Abstract
Oxidative DNA damage is implicated in brain aging, neurodegeneration and neurological diseases. Damage can be created by normal cellular metabolism, which accumulates with age, or by acute cellular stress conditions which create bursts of oxidative damage. Brain cells have a particularly high basal level of metabolic activity and use distinct oxidative damage repair mechanisms to remove oxidative damage from DNA and dNTP pools. Accumulation of this damage in the background of a functional DNA repair response is associated with normal aging, but defective repair in brain cells can contribute to neurological dysfunction. Emerging research strongly associates three common neurodegenerative conditions, Alzheimer’s, Parkinson’s and stroke, with defects in the ability to repair chronic or acute oxidative damage in neurons. This review explores the current knowledge of the role of oxidative damage repair in preserving brain function and highlights the emerging models and methods being used to advance our knowledge of the pathology of neurodegenerative disease.
I. Introduction
DNA damage leads to genomic instability and cellular dysfunction. Oxidative damage is particularly harmful; as over 100 oxidative modifications to DNA have been identified. Many adducts are mutagenic while others block replication or transcription, leading to cancer or cell death (1). Though oxidative damage can arise from external sources such as chemical agents and ionizing radiation, the majority of oxidative damage is caused from internally sourced superoxide anions, hydroxyl radicals and hydrogen peroxide (collectively called reactive oxygen species; ROS), produced through normal cellular respiration and metabolism (2). To protect against their own destructive byproducts, cells have evolved an anti-oxidant defense system consisting of enzymes, such as those involved with base excision repair (BER), and scavenging molecules such as superoxide dismutase, glutathione peroxidase, peroxyredoxins and glutathione (1, 3).
The brain is well protected from external insults due to the presence of factors such as cranium and the blood brain barrier. Presumably, DNA damage in the brain is caused by endogenous metabolic activity. Oxidative damage is particularly prevalent in the brain. The continuous electrochemical transmission between cells in the brain requires a great deal of energy. Brain tissue therefore maintains a particularly high basal metabolic rate to meet the high-energy demand, meaning that brain cells produce high levels of ROS. Interestingly, several factors make the brain additionally vulnerable to oxidative damage. Because of its high metabolic rate, the brain has a decreased ratio of anti-oxidant to pro-oxidant enzymes (4, 5). This imbalance amplifies the level of oxidative damage within brain cells, which increases the demand on DNA repair activity, which in turn requires additional energy, creating a perpetual state of oxidative stress. Add to this the fact that post-mitotic terminally differentiated brain cells lack robust replication-associated DNA damage detection and DNA repair machinery (6). This results in a heavy reliance on the BER mechanism to maintain genetic homeostasis in the brain.
Although brain cells have highly efficient BER mechanisms to deal with the elevated oxidation stress, oxidative DNA damage accumulates with age and is implicated in normal aging. Additionally, excess oxidative damage is implicated in neurodegenerative disorders, and emerging research suggests that deficiencies specifically in the BER pathway perpetuate neuronal dysfunction (3). In this review, we discuss oxidative DNA damage in neurons, focusing on current research with BER glycosylase-deficient mouse models that are being used to explore the role of BER in three neurodegenerative diseases (AD, PD and stroke). In addition, we highlight the potential in applying neuroscience techniques in animal behavior testing with DNA repair models to advance brain neurodegeneration research in the future.
II. Oxidative DNA damage in brain, neurodegeneration and aging
Recent work has demonstrated that abnormal BER protein function may be involved in the pathology of three clinical neurodegenerative conditions: Alzheimer’s disease (AD), Parkinson’s disease (PD) and stroke. Among twelve common neurological diseases analyzed in a recent epidemiological review, AD, PD and stroke had the highest incidence in individuals over 65 years of age (7). Interestingly, what also sets these three neurodegenerative conditions apart from others is that their onset and progression are believed to result from internal sources of oxidative stress, where both AD and PD result from a chronic accumulation of excess oxidative damage and stroke results from an acute burst of oxidative stress. The three conditions are, however, individually distinct in the pathophysiological pathways linking the underlying oxidative stress to the neurodegenerative phenotype.
Alzheimer’s and Parkinson’s disease
Clinically, AD is a form of senile dementia characterized by loss of brain functions affecting memory, thought processing and general behavior (8). The histological hallmarks of AD are oligomerized amyloid-β precursor protein (AβPP) deposits and tau tangles caused by abnormal post-translational processing of AβPP and hyper-phosphorylation of tau peptide, respectively (9). PD is a degenerative disorder of the central nervous system and is associated with the death of substantia nigra cells in the mid brain that produce dopamine (10, 11), leading to motor dysfunction and dementia as the disease progresses. A histological hallmark of PD is the accumulation of Lewy bodies in neuronal cells and these are often proportional to the severity of clinical symptoms (12).
While the etiology of either disease is currently undetermined, much research suggests that both AD and PD have an etiology linked to oxidative stress and dysfunction of the mitochondria and endoplasmic reticulum (ER) (13, 14). Mitochondria are the organelles that produce ATP (chemical energy) and have a critical role in energy metabolism, redox state and Ca2+ homeostasis within the cell and are therefore critical for cell survival. Intracellular Ca2+ stimulates the electron transport chain in mitochondria, producing ATP and, as a byproduct, ROS. The ER is a quality control organelle that oversees protein synthesis, folding and transport. Crosstalk between the two organelles is increased with oxidative stress, and mitochondrial stress can cause ER dysfunction. Specifically in AD, oxidative stress is enhanced by AβPP stimulation of N-methyl-D-aspartate receptor-mediated glutamatergic neurotransmission (15–17), which in turn can create intracellular Ca2+ overload and lead to mitochondrial dysfunction, neuronal excitotoxicity, and ultimately neuronal death (18). In PD, research with post-mortem tissues shows impaired mitochondrial function and elevated oxidative stress caused by alpha-synuclein aggregates, dopamine auto-oxidation and degradation in the substantia nigra(10, 19, 20). Additional factors shared between PD and AD that may contribute to chronic oxidative stress are altered levels of iron and antioxidants (superoxide dismutase and glutathione) in brain cells. Interestingly, ROS has been shown to promote protein aggregation (21), and therefore one theory is that ER dysfunction may lead to increased accumulation of abnormally folded proteins as seen with AβPP in AD and alpha-synuclein in PD cells (21–24).
Stroke
Stroke is defined as a cerebrovascular accident (25), where there is loss of blood flow to the brain as a result of either a blood clot (ischemic stroke) or leakage from a damaged blood vessel (hemorrhagic stroke). In either case, there is rapid neurological damage that causes severe loss of brain function and ultimately cell death in the affected area. With ischemic stroke, neurological damage is caused initially by loss of blood flow (ischemia) which cuts off oxygen supply, followed by the rapid return of the blood supply (reperfusion) to the specific area, creating an influx of ROS and interleukins associated with inflammatory response (26). Thus unlike in AD and PD where neurodegeneration is caused by a slow chronic accumulation of oxidative damage, neurological damage in ischemic stroke is caused by this acute burst of ROS during reperfusion.
III. Neuronal oxidative damage repair
Due to their high rate of energy production and consumption, neurons exist in a state of continuous oxidative stress. Oxidative damage can cause a variety of lesions in DNA (1). These include base modifications, abasic sites, single and double strand breaks in DNA. Aside from double strand breaks, these other modifications are largely repaired by BER (3).
The first step in BER is recognition and removal of the damaged DNA base. DNA base modifications are first recognized and removed by DNA glycosylases and abasic sites are removed by apurinic/apyrimidinicendonucleases (APE)(27). Following additional processing by APE or dRPase, gap filling and nick sealing are completed by polymerases and ligases, respectively. Single strand breaks are processed by DNA polymerase beta (Polβ) in association with a scaffolding enzyme, X-ray repair cross-complementing protein (XRCC1) (Fig 1).
