Abstract
Once biologically available aluminum bypasses gastrointestinal and blood-brain barriers, this environmentally-abundant neurotoxin has an exceedingly high affinity for the large pyramidal neurons of the human brain hippocampus. This same anatomical region of the brain is also targeted by the earliest evidence of Alzheimer’s disease (AD) neuropathology. The mechanism for the selective targeting and transport of aluminum into the hippocampus of the human brain is not well understood. In an effort to improve our understanding of a pathological aluminum entry system into the brain, this study examined the aluminum content of 8 arteries that supply blood to the hippocampus, including the aorta and several cerebral arteries. In contrast to age-matched controls, in AD patients we found a gradient of increasing aluminum concentration from the aorta to the posterior cerebral artery that supplies blood to the hippocampus. Primary cultures of human brain endothelial cells were found to have an extremely high affinity for aluminum when compared to other types of brain cells. Together, these results suggest for the first time that endothelial cells that line the cerebral vasculature may have biochemical attributes conducive to binding and targeting aluminum to selective anatomical regions of the brain, such as the hippocampus, with potential downstream pro-inflammatory and pathogenic consequences.
Keywords: aluminum sulfate, Alzheimer’s disease, endothelial cells, genotoxicity, human brain microvessel endothelial cells, inflammation, magnesium sulfate
Aluminum, the most abundant metallic neurotoxin in the biosphere, is an extremely pro-inflammatory, pathological and genotoxic element that is particularly deleterious to the normal homeostatic operation of brain cells, especially at the level of normal cytoplasmic and genetic activities that utilize phosphate [1–10]. Such activities commonly involve adenine diphosphate or adenine triphosphate (ADP, ATP) nucleotides in cytoplasmic energy transactions, including nuclear metabolism that involves the rapid biosynthesis of phosphate-rich nucleic acids, such as DNA and RNA in all of its forms [1,7–15]. Humans intake an average of 10 mg Al/day (range 10–1000 mg Al/day) chiefly via medicine, water, food and inhalation [11,12]. Fortunately, aluminum insolubility at biological pH and highly effective epithelial, gastrointestinal and blood-brain barriers prevent this ubiquitous neurotoxin from accessing human biological compartments where it appears to contribute to inflammatory degeneration and pathogenic gene expression programs highly characteristic of the Alzheimer’s disease (AD) process [1,7–10,13–16].
These experiments were conducted to improve our understanding of a pathological aluminum entry system into the brain and central nervous system (CNS). We analyzed the aluminum content of the arterial walls that supply blood to the hippocampus, including the aortic artery (AA; aorta), the common carotid artery (CCA), the vertebral artery (VA), the internal cerebral artery (ICA), the basilar artery (BA), the anterior cerebral artery (ACA), the middle cerebral artery (MCA) and the posterior cerebral artery (PCA) (Figure 1). Our major findings were that there is a non-uniform distribution of aluminum in human arteries that serve the brain, and compared to age-matched controls, in late-stage AD patients we found a gradient of increasing aluminum concentration from the aorta to the PCA, with the highest aluminum levels in the PCA that immediately supplies blood to the hippocampus. Because the arterial walls of the vasculature are lined with several different types of endothelial cells, the plasma membranes of primary cultures of human brain microvessel endothelial (hBMECs) cells that line the microvasculature of the brain, were next examined for aluminum content after in vitro aluminum sulfate exposure. hBMEC cells were found to have an extremely high affinity for aluminum when compared to other human endothelial and brain cell types and may act as a kind of endothelial cell ‘sink’ for aluminum sequestration. Taken together, these results for the first time suggest that endothelial cells that line the cerebral vasculature and microvasculature, and direct blood supply to the hippocampus, may have biochemical attributes conducive to directing and targeting aluminum to selective anatomical regions of the brain, such as the hippocampus, with downstream pro-inflammatory and pathogenic consequences [5,10].
Figure 1.
