Abstract
The nuclear envelope not only compartmentalizes the genome but is also home to the SUN-KASH domain proteins, which play essential roles both in genome organization and in linking the nucleus to the cytoskeleton. In interphase fission yeast cells, centromeres are clustered near the nuclear periphery. A recent report demonstrates that the inner nuclear membrane SUN domain protein Sad1 and a novel protein Csi1 connect centromeres to the nuclear envelope and that centromere clustering during interphase is critical for the efficient capture of kinetochores by microtubules during mitosis.
Keywords: nuclear envelope, centromere, kinetochore, SPB, Sad1, Csi1
Introduction
Eukaryotic DNA is highly compacted to fit into the nucleus, but nonetheless each chromosome region tends to occupy its own discrete territory.1,2 Spatial and temporal organization of chromosomes is essential for the regulation of gene expression and the maintenance of genome stability.3-5 Genome organization is also linked to the stabilization of cell fate during differentiation6 as well as dictating chromosome translocation events associated with various forms of cancers.7
DNA elements critical for the maintenance of chromosomes include centromeres, which are the sites of kinetochore assembly8,9 and telomeres, which protect the ends of chromosomes.10,11 During mitosis chromosomes condense, and microtubules originating from microtubule organizing centers (MTOCs) capture kinetochores to drive chromosome segregation, with telomeres trailing behind. During interphase, chromosomes decondense, but in many cases still maintain a polarized arrangement termed Rabl configuration, in which centromeres are clustered in a limited region near the nuclear envelope, and telomeres are located at the opposite hemisphere of the nucleus.12,13 Rabl configuration has been regarded as a direct consequence of mitotic anaphase arrangement of chromosomes persisting through interphase and has been observed in vast varieties of organisms, including yeasts, plants, insects and mammals.12,14-18
In the budding yeast, centromeres are clustered near the spindle pole body (SPB, the counterpart of centrosome in yeast) during interphase, forming a rosette structure.16 Microtubules emanating from the SPB maintain their interactions with kinetochores during this cell cycle stage, and disrupting microtubules results in the declustering of centromeres.19 However, given that microtubules are not attached to kinetochores during interphase in other organisms, such microtubule-based tethering of centromeres is unlikely to be universal, and the mechanisms that regulate Rabl configuration in other organisms are largely unknown.
Centromere Clustering in Fission Yeast
The fission yeast Schizosaccharomyces pombe exhibits strong centromere clustering during interphase, in which the three centromeres are localized near the nuclear periphery at the site of the SPB,15 which is cytoplasmic at this stage20 (Fig. 1). At the onset of mitosis, duplicated SPBs separate and insert into the nuclear membrane.20 Centromeres are first released and then recaptured by microtubules emanating from the SPBs for chromosome segregation.15 Examination of the site of centromere clustering by electron microscopy shows that no microtubules are present between kinetochores and the SPB during interphase,20 and centromere clustering is not sensitive to microtubule destabilizing drugs,21,22 suggesting that interphase centromere clustering is not mediated by microtubules in fission yeast.
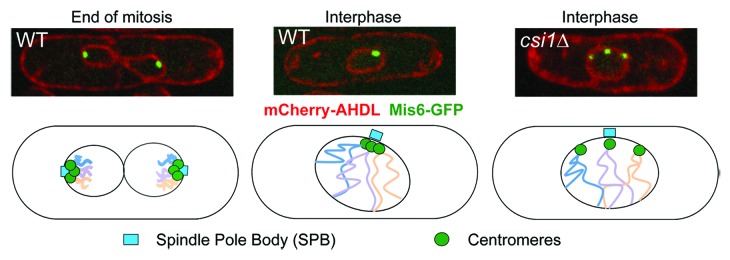
Figure 1. Centromere clustering in fission yeast. Top, live cell imaging of cells expressing AHDL-mCherry (Luminal ER marker indicative of nuclear membrane)52 and Mis6-GFP (kinetochore marker). Bottom, diagrams showing centromere clustering in fission yeast, which is disrupted in csi1∆.
