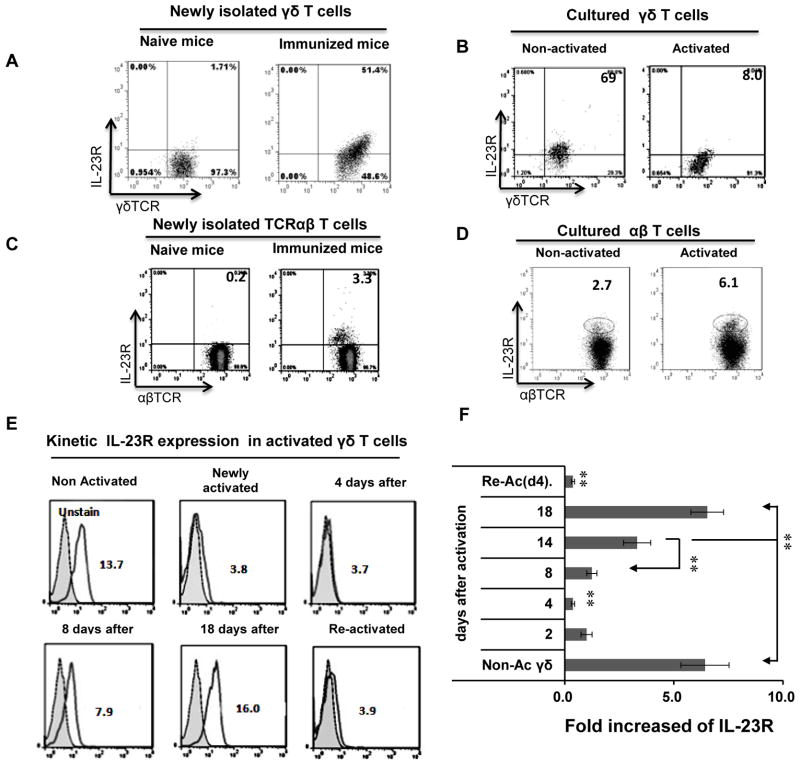
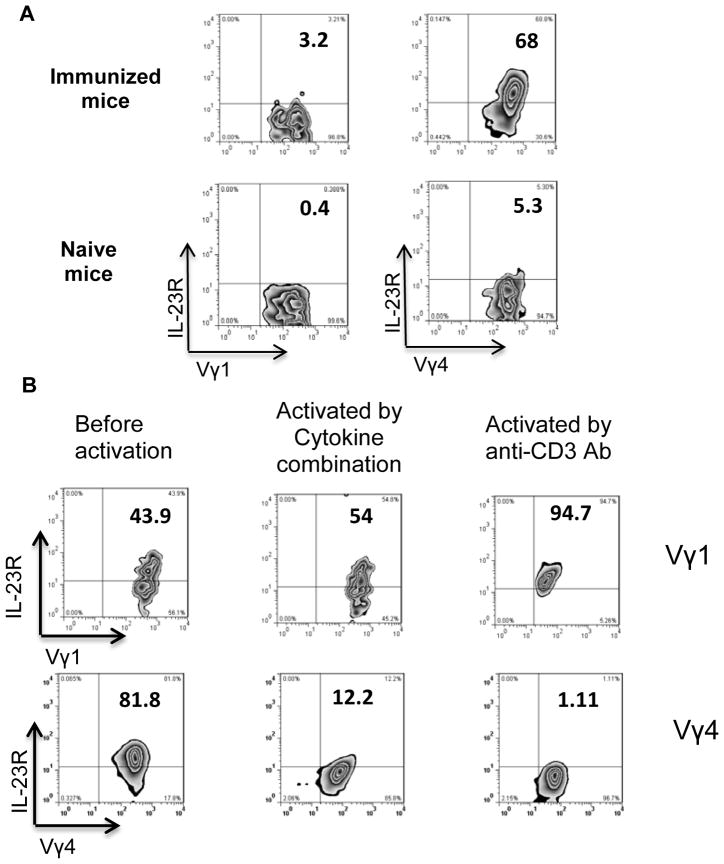
We first measured numbers of IL-23R+ γδ T cells in naïve and immunized mice. As shown in Fig. 1A, none of the γδ T cells in naïve B6 mice expressed appreciable amounts of the IL-23R, whereas this receptor was expressed by a high percentage (51.4%) of γδ T cells from mice immunized with interphotoreceptor retinoid-binding protein (IRBP) in complete Freund’s adjuvant (CFA). We then examined IL-23R expression on γδ T cells isolated from immunized B6 mice and cultured in cytokine-free medium for 5–10 days (resting cells). As shown in Fig. 1B, resting cultured γδ T cells expressed the IL-23R, but activation by 48 h exposure to a combination of cytokines resulted in a marked decrease in IL-23R expression from 69% of cells to 8%. We also compared IL-23R expression by γδ and αβ T cells freshly isolated from naïve and immunized mice and found that it was expressed on 0.2% of αβ T cells from naïve mice and only 3–5% of αβ T cells from immunized mice (Fig. 1C), contrasting with expression on 50–60% of γδ T cells from immunized mice (Fig. 1A). Moreover, only a small percentage of resting cultured αβ T cells expressed the IL-23R following 48h activation by incubation with anti-CD3 antibodies (increase from 2.7 % to 6.1%) (Fig. 1D). A kinetic study (Fig. 1E and 1F) showed that resting γδ T cells expressed high levels of IL-23R protein and mRNA, whereas, two days after in vitro activation the IL-23R was undetectable and remained so for approximately a week, then gradually reappeared and was present at high levels for at least 18 days, the longest time tested, then was again lost on re-activation. Thus, cultured γδ T cells expressed high IL-23R levels until they were exposed to a new activation signal after being rested.
