Abstract
Post-traumatic stress disorder (PTSD) affects the functional recruitment and connectivity between neural regions during autobiographical memory (AM) retrieval that overlap with default and control networks. Whether such univariate changes relate to potential differences in the contribution of large-scale neural networks supporting cognition in PTSD is unknown. In the current functional MRI (fMRI) study we employ independent component analysis to examine the influence the engagement of neural networks during the recall of personal memories in PTSD (15 participants) compared to non-trauma exposed, healthy controls (14 participants). We found that the PTSD group recruited similar neural networks when compared to controls during AM recall, including default network subsystems and control networks, but there were group differences in the spatial and temporal characteristics of these networks. First, there were spatial differences in the contribution of the anterior and posterior midline across the networks, and with the amygdala in particular for the medial temporal subsystem of the default network. Second, there were temporal differences in the relationship of the medial prefrontal subsystem of the default network, with less temporal coupling of this network during AM retrieval in PTSD relative to controls. These findings suggest that spatial and temporal characteristics of the default and control networks potentially differ in PTSD versus healthy controls, and contribute to altered recall of personal memory.
Keywords: PTSD, Memory, Default Network, Control Network, Independent Component Analysis
1. Introduction
Functional neuroimaging studies of post-traumatic stress disorder (PTSD) have observed consistent differences during emotional tasks in the recruitment of several brain regions, such as the medial prefrontal cortex (PFC), medial temporal lobe (MTL) and posterior midline regions (Etkin & Wager, 2007; Shin, Rauch, & Pitman, 2006), which overlap with the typical regions recruited during autobiographical memory (AM) retrieval (Cabeza & St. Jacques, 2007). Further, PTSD studies have also shown that the functional connectivity between these regions is altered (e.g., Bluhm, et al., 2009; Lanius, et al., 2010; St. Jacques, Botzung, Miles, & Rubin, 2011) involving increases and/or decreases between these regions depending upon the nature of the task. Overlap among the functional correlates of AM retrieval and PTSD is consistent with behavioral studies that have reported increased reliving and emotional intensity during retrieval of personal AMs that are not trauma-related in PTSD (Rubin, Boals, & Berntsen, 2008; Rubin, Dennis, & Beckham, 2011). Thus, it is critical to consider the neural mechanisms underlying AM retrieval that includes a wide variety of personal memories in addition to memory for the trauma more specifically. Moreover, avoiding strong emotional and relived memories is a key symptom of PTSD (American Psychiatric Association, 2000). Whether such changes in neural activity and functional connectivity relate to potential differences in the contribution of large-scale neural networks supporting cognition in PTSD is largely unknown.
Several neural networks have been identified in the brain during passive rest states (e.g., Damoiseaux, et al., 2006), and some of these networks are also engaged during memory encoding of naturalistic events (e.g., Anne Botzung, LaBar, Kragel, Miles, & Rubin, 2010) and AM retrieval (St. Jacques, Kragel, & Rubin, 2011). One of the most studied networks is the default network (Gusnard & Raichle, 2001; Raichle, et al., 2001; for review see Buckner, Andrews-Hanna, & Schacter, 2008), a pattern of coherent brain activity first identified during passive rest and shown to be affected in PTSD (Bluhm, et al., 2009; Lanius, et al., 2010). Andrews-Hanna and colleagues (2010) recently showed that the default network comprises a medial PFC subsystem associated with self-relevant and affective processes, and a MTL subsystem linked to memory-based scene construction. Thus, it is not too surprising that the default network overlaps with and is actively recruited during AM retrieval (Spreng & Grady, 2010; St. Jacques, Kragel, et al., 2011), in which self-referential and mnemonic processes are essential components. The default network is not, however, the only neural network that contributes to AM retrieval. For example, we (St. Jacques, Kragel, et al., 2011) found that AM retrieval in healthy young adults is supported by frontoparietal and cingulooperculum networks, in addition to the medial PFC and MTL subsystems of the default network. The frontoparietal and cinguooperculum networks are associated with controlled processes (Dosenbach, et al., 2007; Seeley, et al., 2007), such as flexible and goal-directed cognition, and couple with the default network to support AM functions (Spreng, Stevens, Chamberlain, Gilmore, & Schacter, 2010; St. Jacques, Kragel, et al., 2011). Further, many of the neural regions that show structural and functional changes in PTSD overlap with these control networks (Etkin & Wager, 2007; Shin, et al., 2006).
In the current functional MRI (fMRI) study we report a re-analysis of our previous study that employed a univariate approach (St. Jacques, Botzung, et al., 2011) in order to examine the influence of PTSD on the engagement of neural networks during AM retrieval. The previous study employed a parametric modulation analysis technique to examine how neural activity sensitive to emotional intensity ratings differed during memory construction and elaboration in PTSD versus controls. In the current study we use the multivariate technique independent component analysis (ICA; Calhoun, Adali, Pearlson, & Pekar, 2001b), to specify the temporal and spatial properties of large-scale networks in PTSD versus healthy controls contributing to AM retrieval, collapsed across memories of different emotional quality. Such analyses begin by assembling large-scale networks of voxels that show synchronized activity, rather than the more common univariate analyses that involve first locating areas that differ in activity and then examining connectivity among those areas. While both methods can make valid claims about connectivity, multivariate techniques allow for simultaneous analysis of multiple brain regions comprising a network, rather than focusing on single brain regions in isolation, and more closely resembles how the brain is thought to support cognition (Fuster, 2009; Rubin, 2006). Further, univariate techniques typically rely on subtraction paradigms that emphasize differences from a control task, which may obscure networks that functionally contribute to a particular task (Nyberg & McIntosh, 2001). Importantly, previous functional neuroimaging studies have shown that multivariate approaches can reveal novel information concerning AM retrieval when compared to more standard univariate analyses (Svoboda, McKinnon, & Levine, 2006). At a more basic statistical level, univariate analysis is based on the level of activity in individual voxels whereas multivariate analysis is based on correlations in activity between voxels and so there is no necessary statistical relationship between these two forms of analysis and therefore the conclusions that might be drawn from them. Thus, it is critical to apply such multivariate techniques in order to fully understand the neural mechanisms supporting AM in PTSD. Given the overlap between brain regions affected in PTSD and the default and control networks contributing to AM retrieval, we explored the hypothesis that PTSD would affect both the temporal and spatial extent of these networks.