The prime candidates mediating centromere clustering are kinetochore components and inner nuclear membrane components that reside at the SPB docking site. Indeed, temperature sensitive mutants in kinetochore components, such as mis6 (CENP-I) and nuf2 (NDC80 complex component), result in declustered centromeres at restrictive temperature.21,23,24 However, these mutants also block the cell cycle at mitosis, when centromeres naturally decluster. Other mutations that cause cell cycle arrest at mitosis, such as nda3 (tubulin), cut7 (kinesin) and nuc2 (anaphase promoting complex), also result in declustered centromeres.15 Due to such confounding phenotypes, it is not feasible to identify the kinetochore component directly involved in centromere clustering at interphase.
At the nuclear envelope, inner membrane protein Ima1 has been reported to mediate the association of centromeres with the SPB.25 However, a recent study showed that the original ima1∆ strain25 was mistakenly constructed by deleting a different gene, and the correct ima1∆ does not affect centromere clustering.26 We also did not observe interphase centromere clustering defects in ima1∆ cells (Fig. 2). Thus, the nuclear membrane components involved in centromere clustering remain to be identified.
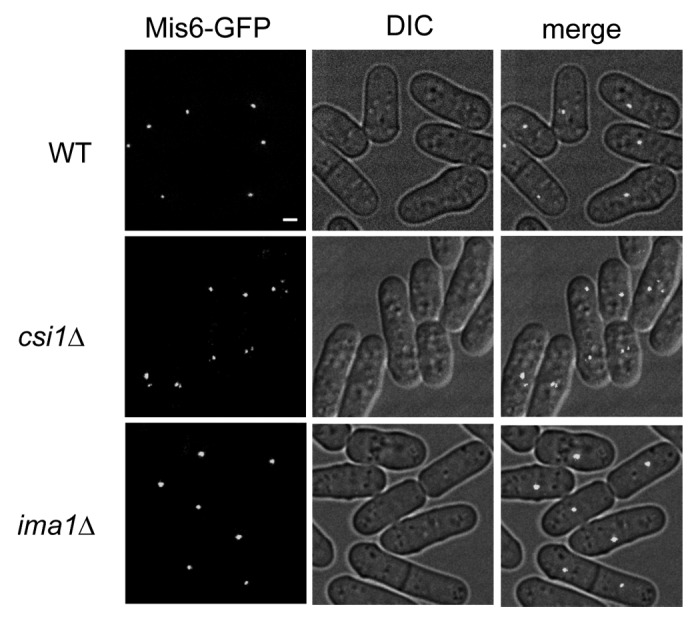
Figure 2. Ima1 is not required for centromere clustering during interphase. Live cell imaging of cells expressing Mis6-GFP. Scale bar is 1 μm. DIC (differential interference contrast microscopy) and merged images are also shown.
Other mutations that affect interphase centromere clustering include crm1, mto1∆ and nsk1∆.15,27,28 Crm1 is a member of the importin family of proteins involved in nuclear-cytoplasmic protein transport.29 It is an essential gene that potentially regulates the nuclear accumulation of diverse proteins, confounding analysis of the mechanism by which it mediates centromere clustering. Mto1 is a γ-tubulin-associated protein localized at the cytoplasmic side of the SPB and is required for the nucleation of cytoplasmic microtubules.30-32 mto1∆ cells have mild defects in interphase centromere clustering, with about 9% of cells showing declustering of only one kinetochore.27 Given that microtubules are observed only in the cytoplasm during interphase,33 the phenotype of mto1∆ in centromere clustering is most likely an indirect effect of a malfunctioning microtubule cytoskeleton. Nsk1 is a protein located at the SPB-kinetochore interface during mitosis.28,34 Loss of Nsk1 results in 9% of cells exhibiting defects in centromere clustering in interphase.28 However, Nsk1 is localized at the nucleolus at this cell cycle stage,28,34 and the effect of nsk1∆ on interphase centromere clustering is likely the result of impaired centromere association with the SPB during late mitosis persisting into interphase.28 Thus the factors that link kinetochores and the SPB during interphase are still unknown, and their identification is crucial for deciphering the mechanism and function of Rabl configuration.