Fig. 1. Biphasic IL-23R expression by γδ T cells.
(A) More than half of γδ T cells from IRBP1–20/CFA-immunized, but not naïve, mice express the IL-23R. Splenic T cells from naïve (left panel) or IRBP1–20/CFA-immunized mice at 13 days after immunization (right panel) were double-stained with antibodies specific for γδ T cells (GL3) and the IL-23R.
(B) Highly activated γδ T cells are IL-23R. Cultured γδ T cells were left untreated (left panel) or exposed to a combination of cytokines (IL-1, IL-7, and IL-23) for 48 h (right panel), then IL-23R expression was examined.
(C) IL-23R expression by αβ T cells newly isolated from naïve and immunized mice.
(D) IL-23R expression by cultured αβ T cells before and after in vitro activation.
(E and F) Kinetic study of IL-23R expression on γδ T cells after a single activation step using a mixture of cytokines (IL-2, IL-7, and IL-23). The cells were cultured in cytokine-free medium containing 10% FCS. IL-23R expression was measured on rested and newly activated γδ T cells and at 4–18 days after activation (E), and IL-23R mRNA expression was measured by real-time PCR (F).
In all panels, the results shown are from a single experiment and are representative of those obtained in >5 experiments.
Weakly activated γδ T cells express the highest IL-23R levels
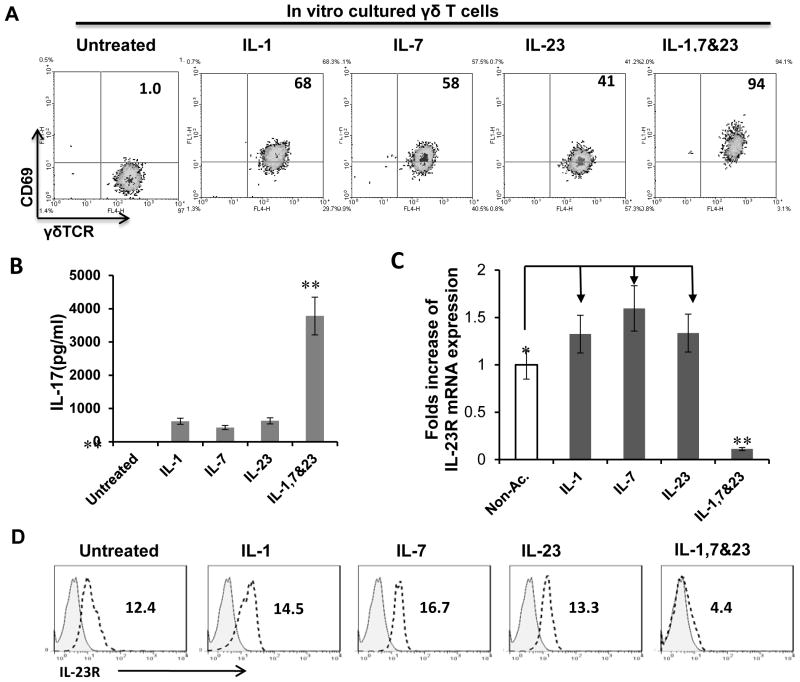
Our studies showed that cytokines, particularly IL-1, 7, and IL-23, are able to activate γδ T cells; in addition, these cytokines have a synergistic effect on γδ activation (Figure 2); therefore, in some cases we used a combination of three cytokines to activate γδ T cells. After exposure of resting cultured γδ T cells to a single cytokine (IL-1, IL-7, or IL-23) or a combination of all three, the activated cells expressed increased levels of CD69 (Fig. 2A) and produced increased amount of IL-17 (Fig. 2B), with the combination of three cytokines having a much greater effect. In contrast, while a single cytokine increased expression of IL-23R mRNA and surface IL-23R, the combination of all three resulted in almost complete loss of expression (Fig. 2C and D).These results suggest that a combination of different cytokines has a synergistic effect on γδ T cell activation, but only weakly activated γδ T cells show increased IL-23R levels.
Fig. 2. Weakly activated γδ T cells are IL-23R+.
(A) γδ T cells express high levels of CD69 after exposure to single cytokine or a combination of cytokines.
(B) ELISA test for IL-17 production. After exposure to a single cytokine or a mixture of cytokines (IL-1, IL-7, IL-23), γδ T cells produce higher amounts of IL-17.
(C) Real-time PCR assay for IL-23R mRNA expression. After exposure to a single cytokine (IL-1, IL-7, or IL-23), γδ T cells express increased IL-23R levels, whereas, after exposure to multiple cytokines, IL-23R expression is suppressed. **p < 0.01; * p<0.05.
(D) Cell surface expression of IL-23R of γδ T cells after exposure to indicated cytokine or cytokines.
