Abstract
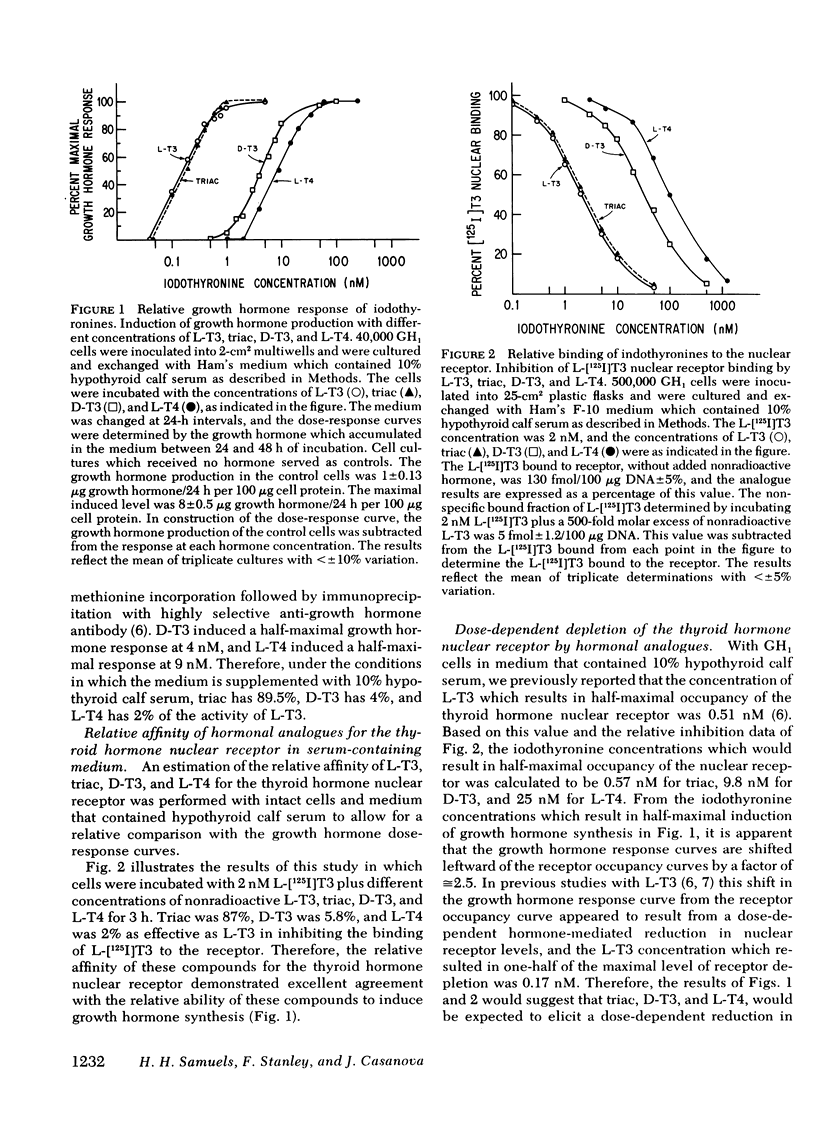
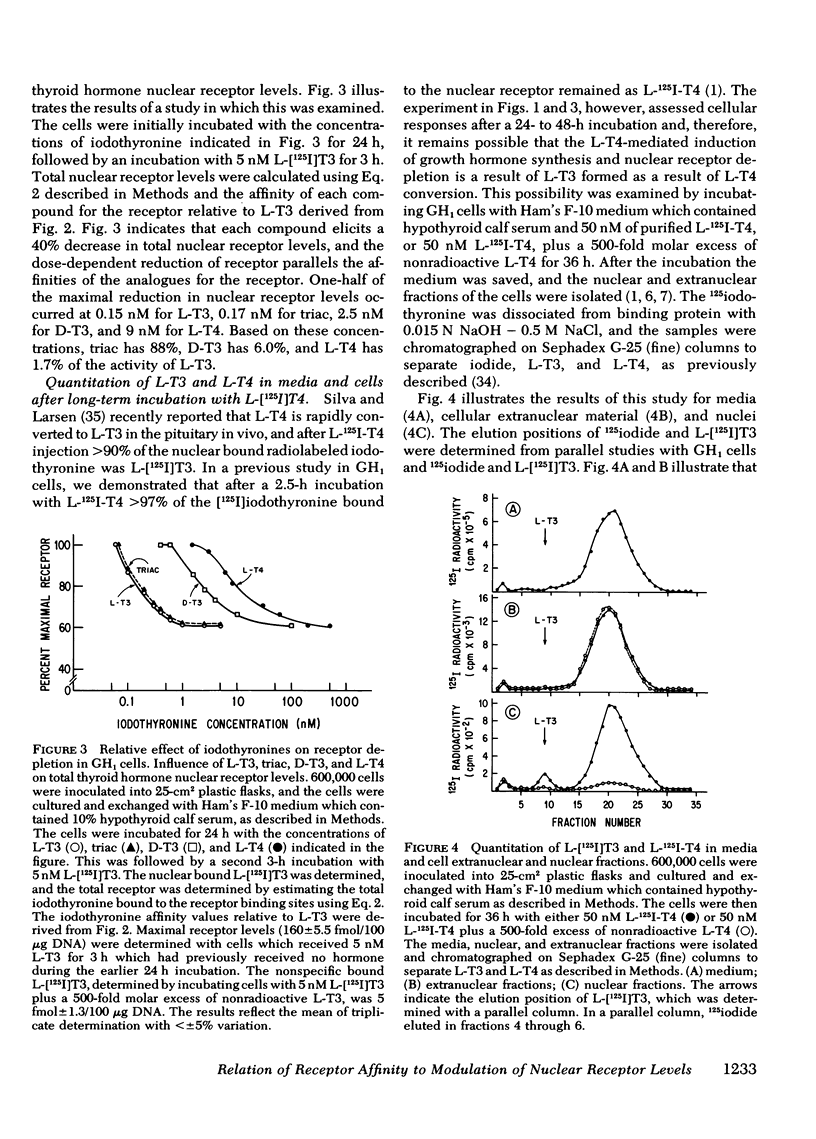
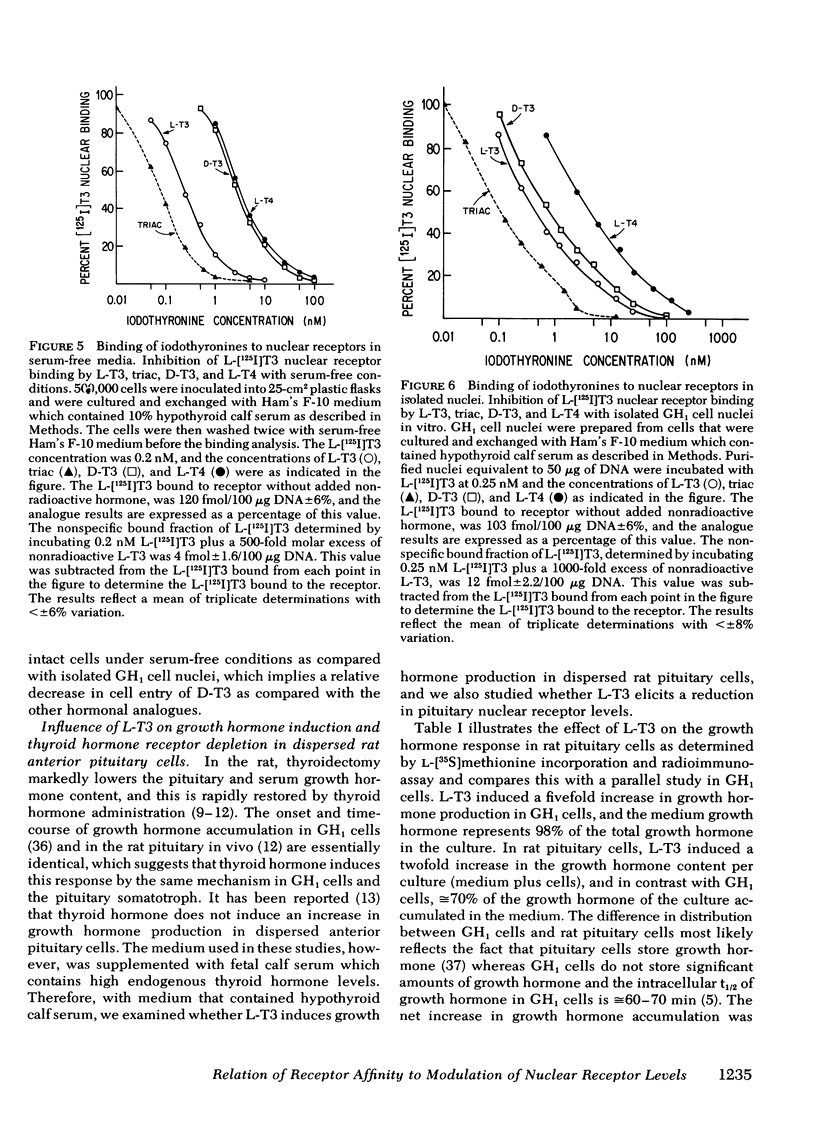
We have previously demonstrated that L-triiodothyronine (L-T3) induces an increase in growth hormone synthesis and messenger RNA in cultured GH1 cells, a rat pituitary cell line. In addition to regulating the growth hormone response, L-T3 elicits a time- and dose-dependent reduction in the level of its nuclear receptor, which is a direct function of the occupancy of the receptor binding site. In this study we have compared the relative affinity of L-T3, triiodothyroacetic acid, D-triiodothyronine (D-T3), and L-thyroxine (L-T4) for the receptor with the induction of the growth hormone synthesis and the ability of these compounds to elicit a reduction in thyroid hormone nuclear receptor levels. Triiodothyroacetic acid and D-T3 were specifically examined because the biologic effect of these compounds in the intact rat is significantly lower than predicted by their affinity for the receptor using isolated rat liver nuclei in vitro. In intact cells each compound demonstrated an excellent relationship between the relative receptor affinity, the induction of growth hormone production, and the concentration-dependent reduction in nuclear receptor levels. With the exception of D-T3, the relative affinity of iodothyronine was identical for the receptor using intact cells in serum-free media, or isolated GH1 cell nuclei in vitro. The apparent receptor affinity of D-T3 with intact cells was 5.5-fold lower than with isolated nuclei, which suggests a decrease in cell entry of D-T3 relative to the other iodothyronines. Quantitation of the [125I]iodothyronine associated with the receptor in GH1 cells after a 36-h incubation with L-125I-T4 was 90% L-T4 and 10% L-T3, which indicates that the major effect of L-T4 in GH1 cells is a result of intrinsic L-T4 activity. Studies with dispersed rat anterior pituitary cells demonstrated that L-T3 induces growth hormone synthesis and elicits a reduction in nuclear receptor levels in the same fashion as GH1 cells. The observation that thyroid hormone influences dispersed rat pituitary cells in a fashion qualitatively similar to GH1 cells may have implications for the growth hormone response of the somatotroph cell in vivo to different thyroidal states.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker B. L., Reel J. R., Van Dewark S. D., Yu Y. Y. Persistence of cell types in monolayer cultures of dispersed cells from the pituitary pars distalis as revealed by immunohistochemistry. Anat Rec. 1974 May;179(1):93–105. doi: 10.1002/ar.1091790107. [DOI] [PubMed] [Google Scholar]