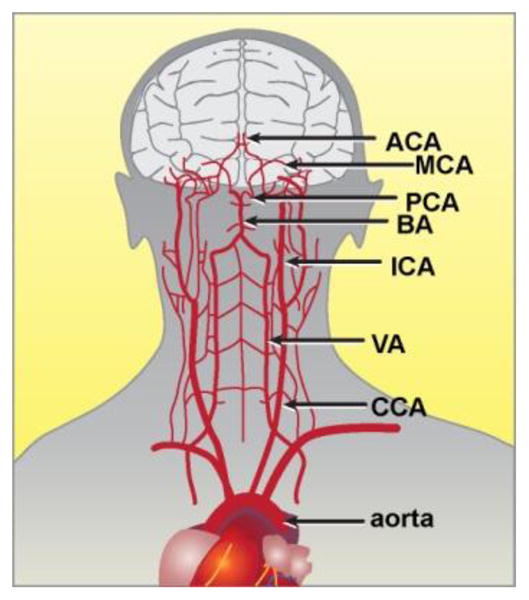
Major arterial circulation that supplies blood to the human brain and central nervous system (CNS). It is remarkable that although the average brain is typically only 3% of total body mass, it demands 20–25% of total cardiac output. Major brain arteries include the aorta (aortic artery, AA), the common carotid artery (CCA), the vertebral artery (VA), the internal cerebral artery (ICA), the basilar artery (BA), the posterior cerebral artery (PCA), the middle cerebral artery (MCA) and the anterior cerebral artery; the PCA provides the major vascular supply to the hippocampus; figure adapted from http://stanfordhospital.org/clinicsmedServices/COE/neuro/stroke/patientEducation/whatIsStroke.html.
The potential contribution of aluminum to the initiation and propagation of AD is widely documented [1–16]. Many key neuropathological features of AD, including beta amyloid precursor protein metabolism and amyloid beta (Aβ) peptide generation, induction of the pro-inflammatory transcription factor NF-kB and pro-inflammatory gene expression, micro RNA (miRNA) up-regulation and ensuing down-regulation of target messenger RNA (mRNA) and gene expression, and interrelated forms of genetic and epigenetic gene control appear to be modulated by physiological realistic amounts of aluminum [2–16]. For example, two major deleterious genotoxic effects of nanomolar amounts of aluminum upon genetic activity in AD-afflicted brain and in vitro, in AD models, appear to be (1) to condense brain chromatin non-randomly through an interaction with H1 linker histones and thereby alter the ability of brain DNA to be effectively transcribed [13,18,23]; and (2) to directly down-regulate the biosynthetic capabilities of RNA polymerase II (RNAPII), a nuclear enzyme that catalyzes the transcription of DNA, and is hence responsible for the biosynthesis of all mRNA and small non-coding miRNA [7,8,13–16,20,22]. Aluminum’s effects on DNA transcription are perhaps not too surprising as RNAPII biosynthetic activities are associated with very high biological concentrations of phosphates as free nucleotide mono-, di- and triphosphates, and polymerized nucleic acid intermediates that include DNA and RNA in all of its forms [13,15–17,20].
In these studies the three major findings were (1) that aluminum does not accumulate uniformly in a number of arteries that provide sustenance to the brain (Figure 1); (2) that there appears to be a gradient of aluminum deposition within the arterial walls from the aorta to the PCA, the major artery of supply to the hippocampal region, and the anatomical region where AD appears to initiate (Table 1) [6,13,18]; and (3) that each endothelial and brain cell plasma membrane appears to have a different affinity for aluminum. The highest aluminum affinity for all endothelial cells studied was in human brain microvessel endothelial cells (hBMECs), a unique type of endothelial cell that line the brain’s microvasculature, and the highest aluminum affinity for brain cells was for human neuronal-glial (HNG) cells in co-culture (Table 2). Interestingly, measurements of aluminum in the celiac artery and superior mesenteric artery and major veins that serve the small intestine, and the main site of dietary nutrient absorption, are not enriched in aluminum, and contain approximately the same amount as was found in the femoral artery (averaging from about 2.5–5.6 ug/g aluminum; Table 1). The gradient of increasing aluminum from the gastrointestinal tract arteries to the PCA is reminiscent of a similar situation in the mammalian brain where the phosphate and polyphosphate content of the plasma (2 mM), the cytoplasm (10 mM), and the nucleoplasm (at least 50 mM) may act as an electrostatic gradient to draw aluminum into the nucleus of nerve cells, where it progressively accumulates [13,15].