Sad1 and Csi1 Play Essential Roles in Centromere Clustering
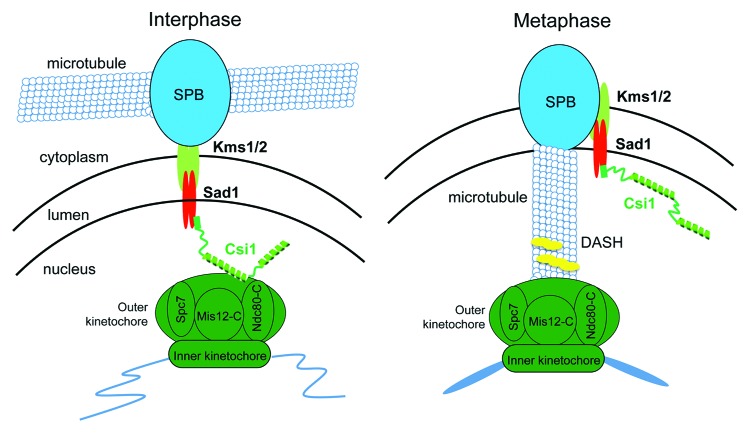
The SUN-KASH domain protein complexes link cytoplasmic structures and the nuclear membrane.5,35,36 KASH domain proteins reside in the outer nuclear membrane and interact with the cytoskeleton and MTOCs while the inner membrane SUN domain proteins directly connect to structures inside the nucleus. In fission yeast, KASH domain proteins Kms1/2 and SUN domain protein Sad1 are critical for docking of the SPB to the nuclear membrane37-39 (Fig. 3). During meiosis, Sad1 mediates interaction between the SPB and telomeres to form a bouquet-like organization critical for the movement of chromosomes.40,41
Figure 3. Diagrams of the interaction between kinetochores and the SPB. During interphase, Sad1-Csi1 forms a molecular link between kinetochores and the SPB to mediate centromere clustering. During mitosis, kinetochores are first released from the SPB and then captured by microtubules emanating from the SPBs in preparation for chromosome segregation. How the interaction between Csi1 and kinetochores is regulated is unknown. Csi1 is phosphorylated during mitosis (our unpublished data), which might contribute to the release of kinetochores.
In a recent study, we showed that Sad1 is also required for centromere clustering.42 Sad1 is an essential gene, and a temperature sensitive mutant of Sad1 (sad1.1)37 shows strong defects in centromere clustering.42 Due to the importance of Sad1 in mediating SPB association with the nuclear membrane, loss of Sad1 results in cell cycle block at mitosis. However, the sad1.1 mutant predominantly blocks the cell cycle at the second cell division after temperature shift,43 while centromere declustering is prominent 90 minutes after temperature shift. Given that one cell cycle of fission yeast is ~2 hours at this temperature, the early appearance of centromere declustering is not the result of a cell cycle block at mitosis. Thus Sad1 directly mediates interphase centromere clustering.
Through a screen of the fission yeast strain library containing about 3,500 deletions of individual genes,44 we identified a viable mutant severely defective in maintaining the artificial mini-chromosome Ch16.42 The gene was therefore designated csi1+ (chromosome segregation impaired 1). csi1∆ cells also show strong declustering of centromeres from the SPB during interphase.42
Further biochemical, genetic and microscopic analyses put Csi1 physically at the interface of kinetochore and the SPB42 (Fig. 3). Csi1-GFP exhibits a single focus in the interphase nucleus, at the site of SPB-kinetochore. Csi1 association with SPB depends on Sad1 as Csi1 shows a diffused staining pattern in sad1.1 cells at the restrictive temperature. Moreover, Csi1 directly binds Sad1 through a N-terminal helix, deletion of which results in diffuse localization of Csi1 and declustered centromeres.
At the kinetochore side, Csi1 is enriched at centromeric DNA, and this enrichment is dependent on kinetochore components, suggesting that a functional kinetochore is essential for the association of Csi1 with centromeres, rather than that Csi1 binds directly to centromeric DNA.42 Moreover, immunoprecipitation of Csi1 co-purifies kinetochore components, suggesting that protein-protein interactions mediate the association of the SPB with the kinetochores. The interaction is mediated by a coiled-coil region in the middle of Csi1 and mutations of this region result in the dissociation of Csi1 from centromeres and defects in centromere clustering. However, the immediate interaction partner of Csi1 at the kinetochore remains to be determined.