- Bellabarba D., Peterson R. E., Sterling K. An improved method for chromatography of iodothyronines. J Clin Endocrinol Metab. 1968 Feb;28(2):305–307. doi: 10.1210/jcem-28-2-305. [DOI] [PubMed] [Google Scholar]
- Burgess J. A., Smith B. R., Merimee T. J. Growth hormone in thyrotoxicosis: effect of insulin-induced hypoglycemia. J Clin Endocrinol Metab. 1966 Nov;26(11):1257–1260. doi: 10.1210/jcem-26-11-1257. [DOI] [PubMed] [Google Scholar]
- Coulombe P., Schwartz H. L., Oppenheimer J. H. Relationship between the accumulation of pituitary growth hormone and nuclear occupancy by triiodothyronine in the rat. J Clin Invest. 1978 Nov;62(5):1020–1028. doi: 10.1172/JCI109206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGroot L. J., Torresani J. Triiodothyronine binding to isolated liver cell nuclei. Endocrinology. 1975 Feb;96(2):357–359. doi: 10.1210/endo-96-2-357. [DOI] [PubMed] [Google Scholar]
- Finkelstein J. W., Boyar R. M., Hellman L. Growth hormone secretion in hyperthyroidism. J Clin Endocrinol Metab. 1974 Apr;38(4):634–637. doi: 10.1210/jcem-38-4-634. [DOI] [PubMed] [Google Scholar]
- GREENBERG C. M., BOCHER C. A., KERWIN J. F., GREENBERG S. M., LIN T. H. Several relative biological activities of D and L triiodothyronine. Am J Physiol. 1961 Oct;201:732–736. doi: 10.1152/ajplegacy.1961.201.4.732. [DOI] [PubMed] [Google Scholar]
- Gaddum J. H. Quantitative observations on thyroxine and allied substances: II. Effects on the oxygen consumption of rats. J Physiol. 1930 Jan 27;68(4):383–405. doi: 10.1113/jphysiol.1930.sp002623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershengorn M. C. Regulation of thyrotropin production by mouse pituitary thyrotropic tumor cells in vitro by physiological levels of thyroid hormones. Endocrinology. 1978 Apr;102(4):1122–1128. doi: 10.1210/endo-102-4-1122. [DOI] [PubMed] [Google Scholar]
- Goldfine I. D., Smith G. J., Simons C. G., Ingbar S. H., Jorgensen E. C. Activities of thyroid hormones and related compounds in an in vitro thymocyte assay. J Biol Chem. 1976 Jul 25;251(14):4233–4238. [PubMed] [Google Scholar]
