Abstract
Background
The PALB2 gene, also known as FANCN, forms a bond and co-localizes with BRCA2 in DNA repair. Germline mutations in PALB2 have been identified in approximately 1% of familial breast cancer and 3–4% of familial pancreatic cancer. The goal of this study was to determine the prevalence of PALB2 mutations in a population of BRCA1/BRCA2 negative breast cancer patients selected from either a personal or family history of pancreatic cancer.
Methods
132 non-BRCA1/BRCA2 breast/ovarian cancer families with at least one pancreatic cancer case were included in the study. PALB2 mutational analysis was performed by direct sequencing of all coding exons and intron/exon boundaries, as well as multiplex ligation-dependent probe amplification.
Results
Two PALB2 truncating mutations, the c.1653T>A (p.Tyr551Stop) previously reported, and c.3362del (p.Gly1121ValfsX3) which is a novel frameshift mutation, were identified. Moreover, several PALB2 variants were detected; some of them were predicted as pathological by bioinformatic analysis. Considering truncating mutations, the prevalence rate of our population of BRCA1/2-negative breast cancer patients with pancreatic cancer is 1.5%.
Conclusions
The prevalence rate of PALB2 mutations in non-BRCA1/BRCA2 breast/ovarian cancer families, selected from either a personal or family pancreatic cancer history, is similar to that previously described for unselected breast/ovarian cancer families. Future research directed towards identifying other gene(s) involved in the development of breast/pancreatic cancer families is required.
Introduction
Hereditary breast cancer accounts for approximately 5–10% of all breast cancer cases. Mutations in the two main susceptibility genes BRCA1 and BRCA2, together with mutations in a number of other high-penetrance genes such as TP53 and PTEN, account for 20% of familial breast cancer cases [1]–[3]. For the remaining 80%, the genetic factors are largely unknown and they are likely to involve mutations in moderate and low penetrance susceptibility genes, plausibly acting together with some environmental or other hereditary factors. Apart from breast and ovarian cancer, BRCA1 and BRCA2 carriers might be at higher risk for additional malignancies such as prostate, colorectal, familial melanoma and pancreatic cancers.
Pancreatic cancers are the fourth most common cause of cancer-related deaths in the Western world. Approximately 5% to 10% of individuals with pancreatic cancer report a history of pancreatic cancer in a close family member. In addition to this, several known genetic syndromes have been shown to be associated with an increased risk of pancreatic cancer. Thus, germline mutations in the BRCA2, p16/CDKN2A, STK11, and PRSS1, that are responsible for familial breast cancer, Familial atypical multiple melanoma, Peutz-Jeghers and Familial pancreatitis, respectively, have been clearly associated with an increase risk of pancreatic cancer [4]–[7]. Additionally, some studies have described pancreatic cancer developing among individuals with HNPCC [8], [9].
The PALB2 (partner and localizer of BRCA2) gene was identified by searching for novel components of endogenous BRCA2-containing complexes [10]. PALB2 supports BRCA2 stability and determines its localization in the nucleus after DNA damage [10]. Relocation of PALB2 and BRCA2 to damaged chromatin is regulated by BRCA1. These three proteins form a complex in which PALB2 acts as a bridge between BRCA1 and BRCA2 [11]. This complex is critical for the initiation of homologous recombination in the DNA-damage response [11], [12]. In cells depleted of PALB2 the DNA repair pathway dependent on the BRCA1/2 is disrupted [10], [11]. Immediately after PALB2 was discovered, evidence showed that it was also a Fanconi anemia gene, known as FANCN [13], [14]. Biallelic inactivation mutations in PALB2/FANCN cause Fanconi anemia subtype N, characterized by a severe predisposition to pediatric malignancies such as Wilms tumor, medulloblastoma, AML and neuroblastoma [14]. Interestingly, the gene underlying the D1 subtype of Fanconi anemia, FANCD1 [15], was found to be BRCA2 and biallelic mutations in BRCA2/FANCD1 originate a phenotype with high risk of childhood malignancies, very similar to that produced by PALB2/FANCN biallelic mutations. This supports the proposal that PALB2 is important for BRCA2 tumor suppression activity [16].
As in other Fanconi anemia genes, monoallelic mutations in PALB2 have been associated with increased breast cancer risk [3]. Thus, PALB2 monoallelic mutations have been identified in approximately 1% of hereditary breast cancer families globally, as summarized by Tischkowitz and Xia [16]. It has recently become clear that the PALB2 gene should not only be considered as a susceptibility gene for breast cancer but also for pancreatic cancer. This pancreatic association was based on the identification of a PALB2 mutation by exomic sequencing and the subsequent PALB2 analysis in additional familial pancreatic cancer patients that revealed a prevalence of 3.1% [17]. A similar prevalence (3.7%) was found by Slater et al [18] in European patients with familial pancreatic cancer, whereby PALB2 carriers also had a history of breast cancer.
Given these findings, we aimed to determine the prevalence of PALB2 mutations in a Spanish population of BRCA1/BRCA2-negative breast/ovarian cancer families with either a personal or family history of pancreatic carcinoma.
Materials and Methods
Patients
Index cases from 132 BRCA1/BRCA2 mutation-negative unrelated Spanish breast/ovarian cancer families with a personal history of both breast and pancreatic cancer, or a family history with pancreatic cancer cases, were screened for mutations within the entire coding sequence and splicing sites as well as large genomic rearrangements of PALB2 gene. Patient and family characteristics are summarised in Table 1. Families were enrolled from 11 different Spanish centres (Table S1). Ethical committee approval and informed consent for all participants in the study were obtained.
