Abstract
Heterochromatin usually is sequestered near the periphery and the nucleoli in mammalian nuclei. However, in terminally differentiated retinal rod cells of nocturnal mammals, heterochromatin instead accumulates in the interior, to give a so-called inside-out nuclear architecture. Solovei et al. now reports that in most cells, the lamin B receptor mediates peripheral localization early during development and that lamin A/C then takes over this tethering function during terminal differentiation. Furthermore, they show that the unique architecture of the nocturnal animal rod cell is caused by the absence of both tethers and can be phenocopied in LBR/lamin A/C double knockouts.
Keywords: heterochromatin, nuclear organization, terminal differentiation, nuclear periphery, muscle development
The nucleus of differentiated cells is generally characterized by the marked clustering of heterochromatin at the periphery and near the nucleoli.1 Both constitutive and facultatively repressed heterochromatin are found in these regions and the segregation of the relatively gene-poor heterochromatin from gene-rich euchromatin is thought to be crucial for differential gene expression and normal development. Peripheral heterochromatin is anchored at least partially via direct and/or indirect interactions with lamins A/C, lamin B and LBR (lamin B receptor) and LEM (lamina-associated polypeptide-emerin-MAN1) proteins, the major constituents of the lamina that lines the inner surface of the nuclear envelope.2
Recently Solovei and colleagues demonstrated that during terminal differentiation of retinal rod cells in nocturnal mammals, nuclei adopt a so-called “inside-out” organization, where heterochromatin is centered internally and euchromatin is displaced to the periphery.3 This post-mitotic process takes several weeks to complete and the specially organized rod cells align in the retina to help to maximize light collection. By contrast, diurnal mammals have conventionally organized rod cell nuclei. Now Solovei et al.4 have followed up on these studies by demonstrating that this inside-out architecture they discovered is due to a hitherto unknown and unanticipated absence of both lamin A/C and LBR in rod nuclei of adult nocturnal mammals (Fig. 1A, top row). Interestingly, in mammals that evolved from nocturnal back to diurnal, either LBR or lamin A/C expression was re-acquired in the rod nuclei, which then again came to possess conventional nuclear architecture. Concordantly, ectopic expression of LBR reverses the nocturnal phenotype, while lamin A/LBR double mutants recapitulate the inside-out nuclear architecture, at least partially, in all other cell types studied.
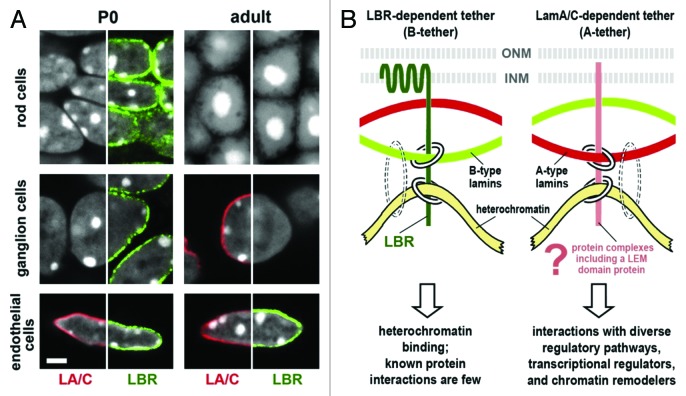
Figure 1. Heterochromatin clusters internally in the absence of peripheral tethers. (A) Temporal expression of LBR and lamin A/C in mouse retinal tissues. Tissues were stained simultaneously for lamin A/C (LA/C, red), LBR (green) and DNA (white, DAPI staining, where heterochromatin stains brightest). The distribution of one peripheral stain combined with DAPI is shown in each half-panel. Rod and ganglion cells express only LBR at stage P0; in adult tissue, ganglion cells have switched to lamin A/C (middle row) while rod cells uniquely express neither tether protein and exhibit the inverted nuclear architecture (top row). Endothelial cells express LBR and lamin A/C at both stages of development (bottom row). Scale bar = 2 µm. (B) Models for lamin A/C and LBR tethering of peripheral heterochromatin. Chromatin/DNA binding by lamins is indicated by dotted circles to show that lamins are not sufficient for heterochromatin tethering. From Solovei et al.4 with permission from Elsevier.
Solovei et al. extended the rod cell studies to look at over 30 other murine tissue types. The majority exhibited sequential production of LBR early in development and then lamin A/C during terminal differentiation, as demonstrated in ganglion cells in Figure 1A (middle row). There are exceptions to this rule, for example, endothelial cells expressed both LBR and lamin A/C throughout their lifetimes (Fig. 1A, bottom row), but all cells examined (except rod cells) expressed LBR or lamin A/C, or both. In LBR null mutants (ichthyosis mice), cells that would normally express LBR and not lamin A/C exhibited the inside-out phenotype. Once lamin A/C expression commenced, normal architecture was re-established. On the other hand, in lamin A/C knockout mice LBR was never turned off, even in terminally differentiated cells, and the inside-out architecture was not established (except in one cell type, fibroblasts in hair follicles). Thus, LBR can substitute for lamin A/C to a certain extent, but not vice-versa. The authors point out that this may be because the transmembrane LBR can interact directly with chromatin, while the membrane-associated lamin A/C probably requires cell-specific mediators to facilitate both binding to the nuclear envelope and heterochromatin binding (Fig. 1B). Indeed, lamin A/C based laminopathies often display cell type specific phenotypes, suggesting that particular lamin A/C mutations affect interactions with different cell-specific LEM proteins.2 Furthermore, in rat retina, where photoreceptor cells were also missing lamin A/C, differential expression of lamin A and C was observed in the other retinal cell types.5 This suggests that changes in the ratios of lamin A and C may also be coupled to terminal differentiation. Taken together then, these results give strong credence to earlier work suggesting that lamin A/C and LBR mediate heterochromatin anchoring at the periphery and provide an important underpinning to the understanding of laminopathies.