- Goslings B., Schwartz H. L., Dillmann W., Surks M. I., Oppenheimer J. H. Comparison of the metabolism and distribution of L-triiodothyronine and triiodothyroacetic acid in the rat: a possible explanation of differential hormonal potency. Endocrinology. 1976 Mar;98(3):666–675. doi: 10.1210/endo-98-3-666. [DOI] [PubMed] [Google Scholar]
- Green W. L. Separation of iodo compounds in serum by chromatography on Sephadex columns. J Chromatogr. 1972 Oct 5;72(1):83–91. doi: 10.1016/0021-9673(72)80010-4. [DOI] [PubMed] [Google Scholar]
- Hervas F., Morreale de Escobar G., Escobar Del Rey F. Rapid effects of single small doses of L-thyroxine and triiodo-L-thyronine on growth hormone, as studied in the rat by radioimmunoassy. Endocrinology. 1975 Jul;97(1):91–101. doi: 10.1210/endo-97-1-91. [DOI] [PubMed] [Google Scholar]
- Iwatsubo H., Omori K., Okada Y., Fukuchi M., Miyai K., Abe H., Kumahara Y. Human growth hormone secretion in primary hypothyroidism before and after treatment. J Clin Endocrinol Metab. 1967 Dec;27(12):1751–1754. doi: 10.1210/jcem-27-12-1751. [DOI] [PubMed] [Google Scholar]
- Koerner D., Schwartz H. L., Surks M. I., Oppenheimer J. H. Binding of selected iodothyronine analogues to receptor sites of isolated rat hepatic nuclei. High correlation between structural requirements for nuclear binding and biological activity. J Biol Chem. 1975 Aug 25;250(16):6417–6423. [PubMed] [Google Scholar]
- Koerner D., Surks M. I., Oppenheimer J. H. In vitro demonstration of specific triiodothyronine binding sites in rat liver nuclei. J Clin Endocrinol Metab. 1974 Apr;38(4):706–709. doi: 10.1210/jcem-38-4-706. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MONEY W. L., KUMAOKA S., RAWSON R. W., KROC R. L. Comparative effects of thyroxine analogues in experimental animals. Ann N Y Acad Sci. 1960 Apr 23;86:512–544. doi: 10.1111/j.1749-6632.1960.tb42827.x. [DOI] [PubMed] [Google Scholar]
- MacGillivray M. H., Aceto T., Jr, Frohman L. A. Plasma growth hormone responses and growth retardation of hypothyroidism. Am J Dis Child. 1968 Feb;115(2):273–276. doi: 10.1001/archpedi.1968.02100010275018. [DOI] [PubMed] [Google Scholar]
- Mitsuma T., Colucci J., Shenkman L., Hollander C. S. Rapid simultaneous radioimmunoassay for triiodothyronine and thyroxine in unextracted serum. Biochem Biophys Res Commun. 1972 Mar 24;46(6):2107–2113. doi: 10.1016/0006-291x(72)90766-8. [DOI] [PubMed] [Google Scholar]