The suppressive effect is restricted to IL-23R+ γδ T cells
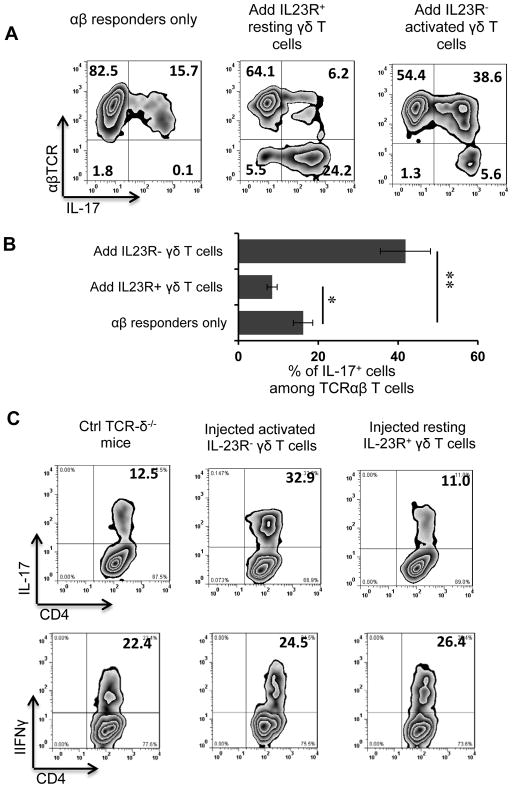
We then compared the regulatory effect of IL-23R+ and IL-23R− γδ T cells on the IL-17+IRBP-specific T cell response in vitro. As we reported previously (17,18), addition of a small number of cultured γδ T cells to IRBP-specific αβ T cells prepared from immunized TCR-δ−/− mice greatly affected the response of the αβ T cells. As shown in Fig. 3A&B, addition of 2% of IL-23R− highly activated γδ T cells enhanced the IL-17+ αβ T cell response from 15.7% to 37.6%, whereas addition of 2% of IL-23R+ resting γδ T cells inhibited the response, which dropped from 15.7 to 6.2%. To determine whether IL-23R+ and IL-23R− γδ T cells had a similar or different regulatory effect on the IL-17+ IRBP-specific T cell response in vivo, groups of TCR-δ−/− mice (n=6) were immunized with the uveitogenic peptide (IRBP1–20/CFA) with or without i.p. transfer of 105 IL-23R+ resting or IL-23R− highly activated γδ T cells 5 days after immunization, then, at 13 days post-immunization, splenic T cells were enriched and subjected to stimulation with the immunizing peptide for 5 days, then IL-17+ αβTCR+ T cells were measured by cytoplasmic staining. As shown in Fig. 3C, mice that received IL-23R− highly activated γδ T cells, but not resting IL-23R+ γδ T cells, showed a significant increase in the percentage of IL-17+ IRBP-specific T cells.
Fig. 3. IL-23R+ γδ T cells show strong suppressive activity.
(A) Addition of 2% IL-23R+, but not IL-23R−, γδ T cells to responder αβ T cells inhibits the Th17 response. Responder αβ T cells were obtained from TCR-δ−/− mice immunized with IRBP1–20/CFA and were stimulated (1 x 106/well) for 5 days with immunizing peptide and APCs without (left panel) or with addition of 2 x 104/well resting IL-23R+ (center panel) or activated IL-23R− (right panel) γδ T cells, then the activated T cells were separated, stained intracellularly for IL-17, and subjected to FACS analysis.
(B) Summarized results for five assays showing the percentage of IL-17+ IRBP-specific T cells in 107 αβ T cells from an immunized mouse after 5 days in vitro expansion in the absence or presence of 2% IL-23R− or IL-23R+ γδ T cells. **p < 0.01; * p<0.05.
(C) Groups of TCR-δ−/− mice (n=6) immunized with the uveitogenic peptide (IRBP1-20/CFA) were left untreated or were injected i.p. with 105 IL-23R+ or IL-23R− γδ T cells 5 days after immunization, then, at 13 days post-immunization, splenic T cells were enriched and stimulated with immunizing peptide under Th17-polarized conditions and the activated T cells separated and stained for intracellular expression of IL-17 (top row) or IFNγ (bottom row).
IL-23R+ γδ T cells treated with anti-IL-23R antibody have a reduced suppressive effect on Th17 cells
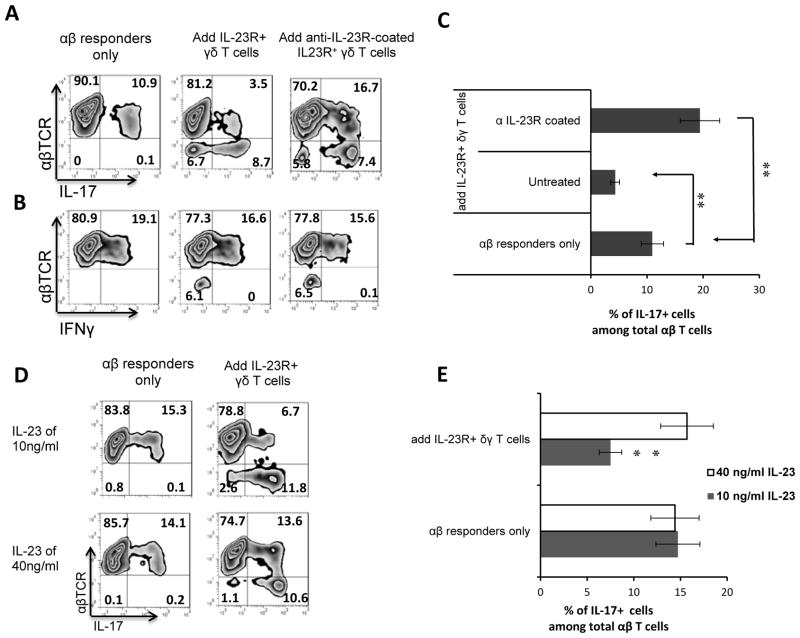
One possible mechanism for the increased suppressive activity of IL-23R+ γδ T cells was that IL-23R+ γδ T cells bind the IL-23 that is needed for the activation of IL-17+ autoreactive T cells. To test this possibility, we assessed the inhibitory effect of IL-23R+ γδ T cells on the activation of both Th17 and Th1 IRBP-specific T cells. The responder T cells were stimulated with the immunization antigen (IRBP) under either Th1- (Fig. 4A) or Th17- (Fig. 4B) polarizing conditions in the presence or absence of 2% γδ T cells that had been previously left untreated or incubated with anti-IL-23R antibody. As shown in Fig. 4, treatment with anti-IL-23R antibody reduced the suppressive activity of IL-23R+ γδ T cells on autoreactive Th17 cells (Fig. 4A and 4C), but only had a very limited effect on autoreactive Th1 T cells (Fig. 4B), supporting the idea that competition for IL-23 is the mechanism by which γδ T cells suppress Th17 cells.