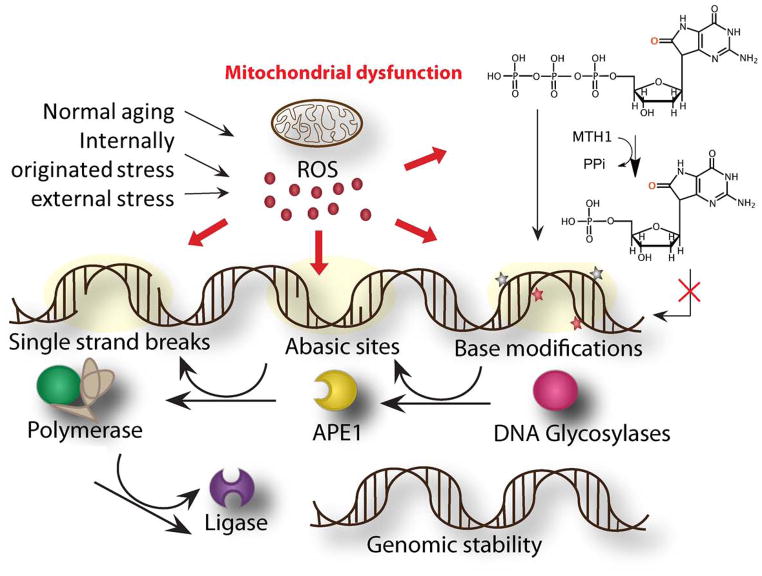
Figure 1.
Oxidative damage repair of ROS-induced DNA damage in normal aging and neurological diseases. Amyloid beta precursor protein (Aβ PP) aggregates, α-synuclein deposits and ischemic reperfusion can cause mitochondrial dysfunction and increase cellular ROS levels. ROS damages DNA and generates various lesions including base modifications, abasic sites and strand breaks. BER proteins repair these three varieties of damages in DNA. The multiple stages of BER are depicted. The base modifications in nucleotide pools significantly contribute to the oxidative lesions in DNA. MTH1 converts oxidatively damaged dGTP into dGMP, which cannot be incorporated into DNA during synthesis.
The BER glycosylases – a team effort
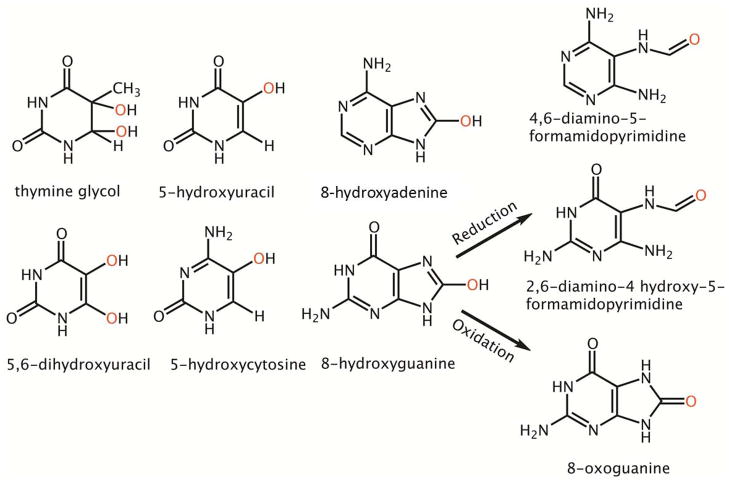
DNA glycosylases are the first DNA repair enzymes recruited to oxidative lesions (28), and there are 11 glycosylases in humans (29); of these, three central glycosylases recognize oxidative damage: 8-oxoguanine DNA glycosylase (OGG1), endonuclease III-like 1 (NTH1), and endonuclease VIII-like 1 (NEIL1) (29). Knockout mouse models have been developed for all three, as well as a NEIL1/NTH1 double knockout model. From these models and cell culture studies combined with in vitro biochemical assays, the type of damage repaired by each glycosylase has been well studied (Table 1). Several lesions are produced from the radical attack on pyrimidine and purine bases (Fig 2). Some of the common oxidative pyrimidine lesions produced include 5-hydroxyuracil (5OHU), 5,6-dihydroxyuracil (DHU), 5-hydroxycytosine (5OHC), thymine glycol (Tg) and formamidopyrimidine (FAPY) lesions (Fig 2). The major oxidative purine base lesions are 8-hydroxyguanine (8OHG) and its metabolic derivatives 8-oxoguanine and FAPY lesions (Fig 2). The pyrimidine base lesions are mainly recognized and repaired by NTH1, a human homolog of endonuclease III in E. coli, whereas the purine base lesions are primarily repaired by OGG1. However, there is some overlap among the enzymes, indicating that multiple glycosylases cooperate with or compensate for each other to maintain genomic stability, particularly during oxidative damage overload. For example, while OGG1 shows specificity for 8-oxoguanine lesions, mutY homolog, MYH, can cleave a mis-paired adenine from across the 8-oxoguanine lesion to suppress mutagenicity (30). There is greater overlap of lesion specificity among the glycosylases for oxidative pyrimidine lesions such as 5-hydroxyuracil (5OHU), 5-hydroxycytosine (5OHC), thymine glycol (TG) and FAPY lesions (30). FAPY lesions, for example, are primarily recognized and repaired by NTH1 and NEIL1, but they may also be repaired by OGG1 (2,6-diamino 5-hydroxy 5-formamidopyrimidine (FAPY G)), NEIL2 (4,6-diamino 5-formamidopyrimidine (FAPY A)) and NEIL3 (FAPY G and FAPY A) (31, 32). Among all of the glycosylases that target oxidative DNA damage, NEIL1, the human homolog of endonuclease VIII in E. coli, seems to have the widest substrate recognition and repair capacity ranging from pyrimidine lesions to purine lesions. Though NEIL1 can recognize a number of oxidized lesions, the most physiologically relevant substrates for NEIL1 are FAPY A, FAPY G, 5OHU, 5OHC, and Tg. Based on this substrate specificity, NEIL1 was originally thought to simply backup NTH1 and OGG1 enzymes. However, emerging research on its splice variants and protein interacting partners indicates that NEIL1 has an ever increasing substrate specificity towards complex substrates such as 2,2,4-triamino-5(2H)-oxazolone, bulky lesions such as 5-(S) or 5-(R) cyclo-2 deoxyadenosines, and that NEIL1 may also be a crucial enzyme in more than one DNA repair pathway, suggesting that NEIL1 has a critical rather than redundant function in oxidative damage repair (33).
Table 1.
| Serial Number | DNA Glycosylase | Substrate specificity | Overlap* |
|---|---|---|---|
| 1 | OGG1 | 8oxoG, FAPY G | NTH1, NEIL1, 2 and 3 |
| 2 | MYH | A paired across 8oxoG | |
| 3 | NTH1 | TG, 5OHU, 5OHC, DHU and FAPY G | OGG1 |
| 4 | NEIL1 | FAPY A and FAPY G, TG, 5OHU, 5OHC | OGG1, NTH1, NEIL2 and 3 |
| 5 | NEIL2 | 5OHU, FAPY A AP-site | NTH1, NEIL1, NEIL3 |
| 6 | NEIL3 | FAPY G, FAPY A, Sp, and Gh | NEILl and 2, NTH1, OGG1 |
Overlap includes all the proteins that share at least one substrate specificity with specified glycosylase in column 2.
Abbreviations : 8-oxoguanine (8oxoG), 2,6-diamino 4-hydroxy 5-formamidopyrimidine (FAPY A),), 4,6-diamino 5-formamidopyrimidine (FAPY G), Thyminegycol (TG), 5-hydroxycytosine, 5-hydroxyuracil, 5,6-dihydroxyuracil, abasic site (AP site), spiroiminodihydantoin (Sp), guanidinohydantoin (Gh), 8-oxoguanine DNA glycosylase (OGG1), mutY homolog (MYH), endonuclease three like1 (NTH1), endonuclease eight like (NEIL).
Figure 2.
Common Oxidative lesions found in DNA. The oxidative damage position is represented by a red colored oxygen molecule. 8-hydroxy guanine can be further metabolized into 4,6-diamino 5-formamidopyrimidine (FAPY G) or 8-oxoguanine.