- OPPENHEIMER J. H., SURKS M. I. DETERMINATION OF FREE THYROXINE IN HUMAN SERUM: A THEORETICAL AND EXPERIMENTAL ANALYSIS. J Clin Endocrinol Metab. 1964 Aug;24:785–793. doi: 10.1210/jcem-24-8-785. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Dillman W., Surks M. I. Effect of thyroid hormone analogues on the displacement of 125I-L-triiodothyronine from hepatic and heart nuclei in vivo: possible relationship to hormonal activity. Biochem Biophys Res Commun. 1973 Dec 10;55(3):544–550. doi: 10.1016/0006-291x(73)91177-7. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I. Nuclear binding capacity appears to limit the hepatic response to L-triiodothyronine (T3). Endocr Res Commun. 1975;2(4-5):309–325. doi: 10.1080/07435807509089004. [DOI] [PubMed] [Google Scholar]
- Peake G. T., Birge C. A., Daughaday W. H. Alterations of radioimmunoassayable growth hormone and prolactin during hypothroidism. Endocrinology. 1973 Feb;92(2):487–493. doi: 10.1210/endo-92-2-487. [DOI] [PubMed] [Google Scholar]
- Root A. W., Rosenfield R. L., Bongiovanni A. M., Eberlein W. R. The plasma growth hormone response to insulin-induced hypoglycemia in children with retardation of growth. Pediatrics. 1967 Jun;39(6):844–852. [PubMed] [Google Scholar]
- SOLOMON J., GREEP R. O. The effect of alterations in thyroid function on the pituitary growth hormone content and acidophil cytology. Endocrinology. 1959 Aug;65(2):158–164. doi: 10.1210/endo-65-2-158. [DOI] [PubMed] [Google Scholar]
- STASILLI N. R., KROC R. L., MELTZER R. I. Antigoitrogenic and calorigenic activities of thyroxine analogues in rats. Endocrinology. 1959 Jan;64(1):62–82. doi: 10.1210/endo-64-1-62. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Shapiro L. E. Thyroid hormone stimulates de novo growth hormone synthesis in cultured GH1 cells: evidence for the accumulation of a rate limiting RNA species in the induction process. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3369–3373. doi: 10.1073/pnas.73.10.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels H. H., Stanley F., Shapiro L. E. Dose-dependent depletion of nuclear receptors by L-triiodothyronine: evidence for a role in induction of growth hormone synthesis in cultured GH1 cells. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3877–3881. doi: 10.1073/pnas.73.11.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels H. H., Stanley F., Shapiro L. E. Modulation of thyroid hormone nuclear receptor levels by 3,5,3'-triiodo-L-thyronine in GH1 cells. Evidence for two functional components of nuclear-bound receptor and relationship to the induction of growth hormone synthesis. J Biol Chem. 1977 Sep 10;252(17):6052–6060. [PubMed] [Google Scholar]