Fig. 4. Anti-IL-23R antibody or a high concentration of IL-23 reduces the suppressive effect of IL-23R+ γδ T cells.
(A&B) αβ T cells (1 x 106/well) from TCR-δ−/− mice immunized with IRBP1–20/CFA were stimulated with immunizing peptide and APCs under Th17 (A) or Th1 (B) polarized conditions. The figure shows the percentage of IL-17+ and IFN-γ+ αβ T cells generated in the absence or presence of 2% (2 x 104/well) IL-23R+ γδ T cells that had been previously left untreated or pretreated with anti-IL-23R antibodies.
(C) Summary statistics of multiple experiments shown in Fig 4A and 4B. **p < 0.01.
(D) Addition of a high concentration of IL-23 reduces the suppressive effect of IL-23R+ γδ T cells. The protocol for in vitro activation of Th17 cells was that described in Fig. 3. The responses shown are those of IL-17+ αβTCR+ T cells with or without addition of IL-23R+ γδ T cells in the presence of either the normally optimal concentration (10 ng/ml) or a high concentration (40 ng/ml) of IL-23.
(E) Summary statistics of multiple experiments shown in Fig 4D. **p < 0.01.
Addition of excess IL-23 inhibits the suppressive effect of IL-23R+ γδ T cells
We hypothesized that the suppressive effect might be due to competitive binding of IL-23 by the earlier responding innate γδ T cells, thus preventing the later responding IL-23-dependent αβ T cells from reacting. In cell activation studies under Th17 polarized conditions, we previously found that the optimal concentration of IL-23 was 10 ng/ml.. If the suppressive effect was due to IL-23 binding by innate γδ T cells, it should be reduced if large amounts of IL-23 were provided. We therefore set up two parallel assay groups in which Th17+ T cell activation was performed under Th17 polarized conditions in the presence of either the standard concentration (10 ng/ml) or a high concentration (40 ng/ml) of IL-23. As shown in Fig. 4D and 4E, addition of a high concentration of IL-23 markedly reduced the suppressive effect of IL-23R+ γδ T cells, suggesting that their ability to bind IL-23 is involved in their inhibitory effect.
IL-23R+, but not IL-23R−, γδ T cells proliferate vigorously when added to αβ responder T cells under Th17 polarized conditions
To further test the possibility that competition for IL-23 was responsible for the γδ T cell suppressive effect, we assessed the number of proliferating αβ and γδ T cells after 5 days of in vitro stimulation of responder αβ T cells derived from IRBP-immunized TCR-δ−/− mice with immunizing peptide in the presence of 2% IL23+ resting or IL23− highly activated γδ T cells under Th17 polarized conditions. As shown in Fig. 5, while IL-23R+ resting γδ T cells did not promote the activation of αβ T cells, the γδ T cells themselves proliferated vigorously, in sharp contrast to the response of cultures with added IL-23R− activated γδ T cells, in which αβ T cell proliferation was dominant.
Fig. 5. IL-23R+ γδ T cells proliferate vigorously while they suppress the αβ T cell response.
In a 24-well plate, 2% (1 x 104/well) IL-23R+ or IL-23R− γδ T cells were added to 106 αβ responder T cells from IRBP/CFA-immunized TCR-δ−/− mice. After 5 days antigenic stimulation under Th17-polarizing conditions, the activated T cells were separated by Ficoll gradient centrifugation, then surface stained with anti-mouse αβTCR or γδTCR antibodies and stained intracellularly for IL-17. The results of two separate experiments are shown.