Table 1. Aluminum content of major arteries that supply the human brain.
All arteries used in these studies were obtained from short post-mortem interval donors; arteries were dissected away and washed using physiological saline made up from ultrapure water (18 megohm, Milli-Q, Millipore or Puriss 95305, Fluka; aluminum content less than 1 ppb). Tubular segments of whole arteries from each anatomical area of interest, approximately 1.0 cm long (~6 gm wet weight), were subjected to HNO3-hydrolysis in platinum crucibles and trace metal analysis was performed using Zeeman-type electrothermal atomic absorption spectrophotometry (ETAAS; PE5000PC system, Perkin-Elmer, Waltham MA, USA), equipped with an automated sampler and IBM/AT-supported analysis package for trace metal analysis, as previously described by our group [8,9,18,20]. Ultrapure HNO3-washed polysulfonate plasticware was used according to the URI-GSO protocols and ultrapure water was employed throughout all isolation and biochemical procedures to stringently exclude trace metal contamination [18,20].
| artery | N* | μg/g** | fold increase*** |
|---|---|---|---|
| celiac (CA) | 8 | 2.5+/−2.1 | 1 |
| femoral (FA) | 6 | 5.1+/−2.2 | 1 |
| aorta (AA) | 6 | 7.0+/−5.1 | 1 |
| vertebral (VA) | 5 | 8.2+/−4.4 | 1 |
| common carotid (CCA) | 5 | 18.5+/−10.1 | 2 |
| internal carotid (ICA) | 8 | 22.5+/−12.3 | 3 |
| basilar artery (BA) | 5 | 27.1+/−15.7 | 3.5 |
| middle cerebral (MCA) | 5 | 35.1+/−16.3 | 4 |
| posterior cerebral (PCA) | 6 | 54.2+/−18.2 | 9 |
N* = number of individual arterial samples analyzed (**μg/g = mean +/− one standard deviation, micrograms of aluminum per gram whole artery (wet weight);
fold increase = mean ratio of AD over age-matched controls for the same artery; the study group of controls (N=6) and AD (N=12) tissues exhibited no significant differences in age (72.2±5.1 vs 73.1±4.8 yr, p~0.87, respectively) or postmortem interval (mean 5.2±2.8 vs 5.4±3.1 hr, p~0.91, respectively); the clinical and drug histories of the donors were not completely known; all AD cases were each case confirmed post-mortem using CERAD criteria and all AD cases were from advanced stages of AD [17,18].
Table 2.
Aluminum content of human endothelial and related brain cell types
| species | cell type | μg/g* | N** |
|---|---|---|---|
| human | hepatocyte | 1.1 +/− 0.8 | 5 |
| human | microglial (HMG) | 1.5 +/− 1.1 | 6 |
| rat | astroglial | 2.5 +/− 1.2 | 3 |
| human | astroglial (HAG) | 6.1 +/− 2 | 6 |
| human | endothelial (HUVEC) | 11 +/− 5 | 6 |
| rhesus | endothelial (RF-6A) | 9.2 +/− 3 | 6 |
| human | endothelial (hBMEC) | 36 +/− 8 | 6 |
| rat | brain, endothelial | 15 +/− 5 | 3 |
| human | neuron, cortical (HNG) | 58 +/− 12 | 6 |
| human | neuron, large pyramidal (HNG) | 104 +/− 18 | 6 |
μg/g* = mean +/− one standard deviation, micrograms of aluminum per gram whole cell plasma membrane (wet weight);
N** = number of individual samples analyzed; primary human brain microvessel endothelial cells (hBMEC; ACBRI 376, Cell Systems, Kirkland WA, USA), human umbilical vein endothelial cells (HUVEC; ATCC® PCS-100-100; ATCC Manassas VA, USA) and rhesus monkey retinal endothelial (RF/6A; Macaca mulatta; ATCC® CRL-1780, ATCC) cells were cultured according to manufacturer’s protocols and from previously published work [8,20,21].