- Samuels H. H., Tsai J. S., Casanova J., Stanley F. Thyroid hormone action: in vitro characterization of solubilized nuclear receptors from rat liver and cultured GH1 cells. J Clin Invest. 1974 Oct;54(4):853–865. doi: 10.1172/JCI107825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels H. H., Tsai J. S., Cintron R. Thyroid hormone action: a cell-culture system responsive to physiological concentrations of thyroid hormones. Science. 1973 Sep 28;181(4106):1253–1256. doi: 10.1126/science.181.4106.1253. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Tsai J. S. Thyroid hormone action in cell culture: domonstration of nuclear receptors in intact cells and isolated nuclei. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3488–3492. doi: 10.1073/pnas.70.12.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels H. H., Tsai J. S. Thyroid hormone action. Demonstration of similar receptors in isolated nuclei of rat liver and cultured GH1 cells. J Clin Invest. 1974 Feb;53(2):656–659. doi: 10.1172/JCI107601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels H. H., Tsai J. S. Thyroid hormone action. Demonstration of similar receptors in isolated nuclei of rat liver and cultured GH1 cells. J Clin Invest. 1974 Feb;53(2):656–659. doi: 10.1172/JCI107601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schooley R. A., Friedkin S., Evans E. S. Re-examination of the discrepancy between acidophil numbers and growth hormone concentration in the anterior pituitary gland following thyroidectomy. Endocrinology. 1966 Dec;79(6):1053–1057. doi: 10.1210/endo-79-6-1053. [DOI] [PubMed] [Google Scholar]
- Shapiro L. E., Samuels H. H., Yaffe B. M. Thyroid and glucocorticoid hormones synergistically control growth hormone mRNA in cultured GH1 cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):45–49. doi: 10.1073/pnas.75.