2. Methods
2.1. Participants
We recruited young adult participants from Duke University (range of 18 - 35 years of age) and all testing occurred at Duke University. A database of healthy young adults who had expressed interest in participating in fMRI research studies was used to recruit participants in the control group. Inclusion criteria for the control group followed procedures that we have routinely used in behavioral studies in the Durham Veteran’s Association Medical Center (VAMC), which do not include previous trauma. This allowed us to examine the effect of PTSD versus a control group that was a random sample rather than one that was resilient to PTSD. Participants in the PTSD group were recruited from advertisements seeking volunteers who were exposed to a traumatic event and from a pre-screen test administered during a large group screening. The Clinician Administered PTSD Scale (CAPs) was used to determine PTSD diagnostic status in this group (Blake, et al., 1995; Weathers, Keane, & Davidson, 2001). All participants in the PTSD group met the DSM-IV-TR criterion as measured by highly trained master’s level clinicians who were experienced in giving the CAPS in a research setting at the Durham VAMC. There were nineteen participants in each group. All participants reported that they were not taking medication known to affect cognitive function (e.g., antidepressants, benzodiazepines, or any other psychiatric medication). Thus, participants in both groups were not taking psychotropic medication. The investigation was carried out in accordance with the latest version of the Declaration of Helsinki, and participants gave written informed consent for a protocol approved by the Duke University Institutional Review Board. Five controls and four participants in the PTSD group were excluded from the analyses because of technical issues (e.g., no key responses recorded) or problems with completing the task as instructed (e.g., falling asleep, not following instructions). Thus, the reported results are based on data from fourteen controls and fifteen PTSD participants.
Demographic and psychometric data were obtained in a separate session within one week of the scanning session (see Table 1; as reported in St. Jacques, Botzung et al., 2011). As expected, the PTSD group had higher scores on the PTSD Check List (PCL; Weathers, Litz, Huska, & Keane, 1994) when compared to the control group. The most common categories of traumatic events that the participants nominated for the PCL were the death, attempted or actual suicide or severe illness of a friend or family member (PTSD = 3, Controls = 4), interpersonal issues (PTSD = 1, Controls = 5), problems in academic or work environment (PTSD = 1, Controls = 3), assault or abuse (PTSD = 4, Controls = 0), motor vehicle accident (PTSD= 2, Controls = 1), other (PSTD = 1, Controls = 1), not enough information to classify (PTSD = 3, Controls = 0). It is important to note that the PCL was not used for diagnostic purposes, but was included for comparison with the control group. The PTSD group also had higher scores on the Beck Depression Index (BDI-II; Beck, Steer, & Brown, 1996) when compared to controls. There were no group differences (all p’s > .05) in the proportion of women (PTSD = 11, Controls = 7), years of age, number of years of education, the full Weschler Abbreviated Scale of Intelligence (WASI;Wechsler, 1999), verbal fluency (FAS), or categorical fluency (animal names and supermarket items).
Table 1.
Participant Variables by Group
| M |
SD |
t (27) | |||
|---|---|---|---|---|---|
| Controls | PTSD | Controls | PTSD | ||
| Age (years) | 24.43 | 22.21 | 3.73 | 4.23 | 1.47 |
| Education (years) | 16.50 | 14.70 | 2.28 | 2.56 | 1.95 |
| PCL† | 27.93 | 49.00 | 7.99 | 12.94 | − 5.19 * |
| BDI | 3.93 | 16.86 | 3.29 | 11.00 | − 4.22 * |
| WASI-Full IQ | 122.36 | 121.50 | 7.66 | 10.14 | 0.25 |
| WASI-Verbal IQ | 120.93 | 123.93 | 7.87 | 11.44 | − 0.81 |
| WASI-Performance IQ | 118.14 | 114.72 | 7.49 | 10.17 | 1.01 |
| Verbal Fluency | 47.43 | 51.36 | 10.87 | 10.49 | − 0.97 |
| Categorical Fluency | 23.57 | 27.50 | 6.69 | 6.00 | − 1.63 |
p < .001,
df = 26
2.2. Materials
Retrieval cues to elicit AMs consisted of 60 emotionally arousing words selected from the affective norms for English words (ANEW) database (Bradley & Lang, 1999), and included 30 positive (Valence = 7.93, SD = 0.45; Arousal = 5.96; SD = 0.83) and 30 negative (Valence = 2.17, SD = 0.52; Arousal = 6.00; SD = 1.03) words that were equally arousing. Auditory cue words were created by recording the words in a female voice and were constrained to an equal duration of 1 s.
2.3. Procedure
A generic cue word method (Rubin, Schrauf, & Greenberg, 2003), frequently used in functional neuroimaging studies in healthy participants (Cabeza & St. Jacques, 2007), was employed to elicit AMs during fMRI scanning. Because the focus here was to examine the neural mechanisms associated with observed changes in the voluntary recall of AM (Rubin, Berntsen, & Bohni, 2008; Rubin, Boals, et al., 2008; Rubin, et al., 2011), rather than to specify differences between trauma and non-trauma related personal memories in PTSD, this method allowed us to query a wide variety of memories. The procedure was similar to Daselaar et al. (2008; also see Greenberg, et al., 2005). During scanning participants heard an auditory cue word and were asked to use it to search for an AM. Participants were instructed to covertly recall a unique AM for each cue word with specific spatiotemporal coordinates. They indicated when a specific AM was found by making a response on the button-box and then continued to elaborate on the retrieved event in as much detail as possible for the rest of the trial. Thirty seconds following the onset of the auditory cue participants were given auditory instructions to rate the amount of emotion (negatively arousing to positively arousing) and reliving (low to high) associated with the memory on an 8-point scale. Rating responses were self-paced (up to 6 s) and separated by at least 0.5 s, and the order was counterbalanced between participants. We included 6 functional runs, with 10 memory cues in each run (5 positive words and 5 negative word), and an inter-trial interval at least 1.5 to 7.5 s. During the duration of the run, participants were instructed to keep their eyes closed so that any potential effects of visual imagery were not confounded by external attention to the stimulus.
Post-scanning, participants were asked to provide a short title for the memory they had retrieved during scanning and then to answer additional questions on a subset of the AM questionnaire (e.g., Rubin, et al., 2003). Participants were asked to date when the event had occurred (e.g., last day to > 10 years ago), to indicate the amount of vividness or how clearly the event was remembered, the perspective or whether the memory was seen through their own eyes or through the eyes of an outside observer, the significance of the memory, and the physiological response during retrieval (e.g., heart pounding, etc.). Also, given that AM comprises different types of events (Brewer, 1986) we asked participants to indicate whether the type of memory retrieved was a unique event (referring to a particular time and place), repeated event (memory for an event with multiple occurrences), extended event (occurring longer than one day), or semantic information (long-standing facts about one’s own life; Williams, 1995).
2.4. fMRI Methods
2.4.1. Image Acquisition
Scanning was conducted using a 4T GE magnet. Auditory stimuli were presented using headphones and behavioral responses were recorded using an eight-button fiber optic response box (Resonance Technology, Northridge, CA). Head motion was minimized using foam pads and a headband. Anatomical scanning started with a T1-weighted sagittal localizer series, and then 3D fast spoiled gradient echo recalled (SPGR) structural images were acquired in the coronal plane (2562 matrix, TR = 12.3 ms, TE = 5.4 ms, flip angle = 20°, FOV = 240, 68 slices, 1.9 mm slice thickness). Functional images were subsequently acquired using an interleaved inverse spiral sequence (642 image matrix, TR = 2000 ms, TE = 6 ms, FOV = 240, flip angle = 60°, 34 slices, 3.8 mm slice thickness).