Table 1. BRCA1/BRCA2 mutation-negative Spanish high risk breast/ovarian cancer families with pancreatic cancer cases.
| Type of case | N° of cases (n = 132) | Mean age at cancer diagnosis | Additional family history | |
| Personal history of BC and PC | 3 | BC:43.6 | PC in FDR:0 | |
| PC:44 | PC in SDR:2 | |||
| BC in FDR:0 | ||||
| BC in SDR:1 | ||||
| Personal history of PC and familiar history of BC and PC | 4 | PC:65 | PC in FDR:1 | |
| PC in FDR: 50 | PC in SDR:0 | |||
| BC in FDR:4 (1BiBC) | ||||
| BC in SDR:1 | ||||
| Personal history of OC and familiar history of PC | 9 (1BiOC) | OC:43.6 | PC in FDR:3 | |
| PC in FDR: 71.5 | PC in SDR:7 | |||
| PC in SDR: 64.7 | BC in FDR:1 | |||
| BC in SDR:2 (1 BiBC) | ||||
| OC in FDR:2 | ||||
| OC in SDR:2 | ||||
| Personal history of PrC and familiar history of BC and PC | 2 | PrC:54.5 | PC in FDR:1 | |
| PC in FDR: 61 | PC in SDR:2 | |||
| PC in SDR: 77 | BC in FDR:2 | |||
| BC in SDR:2 (1 BiBC) | ||||
| OC in SDR:1 | ||||
| Personal history of BC and familiar history of PC | BC iagnosed <50 | 92 (13BiBC (1+leukemia, 1+melanoma; 1+OC), 1 MBC, 1+CCR | BC:39.2 | PC in FDR:25 (1+BC) |
| PC in FDR:58.8 | PC in SDR:50 | |||
| PC IN SDR: 63.8 | BC in FDR:31 (2 BiBC) | |||
| BC in SDR:26 (1 BiBC, 1 MBC) | ||||
| OC in FDR:1 | ||||
| OC in SDR:4 | ||||
| BC diagnosed >50 | 22 (4 BiBC, 1MBC, 1+OC, 1+endometrium) | BC:58.6 | PC in FDR:9 (1+BC) | |
| PC in FDR:64.1 | PC in SDR:12 (1+BC) | |||
| PC in SDR:65.7 | BC in FDR:15 (1 BiBC, 1 MBC, 1+PC) | |||
| BC in SDR:15 | ||||
| OC in FDR:1 | ||||
| OC in SDR:1 | ||||
BC: Breast cancer; PC: Pancreatic cancer; OC: Ovarian cancer; BiOC: Bilateral Ovarian cancer; PrC: Prostate cancer; MBC: Male Breast cancer; BiBC: Bilateral Breast Cancer; CCR: Colorectal cancer; FDR: First degree relative; SDR: Second degree relative.
All index cases had been previously screened for point mutations and large rearrangements in BRCA1 and BRCA2 genes. All were found to be negative.
Mutation analysis of the PALB2 gene
Mutational analysis of PALB2 gene included the complete coding sequencing and flanking intron-exon boundaries along with the analysis of genomic rearrangements, as previously described by Blanco et al [19].
Nomenclature and databases
Sequences used for PALB2 nomenclature were obtained from the NCBI RefSeq database (NG_007406.1 for genomic, NM_024675.3 for mRNA and NP_078951.2 for protein) (http://www.ncbi.nlm.nih.gov). Standardized nomenclature was reported considering the A of the ATG initiation codon of the coding DNA Reference Sequence as nucleotide position +1.
Prediction of pathogenicity of splicing and missense variants
Splicing predictions were performed with Splicing Sequences Finder (SSF) (http://www.umd.be/searchSpliceSite.html), MaxEntScan (http://genes.mit.edu/burgelab/maxent/Xmaxentscan_scoreseq.html), NNSplice (http://www.fruitfly.org/seq_tools/splice.html) and HumanSplicingFinder (HSF) (http://www.umd.be/HSF/) algorithms through the Alamut- Mutation Interpretation Software v.1.54 (http://www.interactivebiosoftware.com/alamut.html). Default thresholds were used for all the analyses.
Potential consequences of missense substitutions were obtained using the prediction software PolyPhen-2 (Polymorphism Phenotyping-2, see http://genetics.bwh.harvard.edu/pph2/), SIFT (Sorting Intolerant From Tolerant, see http://sift.jcvi.org/) and Align-GVGD (Grantham score difference, see http://agvgd.iarc.fr/) tools. Native alignments of each algorithm were used (see Text S1 for a brief description of the tools).
CEU-population data from 1000 Genomes Project database (http://www.1000genomes.org/) was used to obtain allelic frequency of the identified variants in our samples.