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J. E., Larsen P. R. Contributions of plasma triiodothyronine and local thyroxine monodeiodination to triiodothyronine to nuclear triiodothyronine receptor saturation in pituitary, liver, and kidney of hypothyroid rats. Further evidence relating saturation of pituitary nuclear triiodothyronine receptors and the acute inhibition of thyroid-stimulating hormone release. J Clin Invest. 1978 May;61(5):1247–1259. doi: 10.1172/JCI109041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder G., Hymer W. C., Snyder J. Functional heterogeneity in somatotrophs isolated from the rat anterior pituitary. Endocrinology. 1977 Sep;101(3):788–799. doi: 10.1210/endo-101-3-788. [DOI] [PubMed] [Google Scholar]
- Spindler B. J., MacLeod K. M., Ring J., Baxter J. D. Thyroid hormone receptors. Binding characteristics and lack of hormonal dependency for nuclear localization. J Biol Chem. 1975 Jun 10;250(11):4113–4119. [PubMed] [Google Scholar]
- Stachura M. E. Basal and dibutyryl cyclic AMP-stimulated release of newly synthesized and stored growth hormone from perifused rat pituitaries. Endocrinology. 1976 Mar;98(3):580–589. doi: 10.1210/endo-98-3-580. [DOI] [PubMed] [Google Scholar]
- Surks M. I., DeFesi C. R. Determination of the cell number of each cell type in the anterior pituitary of euthyroid and hypothyroid rats. Endocrinology. 1977 Sep;101(3):946–958. doi: 10.1210/endo-101-3-946. [DOI] [PubMed] [Google Scholar]
- TOMICH E. G., WOOLLETT E. A., PRATT M. A. Some biological actions of iodo-L-thyronines. J Endocrinol. 1960 Feb;20:65–68. doi: 10.1677/joe.0.0200065. [DOI] [PubMed] [Google Scholar]
- Tsai J. S., Chen A. Effect of L-triiodothyronine on (--)3H-dihydroalprenolol binding and cyclic AMP response to (--)adrenaline in cultured heart cells. Nature. 1978 Sep 14;275(5676):138–140. doi: 10.1038/275138a0. [DOI] [PubMed] [Google Scholar]
- Tsai J. S., Samuels H. H. Thyroid hormone action: stimulation of growth hormone and inhibition of prolactin secretion in cultured GH1 cells. Biochem Biophys Res Commun. 1974 Jul 10;59(1):420–428. doi: 10.1016/s0006-291x(74)80223-8. [DOI] [PubMed] [Google Scholar]
- Vale W., Grant G., Amoss M., Blackwell R., Guillemin R. Culture of enzymatically dispersed pituitary cells: functional validation of a method. Endocrinology. 1972 Aug;91(2):562–572. doi: 10.1210/endo-91-2-562. [DOI] [PubMed] [Google Scholar]
- Westerfeld W. W., Richert D. A., Ruegamer W. R. New assay procedure for thyroxine analogs. Endocrinology. 1965 Nov;77(5):802–811. doi: 10.1210/endo-77-5-802. [DOI] [PubMed] [Google Scholar]