Fetal calf serum contains a factor or factors that support IL-23R expression by cultured γδ T cells
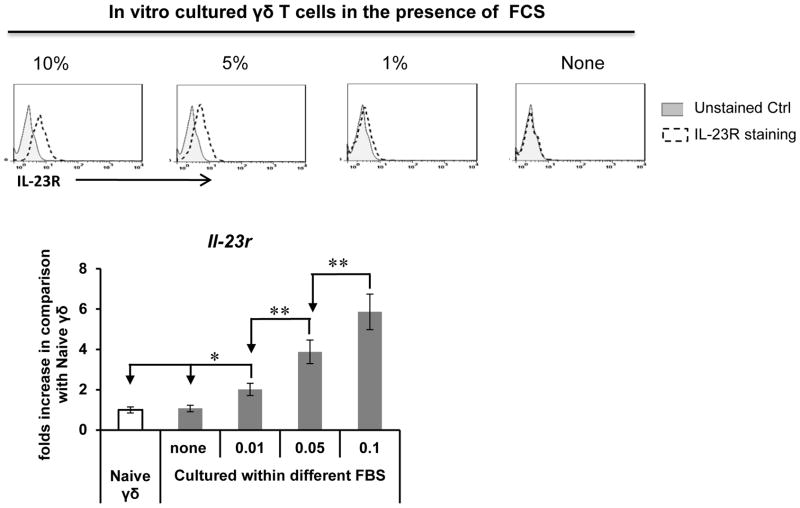
As mentioned earlier, γδ T cells from naïve mice do not express the IL-23R, whereas resting cultured γδ T cells express high IL-23R levels, even after prolonged culture in cytokine-free medium when the cells no longer express activation markers, such as CD69. To examine the possibility that factors in the culture medium other than added cytokines were able to maintain IL-23R expression on γδ T cells, we tested whether fetal calf serum (FCS)-containing medium helped retain IL-23R expression. As show in Fig. 6, when we cultured γδ T cells for 4 days in cytokine-free medium containing 0–10% of heat-inactivated FCS, we found that γδ T cells cultured in FCS-free medium no longer expressed the IL-23R, whereas those cultured in medium containing >3% FCS showed partial or complete retention of IL-23R expression, showing that FCS contains a factor or factors that support IL-23R expression by γδ T cells.
Fig. 6. Fetal calf serum (FCS) contains a factor or factors that help γδ T cells retain IL-23R expression.
Upper panels: A γδ T cell line was cultured for 4 days in cytokine-free medium containing the indicated percentage of FCS, then was stained using a FITC-conjugated anti-IL-23R antibody. Bottom panel: real-time assay for IL-23 mRNA levels in the treated cells expressed relative to that in naïve γδ T cells. **p < 0.01; * p<0.05.
Different IL-23R expression by Vγ1+ and Vγ4+ γδ T cells
A previous study in Listeria monocytogenes-infected mice showed that the IL-23R is expressed by γδ T cells expressing TCR-Vγ4 or TCR-Vγ6, but not by γδ T cells expressing TCR-Vγ1 (21). To determine whether the IL-23R-expressing γδ T cells in B6 mice at 13 days after IRBP immunization belonged to different γδ T cell subsets expressing different TCR segments, we compared IL-23R expression of Vγ1+ and Vγ4+ cells in naïve and immunized mouse spleens. As shown in Fig. 7A, the IL-23R was expressed at high levels in Vγ4+ cells from immunized mice, but only at low levels in these cells from naïve mice, whereas Vγ1+ cells from either naïve or immunized mice only expressed low or very low levels. Similarly, as shown in Fig. 7B, when cultured in vitro, Vγ1+ cells initially expressed the IL-23R at lower levels than Vγ4+ cells, but, on activation with either a cytokine mixture or anti-CD3 antibodies, Vγ1+ cells showed increased IL-23R expression, whereas Vγ4+ cells showed a marked reduction in IL-23R expression.
Fig. 7. Highly activated Vγ4+, but not Vγ1+, γδ T cells lose IL-23R expression.
(A) Enriched splenic T cells prepared by passage through nylon wool from naïve B6 mice or B6 mice 13 days after immunization with IRBP/CFA were double-stained for Vγ1 or Vγ4 and the IL-23R and analyzed by FACS.
(B) IL-23R expression by resting cultured Vγ1+ and Vγ4+ γδ T cell lines measured before and after 48 h exposure to a combination of cytokines (IL-1, IL-7, and IL-23) or anti-CD3 antibodies (2 μg/ml).
Discussion
Although a regulatory effect of γδ T cells on adaptive immunity has been repeatedly observed (15,22–24), knowledge of how these cells regulate the immune response is still limited, and the mechanisms by which they enhance an immune response in some cases, but inhibit it in others remain largely obscure. γδ T cell subsets expressing distinct γδ TCR Vγ segments have been found to show functional diversity (13–16). More recently, we showed that the regulatory effect of γδ T cells fluctuates with their activation status (17,18). Examining the mechanism by which γδ T cells exert enhancing or inhibitory effects on autoimmune responses, we previously showed that activated γδ T cells differ greatly from non-activated γδ T cells in their regulatory effect (17,25). In the present study, we found that IL-23R expression on γδ T cells differed greatly from that on αβ T cells, with a large percentage (~50%) of γδ T cells, but only a small percentage of αβ T cells (3%) in the spleens of immunized mice expressing the IL-23R. Our in vitro studies showed that IL-23R expression was seen on γδ T cells exposed to a single cytokine, which provides only a partial stimulatory effect, but not on highly activated γδ T cells, such as those activated by multiple cytokines or anti-CD3 antibodies. Both in vitro and in vivo functional tests showed that IL-23R+ γδ T cells had a strong suppressive effect, whereas IL-23R− γδ T cells were far less suppressive when non-activated and had an enhancing effect when highly activated. Since both non-activated and highly activated γδ T cells are IL-23R−, the function of the IL-23R− γδ T cells is complex and would depend on their activation status (17). We have determined factors that lead to increased expression IL-23R on γδ cells in immunized mice. Our results show that a synergistic effect between CFA and pertussis toxin (PTX), rather than the immunizing autoantigen, has caused this alteration, and the expression of IL-23R on γδ T cells directly correlates with the activation status of the latter cells.