Results
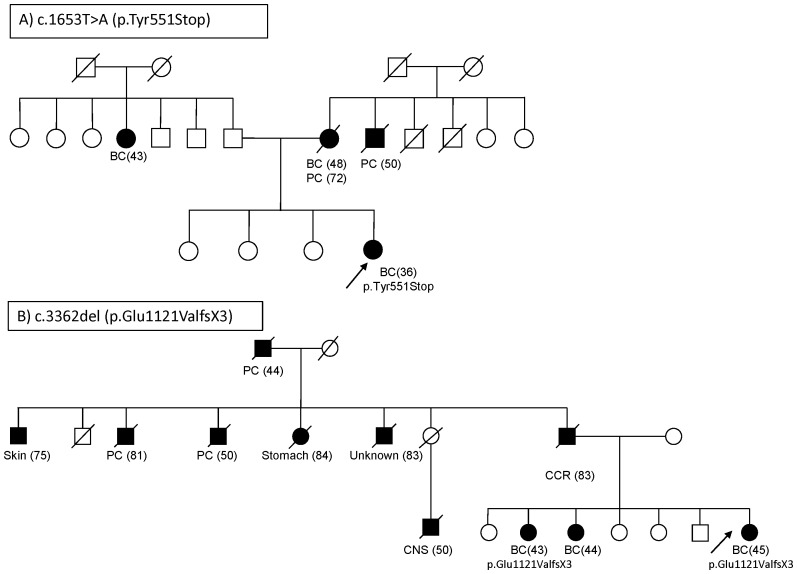
We sequenced all exons and splicing boundaries of PALB2 gene. We also carried out MLPA analysis in 132 index cases from BRCA1/BRCA2-negative families with breast and/or ovarian cancer with either a personal or familial history of pancreatic cancer. Two mutations were identified by sequencing analysis, the nonsense c.1653T>A (p.Tyr551Stop) located at exon 4 with the result of a premature stop codon, and the frameshift c.3362del (p.Gly1121ValfsX3) in exon 13, which is predicted to generate a translation-stop three codons downstream from the first affected amino acid. The PALB2 truncating mutation c.1653T>A (p.Tyr551Stop) was identified in a woman diagnosed with an infiltrating ductal carcinoma (IDC), negative for estrogen receptor (ER), progesterone receptor (PR) and HER2 at the age of 36. Her mother had been diagnosed with breast cancer at 48 and with pancreatic cancer at 72 years of age. Her maternal uncle had been diagnosed with pancreatic cancer at 50 years of age (Figure 1A). Unfortunately, it was not possible to obtain samples from family members to confirm the mutation in the paternal branch of the proband. The frameshift mutation in exon 13, c.3362del produces a stop codon in position 1123 (p.Gly1121ValfsX3) that would cause the loss of the 63 amino acids from the N-terminal PALB2 region. Other truncated mutations in the last codons of the gene have already been described in breast/pancreatic cancer families [3], [20]. It has been shown that residues 836 to 1186 of the PALB2 protein are part of the WD40 repeats C-terminal domain which associates with the N-terminus of BRCA2 [12], [13]. The mutation c.3362del was identified in a woman diagnosed with breast cancer at the age of 45. Likewise, the same mutation was also detected in one of her sisters who was diagnosed with breast cancer at the age of 43. Furthermore, they had another sister who had been diagnosed with breast cancer; however, it was not possible to carry out the genetic analysis. The three of them were diagnosed with ER and PR positive infiltrating ductal carcinoma (IDC). Moreover, two paternal aunts and also the paternal grandfather were diagnosed with pancreatic cancer at the ages of 81, 50 and 44, respectively. We could not, unfortunately, confirm the origin of the mutation in the maternal branch. Other types of cancer present in the family were skin, stomach and CNS in paternal aunts (Figure 1B).
Figure 1. Pedigrees of the families carrying the mutations.
a) c.1653T>A (p.Tyr551Stop); b) c.3362del (p.Glu1121ValfsX3). Cancer diagnoses are indicated in affected patients; in brackets the age at diagnosis or exitus. The arrow indicates the index case analyzed. BC breast cancer, PC Pancreatic Cancer, CCR Colorectal cancer, CNS central nervous system cancer.
In addition to these two mutations, sequence analysis revealed another 21 different PALB2 variants and polymorphims (Table 2), one in the 5′UTR region, 4 in introns and 16 in exons (12 missense and 4 silent coding variants). From the total number of variants identified, seven were novel (c.110G>A (p.Arg37His), c.212-180T>G, c.232G>A (p.Val78Ile), c.262C>T (p.Leu88Phe), c.1431T>C (p. = ), c.2587-59T>C, c.2837C>G (p.Ala946Gly)) and observed only once in our samples, whereas the other variants had been previously reported [3], [19], [21], [22].
Table 2. PALB2 sequence variants identified in 132 Spanish breast/ovarian cancer families with pancreatic cancer cases.
| CASE POPULATION | CONTROL POPULATION | |||||||||
| GENOTYPES | ALLELIC FREQUENCY (%)b | 1000 GENOMES | ||||||||
| NUCLEOTIDE CHANGEa | PROTEIN CHANGE | Name of SNP | AA | AB | BB | A (A%) | B (B%) | A% | B% | |
| 5′ upstream sequence | c.-47G>A | - | rs8053188 | 127 | 5 | 0 | 259(98.1) | 5(1.9) | 98 | 2 |
| EXON 3 | c.110G>A | p.Arg37His | rs202194596 | 131 | 1 | 0 | 263(99.6) | 1(0.4) | 100 | 0 |
| INTRON 3 | c.212-180T>G | - | - | 131 | 1 | 0 | 263(99.6) | 1(0.4) | ||
| c.212-58A>C | - | rs80291632 | 123 | 9 | 0 | 255 (96.6) | 9(3.4) | 96 | 4 | |
| EXON 4 | c.232G>A | p.Val78Ile | - | 131 | 1 | 0 | 263(99.6) | 1(0.4) | ||
| c.262C>T | p.Leu88Phe | - | 131 | 1 | 0 | 263(99.6) | 1(0.4) | |||