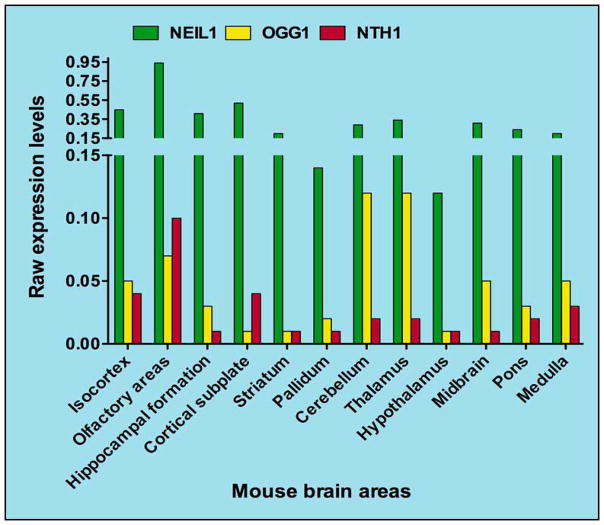
While ROS produce a variety of oxidative lesions, the incidence of each lesion can vary due to factors in the local environment that affect chemical interactions and lesion stability (34). In the brain, the most abundant oxidative lesions are 8-oxoguanine and FAPY G, which are derived from the oxidation and reduction of 8-hydroxyguanine lesions respectively (34). NEIL1 efficiently removes both FAPY G and FAPY A lesions, and the expression levels of NEIL1 are greater than either NTH1 or OGG1 in various brain regions (Fig 3)(35). Noticeably, the loss of NEIL1 led to greater FAPY lesion accumulation in the mouse brain than that observed with the loss of NTH1 (36). NEIL1 may therefore play acritical role in preserving brain function through oxidative DNA damage repair.
Figure 3.
Regional mRNA expression levels of the major DNA glycosylases that repair oxidative lesions in mouse brain. Raw expression levels of mRNA isolated from mouse brain regions are plotted. The data used to create the figure was derived from the online data bank-brain atlas found at the website- (http://mouserain-map.org).
Uracil-DNA glycosylase (UDG) and its family member single-strand-selective monofunctional uracil-DNA glycosylase1 (hSMUG1) collectively remove uracils from DNA. hSMUG1 is also capable of removing 5OHU lesions. In addition to the traditional BER glycosylases, several other enzymes are critical to maintaining DNA base integrity. Just as ROS create lesions directly to DNA, genetic instability also arises from incorporation of oxidized dNTPs into DNA. To minimize this, 7,8-dihydro-8-oxoguanine triphosphatase (MTH1) converts 8-oxoguanine triphosphate (8oxodGTP) to 8-oxoguanine monophosphate (8oxodGMP) which cannot be incorporated into DNA. This activity helps reduce the burden on downstream BER enzymes.
IV. Mouse models of BER glycosylases in neurodegenerative diseases
Homozygous knockout models of mid and late-stage BER proteins are embryonically lethal, making them impractical for functional neurodegeneration models (37). Still, associations between BER deficiencies and neurodegenerative diseases are observed with loss of normal activity of these proteins. Altered activity and expression of Polβ and XRCC1, for example, are observed in brain tissue of AD and stroke patients (38–40). Considering that Polβ knockout embryos show defective neurogenesis (41), alterations of Polβ may have potential role in etiology of neurodegenerative diseases.
More extensively studied in relation to AD, PD and stroke, however, are the BER glycosylases using knockout mouse models. These models do not show drastic phenotypes. This may be due to the compensatory nature of other DNA glycosylases as previously discussed. Nonetheless, given the elevated vulnerability of brain cells to increased oxidative stress, efficient BER is critical for normal brain function, and altered DNA repair efficiency could be directly involved in neurodegeneration in AD, PD and stroke. Transgenic and AβPP mouse models of AD have not exhibited the significant BER defects observed in human AD (42). These models, however, are developed through overexpression of mutant AβPP and other proteins like Tau, assuming that AβPP/Tau deposition is the founding etiology of AD rather than some other factor. Interestingly, these mouse models show cellular resistance to AβPP excitotoxicity (reviewed in (43)); one could speculate that this may be due to retention of an efficient BER mechanism in their genetic background, and that human AD and its associated AβPP/Tau deposition may therefore develop from deficient BER resulting in slow accumulation of damage and neuronal dysfunction. The critical role of BER in neuronal homeostasis hypothesis is strengthened by observations that BER deficiencies in the mitochondria are directly implicated in aging and neurodegeneration (12). ROS generated during oxidative phosphorylation within the mitochondria targets mitochondrial DNA (mtDNA) more than nuclear DNA (nDNA) due to its close proximity. BER is well characterized as a robust mechanism in mitochondria to defend against oxidative DNA damage. Ogg1 knockout mice accumulate 8-oxoguanine lesions in their mtDNA (44). Further, Ogg1 is crucial for the developing mouse brain, and Ogg1 activity steadily decreases with increasing age (45, 46). This indicates that in the brain, at least in mice, there may be additional mechanisms or BER enzymes such as Nth1 or Neil1 that help in 8-oxoguanine surveillance (45). Neil1 knockout mice show higher mtDNA mutations in liver cells (47) and accumulate FAPY A lesions in aged brains. In the mouse, Nth1 is localized more to the mitochondria, and loss of Nth1 abbrogated Tg and 5OHU incision in the mitochondrial compartment (45). Overall, the major DNA glycosylase-deficient mice accumulate oxidative DNA lesions, but whether these features lead to brain related pathology is not well understood. Given that these enzymes have a crucial role in reducing the oxidative damage to DNA, particularly mtDNA, and mtDNA damage is implicated in neurodegeneration, glycosylase mouse models are important tools for exploring neurodegenerative pathology.
Oxidative damage repair in AD and PD
Glycosylase expression and function is known to change in aging neurons, and these changes are more severe in AD (48, 49). AD brain tissue exhibits significantly lower expression of UDG, a nuclear and mitochondrial glycosylase that targets uracil lesions, and cell extracts show decreased uracil incision activity compared with control cells (40). UDG deficiency is also shown to induce apoptosis in rat hippocampal neurons in culture (50). Interestingly, folic acid deficiency, which has been linked to increased susceptibility to AD (51), promoted uracil misincorporation and hypomethylation of DNA in neurons and sensitized them to AβPP toxicity (52). It also resulted in increased DNA damage and hippocampal neurodegeneration in AβPP transgenic Alzheimer’s mouse model (52), suggesting that reduced uracil incision capacity plays a role in neuronal AβPP toxicity in AD. Furthermore, folic acid deficiency also caused increased mitochondrial DNA mutagenesis in aged brains (53), supporting the role of BER in the mitochondrial dysfunction associated with neurodegeneration.
OGG1 is dysregulated in both AD and PD brains (54, 55). There is significant elevation of 8-oxoguanine in frontal, temporal and parietal lobes of nDNA in AD (56) and decreased 8-oxoguanine glycosylase activity in brain tissue extracts (55). Additionally, expression of mitochondrial β-OGG1 in the orbitofrontal gyrus and entorinal cortex of AD brains was decreased when compared with controls. β-OGG1 is a mitochondrial isoform of OGG1 and is associated with neurofibrillary tangles and dystrophic neuritis (57). Unlike in AD, β-OGG1 expression is increased in the substantia nigra and associated dopaminergic neurons in PD brain tissue, as is the MUTY (54, 58, 59). This glycosylase upregulation may be a compensatory response to the elevated levels of 8-oxoguanine in PD brain cells. PD pathology is strongly associated with mitochondrial dysfunction, which can result from increased oxidative stress.
Other factors in brain tissue may contribute to glycosylase dysfunction and neurodegeneration in AD and PD. Close association between metal dys-homeostasis in the brain and the onset and/or progression of AD and PD has been established in a number of studies (60). Iron and copper ions (Fe(II) and Cu(II)) can specifically inhibit the activity of NEIL1 and NEIL2 by stably binding them with high affinity. Fe(II), in particular, inhibited the interaction of NEIL1 with downstream BER proteins Polβ and FEN1 (61). Although not directly studied, based on the above evidence, it is conceivable that the loss of NEIL1 activity in iron loaded AD brains may play an important role in enhancing the oxidative DNA damage thus contributing to the neurodegeneration.
While oxidative damage greatly affects DNA, there are also pools of dNTPs waiting to be incorporated into DNA, which are also damaged during oxidative stress. MTH1 has an indirect but important role in reducing oxidative DNA damage by minimizing the level of oxidative damage in the dNTP precursor pools. Recently, alterations in the expression of MTH1 in postmortem AD brains were found to correlate with neurodegenerative regions. MTH1 was decreased in the hippocampus of AD brains compared with controls, while its expression is increased in the entorinal cortex, one of the most vulnerable regions in the brain of AD patients (62); the increased expression might possibly be a compensatory mechanism for increased oxidation. There is also evidence for MTH1 involvement in PD pathology (59). The level of MTH1 protein localized to mitochondria was uniquely and significantly increased in dopaminergic neurons of the substantia nigra of PD patients compared with this region in other neurodegenerative diseases. In fact, mitochondrial MTH1 expression was difficult to detect in dopaminergic neurons of normal brains, suggesting that increased expression may be a protective response in the surviving dopaminergic neurons of PD brains (63). Indeed, studies in MTH1-knockout rodents have shown that MTH1 protects dopaminergic neurons from oxidative damage caused by the mitochondrial toxin 1-methyl-4-phenyl-1,2,3,6,-tetrahydropyridine (MPTP) (57, 64). MPTP inhibits the mitochondrial electron transport complex 1 causing electron transport chain dysfunction and accumulation of ROS(10). MPTP is able to induce Parkinson-like symptoms in humans and in animal models.