The primary culture of human hepatocytes, human neuronal-glial (HNG) cells in co-culture (human neurons do not culture well without the presence of glia), human astroglial (HAG) cells, and human microglial (HMG) cells have been extensively described by our laboratory [16,20–22]; ultrapure reagents for molecular biology, including MgSO4 (63133) and Al2(SO4)3 (11044; Biochemika MicroSelect©; Fluka Ultraselect©; Fluka Chemical, Milwaukee, WI), freshly prepared as 0.1 M stock solutions [9,15,20], were instilled into either serum-containing or half serum strength respective cell maintenance medium by shaking, followed by filter sterilization using 0.2-μM spin filters (Millipore Corporation, Billerica, MA). All cell media solutions contained a final concentration of 2.0 uM MgSO4 or 2.0 uM of Al2(SO4)3. Details of control, magnesium- and aluminum-sulfate treatment of brain cells have been previously described [15,20]. Statistical procedures for aluminum and magnesium abundance were analyzed using a two-way factorial analysis of variance (p, ANOVA) using programs and procedures in the SAS language (Statistical Analysis Institute, Cary, NC) [13–16,19].
Only p-values less than 0.05 (ANOVA) were considered to be statistically significant. Figures and Tables were generated using Photoshop CS2 ver 9.0.2 (Adobe, San Jose, CA).
As previously mentioned, aluminum has an extremely high affinity for biologically available phosphates, and phosphate groups are covalently linked to lipids to form outward facing phospholipids in the plasma membrane lipid bilayer [1,10,15]. Interestingly, the non-uniform distribution of aluminum accumulation in human arteries may be related to the variable phospholipid composition of the individual endothelial cell types that line the vasculature from the aorta to the PCA. We therefore speculate that different phospholipid compositions of different endothelial cells that line the cerebral vasculature may have different affinities for aluminum, and may thus explain different aluminum concentrations of human arterial blood vessels. As dietary lipid intake including cholesterol, saturated, unsaturated and essential fatty acids can alter or modify the phospholipid composition of arterial walls, this brings up the intriguing possibility that dietary effects may be linked to aluminum accumulation in arterial walls, and hence be a factor to consider in the perils of aluminum exposure and aluminum entry into the brain [24–27]. These results further suggest that aluminum intake may be modified by the lipid or phospholipid content of the diet, especially in the case of essential fatty acids that the body cannot biosynthesize [24,25]. Analysis of more human arterial and venous samples will be required to ascertain if selective aluminum accumulation in certain brain arteries is a common feature of large AD populations when compared to controls. Further investigations will also be required to understand the mechanism of the variability of aluminum accumulation in endothelial cell phospholipids that line the brain vasculature, and the interaction between phospholipids and the aluminum-transferrin transport system in blood serum [1,9].
Lastly, a 12 year old study of aluminum in aging human non-brain arteries by Minami et al. (2001), utilizing inductively coupled plasma emission (ICPE) analysis showed aluminum in only 88% of all Japanese cases (N=26) studied, in contrast to our ability to detect aluminum in 100% of all American cases studied in this report [27]. We routinely find that measuring aluminum using ETAAS is a far more sensitive and reliable analytical technique than ICPE [8,9,15,24–26]. Two other possible explanations are (1) variation in individual aluminum accumulation and sequestration amongst different human populations; and/or (2) the vast amount of aluminum mobilized into our biosphere over the last decade, and hence increased human exposure, which may be responsible for the higher aluminum values in selective arteries as was obtained in the current studies [6,9,10,27].