As expected for a linker between the SPB and kinetochores, disruption of Csi1-kinetochore interaction does not affect the interaction between Csi1 and the SPB.42 However, disrupting Csi1-Sad1 interaction results in diffuse localization of Csi1, mostly around the nuclear membrane, instead of centromere localization. Such results suggest either that localization of Csi1 to Sad1 is critical for its ability to associate with kinetochore components or that additional factors near the SPB are required for Csi1 association with kinetochores.
These results therefore establish a hierarchy of proteins interacting with both centromeres and the SPB during interphase, including kinetochore components, Csi1 and inner membrane protein Sad1 (Fig. 3).
Functions of Rabl Configuration in Interphase
Rabl configuration has been proposed to isolate genes near centromeres or telomeres for regulation and preparing for major chromosomal rearrangements, such as condensation, chromosome segregation and recombination.12,19,45 The csi1 mutants that result in high levels of centromere declustering allow further examination of the biological function of Rabl configuration in fission yeast.
Loss of Csi1 results in high loss rate of a mini-chromosome, suggesting that Rabl configuration during interphase directly regulates mitosis.42 In fission yeast, centromeres are released from the nuclear envelope at the onset of mitosis and then recaptured by intranuclear microtubules emanating from the SPBs to drive chromosome segregation (Fig. 3).15 Computer simulations of mitosis indicate that an unbiased MT search-and-capture mechanism is not efficient enough to complete mitosis in a timely manner.46 It was suggested that the clustering of centromeres during interphase facilitates the rapid capture of kinetochores47 (Fig. 4). We observed that csi1∆ cells spend longer and more variable times to reach anaphase, indicative of defects in kinetochore capture.42 This is consistent with previous observations that nsk1∆ cells with de-clustered centromeres spend longer times in mitosis as well.28 Compared with wild-type cells, csi1∆ cells also show more Bub1-GFP foci, an indicator of defects in microtubule-kinetochore attachment and the activation of spindle assembly checkpoint.42 As a result, csi1∆ is lethal or sick when combined with mutants in the spindle assembly checkpoint, possibly because cells continue mitosis with improperly attached kinetochores, resulting in mis-segregation of chromosomes.
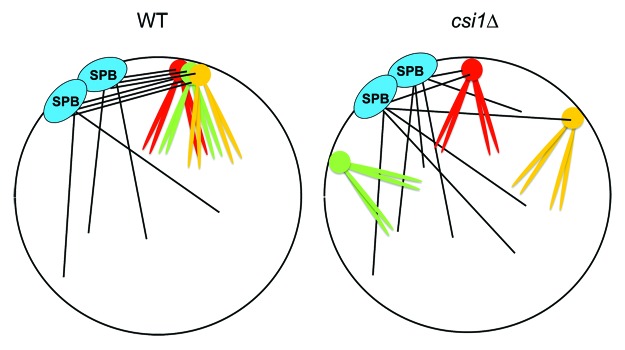
Figure 4. A model showing that centromere clustering during interphase facilitates kinetochore capture by microtubules during mitosis. The clustered centromeres serve as a higher affinity platform for concerted capture by microtubules.
Cells without functional Csi1 are also sensitive to perturbations of microtubule dynamics. For example, csi1∆ cells are highly sensitive to microtubule poison thiabendazole (TBZ) and are lethal or sick when combined with mutants that affect microtubule dynamics such as deletions of microtubule associated proteins dis1∆ and alp14∆. The DASH complex functions to couple kinetochore with microtubules,48,49 and is required for the retrieval of unattached kinetochores during mitosis.27 csi1∆ cells are synthetically lethal with mutations in every component of the DASH complex. Similarly, nsk1∆ cells are also synthetically sick with DASH mutants.28
These data suggest that defective mitosis of csi1∆ cells is due to the difficulty of declustered centromeres to be captured by spindle microtubules. Nevertheless, it is still possible that Csi1 regulates other aspects of mitosis in addition to centromere clustering. As an integral component of the SPB, Csi1 may directly function in regulating microtubule dynamics. The dissociation of centromeres from the SPB in csi1∆ cells may also result in structural changes at the kinetochore. Formally ruling out these possibilities requires artificially tethering centromeres during interphase without affecting their dissociation during mitosis, which is technically very challenging. However, the correlation of the severity of centromere declustering and chromosome segregation defects in mto1∆, nsk1∆ and csi1∆ cells argues that centromere clustering directly contributes to chromosome segregation.27,28,42 Moreover, adding an extra mini-chromosome to csi1∆ cells results in even longer average times to finish mitosis with larger deviations.42 Such a result argues that the capture of declustered centromeres becomes much more stochastic as chromosome number increases and support the idea that centromere clustering directly contributes to the search-and-capture process.