2.4.2. Image Preprocessing
Image preprocessing and analyses were performed using Statistical Parametric Mapping software in MATLAB (SPM5; Wellcome Department of Imaging Neuroscience). Functional images were corrected for slice acquisition order, realigned to correct for motion artifacts, and then spatially normalized to a standard stereotactic space, using the template implemented in SPM5. Subsequently, the functional images were spatially smoothed using an 8 mm FWHM isotropic Gaussian kernel.
2.4.3. Independent Component Analysis (ICA)
ICA was used to determine spatially distinct networks contributing to AM retrieval (for conventional analysis using a general linear model see St Jacques, Botzung, Miles, & Rubin, 2010) as implemented in the Group ICA of fMRI Toolbox (GIFT; Calhoun, Adali, Pearlson, & Pekar, 2001a). This method involves performing ICA on functional data across every participant and then creating a series of spatial maps and associated time courses for the group. Back reconstruction is then used to create individual time courses and spatial maps from each participant’s functional data. This process was performed separately for each group. Conducting separate ICAs on each group strikes a balance between having a common model and combining multiple single subject ICAs. A similar approach has been used to characterize alterations in memory-related networks between healthy aging, mild cognitive impairment, and Alzheimer’s disease (Celone, et al., 2006). The number of components in each group was estimated to be 30 using the minimum description length criteria (Li, Adali, & Calhoun, 2007). The amplitude of individual time courses and spatial maps were calibrated for comparison across participants, by regressing the back reconstructed timecourses from voxels with maximal spatial weights for each component and scaling to reflect percent change from the mean signal as implemented in the GIFT toolbox.
2.4.4. Component Identification
Components with time courses related to the experimental design were identified using multiple regression conducted separately within each group. AM retrieval operations were modeled by convolving stimuli functions with the canonical hemodynamic response. This model, implemented in SPM5, had separate regressors for the Cue, Construction, Response, Elaboration, and Rating aspects of the task. Stimuli functions varied between regressors as either zero duration Dirac delta functions (Cue, Response, Ratings) or as variable duration box-car functions (Construction, Elaboration) that depend on the time of the motor response. The primary goal of the present study was to examine differences in components associated with construction and elaboration phases of AM retrieval in healthy controls compared to those with PTSD. We conducted a regression analysis, using the temporal sorting tool in GIFT, to determine β weights associating the components with construction and elaboration phases separately. One sample t-tests on these β weights showed four components that were significantly related (p < .001) to construction and/or elaboration phases in healthy controls. As our goal was to examine alterations in the spatial and temporal characteristics of networks supporting AM in PTSD, a spatial cross correlation of component averages for each group was performed, yielding four components in the PTSD group that were highly similar (r’s > 0.80) to those in the controls. There were no other components in the PTSD group that were significantly related (p < .001) to the AM task. These four components were subsequently assessed to examine differences in their spatial and temporal characteristics between the PTSD and control groups.
2.4.5. Spatial Comparison
To assess group differences in the spatial aspects of the components the individual spatial maps were examined in a two-sample t-test as implemented in SPM5. To insure that comparisons of spatial maps reflected differences in network weighting, as opposed to differences in mean signal amplitude within a region (which would be better suited to univariate approaches), masks were separately created for each group and then combined using a conjunction approach. This procedure was done in three steps: first a mask of all voxels that exceeded the mean activity were created for each subject, next ICA were performed separately for each group using the methods outlined above, finally the conjunction of the two group level masks was performed to reduce bias from differences in the initial masking procedure. Statistical comparisons of the spatial weights were controlled for multiple comparison using an FDR corrected p-level = .05 (Genovese, Lazar, & Nichols, 2002), and > 5 voxels. Given the a priori role of the amygdala in AM retrieval and our hypothesis regarding its differential recruitment in PTSD (Etkin & Wager, 2007; Shin, et al., 2006), we conducted an additional region of interest analysis on the results of the two-sample t-test using the Talaraich Daemon Atlas (Lancaster, Summerin, Rainey, Freitas, & Fox, 1997; Lancaster, et al., 2000) implemented with PickAtlas software (Maldjian, Laurienti, Kraft, & Burdette, 2003). The multiple comparison correction was relaxed for the amygdala ROI (uncorrected p = .05).
2.4.6. Temporal Comparison
To assess group differences in the temporal aspects of the components we examined the relationship between the independent components and the temporally distinct phases of AM retrieval by conducting a series of Phase (construction, elaboration) × Group ANOVAs on the β weights from the ICA. Additionally, to further interrogate temporal differences we examined the post stimulus time-courses of the components, using a window of 30 seconds to encompass the construction and elaboration phases, by performing cue locked event averaging as implemented in GIFT. Separate temporal regressions conducting a 2 × 2 ANOVA with valence (positive, negative) and phase (construction, elaboration) did not reveal an effect of valence within either group; thus, the results are presented across both positive and negative trials.
2.4.7. Network-PCL Correlation
We conducted a correlation analysis in order to examine the relationship between network activity and individual differences in PTSD symptomology. For each network we computed neural activity based on the area under the curve of the post stimulus time courses and then examined the correlation with PCL scores while controlling for group.
2.4.8. Network-Behavior Regression
In order to examine the relationship between network activity and autobiographical characteristics as they potentially varied across the groups we conducted multiple regression analyses. For each network we computed neural activity based on the area under the curve of the post stimulus time-courses and regressed this against group, emotional intensity, memory perspective, and the two-way interactions. We chose to focus on the contribution of emotional intensity and memory perspective because these variables differ in PTSD (e.g., Rubin, Boals, et al., 2008). Reliving was not included in the multiple regression analyses due to issues of multicolinearity with emotional intensity. We note that substituting intensity by an interaction term with reliving produces nearly identical results, but for simplicity the reported results are shown with intensity only.