| c.656A>G | p.Asp219Gly | rs45594034 | 131 | 1 | 0 | 263(99.6) | 1(0.4) | 100 | 0 | |
| c.1010T>C | p.Leu337Ser | rs45494092 | 131 | 1 | 0 | 263(99.6) | 1(0.4) | 98 | 2 | |
| c.1194G>A | p. = | rs61755173 | 130 | 2 | 0 | 262(99.2) | 2(0.8) | 100 | 0 | |
| c.1431T>C | p. = | - | 131 | 1 | 0 | 263(99.6) | 1(0.4) | |||
| c.1572A>G | p. = | rs45472400 | 131 | 1 | 0 | 263(99.6) | 1(0.4) | 100 | 0 | |
| c.1653T>A | p.Tyr551Stop | rs118203997 | 131 | 1 | 0 | 263(99.6) | 1(0.4) | |||
| c.1676A>G | p.Gln559Arg | rs152451 | 105 | 24 | 3 | 234(88.6) | 30(11.4) | 90 | 10 | |
| EXON 5 | c.2014G>C | p.Glu672Gln | rs45532440 | 122 | 10 | 0 | 254(96.2) | 10(3.8) | 96 | 4 |
| INTRON 6 | c.2586+58C>T | - | rs249954 | 90 | 37 | 5 | 217(82.2) | 47(17.8) | 79 | 21 |
| c.2587-59T>C | - | - | 131 | 1 | 0 | 263(99.6) | 1(0.4) | |||
| EXON 7 | c.2590C>T | p.Pro864Ser | rs45568339 | 129 | 3 | 0 | 261(98.9) | 3(1.1) | 100 | 0 |
| EXON 8 | c.2794G>A | p.Val932Met | rs45624036 | 131 | 1 | 0 | 263(99.6) | 1(0.4) | 99 | 1 |
| c.2816T>G | p.Leu939Trp | rs45478192 | 131 | 1 | 0 | 263(99.6) | 1(0.4) | 100 | 0 | |
| EXON 9 | c.2837C>G | p.Ala946Gly | - | 131 | 1 | 0 | 263(99.6) | 1(0.4) | ||
| c.2993G>A | p.Gly998Gln | rs45551636 | 125 | 7 | 0 | 257(97.3) | 7(2.7) | 98 | 2 | |
| EXON 12 | c.3300T>G | p. = | rs45516100 | 123 | 9 | 0 | 255 (96.6) | 9(3.4) | 96 | 4 |
| EXON 13 | c.3362del | p.Gly1121ValfsX3 | - | 131 | 1 | 0 | 263(99.6) | 1(0.4) | ||
In bold variants not previously reported.
Allelic frequency is the percentage of n/N, where n is the number of minor alleles and N is the total number of alleles.
The results of bioinformatic predictions for intronic and missense variants are represented in Table 3. Four of the missense variants, c.1010T>C (p.Leu337Ser), c.2816T>G (p.Leu939Trp), c.2837C>G (p.Ala946Gly) and c.2993G>A (p.Gly998Gln), were predicted to likely affect PALB2 protein function by all the tested algorithms. Variants c.2816T>G (p.Leu939Trp) and c.2837C>G (p.Ala946Gly) are not present in CEU 1000 Genome Project samples, whereas c.1010T>C (p.Leu337Ser) and c.2993G>A (p.Gly998Gln), have a 2% frequency in CEU population (Table 2). For variant c.2816T>G (p.Leu939Trp), a similar frequency in controls and cases was observed [22]–[24]. For the missense variant c.110 G>A (p.Arg37His) two of the three prediction programs considered the variant as deleterious, whereas only one prediction tool considered variants c.262 C>T (p.Leu88Phe), c.2014 G>C (p.Glu672Gln), c.2590 C>T (p.Pro864Ser) and 2794 G>A (Val932Met) as deleterious (see Table 3). The bioinformatic splicing analyses showed consensus site score variations for variants c.110 G>A (p.Arg37His) (three programs), c.2837C>G (p.Ala946Gly) (two programs) and c.2993G>A (p.Gly998Gln) (one program). A destruction of a cryptic splice site was predicted for variants c.-47G>A and c.2794G>A (p.Val932Met) by three and two programs, respectively, as well as an increase in the score of a cryptic sites by all programs for the variant c.2794G>A (p.Val932Met) and by two programs for the variant c.2590C>T (p.Pro864Ser).
Table 3. Results of bioinformatic analysis for PALB2 variants.
| PREDICTION AMINOACIDIC CHANGE | SPLICE SIGNAL DETECTION | ||||||
| PROTEIN CHANGE | POLYPHEN | SIFT | A-GVGD | Location, SS, Distance† | 5′ or 3′ score modification (% variation) | Proximal Cryptic/De novo (% variation) | |
| c.-47G>A | - | N/A | N/A | N/A | 5′UTR, 5′, 47 | c.-46 MaxEnt: 74.92→- (−100%) | |
| c.-46 SSF: 5.71→- (−100%) | |||||||
| c.-46 HSF: 83.95→- (−100%) | |||||||
| c.110G>A& | p.Arg37His | Probably damaging | Affect protein function | Class C25 | Exon 3, 3′, 2 | MaxEnt: 10.06→9.64 (−4.3%) | |
| NNSPLICE: 0.92→0.91 (−1.0%) | |||||||
| HSF: 82.10→81.93 (−0.2%) | |||||||
| c.212-180T>G | - | N/A | N/A | N/A | Intron 3, 3′, 180 | ||
| c.212-58A>C | - | N/A | N/A | N/A | Intron 3, 3′, 58 | ||
| c.232G>A | p.Val78Ile | Benign | Tolerated | Class C25 | Exon 4, 3′, 21 | ||
| c.262C>T | p.Leu88Phe | Possibly damaging | Tolerated | Class C15 | Exon 4, 3′, 51 | ||
| c.656A>G | p.Asp219Gly | Benign | Tolerated | Class C65 | Exon 4, 3′, 444 | ||
| c.1010T>C | p.Leu337Ser | Probably damaging | Affect protein function | Class C65 | Exon 4, 5′, 674 | ||