In sum, our data support a model in which three-dimensional organization of centromeres in fission yeast facilitates the capture of centromeres by microtubules at the onset of mitosis. In mammalian cells, Rabl configuration is present only in specific cell lineages or developmental stages.16 However, centromeres are transiently arranged in a ring-like structure during mitosis and meiosis, allowing them to be exposed to high concentrations of microtubules for their efficient capture,50,51 suggesting a common theme of using three dimensional chromosome organization to overcome a bottleneck of chromosome segregation.
Perspectives
The identification of Sad1 and Csi1 as critical components of the nuclear envelope that mediate interphase centromere clustering in fission yeast provides mechanistic and functional insights into Rabl configuration. Interestingly, the association of centromeres with the SPB is a highly dynamic process. At the onset of mitosis, centromeres are released from the nuclear membrane to allow kinetochore attachment by nuclear microtubules.15 At the end of mitosis, Csi1 replaces Nsk1 at the kinetochore-SPB interface. At the beginning of meiosis, centromeres dissociate from the SPB, and telomeres are clustered at the SPB to drive chromosome movements.40 We speculate that Csi1 might play an essential role in relaying cellular signals to regulate the interaction between kinetochores and the SPB during mitosis and meiosis.
Besides its importance in chromosome segregation, Rabl configuration may affect genome stability in other ways. Chromosome conformation capture analyses of genome-wide chromosomal contacts in budding yeast have led to a three-dimensional model of chromatin organization, in which centromere clustering likely imposes key constraints for genomic interactions.18 The ability of csi1∆ to disrupt such organization provides an important tool to study the role of Rabl configuration in regulating three-dimensional genome DNA organization, transcription and recombination in fission yeast.
Acknowledgments
We thank Fred Chang for critical reading of the manuscript. Work in the Jia lab was supported by NIH R01-GM085145. S.P.K. was supported by NIH training grant T32-GM008798.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/nucleus/article/24876
References
- 1.Cremer T, Cremer M. Chromosome territories. Cold Spring Harb Perspect Biol. 2010;2:a003889. doi: 10.1101/cshperspect.a003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajapakse I, Groudine M. On emerging nuclear order. J Cell Biol. 2011;192:711–21. doi: 10.1083/jcb.201010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dion V, Gasser SM. Chromatin movement in the maintenance of genome stability. Cell. 2013;152:1355–64. doi: 10.1016/j.cell.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Misteli T. Beyond the sequence: cellular organization of genome function. Cell. 2007;128:787–800. doi: 10.1016/j.cell.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 5.Mekhail K, Moazed D. The nuclear envelope in genome organization, expression and stability. Nat Rev Mol Cell Biol. 2010;11:317–28. doi: 10.1038/nrm2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meister P, Mango SE, Gasser SM. Locking the genome: nuclear organization and cell fate. Curr Opin Genet Dev. 2011;21:167–74. doi: 10.1016/j.gde.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, McCord RP, Ho YJ, Lajoie BR, Hildebrand DG, Simon AC, et al. Spatial organization of the mouse genome and its role in recurrent chromosomal translocations. Cell. 2012;148:908–21. doi: 10.1016/j.cell.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheeseman IM, Desai A. Molecular architecture of the kinetochore-microtubule interface. Nat Rev Mol Cell Biol. 2008;9:33–46. doi: 10.1038/nrm2310. [DOI] [PubMed] [Google Scholar]