Overall, prevailing studies show that elevated DNA oxidative damage and dysfunctional repair might be involved in the pathology of AD and PD (65). Further studies are necessary to investigate and understand the differential expression and activities of DNA glycosylases in both diseases. Better understanding of the discrepancy of DNA repair enzymes in affected neuronal cells could be a potential target for gene therapy aimed at neuroprotection in AD and PD.
Oxidative damage repair in stroke
The role of BER enzymes in ischemic stroke is not well understood. High levels of ROS are known to cause neurodegeneration, and severe bursts of ROS, such as that caused by reperfusion following ischemic stroke, impairs neuronal function. In order to recover from this injury, neurons require a robust DNA repair system to repair the oxidative damage, avoid cell death, and preserve normal brain function. Despite this strong link between BER and the acute oxidative damage produced during ischemia and reperfusion, there is very little data on the role of DNA repair in either human stroke patients or animal stroke models.
When subjected to middle cerebral artery occlusion/reperfusion (MCAO/R) to mimic ischemic stroke, wild-type mice accumulated more FAPY A and FAPY G lesions compared to the sham treated (mice are operated on equally except for the occlusion of the middle cerebral artery) animals but not 8-oxoguanine in both ipsilateral and contralateral sides of the brains (66). Even in Ogg1 null mice, the MCAO/R procedure did not enhance 8-oxoguanine levels relative to sham treated animals, whereas there was a significant increase in FAPY G lesions in the ipsilateral side (66). This indicates that Ogg1 plays a significant role in removal of FAPY G lesions that are generated during ischemic stroke. It is possible that the 8-oxoguanine lesions are repaired more than FAPY G lesions by the combined effort of all backup enzymes present and or alternative mechanisms. Therefore the loss of enzymes that remove FAPY G lesions such as Ogg1 and Neil1 may be more important than others to prevent oxidative stress following ischemia. It has been demonstrated that Ogg1 and Neil1 knockout mice indeed show more motor dysfunction after ischemic stroke. In addition, Neil1 knockout mice showed increased neurological deficit as well as mortality after ischemia/reperfusion. Further, brain sections from these mice showed enhanced infarct volume and neuronal cell death indicating that these enzymes may contribute to brain recovery from ischemic injury (66, 67).
Animal studies show that a process called ischemic preconditioning enhances BER activity. In a manner similar to immunization, ischemic pre-conditioning improves tolerance to the burst of oxidative DNA damage generated during the ischemia and reperfusion events. Although complex, this tolerance mechanism is linked to the up-regulation of DNA repair systems. A recent study observed that ischemic preconditioning resulted in enhanced expression and function of BER proteins Xrcc1, Polβ, and DNA ligase III, and it also increased the physical interaction between Xrcc1 and Polβ specifically after reperfusion (68). These findings suggested a mechanistic model for neuronal cell protection after ischemic stroke. Other studies showed that nuclear Xrcc1 levels are reduced in the caudate putamen area early after focal cerebral ischemia (FCI) (within 10 min)(69)and APE1 levels were reduced (as early as 5 min)(39), however this loss of Xrcc1 expression is correlated with enhanced DNA fragmentation and can be explained through the increased activity of matrix metalloproteinases after FCI, which cleave DNA repair proteins such as Xrcc1, Parp1(39, 69). Along these lines, glutamate, one of the most abundant neurotransmitters and a marker for ischemic pre-conditioning, not only increases oxidative damage but also enhances BER activity by CREB mediated up-regulation of APE1 (70). Overall, animal studies indicate that up-regulation of BER helps tolerate oxidative damage and that loss of BER can cause deficit in brain function.
Although limited, human studies evaluating the association of stroke with various small nuclear polymorphisms in BER genes have been conducted. Association with stroke indicates a poor response to the acute oxidative burst that follows ischemia. Two population studies, from Lixian, China and Edison, New Jersey, showed that polymorphisms in BER genes APE1 and XRCC1 had no association with large artery atherosclerotic (LAA) stroke (71, 72). Interestingly, a polymorphism in XPD, a nucleotide excision repair gene, was associated with a significantly increased risk of stroke. In agreement, a second population study in Taiwan involving cigarette smoking and non-smoking groups showed that polymorphisms of ERCC2 and XPD, were associated with LAA stroke risk, and expression of both OGG1 and ERCC2 polymorphisms concurrently elevated the risk of LAA stroke; additionally, this risk was elevated with cigarette smoking in all groups (73). Although there is no association of APE/ref-1, XRCC1, OGG1 gene polymorphisms with stroke by themselves, combinations of these three polymorphisms increased the risk for LAA stroke in smokers, indicating the protective role of the BER pathway in an increased oxidative stress environment.
V. Studying oxidative damage repair in brain function – DNA repair meets neuroscience
In the DNA repair field, a majority of research is done using mitotic cell cultures and biochemical assays to study the cellular and molecular consequences of DNA repair deficiency. Brain dysfunction as a specific consequence of DNA repair deficiency is less explored. Adopting neuroscience techniques that measure changes in behavior to investigate these consequences in vivo is essential to uncover the role of DNA repair in brain function and neurodegenerative pathology.
Behavior testing methods
The brain is the command center of the nervous system, controlling functions that are broadly classified as learning and memory, motor control, arousal, homeostasis, motivation, information processing and perception. Various brain regions independently or cooperatively perform these functions (Table 2 and Fig 5) (74). The cumulative response to a stimulus is defined as behavior, and all animals are capable of changing their behavior in response to a repeated stimulus. This change in behavior may be monitored or studied using well established testing methods.
Table 2.
| Serial # | * Area/s of Brain | General function | Specific function | Measured parameter | Behavioural test |
|---|---|---|---|---|---|
| 1 | Hippocampus and Cerebellum | Learning and Memory | Spatial memory | Time spent in the quadrant with a platform | Water maze |
| 2 | Amygdalyda and Cerebellum | Anxiety | Locomotion | Zonal distance and Zonal time | Open field |
| 3 | Cerebellum and Cortex | Motor skill | Grip strength and Motor precision | Latency of first fall and Number of falls | Rotarod |
| 4 | Hippocampus and Cerebral cortex | Inquisitive learning | Semantic memory | Novel PI | Novel object recognition |
| 5 | Amygdalyda | Fear based learning and Memory | Instrumental memory | Time of freezing | Fear conditioning |
Brain areas column represents major but not the absolute components that perform the specified general functions. There is more overlap in the brain regions in performing a specific function than as listed above.
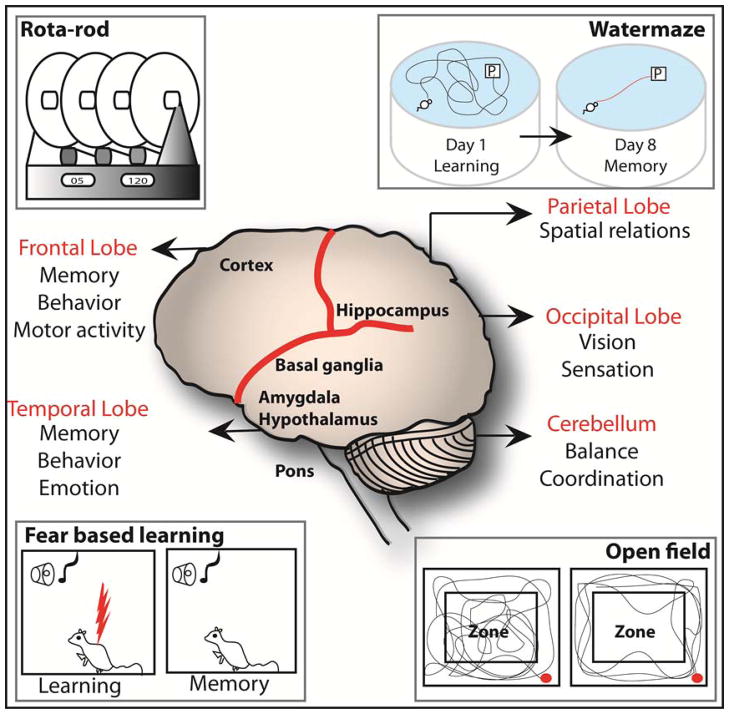
Figure 5.