Highlights of this study.
In these studies the 5 major findings were that
aluminum does not accumulate uniformly within the arteries that provide sustenance to the brain;
there appears to be a gradient of aluminum deposition within the arterial walls from the aorta to the PCA, the major artery that supplies blood to the hippocampal region;
the hippocampal region is the anatomical region where Alzheimer’s disease appears to initiate;
each endothelial and brain cell plasma membrane appears to have a different affinity for aluminum;
the highest aluminum affinity for all endothelial cells studied was in human brain microvessel endothelial cells, a unique type of cell that lines the human brain’s microvasculature.
Acknowledgments
Human brain and/or vascular tissues or extracts were provided by the Oregon State University Health Science Center, the University of Toronto, the Louisiana State University Health Sciences Center Brain Bank, and by the Memory Impairments and Neurological Disorders (MIND) Institute at the University of California, Irvine Alzheimer’s Disease Research Center (UCI-ADRC; funded in part though NIA P50 AG16573). Thanks are also extended to the physicians, neuropathologists and families who have kindly consented to provide human brain and vascular tissues for research purposes. Research on the structure and function of aluminum, cerebral vascularization, NF-κB and miRNA expression in AD brain in the Lukiw laboratory were supported through Grant Number P20RR016456 from the National Center for Research Resources (NCRR), Translational Research Initiative (TRI) Grants from LSU Health Sciences Center New Orleans (WJL), an Alzheimer Association Investigator-Initiated Research Grant IIRG-09-131729 (WJL), and NIH NIA Grants AG18031 and AG038834 (WJL). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging, National Center for Research Resources, or the National Institutes of Health.
Abbreviations
- AA
aortic artery (aorta)
- ACA
anterior cerebral artery
- AD
Alzheimer’s disease
- ANOVA
analysis of variance
- ADP
adenine diphosphate
- ATP
adenine triphosphate
- BA
basilar artery
- CCA
common carotid artery
- CERAD
consortium to establish a registry for Alzheimer’s disease
- ETAAS
electrothermal atomic absorption spectroscopy
- HAG cells
human astroglial cells
- hBMEC
human brain microvessel endothelial cells
- HNG
human microglial cells
- HNG
human neuronal-glial cells
- HUVEC
human umbilical vein endothelial cells
- ICA
internal cerebral artery
- ICPE
inductively coupled plasma emission
- MCA
middle cerebral artery
- PCA
posterior cerebral artery
- RF6a
rhesus monkey retinal endothelial cells
- RNA Pol II
RNA polymerase type II
- VA
vertebral artery
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martin RB. Ciba Found Symp. 1992;169:5–18. doi: 10.1002/9780470514306.ch2. [DOI] [PubMed] [Google Scholar]
- 2.Becaria A, Lahiri DK, Bondy SC, Chen D, Hamadeh A, Li H, Taylor R, Campbell A. J Neuroimmunol. 2006;176:16–23. doi: 10.1016/j.jneuroim.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 3.Walton JR, Wang MX. J Inorg Biochem. 2009;103:1548–1554. doi: 10.1016/j.jinorgbio.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 4.Exley C. Subcell Biochem. 2005;38:225–234. doi: 10.1007/0-387-23226-5_11. [DOI] [PubMed] [Google Scholar]
- 5.Campbell A. J Alzheimers Dis. 2006;10:165–72. doi: 10.3233/jad-2006-102-304. [DOI] [PubMed] [Google Scholar]
- 6.Bondy SC. Neurotoxicology. 2010;31:575–581. doi: 10.1016/j.neuro.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lukiw WJ, Pogue AI. J Inorg Biochem. 2007;101:1265–1269. doi: 10.1016/j.jinorgbio.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pogue AI, Li YY, Cui JG, Zhao Y, Kruck TP, Percy ME, Tarr MA, Lukiw WJ. J Inorg Biochem. 2009;103:1591–1595. doi: 10.1016/j.jinorgbio.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Alexandrov PN, Zhao Y, Pogue AI, Tarr MA, Kruck TPA, Percy ME, Cui JG, Lukiw WJ. J Alzheimer’s Dis. 2005;8:117–127. doi: 10.3233/jad-2005-8204. [DOI] [PubMed] [Google Scholar]
- 10.Lukiw WJ, Bazan NG. Neurochemical Research. 2000;25:1173–1184. doi: 10.1023/a:1007627725251. [DOI] [PubMed] [Google Scholar]
- 11.Cuciureanu R, Urzică A, Voitcu M, Antoniu A. A Rev Med Chir Soc Med Nat. 2000;104:107–112. [PubMed] [Google Scholar]
- 12.Centers for disease control (CDC) Atlanta GA, USA; background and environmental exposures to aluminum in the United States. 2012 http://www.atsdr.cdc.gov/toxprofiles/tp22-c2.pdf.