| c.1194G>A | p. = | N/A | N/A | N/A | Exon 4,5′, 490 | ||
| c.1431T>C | p. = | N/A | N/A | N/A | Exon 4, 5′, 253 | ||
| c.1572A>G | p. = | N/A | N/A | N/A | Exon 4, 5′, 111 | ||
| c.1653T>A | p.Tyr551Stop | N/A | N/A | N/A | Exon 4, 5′, 31 | ||
| c.1676A>G | p.Gln559Arg | Benign | Tolerated | Class C35 | Exon 4, 5′, 8 | ||
| c.2014G>C | p.Glu672Gln | Probably damaging | Tolerated | Class C25 | Exon 4, 3′, 329 | ||
| c.2586+58C>T | - | N/A | N/A | N/A | Intron 6, 5′, 58 | ||
| c.2587-59T>C | - | N/A | N/A | N/A | Intron 6, 3′, 59 | ||
| c.2590C>T | p.Pro864Ser | Benign | Tolerated | Class C65 | Exon 7, 3′, 4 | c.2597 MaxEnt: 1.70→1.89 (+11.2%) | |
| c.2597 NNSPLICE: 0.53→0.65 (+22.3%) | |||||||
| c.2597 HSF: 85.31→84.89 (−0.5%) | |||||||
| c.2794G>A | p.Val932Met | Probably damaging | Tolerated | Class C15 | Exon 8, 5′, 46 | c.2793 MaxEnt: 1.44→- (−100%) | |
| c.2793 HSF: 74.27→- (−100%) | |||||||
| c.2795 SSF: −→72.85 (+100%) | |||||||
| c.2795 MaxEnt: 1.30→2.60 (+100.7%) | |||||||
| c.2795 HSF: 74.31→79.17 (+6.5%) | |||||||
| c.2816T>G | p.Leu939Trp | Probably damaging | Affect protein function | Class C55 | Exon 8, 5′, 18 | ||
| c.2837C>G | p.Ala946Gly | Possibly damaging | Affect protein function | Class C55 | Exon 9, 3′, 3 | MaxEnt: 8.14→7.33 (−9.9%) | |
| NNSPLICE: 0.54→- (−100%) | |||||||
| c.2993G>A | p.Gly998Gln | Probably damaging | Affect protein function | Class C65 | Exon 9, 5′, 4 | NNSPLICE: 0.99→1.00 (+1.0%) | |
| c.3300T>G | p. = | N/A | N/A | N/A | Exon 12, 5′, 50 | ||
| c.3362del | p.Gly1121ValfsX3 | N/A | N/A | N/A | Exon 13, 3′, 12 | ||
The table reports 5′ or 3′ score modifications due to the detected variants in PALB2 (for greater clarity, when the variants didn't change the score, the corresponding tool is not indicated). Proximal cryptic sites are indicated with the corresponding tool, when the variants led to de novo site it is also indicated. N/A = not applicable as the change is synonymous or intronic.
Location indicates exon/intron, SS stands for splice site and distance to the nearest splice site is indicated in base pairs.
cDNA analysis was performed. No additional products in the carrier sample compared to control samples has been identified (data not shown).
Discussion
Mutations in PALB2 gene were originally associated with an increased risk for breast cancer and later, with pancreatic cancer. We analyzed a large series of hereditary breast/pancreatic cancer families analysed for PALB2 mutations. We identified two germline truncating mutations, the nonsense c.1653T>A (p.Tyr551Stop) located at exon 4 and the novel frameshift mutation c.3362del (p.Gly1121ValfsX3) in exon 13. These mutations were considered to be pathogenic, since they all create a stop codon that is predicted to cause a truncation of the PALB2 protein. The nonsense mutation c.1653T>A (p.Tyr551Stop) had been previously reported in a Fanconi anemia patient as well as in a familial breast cancer case [13], [25]. Truncating mutations in PALB2 are rare in individuals without cancer. In fact, they had not been identified in 1084 healthy individuals analysed [3]. In our series, c.1653T>A (p.Tyr551Stop) and c.3362del (p.Gly1121ValfsX3) were identified in index cases diagnosed with breast cancer under 50 years of age and at least one first degree relative diagnosed with pancreatic cancer. No ovarian cancer was present in these families. Considering these two PALB2 variants as causal mutations, the prevalence of PALB2 mutation in our BRCA1/BRCA2 breast and pancreatic cancer series is 1.5% (2/132). Previous studies of breast/pancreatic cancer families have described prevalences from 0% (77 families analysed in Stadler et al [26], 45 in Adank et al [27], 29 in Guiorzo et al [28] and 28 in Harinck et al [29]), 2.1% (Hofstatter et al [24], 2 mutations in 94 families), to 4.8% (Peterlongo et al [20], 3 mutations in 62 families) reviewed in Table 4. Considering these studies with our data, the global prevalence of PALB2 mutation in breast/pancreatic cancer families is 1.5% (7 mutations in 467 families).
Table 4. Overview of literature on role of PALB2 mutations in FPC and in BC families affected by PC (BC-PC families).