Brain components and related behavior tests: Figure 4. Various brain regions and functions are highlighted. Behavioral tests that test specific brain regions are shown. The hippocampus in the parietal lobe is important for learning spatial relationships and can be studied using a water maze. The amygdala mainly creates emotions including fear that can be measured using fear-conditioning test. Fear based learning utilizes freezing time as a measure of change in behavior to a repeated and alternative aversive and neutral stimuli given to the animal on the training day. Open field test is used to measure anxiety and general locomotion and these actions are controlled by amygdala and cerebellum. The rota-rod machine measures the motor function including balance and coordination of movement. Control of motor function is performed by the combine action of the cerebellum, basal ganglia and cortex in the frontal lobe.
A battery of tests exists to study the functions of various parts of the brain using animal models, most commonly mice and rats (Table 2/Fig 5). For example, the cortex, cerebellum, hippocampus and basal ganglia together coordinate motor activity (Table 2), which includes grip strength, precision, co-ordination of movement and endurance. Two tests are mainly designed to measure motor activity: the rota-rod test, and the hanging wire test. The rota-rod test is designed to measure several parameters of motor activity. The rota-rod machine has adjustable (fixed and accelerating) speed settings, where the number of falls over a pre-determined time is counted to provide a measure of the subject’s motor precision, and the latency (time) to the subject’s first fall is recorded to serve as a measure of its co-ordination, or ability to adjust to a changing speed. The hanging wire test, in which the subject is timed while it hangs from a wire, measures grip and endurance as a factor of muscle strength. Elsewhere in the brain, the hypothalamus, amygdala and hippocampus coordinate the emotions including anxiety and fear. Anxiety and fear-based learning manifest behavior which can be measured using the fear conditioning and open field tests, respectively (Table 2). While an indicative measure of neurological function in its own right, anxiety can also affect other aspects of behavior; therefore, the open field test is conducted prior to the other tests as a control. In the open field test, parameters measured are distance travelled and the time spent in the center and peripheral zones during a period of time. Anxious animals tend to be more active and spend less time in the center zone relative to normal animals, for example. In the fear conditioning test, an aversive stimulus (an electric shock) is associated with a neutral stimulus (a tone), whereby the shock follows closely behind the tone in a training session, involving multiple conditioning trials on day one. On day two when exposed to the tone, animals that show fear conditioning freeze in expectation of the shock. The time the animal spends frozen in fear is the parameter that indicates fear-based learning in this test (75). Lastly, various parts of the brain coordinate learning and memory, but the hippocampus in particular plays a major role in spatial memory retention in a process called long-term potentiation via enhanced synaptic plasticity and navigation. The classic test for learning and memory is the Morris water maze test. In the water maze test, animal subjects are trained to find a hidden platform in a pool; they are provided with clues and latencies to reach the platform are recorded each day until the goal latencies are significantly reduced. From the next day, the platform and clues are removed and probed for the memory gained during the training period, and the subject’s memory retention is measured as directly proportional to the time spent swimming in the quadrant where the platform was located during training. Other learning and memory tests include an elevated plus maze and a radial maze which are not discussed here (76).
Inter-laboratory variation in mouse behavior analyses can result from different experimental approaches and data interpretation. Sources of experimental variation include genotype manipulation, chemical treatment, variable handling and surgical techniques, inconsistent housing conditions, order of test administration. Bailey et al provide a comprehensive review of strategies that may help to streamline animal behavior test analyses (77).
Loss of certain genes may disable the animal physically or mentally, complicating comparison with their wild type counterparts. For instance, knocking out a DNA damage response gene, ataxia telangiectasia mutated (ATM) has been reported to severely dysregulate an animal’s gait and decrease motor skill efficiency (78). Therefore, one cannot accurately compare spatial memory of ATM−/− mice with wild-type mice using a water maze test because the ATM−/− mice are likely less efficient at swimming due to impaired motor skills. Therefore additional stringency should be applied while conducting experiments in the behavioral domain. New mutant line sinitially should be assessed for general health, examining the fur condition for dermatitis, body weight for irregular metabolism, muscle and skeletal development for abnormal growth, general activity level, and social interactions of the mice for psychiatric health. All of these factors may dramatically change the behavior of an animal which can ultimately affect the observed data.
In addition, to strengthen the accuracy of data interpretation, multiple methods should be utilized. A wide range of behavioral paradigms are available to corroborate an observed phenomenon and avoid the pitfalls associated with drawing conclusions from a single test. For example, anxiety may be tested using open field exploration test, elevated plus-maze/elevated zero-maze, light ↔ dark exploration test, and a social interaction test, and consideration of data from more than one test gives a more accurate profile of the behavior (79).
Current progress in understanding the effect of oxidative damage repair on brain function using mouse behavior tests
Using the above tests one can study brain function in different genetic backgrounds and in the presence or absence of external stress. The potential of these tests to uncover the functional impact of DNA repair in neurodegenerative disease pathology is just beginning to be recognized.
Using the rota-rod test, Ogg1 knockout mice were observed to have significant motor dysfunction compared with wild-type mice in the absence of external oxidative stress (66). Ogg1−/− mice had a significant increase in the number of falls from the rota-rod machine over a fixed time period, indicating motor coordination defects. The motor tests suggest a functional deficit in the cortex, cerebellum, hippocampus and/or basal ganglia, the regions which control this activity, and possible pathological etiology for motor dysfunction in PD and stroke (Fig 4). The Ogg1 glycosylase is shown to protect neuronal cells from oxidative damage induced after ischemic stroke, and the motor dysfunction observed in Ogg1 knockout mice was further increased after MCAO/R ischemic stroke compared to both wild-type stroke mice and untreated Ogg1 knockouts (66). In addition, the loss of Neil1 also exacerbated the motor dysfunction in mice after MCAO/R procedure, indicating a role for Neil1 in neuronal recovery following stroke (81). Interestingly, under no external stress, Neil1 knockout mice showed decreased spatial memory retention in the water maze test (81); from this observation, one could speculate that Neil1 deficit may play a role in impaired memory retention observed in AD.
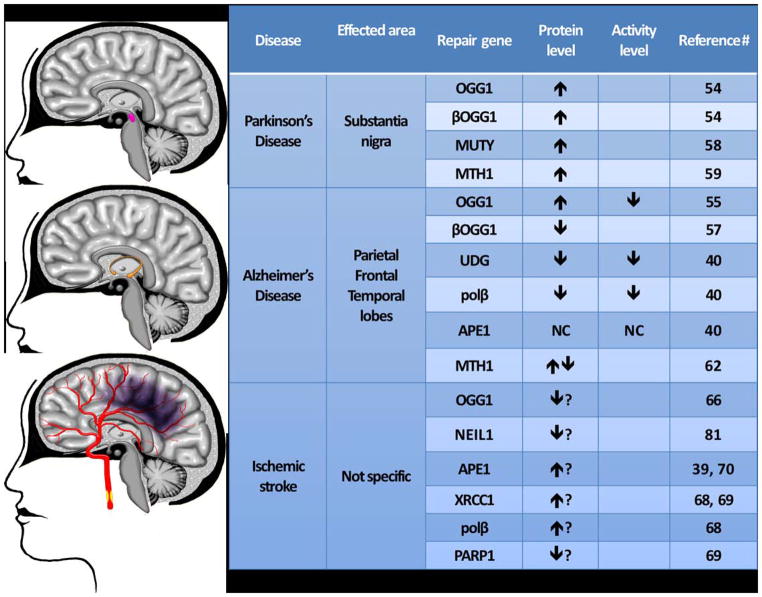
Figure 4.
Expression levels of oxidative damage repair enzymes in AD, PD, and stroke. Most effected brain regions in each disease are highlighted and the relative dysregulation of various repair proteins is indicated with upward or downward arrows. No change is indicated by symbol.