It appears that γδ T cells can readily alter their regulatory activity in response to environmental factors. Given that IL-23 is an important growth factor for pathogenic IL-17-producing αβ T (Th17) cells (21,26–28) and that it is produced only in small amounts and only by myeloid cells activated by certain ligands (29,30), binding of IL-23 to the earlier responding innate γδ T cells might have a significant impact on subsequent αβ T cell responses. This idea was supported by experiments showing the suppressive effect was abolished by blockade of the IL-23R on γδ T cells using anti-IL-23R antibody and attenuated in the presence of a high concentration of exogenous IL-23. In addition, IL-23R+ γδ T cells proliferated vigorously when added to responder αβ T cells under Th17 polarized conditions (culture medium containing IL-23), whereas IL-23R− γδ T cells only proliferated poorly.
γδ T cells can be activated by multiple pathways in the absence of TCR ligation (1,31–35). We have previously shown that γδ T cells in mice with induced EAU are not activated by the immunizing antigen, but by cytokines (20). Here, we showed that cultured γδ T cells could be readily activated by exposure to various cytokines. The degree of activation of γδ T cells after exposure to different stimulatory factors varied, as judged by expression of activation surface molecules (CD69, CD62L, and CD44) and the production of cytokines (IL-17, IFN-γ, and IL-22). A synergistic effect was seen when γδ T cells were exposed to a combination of stimulatory factors. An inflammatory condition might result in partially or highly activated γδ T cells. In a weak inflammatory environment, γδ T cells might be modestly activated and become IL-23R+ and gain inhibitory activity, whereas, in strong inflammatory conditions or when larger amounts of pro-inflammatory cytokines are available, they might become highly activated and become IL-23R− and lose inhibitory activity. Our findings agree with a previous observation that IL-23-activated γδ T cells show increased suppressive activity (1) and further support the notion that IL-23 plays a major role in modulating the regulatory activity of γδ T cells on subsequently induced adaptive responses.
We have observed that fetal calf serum contain factor(s) that helped retain IL-23R expression of cultured γδ T cells, in that γδ T cells cultured in FCS-free medium gradually lost surface IL-23R whereas those cultured in medium containing >3% FCS showed partial or complete retention of IL-23R expression. To overcome potentially decreased cell viability of cultured cells in serum-free conditions, we have replaced 10% FCS in culture with small amount (1%) of mouse serum. Remarkably, γδ T cells survived much better in medium containing 1% mouse serum and the results repeated those obtained with cells cultured in serum-free medium (data not shown). These results further supported our conclusion that high FCS-containing medium are able to retain γδ T cell activation status.
We observed that Vγ1+ and Vγ4+ γδ T cells differed significantly in IL-23R expression. For example, Vγ4+, but not Vγ1+, γδ T cells in immunized mice expressed the IL-23R in vivo, even though both cell populations were able to express it after anti-CD3 activation in vitro, suggesting that these two cell populations have different requirements or thresholds for activation. Further studies should clarify how differences in activation requirements contribute to the different dominance and regulatory effects of different γδ T cell subsets in infections (36,37), tumors (6,38) and induced autoimmune diseases (39). In the present study, we observed that cultured resting γδ T cells retained the IL-23R in the absence of additional stimulation by cytokines and that FCS contained a factor or factors that are able to maintain IL-23R expression on γδ T cells, suggesting that γδ T cells grown in FCS-containing culture medium are constantly partially activated, even though they fail to express other activation markers, such as CD69. Expression of the IL-23R may represent an early biomarker for minimally activated γδ T cells. It is likely that a large number of environmental factors are capable of causing γδ T cell activation and thus modulating the regulatory effects of γδ T cells.
Acknowledgments
This work was supported in part by NIH grants EY 0022403, EY018827, and EY003040.
Abbreviations
- EAU
experimental autoimmune uveitis
- DC
dendritic cell
- IL-23R
interleukin-23 receptor
- IRBP
interphotoreceptor retinoid-binding protein
References
- 1.Petermann F, Rothhammer V, Claussen MC, Haas JD, Blanco LR, Heink S, Prinz I, Hemmer B, Kuchroo VK, Oukka M, Korn T. γδ T Cells Enhance Autoimmunity by Restraining Regulatory T Cell Responses via an Interleukin-23-Dependent Mechanism. Immunity. 2010;33:351–363. doi: 10.1016/j.immuni.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Born W, Cady C, Jones-Carson J, Mukasa A, Lahn M, O’Brien R. Immunoregulatory functions of γδ T cells. Adv Immunol. 1999;71:77–144. [PubMed] [Google Scholar]