3. Results
3.1. Behavioral Results
Participants were able to recall an event matching the cue on more than 97% of trials. The PTSD group (Mean = .11, SD = .11) recalled a greater proportion of memories associated with the stressful event identified in the PCL than did the control group (Mean = .04, SD = .03), t (25) = −2.35, p < .05.1 There were no group differences (all p’s < .05) in reaction time to retrieve an AM (PTSD = 6.53 s, SD = 1.83; Controls = 6.51 s SD = 2.16), in the online ratings of reliving (PTSD = 5.35, SD = 0.68; Controls = 5.03, SD = 1.06) or emotional intensity (PTSD = 2.54, SD = 0.10; Controls = 2.50, SD = 0.08). Post-scan ratings of vividness (PTSD = 4.68, SD = 0.71; Controls = 4.67, SD = 0.78), significance (PTSD = 3.40, SD = 0.53; Controls = 3.00, SD = 0.81), physiological response (PTSD = 2.61, SD = 0.78; Controls = 2.30, SD = 0.98) did not differ between groups. Further, the date and type (i.e. memory specificity) of the memory did not differ between the groups (for full report of behavioral results see; St. Jacques, Botzung, et al., 2011). However, as expected (Rubin, Berntsen, et al., 2008), the perspective from which memories retrieved differed between the two groups. Compared to the control group (Own Eyes = 5.75, SD = 0.84; Observer = 2.39, SD = 0.92), memories retrieved by the PTSD group (Own Eyes = 4.86, SD = 1.22; Observer = 3.23, SD = 1.18) were recalled less from the perspective of one’s own eyes, t (27) = 2.29, p <. 05, and more from an observer’s perspective, t (27) = −2.13, p < .05.
3.2. fMRI Results
3.2.1. Independent Component Analysis
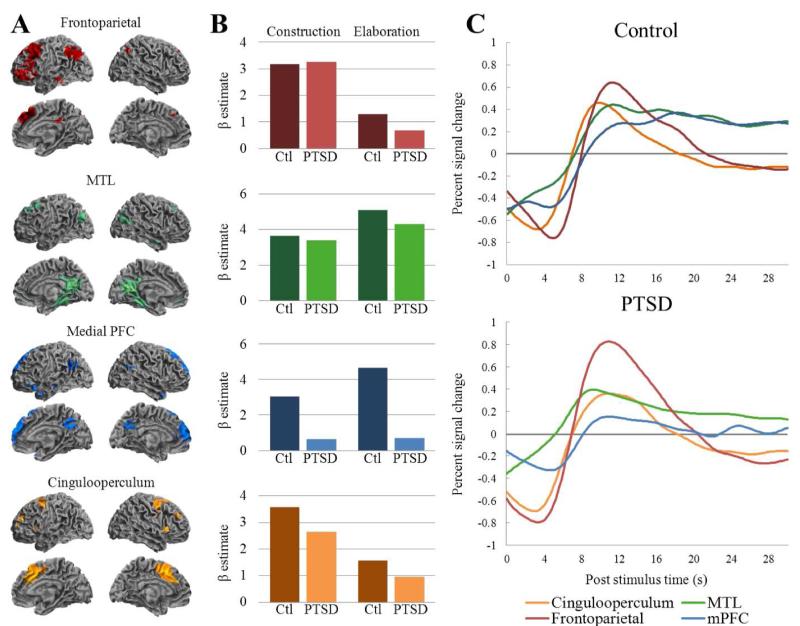
ICA revealed four components that were related to the construction and elaboration of AMs in the control group (also see St Jacques, Kragel, & Rubin, 2011). Components were labeled based on their anatomical location and included frontoparietal, cingulooperculum, medial prefrontal cortex, and medial temporal lobe networks (see Figure 1A). The four networks correspond to large-scale neural networks previously observed using both ICA (e.g., Anne Botzung, et al., 2010) and other task-related (e.g., St. Jacques, Conway, Lowder, & Cabeza, 2010) and resting state (e.g., Andrews-Hanna, et al., 2010; Dosenbach, et al., 2007) functional connectivity analyses. Importantly, the spatial cross correlation values for each of the individual components revealed a high degree of similarity with four components identified independently in the PTSD group (see Table 2). Thus, similar networks contributing to AM retrieval were recruited in both the control and PTSD groups.
Figure 1.
Temporal comparison of the networks and task in PTSD and control groups. (A) Spatial distributions of the four components. Activation reflects a conjunction of network pattern of activation in each group at p < .05 corrected for multiple comparisons. (B) Task relatedness of component activity to construction and elaboration task phases. Vertical axis depicts z scores of β weights indicating similarity between component time courses and modeled activity for each phase. ANOVAs on β weights reveal a main effect of phase in cingulooperculum and frontoparietal components, and a main effect of group in medial prefrontal cortex (PFC) component. (C) Cue locked component time-courses averaged across participants for each group in units of percent signal. MTL = medial tempora lobe; mPFC = medial prefrontal cortex; Ctl = Control group; PTSD = Post-traumatic stress disorder group.
Table 2.
Independent Component Analysis
| Control | PTSD | ||||
|---|---|---|---|---|---|
| Network | r | Construction | Elaboration | Construction | Elaboration |
| Cingulooperculum | 0.83 | 5.03 ** | 2.06 | 3.42 * | 1.45 |
| MTL | 0.82 | 5.19 *** | 9.53 *** | 4.69 ** | 6.88 *** |
| Medial PFC | 0.80 | 4.08 * | 7.95 *** | 0.33 | 1.22 |
| Frontoparietal | 0.84 | 4.27 ** | 1.77 | 4.45 ** | 0.32 |
T-values reported.
p < .01,
p < .001,
p < .0001
3.2.2. Spatial Comparisons
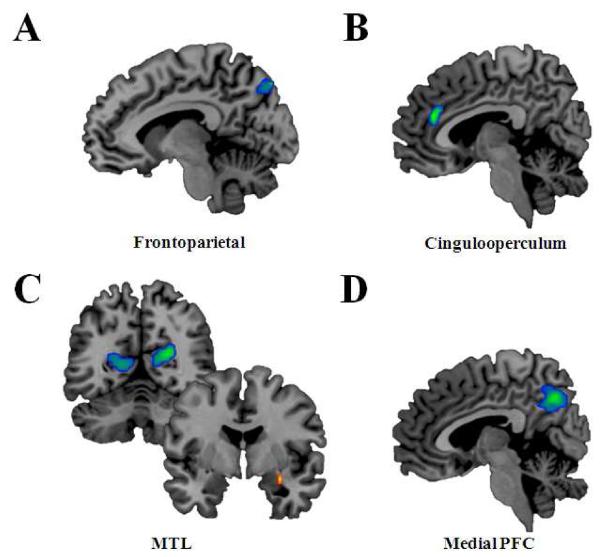
Although both groups recruited similar networks during AM retrieval the comparison of the spatial weightings within each network revealed several group differences (see Table 3 and Figure 2). First, within the frontoparietal network the PTSD group showed decreased component weights in the precuneus relative to controls. Second, within the cinguloperculum network the PTSD group had decreased spatial weightings in the dorsal anterior cingulate cortex (dACC) compared to controls. Third, within the medial PFC network the PTSD group showed decreased spatial weightings in posterior midline and superior temporal cortex compared to controls. Finally, spatial comparison within the MTL network revealed differential spatial weighting in the right amygdala, parietal cortex, and occipital cortex. The PTSD group had increased spatial weighting in the amygdala, but decreased weighting in the retrosplenial cortex, posterior cingulate, and parieto-occipital cortex when compared to controls.