Using the rota-rod, water maze and fear-based learning tests, a senescence-accelerated aging mouse prone model (SAMP8) was used to show an age related loss of brain functions. It was later discovered that this was associated with decreased Mth2 levels (80). Mth2, along with Mth1, is a mutT-related 7,8-dihydro-8-oxoguanine triphosphatase that eliminates 8-oxoguanine-containing nucleotides from the dNTP pool. Dysfunction in learning and motor coordination in this mouse model suggests that Mth2 and perhaps Mth1 have a role in preserving the function of multiple brain regions. This is supported by observations of concomitant increase in 8-oxoguanine levels and reduced expression of Mth2 associated with age in the brain tissue of SAMP8 mice. From the behavior tests, one could speculate that Mth2 deficiency may be involved in the underlying pathology of AD which shows deficits in learning and memory function (81). Additional support for these proteins in brain function is that MTH1 has been shown to suppress the accumulation of oxidative damage of nucleic acids in the hippocampal microglia after kainate-induced excitotoxicity (82).
Summary and future prospects
In this review, we discussed the role of oxidative DNA damage repair in preserving normal brain function from the endogenous oxidative damage and in response to altered physiology, causing neurodegeneration. Imbalance in the redox state in the brain may be responsible for the three major neurodegenerative diseases. In AD and PD a slow buildup of oxidative damage in the non-proliferative neuronal cells accompanied by the loss of anti-oxidant molecules may cause the disease, whereas in stroke, a sudden burst of ROS causes acute oxidative damage. In each case there is evidence of increased oxidative damage and this was studied in mouse models and post-mortem human material. The deficiencies and alterations in the oxidative damage repair observed in these diseases suggests that the glycosylases and 7,8-dihydro-8-oxoguanine triphosphatases which remove oxidized bases from DNA and dNTP pools, respectively, play an important role in neurodegeneration.
Presently, neurodegenerative diseases and stroke dominate the list of diseases that cause high incidence of death and disability. Neurodegenerative diseases are considered as sporadic, idiopathic or non-genetic in nature as only ~5% of the AD or PD cases are recorded as familial (83, 84). The etiology of AD, PD and stroke neurodegeneration is unknown, but recent research suggests that age-related and oxidative DNA damage-dependent changes in the BER proteins may be major contributors. Further research is needed to investigate the impact of DNA repair deficiency on brain function relevant to AD, PD and stroke, and to explore possible therapeutic options. These questions may be answered by merging neuroscience behavior testing techniques for studying brain function in the background of DNA repair mouse models. This research will also provide insight into DNA repair-related mechanisms in the process of normal aging, which is still not well understood.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dizdaroglu M. Base-excision repair of oxidative DNA damage by DNA glycosylases 13. Mutat Res. 2005;591(1–2):45–59. doi: 10.1016/j.mrfmmm.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 2.Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411(6835):366–74. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 3.Wilson DM, 3rd, Bohr VA. The mechanics of base excision repair, and its relationship to aging and disease. DNA repair. 2007;6(4):544–59. doi: 10.1016/j.dnarep.2006.10.017. Epub 2006/11/23. [DOI] [PubMed] [Google Scholar]
- 4.Floyd RA, Hensley K. Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative diseases. Neurobiol Aging. 2002;23(5):795–807. doi: 10.1016/s0197-4580(02)00019-2. [DOI] [PubMed] [Google Scholar]
- 5.Halliwell B. Reactive oxygen species and the central nervous system. J Neurochem. 1992;59(5):1609–23. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- 6.Barnes DE, Lindahl T. Repair and genetic consequences of endogenous DNA base damage in mammalian cells. Annu Rev Genet. 2004;38:445–76. doi: 10.1146/annurev.genet.38.072902.092448. [DOI] [PubMed] [Google Scholar]
- 7.Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the “common” neurologic disorders? Neurology. 2007;68(5):326–37. doi: 10.1212/01.wnl.0000252807.38124.a3. [DOI] [PubMed] [Google Scholar]
- 8.Berchtold NC, Cotman CW. Evolution in the conceptualization of dementia and Alzheimer’s disease: Greco-Roman period to the 1960s. Neurobiol Aging. 1998;19(3):173–89. doi: 10.1016/s0197-4580(98)00052-9. [DOI] [PubMed] [Google Scholar]
- 9.Tiraboschi P, Hansen LA, Thal LJ, Corey-Bloom J. The importance of neuritic plaques and tangles to the development and evolution of AD. Neurology. 2004;62(11):1984–9. doi: 10.1212/01.wnl.0000129697.01779.0a. [DOI] [PubMed] [Google Scholar]
- 10.Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39(6):889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 11.Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79(4):368–76. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- 12.Schulz-Schaeffer WJ. The synaptic pathology of alpha-synuclein aggregation in dementia with Lewy bodies, Parkinson’s disease and Parkinson’s disease dementia. Acta Neuropathol. 2010;120(2):131–43. doi: 10.1007/s00401-010-0711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kulic L, Wollmer MA, Rhein V, Pagani L, Kuehnle K, Cattepoel S, et al. Combined expression of tau and the Harlequin mouse mutation leads to increased mitochondrial dysfunction, tau pathology and neurodegeneration. Neurobiol Aging. 2011;32(10):1827–38. doi: 10.1016/j.neurobiolaging.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 14.Pagani L, Eckert A. Amyloid-Beta interaction with mitochondria. Int J Alzheimers Dis. 2011;2011:925050. doi: 10.4061/2011/925050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butterfield DA, Boyd-Kimball D. Amyloid beta-peptide(1-42) contributes to the oxidative stress and neurodegeneration found in Alzheimer disease brain. Brain Pathol. 2004;14(4):426–32. doi: 10.1111/j.1750-3639.2004.tb00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nizzari M, Thellung S, Corsaro A, Villa V, Pagano A, Porcile C, et al. Neurodegeneration in Alzheimer disease: role of amyloid precursor protein and presenilin 1 intracellular signaling. J Toxicol. 2012;2012:187297. doi: 10.1155/2012/187297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russo C, Venezia V, Repetto E, Nizzari M, Violani E, Carlo P, et al. The amyloid precursor protein and its network of interacting proteins: physiological and pathological implications. Brain Res Brain Res Rev. 2005;48(2):257–64. doi: 10.1016/j.brainresrev.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 18.Ferreiro E, Baldeiras I, Ferreira IL, Costa RO, Rego AC, Pereira CF, et al. Mitochondrial- and endoplasmic reticulum-associated oxidative stress in Alzheimer’s disease: from pathogenesis to biomarkers. Int J Cell Biol. 2012;2012:735206. doi: 10.1155/2012/735206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirsch EC. Biochemistry of Parkinson’s disease with special reference to the dopaminergic systems. Mol Neurobiol. 1994;9(1–3):135–42. doi: 10.1007/BF02816113. [DOI] [PubMed] [Google Scholar]
- 20.Nagatsu T, Sawada M. Biochemistry of postmortem brains in Parkinson’s disease: historical overview and future prospects. J Neural Transm Suppl. 2007;(72):113–20. doi: 10.1007/978-3-211-73574-9_14. [DOI] [PubMed] [Google Scholar]
- 21.Benzi G, Moretti A. Are reactive oxygen species involved in Alzheimer’s disease? Neurobiol Aging. 1995;16(4):661–74. doi: 10.1016/0197-4580(95)00066-n. [DOI] [PubMed] [Google Scholar]
- 22.Paschen W. Endoplasmic reticulum: a primary target in various acute disorders and degenerative diseases of the brain. Cell Calcium. 2003;34(4–5):365–83. doi: 10.1016/s0143-4160(03)00139-8. [DOI] [PubMed] [Google Scholar]
- 23.Paschen W. Endoplasmic reticulum dysfunction in brain pathology: critical role of protein synthesis. Curr Neurovasc Res. 2004;1(2):173–81. doi: 10.2174/1567202043480125. [DOI] [PubMed] [Google Scholar]
- 24.Paschen W, Mengesdorf T. Endoplasmic reticulum stress response and neurodegeneration. Cell Calcium. 2005;38(3–4):409–15. doi: 10.1016/j.ceca.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 25.http://www.stepuptoahealthieryou.com/Strokes/8/9 63. 2012.