- 3.Dodd J, Riffault S, Kodituwakku JS, Hayday AC, Openshaw PJM. Pulmonary Vγ4+ γδ T Cells Have Proinflammatory and Antiviral Effects in Viral Lung Disease. J Immunol. 2009;182:1174–1181. doi: 10.4049/jimmunol.182.2.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayday A, Geng L. γδ cells regulate autoimmunity. Curr Opin Immunol. 1997;9:884–889. doi: 10.1016/s0952-7915(97)80193-8. [DOI] [PubMed] [Google Scholar]
- 5.Nian H, Shao H, Zhang G, Born WK, O’Brien R, Kaplan HJ, Sun D. Regulatory effect of γδ T cells on IL-17+ uveitogenic T cells. Invest Ophthalmol Vis Sci. 2010;51:4661–4667. doi: 10.1167/iovs.09-5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng G, Wang HY, Peng W, Kiniwa Y, Seo KH, Wang RF. Tumor-Infiltrating γδ T Cells Suppress T and Dendritic Cell Function via Mechanisms Controlled by a Unique Toll-like Receptor Signaling Pathway. Immunity. 2007;27:334–348. doi: 10.1016/j.immuni.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 7.Rajan AJ, V, Asensio C, Campbell IL, Brosnan CF. Experimental autoimmune encephalomyelitis on the SJL mouse: effect of γδ T cell depletion on chemokine and chemokine receptor expression in the central nervous system. J Immunol. 2000;164:2120–2130. doi: 10.4049/jimmunol.164.4.2120. [DOI] [PubMed] [Google Scholar]
- 8.Spahn TW, Issazadah S, Salvin AJ, Weiner HL. Decreased severity of myelin oligodendrocyte glycoprotein peptide 33–35-induced experimental autoimmune encephalomyelitis in mice with a disrupted TCR δ chain gene. Eur J Immunol. 1999;29:4060–4071. doi: 10.1002/(SICI)1521-4141(199912)29:12<4060::AID-IMMU4060>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 9.Tagawa T, Nishimura H, Yajima T, Hara H, Kishihara K, Matsuzaki G, Yoshino I, Maehara Y, Yoshikai Y. Vδ1+ γδ T Cells Producing CC Chemokines May Bridge a Gap between Neutrophils and Macrophages in Innate Immunity during Escherichia coli Infection in Mice. J Immunol. 2004;173:5156–5164. doi: 10.4049/jimmunol.173.8.5156. [DOI] [PubMed] [Google Scholar]
- 10.D’Souza CD, Cooper AM, Frank AA, Mazzaccaro RJ, Bloom BR, Orme IM. An anti-inflammatory role for γδ T lymphocytes in acquired immunity to Mycobacterium tuberculosis. J Immunol. 1997;158:1217–1221. [PubMed] [Google Scholar]
- 11.Tsuchiya T, Fukuda S, Hamada H, Nakamura A, Kohama Y, Ishikawa H, Tsujikawa K, Yamamoto H. Role of γδ T cells in the inflammatory response of experimental colitis mice. J Immunol. 2003;171:5507–5513. doi: 10.4049/jimmunol.171.10.5507. [DOI] [PubMed] [Google Scholar]
- 12.Uezu K, Kawakami K, Miyagi K, Kinjo Y, Kinjo T, Ishikawa H, Saito A. Accumulation of γδ T Cells in the Lungs and Their Regulatory Roles in Th1 Response and Host Defense against Pulmonary Infection with Cryptococcus neoformans. J Immunol. 2004;172:7629–7634. doi: 10.4049/jimmunol.172.12.7629. [DOI] [PubMed] [Google Scholar]
- 13.Dalton JE, Pearson J, Scott P, Carding SR. The Interaction of γδ T Cells with Activated Macrophages Is a Property of the Vg1 Subset. J Immunol. 2003;171:6488–6494. doi: 10.4049/jimmunol.171.12.6488. [DOI] [PubMed] [Google Scholar]
- 14.Hahn YS, Taube C, Jin N, Sharp L, Wands JM, Aydintug MK, Lahn M, Huber SA, O’Brien RL, Gelfand EW, Born WK. Different Potentials of gd T Cell Subsets in Regulating Airway Responsiveness: Vγ1+ Cells, but Not Vγ4+ Cells, Promote Airway Hyperreactivity, Th2 Cytokines, and Airway Inflammation. J Immunol. 2004;172:2894–2902. doi: 10.4049/jimmunol.172.5.2894. [DOI] [PubMed] [Google Scholar]
- 15.Huber SA, Graveline D, Newell MK, Born WK, O’Brien RL. Vγ1+ T Cells Suppress and Vγ4+ T Cells Promote Susceptibility to Coxsackievirus B3-Induced Myocarditis in Mice. J Immunol. 2000;165:4174–4181. doi: 10.4049/jimmunol.165.8.4174. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien RL, Yin X, Huber SA, Ikuta K, Born WK. Depletion of a γδ T Cell Subset Can Increase Host Resistance to a Bacterial Infection. J Immunol. 2000;165:6472–6479. doi: 10.4049/jimmunol.165.11.6472. [DOI] [PubMed] [Google Scholar]
- 17.Nian H, Shao H, O’Brien BA, Born WK, Kaplan HJ, Sun D. Activated γδ cells promote the activation of uveitogenic T cells and exacerbate EAU development. Invest Ophthalmol Vis Sci. 2011;52:5920–5927. doi: 10.1167/iovs.10-6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nian H, Liang D, Zuo A, Wei R, Shao H, Born WK, Kaplan HJ, Sun D. Characterization of autoreactive and bystander IL-17+ T cells induced in immunized C57BL/6 mice. Invest Ophthalmol Vis Sci. 2012;53:897–905. doi: 10.1167/iovs.11-8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng L, Cui Y, Shao H, Han G, Zhu L, Huang Y, O’Brien RL, Born WK, Kaplan HJ, Sun D. Mouse γδ T cells are capable of expressing MHC class II molecules, and of functioning as antigen-presenting cells. J Neuroimmunol. 2008;203:3–11. doi: 10.1016/j.jneuroim.