- 9.Verdaasdonk JS, Bloom K. Centromeres: unique chromatin structures that drive chromosome segregation. Nat Rev Mol Cell Biol. 2011;12:320–32. doi: 10.1038/nrm3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain D, Cooper JP. Telomeric strategies: means to an end. Annu Rev Genet. 2010;44:243–69. doi: 10.1146/annurev-genet-102108-134841. [DOI] [PubMed] [Google Scholar]
- 11.Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–34. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- 12.Cowan CR, Carlton PM, Cande WZ. The polar arrangement of telomeres in interphase and meiosis. Rabl organization and the bouquet. Plant Physiol. 2001;125:532–8. doi: 10.1104/pp.125.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zimmer C, Fabre E. Principles of chromosomal organization: lessons from yeast. J Cell Biol. 2011;192:723–33. doi: 10.1083/jcb.201010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang Y, Spector DL. Centromere positioning and dynamics in living Arabidopsis plants. Mol Biol Cell. 2005;16:5710–8. doi: 10.1091/mbc.E05-08-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Funabiki H, Hagan I, Uzawa S, Yanagida M. Cell cycle-dependent specific positioning and clustering of centromeres and telomeres in fission yeast. J Cell Biol. 1993;121:961–76. doi: 10.1083/jcb.121.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin Q, Trelles-Sticken E, Scherthan H, Loidl J. Yeast nuclei display prominent centromere clustering that is reduced in nondividing cells and in meiotic prophase. J Cell Biol. 1998;141:21–9. doi: 10.1083/jcb.141.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sexton T, Yaffe E, Kenigsberg E, Bantignies F, Leblanc B, Hoichman M, et al. Three-dimensional folding and functional organization principles of the Drosophila genome. Cell. 2012;148:458–72. doi: 10.1016/j.cell.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Duan Z, Andronescu M, Schutz K, McIlwain S, Kim YJ, Lee C, et al. A three-dimensional model of the yeast genome. Nature. 2010;465:363–7. doi: 10.1038/nature08973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin QW, Fuchs J, Loidl J. Centromere clustering is a major determinant of yeast interphase nuclear organization. J Cell Sci. 2000;113:1903–12. doi: 10.1242/jcs.113.11.1903. [DOI] [PubMed] [Google Scholar]
- 20.Ding R, West RR, Morphew DM, Oakley BR, McIntosh JR. The spindle pole body of Schizosaccharomyces pombe enters and leaves the nuclear envelope as the cell cycle proceeds. Mol Biol Cell. 1997;8:1461–79. doi: 10.1091/mbc.8.8.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Appelgren H, Kniola B, Ekwall K. Distinct centromere domain structures with separate functions demonstrated in live fission yeast cells. J Cell Sci. 2003;116:4035–42. doi: 10.1242/jcs.00707. [DOI] [PubMed] [Google Scholar]
- 22.Castagnetti S, Oliferenko S, Nurse P. Fission yeast cells undergo nuclear division in the absence of spindle microtubules. PLoS Biol. 2010;8:e1000512. doi: 10.1371/journal.pbio.1000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saitoh S, Takahashi K, Yanagida M. Mis6, a fission yeast inner centromere protein, acts during G1/S and forms specialized chromatin required for equal segregation. Cell. 1997;90:131–43. doi: 10.1016/S0092-8674(00)80320-7. [DOI] [PubMed] [Google Scholar]
- 24.Asakawa H, Hayashi A, Haraguchi T, Hiraoka Y. Dissociation of the Nuf2-Ndc80 complex releases centromeres from the spindle-pole body during meiotic prophase in fission yeast. Mol Biol Cell. 2005;16:2325–38. doi: 10.1091/mbc.E04-11-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King MC, Drivas TG, Blobel G. A network of nuclear envelope membrane proteins linking centromeres to microtubules. Cell. 2008;134:427–38. doi: 10.1016/j.cell.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hiraoka Y, Maekawa H, Asakawa H, Chikashige Y, Kojidani T, Osakada H, et al. Inner nuclear membrane protein Ima1 is dispensable for intranuclear positioning of centromeres. Genes Cells. 2011;16:1000–11. doi: 10.1111/j.1365-2443.2011.01544.x. [DOI] [PubMed] [Google Scholar]