Table 3.
Spatial Comparison of Networks
| MNI |
% Signal Change |
|||||||
|---|---|---|---|---|---|---|---|---|
| Region | BA | X | y | z | voxels | z-value | Control | PTSD |
| Medial PFC Network | ||||||||
| PTSD < Controls | ||||||||
| Posterior Midline | 7/31 | 0 | −68 | 38 | 89 | 5.01 | 2.20 | 0.22 |
| Superior Temporal Cortex | 22/21 | 60 | −49 | 8 | 18 | 4.93 | 0.72 | −0.17 |
| PTSD > Controls | ||||||||
| No Significant Differences | ||||||||
| MTL Network | ||||||||
| PTSD < Controls | ||||||||
| Retrosplenial Cortex | 29/30 | 4 | −45 | 0 | 22 | 5.09 | 1.34 | −0.15 |
| Parieto-Occipital Cortex | 31/18 | 23 | −64 | 19 | 37 | 4.94 | 1.26 | 0.20 |
| 31/18 | −23 | −68 | 19 | 14 | 4.06 | 0.84 | 0.11 | |
| Posterior Cingulate | 31 | −8 | −68 | 19 | 6 | 3.86 | 2.52 | 1.16 |
| PTSD > Controls | ||||||||
| Amygdala* | - | 30 | −4 | −19 | 9 | 2.64 | −0.17 | 0.12 |
| Frontoparietal Network | ||||||||
| PTSD < Controls | ||||||||
| Precuneus | 7 | −15 | −79 | 46 | 6 | 4.45 | −0.05 | 0.69 |
| PTSD > Controls | ||||||||
| No Significant Differences | ||||||||
| Cingulooperculum Network | ||||||||
| PTSD < Controls | ||||||||
| Dorsal ACC | 32 | 4 | 38 | 23 | 11 | 5.34 | 0.24 | 1.34 |
| PTSD > Controls | ||||||||
| No Significant Differences | ||||||||
BA= Brodmann’s Area; ACC = Anterior Cingulate Cortex; MTL = Medial Temporal Lobe; PFC = Prefrontal Cortex; PTSD = Post-Traumatic Stress Disorder
Region of Interest Analysis
Figure 2.
Differences in spatial weighting of the networks between PTSD and control groups. Contrasts in red depict spatial weighting where PTSD is greater than controls, whereas contrasts in blue depict spatial weighting where PTSD is less than controls. Statistical maps are thresholded at p <. 05 corrected for multiple comparisons. Multiple comparison correction was relaxed the amygdala. (A) In the frontoparietal network there was decreased spatial weighting in the precuneus in PTSD versus controls. (B) In the cingulooperculum network there was decreased spatial weighting in the anterior cingulate in PTSD versus controls. (C) In the MTL (medial temporal lobe) network, compared to controls, the PTSD group had decreased spatial weighting in bilateral parieto-occipital and increased spatial weighting in the right amygdala. (D) In the Medial PFC (prefrontal cortex) network there was decreased spatial weighting in the posterior cingulate in PTSD compared to controls.
3.2.3. Temporal Comparisons
Examining differences in task relatedness showed two networks that had a main effect of phase, one with a main effect of group, and no interactions (see Figure 1B). The frontoparietal network showed a main effect of phase, F (1, 28) = 5.55, p < .05, indicating that activity in this component was associated more with construction than elaboration. Similarly, the cingulooperculum network showed a main effect of phase, F (1, 28) = 3.68, p < .05, indicating that this component contributed during construction but not elaboration. There were no main effects or interactions in the MTL network, indicating that activity within this network was equally associated with construction and elaboration phases in both groups. Finally, in the medial PFC network there was no main effect of phase or interaction, indicating that activity here was equally associated with construction and elaboration phases across the groups. However, there was a main effect of group, F (1, 28) = 12.58, p < .001, which showed that the medial PFC network was less associated with AM retrieval in PTSD when compared to controls. Cue locked component time-courses averaged across participants for each group are shown in Figure 1C.
3.2.4. Network-PCL Correlation
Correlation analyses did not reveal any significant relationships between PCL scores and network activity after controlling for group (all p’s > .05). These results suggest that individual differences in PTSD syptomology were not related to network activity.
3.2.5. Network-Behavior Regression
Multiple regression analyses revealed that there was a significant relationship between the characteristics of autobiographical experience and neural activity in two of the networks. Within the frontoparietal network multiple R for regression was statistically significant, F (5, 23) = 3.65, p < .05, R2 adjusted = .32. We found a significant group × emotional intensity interaction, β = −59.15, t (23) = −3.06, p < .01, that was reflected by a significant effect of emotional intensity in the control group, β = 47.27, t (23) = 3.18, p < .005, but not in the PTSD group, β = −11.88, t (23) = 1.55, p = .35. Thus, within the control group increases in emotional intensity predicted neural activity in the frontoparietal network, but a similar relationship between emotional intensity and frontoparietal network activity was not found in the PTSD group. The perspective × group interaction and main effects of group and perspective were not significant predictors of neural activity within the frontoparietal network (all p’s > .05).
Within the MTL network multiple R for regression was also statistically significant, F (5, 23) = 4.36, p < .01, R2 adj = .38. We found that memory perspective predicted neural activity equally across both groups, β = 13.98, t (23) = 2.52, p < .05, which indicated that memories retrieved from a first person versus a third person perspective recruited greater network activity in the MTL network. Group also predicted neural activity in the MTL network, β = −14.83, t (23) = −2.07, p = .05, with less neural recruitment of the MTL network in the PTSD group versus the control group. Emotional intensity, and emotional intensity × group interactions were not significant. There were no significant relationships between the AM characteristics and the remaining networks. However, within the medial PFC network, multiple R for regression was statistically significant, F (5, 23) = 5.26, p < .005, R2 adjusted = .43. This was reflected by a significant main effect of group, β = −42.36, t (23) = −3.87, p < .001, indicating that network activity in the medial PFC was reduced in the PTSD group when compared to controls.
4. Discussion
The current study examines the large-scale neural networks contributing to AM retrieval in PTSD and controls. The data revealed that the PTSD group recruited similar neural networks during the recall of personal memories, including default network subsystems (medial PFC and MTL network) and control networks (frontoparietal and cinguooperculum network). Despite general similarities in network identification, however, there were differences in the spatial and temporal characteristics of these networks in PTSD. First, there were spatial differences in the contribution of the anterior and posterior midline across the networks, and with the amygdala in particular for the MTL network. Second, there were temporal differences in the relationship between the medial PFC network and AM retrieval in PTSD relative to controls. We discuss each of these findings below.