- 26.Brait VH, Arumugam TV, Drummond GR, Sobey CG. Importance of T lymphocytes in brain injury, immunodeficiency, and recovery after cerebral ischemia. J Cereb Blood Flow Metab. 2012;32(4):598–611. doi: 10.1038/jcbfm.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dianov GL, Sleeth KM, Dianova II, Allinson SL. Repair of abasic sites in DNA. Mutat Res. 2003;531(1–2):157–63. doi: 10.1016/j.mrfmmm.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Hegde ML, Hazra TK, Mitra S. Early steps in the DNA base excision/single-strand interruption repair pathway in mammalian cells. Cell Res. 2008;18(1):27–47. doi: 10.1038/cr.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu J, de Souza-Pinto NC, Haraguchi K, Hogue BA, Jaruga P, Greenberg MM, et al. Repair of formamidopyrimidines in DNA involves different glycosylases: role of the OGG1, NTH1, and NEIL1 enzymes 2. J Biol Chem. 2005;280(49):40544–51. doi: 10.1074/jbc.M508772200. [DOI] [PubMed] [Google Scholar]
- 30.Ide H, Kotera M. Human DNA glycosylases involved in the repair of oxidatively damaged DNA 1. Biol Pharm Bull. 2004;27(4):480–5. doi: 10.1248/bpb.27.480. [DOI] [PubMed] [Google Scholar]
- 31.Das S, Chattopadhyay R, Bhakat KK, Boldogh I, Kohno K, Prasad R, et al. Stimulation of NEIL2-mediated oxidized base excision repair via YB-1 interaction during oxidative stress 4. J Biol Chem. 2007;282(39):28474–84. doi: 10.1074/jbc.M704672200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Popuri V, Croteau DL, Bohr VA. Substrate specific stimulation of NEIL1 by WRN but not the other human RecQ helicases. DNA repair. 2010;9(6):636–42. doi: 10.1016/j.dnarep.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grin IR, Zharkov DO. Eukaryotic endonuclease VIII-like proteins: new components of the base excision DNA repair system 1. Biochemistry (Mosc) 2011;76(1):80–93. doi: 10.1134/s000629791101010x. [DOI] [PubMed] [Google Scholar]
- 34.Kalam MA, Haraguchi K, Chandani S, Loechler EL, Moriya M, Greenberg MM, et al. Genetic effects of oxidative DNA damages: comparative mutagenesis of the imidazole ring-opened formamidopyrimidines (Fapy lesions) and 8-oxo-purines in simian kidney cells 4. Nucleic Acids Res. 2006;34(8):2305–15. doi: 10.1093/nar/gkl099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atlas AB. http://mouse.brain-map.org/
- 36.Chan MK, Ocampo-Hafalla MT, Vartanian V, Jaruga P, Kirkali G, Koenig KL, et al. Targeted deletion of the genes encoding NTH1 and NEIL1 DNA N-glycosylases reveals the existence of novel carcinogenic oxidative damage to DNA 2. DNA repair. 2009;8(7):786–94. doi: 10.1016/j.dnarep.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larsen E, Meza TJ, Kleppa L, Klungland A. Organ and cell specificity of base excision repair mutants in mice. Mutat Res. 2007;614(1–2):56–68. doi: 10.1016/j.mrfmmm.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 38.Calafiore M, Copani A, Deng W. DNA polymerase-beta mediates the neurogenic effect of beta-amyloid protein in cultured subventricular zone neurospheres. J Neurosci Res. 2012;90(3):559–67. doi: 10.1002/jnr.22780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujimura M, Morita-Fujimura Y, Kawase M, Chan PH. Early decrease of apurinic/apyrimidinic endonuclease expression after transient focal cerebral ischemia in mice 31. J Cereb Blood Flow Metab. 1999;19(5):495–501. doi: 10.1097/00004647-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Weissman L, Jo DG, Sorensen MM, de Souza-Pinto NC, Markesbery WR, Mattson MP, et al. Defective DNA base excision repair in brain from individuals with Alzheimer’s disease and amnestic mild cognitive impairment 5. Nucleic Acids Res. 2007;35(16):5545–55. doi: 10.1093/nar/gkm605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gu H, Marth JD, Orban PC, Mossmann H, Rajewsky K. Deletion of a DNA polymerase beta gene segment in T cells using cell type-specific gene targeting 6. Science. 1994;265(5168):103–6. doi: 10.1126/science.8016642. [DOI] [PubMed] [Google Scholar]
- 42.Weissman L, de Souza-Pinto NC, Mattson MP, Bohr VA. DNA base excision repair activities in mouse models of Alzheimer’s disease. Neurobiol Aging. 2009;30(12):2080–1. doi: 10.1016/j.neurobiolaging.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morrissette DA, Parachikova A, Green KN, LaFerla FM. Relevance of transgenic mouse models to human Alzheimer disease. J Biol Chem. 2009;284(10):6033–7. doi: 10.1074/jbc.R800030200. [DOI] [PubMed] [Google Scholar]
- 44.de Souza-Pinto NC, Wilson DM, 3rd, Stevnsner TV, Bohr VA. Mitochondrial DNA, base excision repair and neurodegeneration. DNA repair. 2008;7(7):1098–109. doi: 10.1016/j.dnarep.2008.03.011. Epub 2008/05/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klungland A, Rosewell I, Hollenbach S, Larsen E, Daly G, Epe B, et al. Accumulation of premutagenic DNA lesions in mice defective in removal of oxidative base damage 2. Proc Natl Acad Sci USA. 1999;96(23):13300–5. doi: 10.1073/pnas.96.23.13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leonard S, Logel J, Luthman D, Casanova M, Kirch D, Freedman R. Biological stability of mRNA isolated from human postmortem brain collections 14. Biol Psychiatry. 1993;33(6):456–66. doi: 10.1016/0006-3223(93)90174-c. [DOI] [PubMed] [Google Scholar]
- 47.Vartanian V, Lowell B, Minko IG, Wood TG, Ceci JD, George S, et al. The metabolic syndrome resulting from a knockout of the NEIL1 DNA glycosylase 3. Proc Natl Acad Sci USA. 2006;103(6):1864–9. doi: 10.1073/pnas.0507444103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Focher F, Mazzarello P, Verri A, Hubscher U, Spadari S. Activity profiles of enzymes that control the uracil incorporation into DNA during neuronal development. Mutat Res. 1990;237(2):65–73. doi: 10.1016/0921-8734(90)90012-g. [DOI] [PubMed] [Google Scholar]
- 49.Swain U, Rao KS. Age-dependent decline of DNA base excision repair activity in rat cortical neurons. Mech Ageing Dev. 2012;133(4):186–94. doi: 10.1016/j.mad.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 50.Kruman II, Schwartz E, Kruman Y, Cutler RG, Zhu X, Greig NH, et al. Suppression of uracil-DNA glycosylase induces neuronal apoptosis. J Biol Chem. 2004;279(42):43952–60. doi: 10.1074/jbc.M408025200. [DOI] [PubMed] [Google Scholar]
- 51.Ravaglia G, Forti P, Maioli F, Martelli M, Servadei L, Brunetti N, et al. Homocysteine and folate as risk factors for dementia and Alzheimer disease. Am J Clin Nutr. 2005;82(3):636–43. doi: 10.1093/ajcn.82.3.636. [DOI] [PubMed] [Google Scholar]
- 52.Kruman II, Kumaravel TS, Lohani A, Pedersen WA, Cutler RG, Kruman Y, et al. Folic acid deficiency and homocysteine impair DNA repair in hippocampal neurons and sensitize them to amyloid toxicity in experimental models of Alzheimer’s disease. J Neurosci. 2002;22(5):1752–62. doi: 10.1523/JNEUROSCI.22-05-01752.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kronenberg G, Gertz K, Overall RW, Harms C, Klein J, Page MM, et al. Folate deficiency increases mtDNA and D-1 mtDNA deletion in aged brain of mice lacking uracil-DNA glycosylase. Exp Neurol. 2011;228(2):253–8. doi: 10.1016/j.expneurol.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 54.Fukae J, Takanashi M, Kubo S, Nishioka K, Nakabeppu Y, Mori H, et al. Expression of 8-oxoguanine DNA glycosylase (OGG1) in Parkinson’s disease and related neurodegenerative disorders. Acta Neuropathol. 2005;109(3):256–62. doi: 10.1007/s00401-004-0937-9. [DOI] [PubMed] [Google Scholar]
- 55.Lovell MA, Xie C, Markesbery WR. Decreased base excision repair and increased helicase activity in Alzheimer’s disease brain. Brain Res. 2000;855(1):116–23. doi: 10.1016/s0006-8993(99)02335-5. [DOI] [PubMed] [Google Scholar]