| STUDY | COUNTRY/ETHNICITY | PATIENTS | PALB2 MUTATIONS | FAMILIAR HISTORY |
| Jones et al [17] | USA | 96 FPC families | c.172-175delTTGT: 1(PC) c.3116delA: 1(PC+PrC) c.3256C>T: 1(PC+BC) | c.172-175delTTGT: family history of BC c.3116delA: family history of BC-PC c.3256C>T: family history of BC-PC |
| Tischkowitz et al [23] | Canada | 254 PC (114 sporadic, 80 FPC, 21 FPC with breast/ovary cases; 39 sporadic PC with breast/ovary cases) | 6.7-kb germline deletion (exons12–13): 1 (BC+PC) | 6.7-kb germline deletion (exons12–13): family history of PC |
| Hofstatter et al [25] | USA | 94 BC-PC families | c.2962C>T: 1BC(49) c.3549C>CA: 1BC(43) | c.2962C>T: family history of BC-PC c.3549C>CA: family history of BC-PC |
| Slater et al [18] | Europe | 81 FPC families | c.1240C>T: 1(PC) c.508-509delAG: 1(PC) c.3116delA: 1(PC) | c.1240C>T: family history of BC-PC c.508-509delAG: family history of BC-PC c.3116delA: family history of BC-PC |
| Adank et al [28] | The Netherlands | 45 BC-PC families (all index cases BC) | ||
| Peterlongo et al [21] | Italy | 62 BC-PC families | c.72delG: 1(BC) c.1027C>T: 1(BC) c.3497delG: 1(BC) | c.72delG: family history of BC-PC c.1027C>T: family history of BC-PC c.3497delG: family history of BC-PC |
| Stadler et al [27] | USA | 77 BC-PC families | ||
| Guiorzo et al [29] | Italy | 29 BC-PC famililes | ||
| Harinck et al [30] | The Netherlands | 56 (28 FPC families as 28 BC-PC families) | ||
| Current study | Spain | 132 BC-PC families | c.1653T>A: 1(BC) c.3362del: 1(BC) | c.1653T>A: family history of BC-PC c.3362delG: family history of BC-PC |
BC (breast cancer); PC (pancreatic cancer); FPC (familial pancreatic cancer); PrC (prostate cancer).
We recently, estimated a PALB2 mutation prevalence of 0.75% for Spanish breast/ovarian cancer families with at least one male breast cancer case [19]. Although we identified twice as many carriers in families with pancreatic cancer cases than in families with male breast cancer cases, both prevalences are similar to the 1% reported for families with breast cancer unselected for other cancers [3], [30], [31]. Importantly, for most of the breast/pancreatic cancer series analysed, index cases were breast cancers. Similarly, in our study only 7 from the 132 index cases were pancreatic cancer. The selection of index cases could therefore be introducing a bias in the estimate of the prevalence of PALB2 mutations in these families.
The selection of a gene to be included in a routine genetic test would be, in part, based on the risk it confers. However, the risk associated with deleterious mutations in genes like PALB2 is not easily determined since deleterious alleles are extremely rare in the population, and the number of mutation carriers in published studies are small [32]. Mutations in PALB2 gene were originally associated with a moderate (2–3 fold) increase risk for breast cancer [3]. As higher risks were increasingly suggested, at least for specific mutations [33], [34], the inclusion of this gene in hereditary breast cancer tests would be justified.
In our study both breast tumors from our PALB2 families were IDC, even though one of them had a triple negative phenotype (c.1653T>A, p.Tyr551Stop), while the other was ER+ and PR+ (c.3362del, p.Gly1121ValfsX3). It has been shown that some PALB2-associated breast cancers display a more aggressive tumor phenotype, including triple-negative disease, higher tumor grade and higher Ki67 expression [35]. Thus, tumors of the 1592delT PALB2 mutation carriers presented triple negative phenotype more often (54.5%, P<0.0001) than those of other familial (12.2%) or sporadic (9.4%) breast cancer patients [35]. In fact, nearly 40% of the PALB2-associated breast tumors identified to date displaied a triple-negative phenotype, regardless of the specific PALB2 mutation [16]. This representation of triple negative tumors, more akin to BRCA1- than BRCA2-related tumors, could be related to the nature of the interaction and/or certain functional similarities between PALB2 and BRCA1 [11], [12], or a direct transcriptional activation of the estrogen receptor by PALB2 as has been shown for BRCA1 [36]. However, larger numbers of PALB2-related tumors will need to be studied before any firm conclusions can be drawn.
As shown in Table 3, different prediction tools gave contradictory results. For instance, missense variants c.656 A>G (p.Asp219Gly) and c.2590 C>T (p.Pro864Ser) were predicted as C65 (likely to be pathogenic) by A-GVGD and as Benign and Tolerated by Polyphen and Sift. Prediction of individual variants can be sensitive to the aligment used; both the number and type of orthologues aligned at the mutation site can affect prediction of pathogenicity [37]. Since we used default alignments for each tool, we cannot rule out that the different outcomes we have for these variants is related to this. However, an optimum alignment is difficult to identify and is likely to vary depending on the variant tested [37]. It has been previously shown that the accuracy of the result improves when multiple algorithms give the same prediction [38]. Considering this as well as such consensus predictions for all tools, four of the missense variants identified in our patients were classified as probably deleterious (Table 3). Three of them (c.1010C>T, c.2816T>G and c.2993G>A), had not been previously related with breast cancer risk [3], [19], [21], [24]. Although a recent study did not showed evidence of the influence of rare PALB2 missense mutations and breast cancer risk [34], we think that multifactorial analysis incorporating different approaches to obtain a final pathogenic probability, are required to assess the implication of each of these missense PALB2 variants.
Results from the in silico splicing analysis show three variants predicted to modify the scores for the canonical AG/GT dinucleotides, and three other variants that would modify the score of cryptic splice sites. Recently published guidelines for splicing analysis [39] indicate that only variants located in the consensus sites described by Cartegni et al [40], 11 bases for the 5′ site (from the 3 last exonic to the 8 first intronic bases) and 14 bases for the 3′ site (from the 12 last intronic to the first 2 exonic bases), would have reliable predictions with these bioinformatic tools [39]. In our study, this would mean that the unique reliable prediction is for variant c.110 G>A (p.Arg37His), at the second exonic base from the 3′ site. However, the reduction in the score predicted by the algorithms, all lower than 10%, and the absence of variations near cryptic splice sites would suggest that c.110 G>A (p.Arg37His) is not a variant producing a major impact in splicing process. The RNA analysis of the variant confirmed this prediction (Table 3).