4.1. Spatial Comparison of Networks
Although the networks identified in the PTSD group were largely similar to those identified in the control group, there were important group differences in the spatial characteristics of these networks. Within the MTL network we found greater contribution of the amygdala in PTSD when compared to controls. Previous functional neuroimaging studies of PTSD using univariate analysis have observed hyperactivity in the amygdala and changes in amygdalar-medial PFC coupling (for a review see Shin, et al., 2006). For example, in a univariate analysis on these same participants, we (St. Jacques, Botzung, et al., 2011) observed greater right amygdala recruitment during the construction of negatively intense AMs. Further, there was greater functional coupling between the amygdala and ventral medial PFC in emotionally intense negative AMs in PTSD versus controls. Here we show that the increased coupling with the amygdala in PTSD extends more widely across the entire MTL network and that greater coactivation with the amygdala in PTSD when compared to controls is also evident across a wide variety of AMs (also see Gilboa, et al., 2004). Despite the amygdalar differences, emotional intensity did not differ between the groups as was expected (e.g., Rubin, Boals, et al., 2008).
Although emotion is often considered an important component of AM, the amygdala is not frequently recruited during retrieval of autobiographical experiences (Svoboda, et al., 2006). Indeed, in the current study, despite using emotional word cues to elicit AMs, the amygdala did not contribute to the MTL network in healthy controls. One reason may be that the amygdala is selectively recruited only for particular phases of memory retrieval and only for particularly strong emotional memories (Botzung, Rubin, Miles, Cabeza, & Labar, 2010; Daselaar, et al., 2008; Holland & Kensinger, 2013). For example, Daselaar et al. (2008) found that amygdala activity was modulated by the emotional intensity of AM retrieval during construction, but not during elaboration. This may be because, in healthy individuals, emotional responses that occur early during the course of AM retrieval are down-regulated or inhibited in order to access specific memory details during elaboration (Conway & Pleydell-Pearce, 2000; Philippot, Baeyens, Douilliez, & Francart, 2004). Thus, lack of amygdalar recruitment within the MTL network in healthy controls compared to PTSD may reflect differences in the ability to regulate emotional responses during the time course of AM retrieval.
Spatial comparison of the MTL network also revealed less contribution from several posterior midline regions including the retrosplenial cortex and parieto-occipital cortex in the PTSD group. These regions contribute to visual details that enable the construction of a coherent scene (Hassabis & Maguire, 2007). In our previous study (St. Jacques, Botzung et al., 2011) we observed reduced neural recruitment of the occipital cortex in PTSD versus controls when retrieving emotionally intense AMs, and less involvement of the retrosplenial cortex in PTSD specifically during memory elaboration (but greater involvement in PTSD during construction). In the current study, we show that retrieval of a wide variety of AMs-not necessarily emotionally intense memories-decreases coactivation of the retrosplenial cortex and parieto-occipital brain regions within the MTL network in PTSD. In sum, enagagment of the MTL network in PTSD involves greater amygdala involvement coupled with decreased visual cortex involvement, suggesting that emotional and visual processes contributing to memory retrieval are altered.
In both the MTL and medial PFC subystems of the default network we found reduced contribution of the posterior cingulate in PTSD when compared to controls. The posterior cingulate cortex is an important convergence zone allowing interaction and integration among the medial PFC and MTL subsystems (Buckner, et al., 2008; also see St. Jacques, Kragel, et al., 2011). Several lines of evidence suggest that the posterior cingulate is a pivotal node within the default network. For example, compared to other regions within the default network, the posterior cingulate is the only region that directly interacts with all other nodes in the network (Fransson & Marrelec, 2008), shows the highest metabolic activity (Gusnard & Raichle, 2001), and is one of the first regions to be affected in disease (Minoshima, et al., 1997). Perhaps because of its centrality, the posterior cingulate cortex is one of the most common seeds chosen in resting state functional connectivity analysis. Previous PTSD studies have shown that there is reduced resting state functional connectivity between the posterior cingulate and several regions of the default network, and that such changes are related to symptom severity (Bluhm, et al., 2009; Lanius, et al., 2010). The current results add to this literature by showing that PTSD-related alterations in the posterior cingulate reflect the involvement of both subsystems of the default network and its active engagement during an AM retrieval task. The reduced involvement of the posterior cingulate in the default network recruited by the PTSD group may contribute to differences in the function of this network during AM retrieval, perhaps by altering its interactions with other networks.
Interestingly, spatial comparisons among the networks also revealed differences within the control networks contributing to AM retrieval in PTSD. Within the frontoparietal network there was decreased contribution of the precuneus, whereas the cinguoloperculum network showed decreased involvement of the anterior cingulate cortex. Functional changes in the recruitment of the posterior cingulate and precuneus were not evident in the previous univariate analysis, suggesting that the multivariate approach can provide additional evidence regarding functional regions contributing to PTSD. Group differences in the spatial characteristics of the neural networks may influence their temporal engagement during AM retrieval. We turn to the discussion of the temporal comparisons of the networks next.
4.2. Temporal Comparison of Networks
Temporal comparisons of network contributions to phases of AM retrieval were very similar across the groups, except for the medial PFC network. Similar to our previous analysis in healthy participants (St. Jacques, Kragel, et al., 2011), the frontoparietal and cinguloperculum networks also contributed a greater extent to memory construction than elaboration in the PTSD group. Frontoparietal and cingulooperculum networks support the adaptive deployment of controlled processes and the maintenance of goals (Dosenbach, Fair, Cohen, Schlaggar, & Petersen, 2008; Dosenbach, et al., 2007; Vincent, Kahn, Snyder, Raichle, & Buckner, 2008), both of which may be particularly important during the cue specification, and memory search and construction components of the initial phase of AM retrieval. We also found that the MTL network was equivalently recruited in both groups across both construction and elaboration. Thus, it appears that PTSD does not generally affect either the engagement of controlled networks during construction, or the MTL network supporting mnemonic processes across construction and elaboration of AM. However, temporal engagement of regions within these networks, such as the amygdala and retrosplenial cortex, may be differentially associated with construction versus elaboration of AMs on a trial-by-trial basis according to the emotional response associated with memory retrieval (St. Jacques, Botzung, et al., 2011).
Group differences were only observed when considering the contribution of the medial PFC network to AM retrieval. We found that the PTSD group showed less engagement of the medial PFC network across both construction and elaboration phases of AM retrieval. The medial PFC network supports self-referential and affective processes (Andrews-Hanna, et al., 2010; St. Jacques, Conway, Lowder, & Cabeza, 2011) that are closely linked to memory for autobiographical experiences (e.g., Brewer, 1986; Cabeza, et al., 2004; Conway, 2005; Muscatell, Addis, & Kensinger, 2010). In healthy controls, the medial PFC network drives neural activations during AM retrieval (St. Jacques, Kragel, et al., 2011), a finding consistent with the importance of the self in AM construction (Conway & Pleydell-Pearce, 2000).