- 56.Gabbita SP, Lovell MA, Markesbery WR. Increased nuclear DNA oxidation in the brain in Alzheimer’s disease. J Neurochem. 1998;71(5):2034–40. doi: 10.1046/j.1471-4159.1998.71052034.x. [DOI] [PubMed] [Google Scholar]
- 57.Iida T, Furuta A, Nishioka K, Nakabeppu Y, Iwaki T. Expression of 8-oxoguanine DNA glycosylase is reduced and associated with neurofibrillary tangles in Alzheimer’s disease brain. Acta Neuropathol. 2002;103(1):20–5. doi: 10.1007/s004010100418. [DOI] [PubMed] [Google Scholar]
- 58.Arai T, Fukae J, Hatano T, Kubo S, Ohtsubo T, Nakabeppu Y, et al. Up-regulation of hMUTYH, a DNA repair enzyme, in the mitochondria of substantia nigra in Parkinson’s disease. Acta Neuropathol. 2006;112(2):139–45. doi: 10.1007/s00401-006-0081-9. [DOI] [PubMed] [Google Scholar]
- 59.Shimura-Miura H, Hattori N, Kang D, Miyako K, Nakabeppu Y, Mizuno Y. Increased 8-oxo-dGTPase in the mitochondria of substantia nigral neurons in Parkinson’s disease. Ann Neurol. 1999;46(6):920–4. [PubMed] [Google Scholar]
- 60.Cuajungco MP, Frederickson CJ, Bush AI. Amyloid-beta metal interaction and metal chelation. Subcell Biochem. 2005;38:235–54. doi: 10.1007/0-387-23226-5_12. [DOI] [PubMed] [Google Scholar]
- 61.Hegde ML, Hegde PM, Holthauzen LM, Hazra TK, Rao KS, Mitra S. Specific Inhibition of NEIL-initiated repair of oxidized base damage in human genome by copper and iron: potential etiological linkage to neurodegenerative diseases. J Biol Chem. 2010;285(37):28812–25. doi: 10.1074/jbc.M110.126664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Furuta A, Iida T, Nakabeppu Y, Iwaki T. Expression of hMTH1 in the hippocampi of control and Alzheimer’s disease. Neuroreport. 2001;12(13):2895–9. doi: 10.1097/00001756-200109170-00028. [DOI] [PubMed] [Google Scholar]
- 63.Nakabeppu Y, Tsuchimoto D, Yamaguchi H, Sakumi K. Oxidative damage in nucleic acids and Parkinson’s disease. J Neurosci Res. 2007;85(5):919–34. doi: 10.1002/jnr.21191. [DOI] [PubMed] [Google Scholar]
- 64.Yamaguchi H, Kajitani K, Dan Y, Furuichi M, Ohno M, Sakumi K, et al. MTH1, an oxidized purine nucleoside triphosphatase, protects the dopamine neurons from oxidative damage in nucleic acids caused by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Cell Death Differ. 2006;13(4):551–63. doi: 10.1038/sj.cdd.4401788. [DOI] [PubMed] [Google Scholar]
- 65.Lee HP, Pancholi N, Esposito L, Previll LA, Wang X, Zhu X, et al. Early induction of oxidative stress in mouse model of Alzheimer disease with reduced mitochondrial superoxide dismutase activity. PLoS One. 2012;7(1):e28033. doi: 10.1371/journal.pone.0028033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu D, Croteau DL, Souza-Pinto N, Pitta M, Tian J, Wu C, et al. Evidence that OGG1 glycosylase protects neurons against oxidative DNA damage and cell death under ischemic conditions 2. J Cereb Blood Flow Metab. 2011;31(2):680–92. doi: 10.1038/jcbfm.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Canugovi C, Yoon JS, Feldman NH, Croteau DL, Mattson MP, Bohr VA. Endonuclease VIII-like 1 (NEIL1) promotes short-term spatial memory retention and protects from ischemic stroke-induced brain dysfunction and death in mice. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(37):14948–53. doi: 10.1073/pnas.1204156109. Epub 2012/08/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li N, Wu H, Yang S, Chen D. Ischemic preconditioning induces XRCC1, DNA polymerase-beta, and DNA ligase III and correlates with enhanced base excision repair 31. DNA repair. 2007;6(9):1297–306. doi: 10.1016/j.dnarep.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 69.Yang Y, Candelario-Jalil E, Thompson JF, Cuadrado E, Estrada EY, Rosell A, et al. Increased intranuclear matrix metalloproteinase activity in neurons interferes with oxidative DNA repair in focal cerebral ischemia 6. J Neurochem. 2010;112(1):134–49. doi: 10.1111/j.1471-4159.2009.06433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang JL, Tadokoro T, Keijzers G, Mattson MP, Bohr VA. Neurons efficiently repair glutamate-induced oxidative DNA damage by a process involving CREB-mediated up-regulation of apurinic endonuclease 1 3. J Biol Chem. 2010;285(36):28191–9. doi: 10.1074/jbc.M109.082883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dutra AV, Lin HF, Juo SH, Mohrenweiser H, Sen S, Grewal RP. Analysis of the XRCC1 gene as a modifier of the cerebral response in ischemic stroke 4. BMC Med Genet. 2006;7:78. doi: 10.1186/1471-2350-7-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mahabir S, Abnet CC, Qiao YL, Ratnasinghe LD, Dawsey SM, Dong ZW, et al. A prospective study of polymorphisms of DNA repair genes XRCC1, XPD23 and APE/ref-1 and risk of stroke in Linxian, China 1. J Epidemiol Community Health. 2007;61(8):737–41. doi: 10.1136/jech.2006.048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shyu HY, Shieh JC, Ji-Ho L, Wang HW, Cheng CW. Polymorphisms of DNA repair pathway genes and cigarette smoking in relation to susceptibility to large artery atherosclerotic stroke among ethnic Chinese in Taiwan. J Atheroscler Thromb. 2012;19(4):316–25. doi: 10.5551/jat.10967. [DOI] [PubMed] [Google Scholar]
- 74.Moser VC. Functional assays for neurotoxicity testing. Toxicol Pathol. 2011;39(1):36–45. doi: 10.1177/0192623310385255. [DOI] [PubMed] [Google Scholar]
- 75.Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- 76.Okun E, Griffioen K, Barak B, Roberts NJ, Castro K, Pita MA, et al. Toll-like receptor 3 inhibits memory retention and constrains adult hippocampal neurogenesis 7. Proc Natl Acad Sci USA. 2010;107(35):15625–30. doi: 10.1073/pnas.1005807107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bailey KR, Rustay NR, Crawley JN. Behavioral phenotyping of transgenic and knockout mice: practical concerns and potential pitfalls. ILAR journal/National Research Council, Institute of Laboratory Animal Resources. 2006;47(2):124–31. doi: 10.1093/ilar.47.2.124. Epub 2006/03/21. [DOI] [PubMed] [Google Scholar]
- 78.Nelson RJ, Young KA. Behavior in mice with targeted disruption of single genes. Neuroscience and biobehavioral reviews. 1998;22(3):453–62. doi: 10.1016/s0149-7634(97)00053-5. Epub 1998/05/14. [DOI] [PubMed] [Google Scholar]
- 79.Bailey KR, Crawley JN. Anxiety-Related Behaviors in Mice. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. 2. Boca Raton (FL): 2009. [Google Scholar]
- 80.Zheng JD, Hei AL, Zuo PP, Dong YL, Song XN, Takagi Y, et al. Age-related alterations in the expression of MTH2 in the hippocampus of the SAMP8 mouse with learning and memory deterioration. J Neurol Sci. 2009;287(1–2):188–96. doi: 10.1016/j.jns.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 81.Song XN, Zhang LQ, Liu DG, Lin J, Zheng JD, Dai DP, et al. Oxidative damage to RNA and expression patterns of MTH1 in the hippocampi of senescence-accelerated SAMP8 mice and Alzheimer’s disease patients. Neurochem Res. 2011;36(8):1558–65. doi: 10.1007/s11064-011-0484-4. [DOI] [PubMed] [Google Scholar]
- 82.Kajitani K, Yamaguchi H, Dan Y, Furuichi M, Kang D, Nakabeppu Y. MTH1, an oxidized purine nucleoside triphosphatase, suppresses the accumulation of oxidative damage of nucleic acids in the hippocampal microglia during kainate-induced excitotoxicity. J Neurosci. 2006;26(6):1688–98. doi: 10.1523/JNEUROSCI.4948-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet. 2006;368(9533):387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 84.Davie CA. A review of Parkinson’s disease. Br Med Bull. 2008;86:109–27. doi: 10.1093/bmb/ldn013. [DOI] [PubMed] [Google Scholar]