As mentioned, when both lamin A/C and LBR were absent in differentiated cells, nuclear architecture was at least partially inverted in most cell types. The double lamin A/LBR mutant died shortly after birth, consistent with the notion that peripheral tethering is critical for proper nuclear organization and normal development. In cells missing both tethering proteins, heterochromatin became clustered in one or a few central chromocenters, rather than appearing fragmented and spread throughout the nucleus. Such self-association by untethered chromatin is consistent with a “birds of a feather flock together” model, which postulates that heterochromatin and euchromatin self-association is a driving force for nuclear organization.6 However, although the double mutant died shortly after birth, the lamin A/C knockout survived a few weeks and the LBR null mouse survived to adulthood, even though many cell types showed a partially inverted architecture at birth. This implies that heterochromatin tethering to the periphery is important but not crucial to early development. Indeed, it has been thought that neither lamin A/C nor LBR is present in single cell embryos (although this has been brought into question recently7) and that this might help explain the greater plasticity of chromatin and lower prominence of organized heterochromatic regions in these pluripotent cells. However, the results discussed here show that the lack of both lamin A/C and LBR does not cause the loss of heterochromatin; rather it induces its reorganization.
Although Solovei et al. found that LBR can substitute for lamin A in a general sense, transcriptome analysis on muscle gene expression during development showed that inappropriate LBR synthesis caused changes in muscle transcript levels. In myoblasts, expression was upregulated in LBR knockouts and downregulated in lamin A/C knockouts (where LBR remained expressed during differentiation). The absence of both tethers in the LBR knockout likely led to upregulation of muscle genes in myoblasts as lamin A/C was expressed only later during differentiation. These results suggest that in normal cells, facultatively-repressed muscle genes were peripherally anchored by LBR (and repressed) until differentiation began. Neither mutant showed a significant effect on muscle gene expression in terminally differentiated cells, presumably because in the fully differentiated cells at least one of the two heterochromatin anchors was present to ensure the appropriate sequestration of peripheral heterochromatin. Thus, this report links expression changes in a large number of muscle genes to the deletion of either of two peripheral heterochromatin anchoring proteins, strongly supporting the idea that selective peripheral sequestration of heterochromatin contributes to differentiation.
A recent report by Clowney et al.8 further underscores the importance of peripheral heterochromatin tethers for correct cell differentiation and expression. Clowney et al. showed that in normal olfactory sensory neurons (OSN), silent olfactory receptor genes specifically clustered near centrally organized heterochromatin. In fact, the architecture of these nuclei resembled that of the inverted retinal rod nuclei of nocturnal mammals.3,4 Not surprisingly, these mature OSNs did not express LBR, and although the presence of lamin A/C was not tested, it was likely not being expressed either. Adjoining tissue and immature OSN exhibited conventional nuclear organization and olfactory receptor genes did not cluster, commensurate with maintained LBR expression. Furthermore, forced expression of LBR reversed the inverted nuclear architecture and olfactory receptor genes not only no longer clustered together, but their expression also became deregulated (i.e., more than one gene became active). This scenario is remarkably similar to that of the terminally differentiated rod cells. It appears then, that the inverted organization observed in these cell types is an intriguing example of a special type of terminal nuclear organization, where a lack of peripheral tethering proteins and the resultant internal clustering of heterochromatin is necessary for proper terminal differentiation and expression control. It will be interesting indeed to learn more about how temporal variations in the interactions between the lamina and chromatin might help to shape both early development and terminal differentiation.
Acknowledgments
The authors thank David Scalzo for insightful comments on the manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/nucleus/article/24999
References
- 1.Rajapakse I, Groudine M. On emerging nuclear order. J Cell Biol. 2011;192:711–21. doi: 10.1083/jcb.201010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butin-Israeli V, Adam SA, Goldman AE, Goldman RD. Nuclear lamin functions and disease. Trends Genet. 2012;28:464–71. doi: 10.1016/j.tig.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solovei I, Kreysing M, Lanctôt C, Kösem S, Peichl L, Cremer T, et al. Nuclear architecture of rod photoreceptor cells adapts to vision in mammalian evolution. Cell. 2009;137:356–68. doi: 10.1016/j.cell.2009.01.052. [DOI] [PubMed] [Google Scholar]
- 4.Solovei I, Wang AS, Thanisch K, Schmidt CS, Krebs S, Zwerger M, et al. LBR and lamin A/C sequentially tether peripheral heterochromatin and inversely regulate differentiation. Cell. 2013;152:584–98. doi: 10.1016/j.cell.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Wakabayashi T, Mori T, Hirahara Y, Koike T, Kubota Y, Takamori Y, et al. Nuclear lamins are differentially expressed in retinal neurons of the adult rat retina. Histochem Cell Biol. 2011;136:427–36. doi: 10.1007/s00418-011-0853-8. [DOI] [PubMed] [Google Scholar]
- 6.Gibcus JH, Dekker J. The hierarchy of the 3D genome. Mol Cell. 2013;49:773–82. doi: 10.1016/j.molcel.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eckersley-Maslin MA, Bergmann JH, Lazar Z, Spector DL. Lamin A/C is expressed in pluripotent mouse embryonic stem cells. Nucleus. 2013;4:53–60. doi: 10.4161/nucl.23384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clowney EJ, LeGros MA, Mosley CP, Clowney FG, Markenskoff-Papadimitriou EC, Myllys M, et al. Nuclear aggregation of olfactory receptor genes governs their monogenic expression. Cell. 2012;151:724–37. doi: 10.1016/j.cell.2012.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]