- 13.Lukiw WJ, Kruck TPA, McLachlan DRC. Lancet. 1989;1:781–782. doi: 10.1016/s0140-6736(89)92596-8. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Y, Cui JG, Lukiw WJ. Mol Neurobiol. 2006;34:181–192. doi: 10.1385/MN:34:3:181. [DOI] [PubMed] [Google Scholar]
- 15.Lukiw WJ, LeBlanc HJ, Carver LA, McLachlan DR, Bazan NG. J Molec Neurosci. 1998;11:67–78. doi: 10.1385/JMN:11:1:67. [DOI] [PubMed] [Google Scholar]
- 16.Cui JG, Li YY, Zhao Y, Bhattacharjee S, Lukiw WJ. J Biol Chem. 2010;285:38951–38960. doi: 10.1074/jbc.M110.178848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bancher C, Paulus W, Paukner K, Jellinger K. CERAD Alzheimer Dis Assoc Disord. 1997;11:207–219. [PubMed] [Google Scholar]
- 18.Lukiw WJ, Krishnan B, Wong L, Kruck TP, Bergeron C, McLachlan DR. Neurobiology of Aging. 1992;13:115–121. doi: 10.1016/0197-4580(92)90018-s. [DOI] [PubMed] [Google Scholar]
- 19.Moody JR, Lindstrom RM. Analytical Chemistry. 1977;49:2264–2268. [Google Scholar]
- 20.Pogue AI, Jones BM, Bhattacharjee S, Percy ME, Zhao Y, Lukiw WJ. Int J Mol Sci. 2012;13:9615–9626. doi: 10.3390/ijms13089615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lukiw WJ, Ottlecz A, Lambrou G, Grueninger M, Finley J, Thompson HW, Bazan NG. Invest Ophthalmol Vis Sci. 2003;44:4163–4170. doi: 10.1167/iovs.02-0655. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Y, Bhattacharjee S, Jones BM, Dua P, Alexandrov PN, Hill JM, Lukiw WJ. Neuroreport. 2013 Mar 4; doi: 10.1097/WNR.0b013e32835fb6b0. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lukiw WJ. J Inorg Biochem. 2010;104:1010–1012. [PubMed] [Google Scholar]
- 24.Bradbury J. J Nutrients. 2011;3:529–54. doi: 10.3390/nu3050529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marteinsdottir I, Horrobin DF, Stenfors C, Theodorsson E, Mathé AA. Prog Neuro-psychopharmacol Biol Psychiatry. 1998;22:1007–1021. doi: 10.1016/s0278-5846(98)00052-9. [DOI] [PubMed] [Google Scholar]
- 26.Walton JR. J Alzheimers Dis. 2013 Feb 4; [Epub ahead of print] [Google Scholar]
- 27.Minami T, Tohno S, Utsumi M, Moriwake Y, Yamada MO, Tohno Y. Biol Trace Elem Res. 2001;79:29–38. doi: 10.1385/BTER:79:1:29. [DOI] [PubMed] [Google Scholar]