It is important to point out that the lack of task-related correspondence with the medial PFC network does not necessarily suggest that the PTSD group did not activate this network during AM retrieval, but rather it reflects a failure to activate this network greater than the implicit baseline, the fixation period between trials. One potential explanation could be that the PTSD group activates the medial PFC network during fixation intervals due to continued self-reflection and affective processes related to previously retrieved memories. However, prior research examining resting state functional connectivity suggests that the integrity of the default network, encompassing the medial PFC subnetwork, is impaired in patients with PTSD (Bluhm et al., 2009). Further research is needed to determine the exact nature of this failure of the PTSD group to activate the medial PFC network during functional tasks, such as AM retrieval, and their relationship to similar impairments in large-scale networks observed during rest.
4.3. Network-Behavior Regression
Individual differences in the characteristics of AM retrieval were found to predict recruitment of the MTL and frontoparietal networks. First, we found that the memory perspective was associated with increased engagement of the MTL network, such that participants who experienced memory retrieval from the perspective of their own eyes recruited this network more. The association between perspective and MTL network recruitment was similar across both groups and potentially reflects the involvement of this network in scene construction because memories that are viewed from one’s own perspective are more vivid and detailed (Rice & Rubin, 2009). Second, we found that the emotional intensity of memory was associated with increased engagement of the frontoparietal network in controls but not in PTSD. Emotion tends to be experienced earlier during AM retrieval (Daselaar, et al., 2008), perhaps serving as a warning signal that triggers deployment of control processes that regulate affective responses in order to allow for detailed memory retrieval. Given the role of the frontoparietal network in adaptive control processes during memory construction, one interpretation of this finding is that PTSD participants do not modulate recruitment of top-down control processes based on the emotional intensity experienced during memory retrieval. Thus, despite the lack of overall group differences during AM retrieval, emotional intensity may differentially contribute to the engagement of frontoparietal network in PTSD versus controls.
4.4 Limitations
It is important to note that one potential limitation of the current study is that an additional trauma exposed non-PTSD group was not included. This may be particularly relevant for interpreting group differences observed in the MTL network, because previous evidence has suggested that pre-existing structural abnormalities in the hippocampus may predict vulnerability to PTSD following trauma exposure (Gilbertson, et al., 2002) and functional activity in the hippocampus predicts recovery from PTSD (Dickie, Brunet, Akerib, & Armony, 2011). An additional limitation is that the small sample size precluded the possibility of examining interactions with other factors that may contribute to these findings such as sex and may have reduced power for observing network correlations with individual differences in PTSD symptomology. Future studies should include an additional control non-PTSD group that was trauma-exposed, as well as increasing the sample size of all groups in order to address these issues.
5. Conclusions
In the current study, we found differences in both the spatial extent and the task related activation of neural networks contributing to AM retrieval in PTSD. In particular, during AM retrieval, participants with PTSD activated the same four networks as did controls, including two that together comprise the default network, however there were theoretically relevant differences in the extent and task-related timing of these networks. First, there were spatial differences in the contribution of the anterior and posterior midline across the networks, and with the amygdala in particular for the medial temporal subsystem of the default network. Second, there were temporal differences in the relationship between the medial prefrontal subsystem of the default network, with less temporal coupling between this network and AM retrieval in PTSD relative to controls. These findings contribute to our understanding of the neural mechanisms supporting AM retrieval and their relationship to PTSD by revealing functional differences in the spatial and temporal properties of the multiple neural networks that underlie AM.
Acknowledgments
This research was supported by a National Institute of Aging RO1 AG023123 and National Institute of Mental Health R01 MH066079 grant to DCR. We thank Amanda Miles and Anne Botzung for assistance with scanning, and Polly van de Velde and Gustavo Araujo for assistance with participant recruitment and screening.
Footnotes
Data were missing from two participants in the PTSD group due to experimenter error. Thus the reported results for this analysis are based on 13 participants in the PTSD group and 14 participants in the controls.
References
- American Psychatric Association . In: Diagnostic and statistical manual of mental disorders. Fourth Edition Text Revision (DSM-IV-TR), editor. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65(4):550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory. Psychology Coporation; San Antonio, TX: 1996. [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bluhm RL, Williamson PC, Osuch EA, Frewen PA, Stevens TK, Boksman K, et al. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. J Psychiatry Neurosci. 2009;34(3):187–194. [PMC free article] [PubMed] [Google Scholar]
- Botzung A, LaBar KS, Kragel P, Miles A, Rubin DC. Component neural systems for the creation of emotional memories during free viewing of a complex, real-world event. [Original Research] Frontiers in Human Neuroscience. 2010;4 doi: 10.3389/fnhum.2010.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botzung A, Rubin DC, Miles A, Cabeza R, Labar KS. Mental hoop diaries: emotional memories of a college basketball game in rival fans. J Neurosci. 2010;30(6):2130–2137. doi: 10.1523/JNEUROSCI.2481-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Affective norms for English words (ANEW) The NIMH Center for the Study of Emotion and Attention, University of Florida; Gainesville, FL: 1999. [Google Scholar]
- Brewer WF. What is autobiographical memory? In: Rubin DC, editor. Autobiographical Memory. Cambridge UP; NY: 1986. pp. 25–49. [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Prince SE, Daselaar SM, Greenberg DL, Budde M, Dolcos F, et al. Brain activity during episodic retrieval of autobiographical and laboratory events: An fMRI study using a novel photo paradigm. Journal of Cognitive Neuroscience. 2004;16(9):1583–1594. doi: 10.1162/0898929042568578. [DOI] [PubMed] [Google Scholar]
- Cabeza R, St. Jacques PL. Functional neuroimaging of autobiographical memory. Trends Cogn Sci. 2007;11(5):219–227. doi: 10.1016/j.tics.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001a;14(3):140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. Spatial and temporal independent component analysis of functional MRI data containing a pair of task-related waveforms. Hum Brain Mapp. 2001b;13(1):43–53. doi: 10.1002/hbm.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celone KA, Calhoun VD, Dickerson BC, Atri A, Chua EF, Miller SL, et al. Alterations in memory networks in mild cognitive impairment and Alzheimer’s disease: an independent component analysis. J Neurosci. 2006;26(40):10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway MA. Memory and the self. Journal of Memory and Language. 2005;53:594–628. [Google Scholar]