Conclusions
In summary, we found that PALB2 mutations occur with a prevalence of 1.5% in a population of BRCA1/2-negative breast cancer patients specifically selected from a personal and/or familiar history of pancreatic cancer. This is not much different from the prevalence described for families not selected for the presence of pancreatic cancer. However, we cannot rule out a higher PALB2 prevalence in series with more pancreatic cancer index cases.
PALB2 mutations seem to explain only a small fraction of the clustering of both pancreatic and breast cancer. It is therefore, crucial that future research aims to identify other gene(s) that are involved in the development of familiar breast/pancreatic cancer cases.
Supporting Information
Participating centers and families from Spain.
(DOCX)
This document summarizes the meanings of scores of bioinformatic programs used.
(DOCX)
Acknowledgments
We are grateful to the families for their cooperation and to the clinical personnel involved in aspects of recruitment and clinical data collection. We thank Anna Tenés and Miriam Masas for technical assistance.
Funding Statement
This research was supported by grants from the Xunta de Galicia (10PXIB 9101297PR) and FMM Foundation given to AV. MH was supported from the Instituto de Salud Carlos III, Fondo de Investigación Sanitaria (FIS) Research Grant 09/00859, and Fundación Mutua Madrileña (FMM) Research Grant FMM-08. SGE is supported by a Miguel Servet contract of the Instituto de Salud Carlos III. EAV was supported in part by grants BIO39/VA27/10 (Consejería de Sanidad) and CSI004A10-2 (Consejería de Educación) from the Junta de Castilla y León. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ford D, Easton DF, Stratton M, Narod S, Goldgar D, et al. (1998) Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. Am J Hum Genet 62: 676–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Walsh T, King MC (2007) Ten genes for inherited breast cancer. Cancer Cell 11: 103–105. [DOI] [PubMed] [Google Scholar]
- 3. Rahman N, Seal S, Thompson D, Kelly P, Renwick A, et al. (2007) PALB2, which encodes a BRCA2-interacting protein, is a breast cancer susceptibility gene. Nat Genet 39: 165–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Giardiello FM, Brensinger JD, Tersmette AC, Goodman SN, Petersen GM, et al. (2000) Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology 119: 1447–1453. [DOI] [PubMed] [Google Scholar]
- 5. Goldstein AM, Fraser MC, Struewing JP, Hussussian CJ, Ranade K, et al. (1995) Increased risk of pancreatic cancer in melanoma-prone kindreds with p16INK4 mutations. N Engl J Med 333: 970–974. [DOI] [PubMed] [Google Scholar]
- 6. Lowenfels AB, Maisonneuve P, Cavallini G, Ammann RW, Lankisch PG, et al. (1993) Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med 328: 1433–1437. [DOI] [PubMed] [Google Scholar]
- 7. Whitcomb DC, Gorry MC, Preston RA, Furey W, Sossenheimer MJ, et al. (1996) Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet 14: 141–145. [DOI] [PubMed] [Google Scholar]
- 8. Abraham SC, Wu TT, Klimstra DS, Finn LS, Lee JH, et al. (2001) Distinctive molecular genetic alterations in sporadic and familial adenomatous polyposis-associated pancreatoblastomas : frequent alterations in the APC/beta-catenin pathway and chromosome 11p. Am J Pathol 159: 1619–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yamamoto H, Itoh F, Nakamura H, Fukushima H, Sasaki S, et al. (2001) Genetic and clinical features of human pancreatic ductal adenocarcinomas with widespread microsatellite instability. Cancer Res 61: 3139–3144. [PubMed] [Google Scholar]
- 10. Xia B, Sheng Q, Nakanishi K, Ohashi A, Wu J, et al. (2006) Control of BRCA2 cellular and clinical functions by a nuclear partner, PALB2. Mol Cell 22: 719–729. [DOI] [PubMed] [Google Scholar]
- 11. Zhang F, Ma J, Wu J, Ye L, Cai H, et al. (2009) PALB2 links BRCA1 and BRCA2 in the DNA-damage response. Curr Biol 19: 524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sy SM, Huen MS, Zhu Y, Chen J (2009) PALB2 regulates recombinational repair through chromatin association and oligomerization. J Biol Chem 284: 18302–18310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xia B, Dorsman JC, Ameziane N, de Vries Y, Rooimans MA, et al. (2007) Fanconi anemia is associated with a defect in the BRCA2 partner PALB2. Nat Genet 39: 159–161. [DOI] [PubMed] [Google Scholar]
- 14. Reid S, Schindler D, Hanenberg H, Barker K, Hanks S, et al. (2007) Biallelic mutations in PALB2 cause Fanconi anemia subtype FA-N and predispose to childhood cancer. Nat Genet 39: 162–164. [DOI] [PubMed] [Google Scholar]
- 15. Howlett NG, Taniguchi T, Olson S, Cox B, Waisfisz Q, et al. (2002) Biallelic inactivation of BRCA2 in Fanconi anemia. Science 297: 606–609. [DOI] [PubMed] [Google Scholar]
- 16. Tischkowitz M, Xia B (2010) PALB2/FANCN - recombining cancer and Fanconi anemia. Cancer Res 70: 7353–7359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jones S, Hruban RH, Kamiyama M, Borges M, Zhang X, et al. (2009) Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science 324: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Slater EP, Langer P, Niemczyk E, Strauch K, Butler J, et al. (2010) PALB2 mutations in European pancreatic cancer families. Clin Genet 78: 490–494. [DOI] [PubMed] [Google Scholar]