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cui Y, Shao H, Lan C, Nian H, O’Brien RL, Born WK, Kaplan HJ, Sun D. Major Role of γδ T Cells in the Generation of IL-17+ Uveitogenic T Cells. J Immunol. 2009;183:560–567. doi: 10.4049/jimmunol.0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riol-Blanco L, Lazarevic V, Awasthi A, Mitsdoerffer M, Wilson BS, Croxford A, Waisman A, Kuchroo VK, Glimcher LH, Oukka M. IL-23 Receptor Regulates Unconventional IL-17-Producing T Cells That Control Bacterial Infections. J Immunol. 2010;184:1710–1720. doi: 10.4049/jimmunol.0902796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kabelitz D, Wesch D, He W. Perspectives of γδ T Cells in Tumor Immunology. Cancer Res. 2007;67:5–8. doi: 10.1158/0008-5472.CAN-06-3069. [DOI] [PubMed] [Google Scholar]
- 23.Girardi M. Immunosurveillance and Immunoregulation by γδ T Cells. J Invest Dermatol. 2006;126:25–31. doi: 10.1038/sj.jid.5700003. [DOI] [PubMed] [Google Scholar]
- 24.Poccia F, Agrati C, Martini F, Mejia G, Wallace M, Malkovsky M. Vγ9Vδ2 T cell-mediated non-cytolytic antiviral mechanisms and their potential for cell-based therapy. Immunol Lett. 2005;100:14–20. doi: 10.1016/j.imlet.2005.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang D, Zuo A, Shao H, Born WK, O’Brien RL, Kaplan HJ, Sun D. Role of CD25+ Dendritic Cells in the Generation of Th17 Autoreactive T Cells in Autoimmune Experimental Uveitis. J Immunol. 2012;188:5785–5791. doi: 10.4049/jimmunol.1200109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doisne JM, Soulard V, Becourt C, Amniai L, Henrot P, Havenar-Daughton C, Blanchet C, Zitvogel L, Ryffel B, Cavaillon JM, Marie JC, Couillin I, Benlagha K. Cutting Edge: Crucial Role of IL-1 and IL-23 in the Innate IL-17 Response of Peripheral Lymph Node NK1.1− Invariant NKT Cells to Bacteria. J Immunol. 2011;186:662–666. doi: 10.4049/jimmunol.1002725. [DOI] [PubMed] [Google Scholar]
- 27.Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N, Grainger JR, Chen Q, Kanno Y, Watford WT, Sun Hw, Eberl G, Shevach EM, Belkaid Y, Cua DJ, Chen W, O’Shea JJ. Generation of pathogenic TH17 cells in the absence of TGF-β signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Ann Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 29.Gerosa F, Baldani-Guerra B, Lyakh LA, Batoni G, Esin S, Winkler-Pickett RT, Consolaro MR, De Marchi M, Giachino D, Robbiano A, Astegiano M, Sambataro A, Kastelein RA, Carra G, Trinchieri G. Differential regulation of interleukin 12 and interleukin 23 production in human dendritic cells. J Exp Med. 2008;205:1447–1461. doi: 10.1084/jem.20071450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roses RE, Xu S, Xu M, Koldovsky U, Koski G, Czerniecki BJ. Differential Production of IL-23 and IL-12 by Myeloid-Derived Dendritic Cells in Response to TLR Agonists. J Immunol. 2008;181:5120–5127. doi: 10.4049/jimmunol.181.7.5120. [DOI] [PubMed] [Google Scholar]
- 31.Aydintug MK, Roark CL, Chain JL, Born WK, O’Brien RL. Macrophages express multiple ligands for γδ TCRs. Mol Immunol. 2008;45:3253–3263. doi: 10.1016/j.molimm.2008.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Havlir DV, Ellner JJ, Chervenak KA, Boom WH. Selective expansion of human γδ T cells by monocytes infected with live Mycobacterium tuberculosis. J Clin Invest. 1991;87:729–733. doi: 10.1172/JCI115053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rincon-Orozco B, Kunzmann V, Wrobel P, Kabelitz D, Steinle A, Herrmann T. Activation of Vγ9Vδ2 T cells by NKG2D. J Immunol. 2005;175:2144–2151. doi: 10.4049/jimmunol.175.4.2144. [DOI] [PubMed] [Google Scholar]
- 34.Wesch D, Beetz S, Oberg HH, Marget M, Krengel K, Kabelitz D. Direct costimulatory effect of TLR3 ligand poly(I:C) on human gd T lymphocytes. J Immunol. 2006;176:1348–1354. doi: 10.4049/jimmunol.176.3.1348. [DOI] [PubMed] [Google Scholar]
- 35.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KHG. Interleukin-1 and IL-23 Induce Innate IL-17 Production from γδ T Cells, Amplifying Th17 Responses and Autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Leclercq G, Plum J. Stimulation of TCR Vγ3 cells by gram-negative bacteria. J Immunol. 1995;154:5313–5319. [PubMed] [Google Scholar]
- 37.Mokuno Y, Matsuguchi T, Takano M, Nishimura H, Washizu J, Ogawa T, Takeuchi O, Akira S, Nimura Y, Yoshikai Y. Expression of Toll-Like Receptor 2 on γδ T Cells Bearing Invariant Vγ6/Vδ1 Induced by Escherichia coli Infection in Mice. J Immunol. 2000;165:931–940. doi: 10.4049/jimmunol.165.2.931. [DOI] [PubMed] [Google Scholar]
- 38.Hayday A, Tigelaar R. Immunoregulation in the tissues by γδ T cells. Nat Rev Immunol. 2003;3:233–242. doi: 10.1038/nri1030. [DOI] [PubMed] [Google Scholar]
- 39.Peng Y, Han G, Shao H, Wang Y, Kaplan HJ, Sun D. Characterization of IL-17+ Interphotoreceptor Retinoid-Binding Protein-Specific T Cells in Experimental Autoimmune Uveitis. Invest Ophthalmol Vis Sci. 2007;48:4153–4161. doi: 10.1167/iovs.07-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]