- Conway MA, Pleydell-Pearce CW. The construction of autobiographical memories in the self-memory system. Psychological Review. 2000;107(2):261–288. doi: 10.1037/0033-295x.107.2.261. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103(37):13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Rice HJ, Greenberg DL, Cabeza R, LaBar KS, Rubin DC. The spatiotemporal dynamics of autobiographical memory: neural correlates of recall, emotional intensity, and reliving. Cereb Cortex. 2008;18(1):217–229. doi: 10.1093/cercor/bhm048. [DOI] [PubMed] [Google Scholar]
- Dickie EW, Brunet A, Akerib V, Armony JL. Neural correlates of recovery from post-traumatic stress disorder: a longitudinal fMRI investigation of memory encoding. Neuropsychologia. 2011;49(7):1771–1778. doi: 10.1016/j.neuropsychologia.2011.02.055. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12(3):99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104(26):11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. Neuroimage. 2008;42(3):1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Cortex and memory: emergence of a new paradigm. J Cogn Neurosci. 2009;21(11):2047–2072. doi: 10.1162/jocn.2009.21280. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15(4):870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5(11):1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa A, Shalev AY, Laor L, Lester H, Louzoun Y, Chisin R, et al. Functional connectivity of the prefrontal cortex and the amygdala in posttraumatic stress disorder. Biol Psychiatry. 2004;55(3):263–272. doi: 10.1016/j.biopsych.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Greenberg DL, Rice HJ, Cooper JJ, Cabeza R, Rubin DC, LaBar KS. Co-activation of the amygdala, hippocampus and inferior frontal gyrus during autobiographical memory retrieval. Neuropsychologia. 2005;43(5):659–674. doi: 10.1016/j.neuropsychologia.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2(10):685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hassabis D, Maguire EA. Deconstructing episodic memory with construction. Trends in Cognitive Science. 2007;11(7):299–306. doi: 10.1016/j.tics.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Holland AC, Kensinger EA. The Neural Correlates of Cognitive Reappraisal during Emotional Autobiographical Memory Recall. J Cogn Neurosci. 2013;25(1):87–108. doi: 10.1162/jocn_a_00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Summerin JL, Rainey L, Freitas CS, Fox PT. The Talairach Daemon, a database server for Talairach Atlas Labels. Neuroimage. 1997;5(4):S633. [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talaraich atlas labels for functional brain mapping. Human Brain Mapping. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius RA, Bluhm RL, Coupland NJ, Hegadoren KM, Rowe B, Theberge J, et al. Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatr Scand. 2010;121(1):33–40. doi: 10.1111/j.1600-0447.2009.01391.x. [DOI] [PubMed] [Google Scholar]
- Li YO, Adali T, Calhoun VD. Estimating the number of independent components for functional magnetic resonance imaging data. Hum Brain Mapp. 2007;28(11):1251–1266. doi: 10.1002/hbm.20359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroantomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. WFU Pickatlas, version 1232.1233. [DOI] [PubMed] [Google Scholar]
- Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol. 1997;42(1):85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- Muscatell KA, Addis DR, Kensinger EA. Self-involvement modulates the effective connectivity of the autobiographical memory network. Soc Cogn Affect Neurosci. 2010;5(1):68–76. doi: 10.1093/scan/nsp043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L, McIntosh A. Functional neuroimaging: Network analysis. In: Cabeza R, Kingstone A, editors. Handbook of functional neuroimaging of cognition. MIT Press; London: 2001. pp. 49–72. [Google Scholar]
- Philippot P, Baeyens C, Douilliez C, Francart B. Cognitive regulation of emotion. In: Philippot P, Feldman RS, editors. The regulation of emotion. Laurence Erlbaum Associates; New York: 2004. [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice HJ, Rubin DC. I can see it both ways: first- and third-person visual perspectives at retrieval. Conscious Cogn. 2009;18(4):877–890. doi: 10.1016/j.concog.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DC. The basic-systems model of episodic memory. Perspectives in Psychological Science. 2006;1(1):277–311. doi: 10.1111/j.1745-6916.2006.00017.x. [DOI] [PubMed] [Google Scholar]
- Rubin DC, Berntsen D, Bohni MK. A memory-based model of posttraumatic stress disorder: evaluating basic assumptions underlying the PTSD diagnosis. Psychol Rev. 2008;115(4):985–1011. doi: 10.1037/a0013397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DC, Boals A, Berntsen D. Memory in posttraumatic stress disorder: properties of voluntary and involuntary, traumatic and nontraumatic autobiographical memories in people with and without posttraumatic stress disorder symptoms. J Exp Psychol Gen. 2008;137(4):591–614. doi: 10.1037/a0013165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DC, Dennis MF, Beckham JC. Autobiographical memory for stressful events: the role of autobiographical memory in posttraumatic stress disorder. Conscious Cogn. 2011;20(3):840–856. doi: 10.1016/j.concog.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DC, Schrauf RW, Greenberg DL. Belief and recollection of autobiographical memories. Mem Cognit. 2003;31(6):887–901. doi: 10.3758/bf03196443. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Grady CL. Patterns of Brain Activity Supporting Autobiographical Memory, Prospection, and Theory-of-Mind and Their Relationship to the Default Mode Network. Journal of Cognitive Neuroscience. 2010;22(6):1112–1123. doi: 10.1162/jocn.2009.21282. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage. 2010;53(1):303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques PL, Botzung A, Miles A, Rubin DC. Functional neuroimaging of emotionally intense autobiographical memories in post-traumatic stress disorder. Journal of Psychiatric Research. 2010 doi: 10.1016/j.jpsychires.2010.10.011. doi:10.1016/j.jpsychires.2010.1010.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques PL, Kragel PA, Rubin DC. Dynamic neural networks supporting memory retrieval. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2011.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Jacques PL, Botzung A, Miles A, Rubin DC. Functional neuroimaging of emotionally intense autobiographical memories in post-traumatic stress disorder. Journal of Psychiatric Research. 2011;45:630–7. doi: 10.1016/j.jpsychires.2010.10.011. doi:10.1016/j.jpsychires.2010.1010.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Jacques PL, Conway MA, Lowder MW, Cabeza R. Watching my mind unfold versus yours: An fMRI study using a novel camera technology to examine neural differences in self-projection of self versus other perspectives. Journal of Cognitive Neuroscience. 2010;23(6):1275–1284. doi: 10.1162/jocn.2010.21518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Jacques PL, Conway MA, Lowder MW, Cabeza R. Watching my mind unfold versus yours: An fMRI study using a novel camera technology to examine neural differences in self-projection of self versus other perspectives. Journal of Cognitive Neuroscience. 2011;23(6):1275–1284. doi: 10.1162/jocn.2010.21518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Jacques PL, Kragel PA, Rubin DC. Dynamic neural networks supporting memory retrieval. Neuroimage. 2011;57:608–616. doi: 10.1016/j.neuroimage.2011.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;44(12):2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100(6):3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety. 2001;13(3):132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Litz BT, Huska JA, Keane TM. The PTSD Checklist (PCL) 1994 [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. The Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- Williams JMG. Depression and the specificity of autobiographical memory. In: Rubin DC, editor. Remembering Our Past: Studies in Autobiographical Memory. Cambridge UP; NY: 1995. pp. 244–267. [Google Scholar]