- 19. Blanco A, de la Hoya M, Balmaña J, Ramon y Cajal T, Teule A, et al. (2012) Detection of a large rearrangement in PALB2 in Spanish breast cancer families with male breast cancer. Breast Cancer Res Treat 132: 307–315. [DOI] [PubMed] [Google Scholar]
- 20. Peterlongo P, Catucci I, Pasquini G, Verderio P, Peissel B, et al. (2011) PALB2 germline mutations in familial breast cancer cases with personal and family history of pancreatic cancer. Breast Cancer Res Treat 126: 825–828. [DOI] [PubMed] [Google Scholar]
- 21. Garcia MJ, Fernandez V, Osorio A, Barroso A, Llort G, et al. (2009) Analysis of FANCB and FANCN/PALB2 fanconi anemia genes in BRCA1/2-negative Spanish breast cancer families. Breast Cancer Res Treat 113: 545–551. [DOI] [PubMed] [Google Scholar]
- 22. Tischkowitz M, Sabbaghian N, Hamel N, Borgida A, Rosner C, et al. (2009) Analysis of the gene coding for the BRCA2-interacting protein PALB2 in familial and sporadic pancreatic cancer. Gastroenterology 137: 1183–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rahman N, Scott RH (2007) Cancer genes associated with phenotypes in monoallelic and biallelic mutation carriers: new lessons from old players. Hum Mol Genet 16: R60–R66. [DOI] [PubMed] [Google Scholar]
- 24. Hofstatter EW, Domchek SM, Miron A, Garber J, Wang M, et al. (2011) PALB2 mutations in familial breast and pancreatic cancer. Fam Cancer 10: 225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Casadei S, Norquist BM, Walsh T, Stray S, Mandell JB, et al. (2011) Contribution of inherited mutations in the BRCA2-interacting protein PALB2 to familial breast cancer. Cancer Res 71: 2222–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stadler ZK, Salo-Mullen E, Sabbaghian N, Simon JA, Zhang L, et al. (2011) Germline PALB2 mutation analysis in breast-pancreas cancer families. J Med Genet 48: 523–525. [DOI] [PubMed] [Google Scholar]
- 27. Adank MA, van Mil SE, Gille JJ, Waisfisz Q, Meijers-Heijboer H, et al. (2011) PALB2 analysis in BRCA2-like families. Breast Cancer Res Treat 127: 357–362. [DOI] [PubMed] [Google Scholar]
- 28. Ghiorzo P, Pensotti V, Fornarini G, Sciallero S, Battistuzzi, et al. (2012) Contribution of germline mutations in the BRCA and PALB2 genes to pancreatic cancer in Italy. Fam Cancer 11: 41–47. [DOI] [PubMed] [Google Scholar]
- 29. Harinck F, Kluijt I, van Mil SE, Waisfisz Q, van Os TA, et al. (2012) Routine testing for PALB2 mutations in familial pancreatic cancer families and breast cancer families with pancreatic cancer is not indicated. Eur J Hum Genet 20: 577–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cao AY, Huang J, Hu Z, Li WF, Ma ZL, et al. (2009) The prevalence of PALB2 germline mutations in BRCA1/BRCA2 negative Chinese women with earlyonset breast cancer or affected relatives. Breast Cancer Res Treat 114: 457–462. [DOI] [PubMed] [Google Scholar]
- 31. Balia C, Sensi E, Lombardi G, Roncella M, Bevilacqua G, et al. (2010) PALB2: a novel inactivating mutation in a Italian breast cancer family. Fam Cancer 9: 531–536. [DOI] [PubMed] [Google Scholar]
- 32. Foulkes WD, Ghadirian P, Akbari MR, Hamel N, Giroux S, et al. (2007) Identification of a novel truncating PALB2 mutation and analysis of its contribution to early-onset breast cancer in French-Canadian women. Breast Cancer Res 9: R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Southey MC, Teo ZL, Dowty JG, Odefrey FA, Park DJ, et al. (2010) A PALB2 mutation associated with high risk of breast cancer. Breast Cancer Res 12: R109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tischkowitz M, Capanu M, Sabbaghian N, Li L, Liang X, et al. (2012) Rare germline mutations in PALB2 and breast cancer risk: a population-based study. Hum Mutat 33: 674–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heikkinen T, Karkkainen H, Aaltonen K, Milne RL, Heikkila P, et al. (2009) The breast cancer susceptibility mutation PALB2 1592delT is associated with an aggresive tumor phenotype. Clin Cancer Res 15: 3214–3222. [DOI] [PubMed] [Google Scholar]
- 36. Hosey AM, Gorski JJ, Murray MM, Quinn JE, Chung WY, et al. (2007) Molecular basis for estrogen receptor alpha deficiency in BRCA1-linked breast cancer. J Natl Cancer Inst 99: 1683–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Williams S (2012) Analysis of in silico tools for evaluating missense variants. National Genetics Reference Laboratory (Manchester) [Google Scholar]
- 38. Chan PA, Duraisamy S, Miller PJ, Newell JA, McBride C, et al. (2007) Interpreting missense variants: comparing computational methods in human disease genes CDKN2A, MLH1, MSH2, MECP2, and tyrosinase (TYR). Hum Mutat 28: 683–693. [DOI] [PubMed] [Google Scholar]
- 39. Houdayer C, Caux-Moncoutier V, Krieger S, Barrois M, Bonnet F, et al. (2012) Guidelines for splicing analysis in molecular diagnosis derived from a set of 327 combined in silico/in vitro studies on BRCA1 and BRCA2 variants. Hum Mutat 33: 1228–1238. [DOI] [PubMed] [Google Scholar]
- 40. Cartegni L, Chew SL, Krainer AR (2002) Listening to silence and understanding nonsense: exonic mutations that affect splicing. Nat Rev Genet 3: 285–298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Participating centers and families from Spain.
(DOCX)
This document summarizes the meanings of scores of bioinformatic programs used.
(DOCX)