- 27.Franco A, Meadows JC, Millar JB. The Dam1/DASH complex is required for the retrieval of unclustered kinetochores in fission yeast. J Cell Sci. 2007;120:3345–51. doi: 10.1242/jcs.013698. [DOI] [PubMed] [Google Scholar]
- 28.Buttrick GJ, Meadows JC, Lancaster TC, Vanoosthuyse V, Shepperd LA, Hoe KL, et al. Nsk1 ensures accurate chromosome segregation by promoting association of kinetochores to spindle poles during anaphase B. Mol Biol Cell. 2011;22:4486–502. doi: 10.1091/mbc.E11-07-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukuda M, Asano S, Nakamura T, Adachi M, Yoshida M, Yanagida M, et al. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature. 1997;390:308–11. doi: 10.1038/36894. [DOI] [PubMed] [Google Scholar]
- 30.Sawin KE, Lourenco PC, Snaith HA. Microtubule nucleation at non-spindle pole body microtubule-organizing centers requires fission yeast centrosomin-related protein mod20p. Curr Biol. 2004;14:763–75. doi: 10.1016/j.cub.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 31.Venkatram S, Tasto JJ, Feoktistova A, Jennings JL, Link AJ, Gould KL. Identification and characterization of two novel proteins affecting fission yeast gamma-tubulin complex function. Mol Biol Cell. 2004;15:2287–301. doi: 10.1091/mbc.E03-10-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zimmerman S, Chang F. Effects of gamma-tubulin complex proteins on microtubule nucleation and catastrophe in fission yeast. Mol Biol Cell. 2005;16:2719–33. doi: 10.1091/mbc.E04-08-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hagan IM. The fission yeast microtubule cytoskeleton. J Cell Sci. 1998;111:1603–12. doi: 10.1242/jcs.111.12.1603. [DOI] [PubMed] [Google Scholar]
- 34.Chen JS, Lu LX, Ohi MD, Creamer KM, English C, Partridge JF, et al. Cdk1 phosphorylation of the kinetochore protein Nsk1 prevents error-prone chromosome segregation. J Cell Biol. 2011;195:583–93. doi: 10.1083/jcb.201105074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gundersen GG, Worman HJ. Nuclear positioning. Cell. 2013;152:1376–89. doi: 10.1016/j.cell.2013.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Starr DA, Fridolfsson HN. Interactions between nuclei and the cytoskeleton are mediated by SUN-KASH nuclear-envelope bridges. Annu Rev Cell Dev Biol. 2010;26:421–44. doi: 10.1146/annurev-cellbio-100109-104037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hagan I, Yanagida M. The product of the spindle formation gene sad1+ associates with the fission yeast spindle pole body and is essential for viability. J Cell Biol. 1995;129:1033–47. doi: 10.1083/jcb.129.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miki F, Kurabayashi A, Tange Y, Okazaki K, Shimanuki M, Niwa O. Two-hybrid search for proteins that interact with Sad1 and Kms1, two membrane-bound components of the spindle pole body in fission yeast. Mol Genet Genomics. 2004;270:449–61. doi: 10.1007/s00438-003-0938-8. [DOI] [PubMed] [Google Scholar]
- 39.Shimanuki M, Miki F, Ding DQ, Chikashige Y, Hiraoka Y, Horio T, et al. A novel fission yeast gene, kms1+, is required for the formation of meiotic prophase-specific nuclear architecture. Mol Gen Genet. 1997;254:238–49. doi: 10.1007/s004380050412. [DOI] [PubMed] [Google Scholar]
- 40.Chikashige Y, Ding DQ, Funabiki H, Haraguchi T, Mashiko S, Yanagida M, et al. Telomere-led premeiotic chromosome movement in fission yeast. Science. 1994;264:270–3. doi: 10.1126/science.8146661. [DOI] [PubMed] [Google Scholar]
- 41.Chikashige Y, Tsutsumi C, Yamane M, Okamasa K, Haraguchi T, Hiraoka Y. Meiotic proteins bqt1 and bqt2 tether telomeres to form the bouquet arrangement of chromosomes. Cell. 2006;125:59–69. doi: 10.1016/j.cell.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 42.Hou H, Zhou Z, Wang Y, Wang J, Kallgren SP, Kurchuk T, et al. Csi1 links centromeres to the nuclear envelope for centromere clustering. J Cell Biol. 2012;199:735–44. doi: 10.1083/jcb.201208001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hagan I, Yanagida M. The product of the spindle formation gene sad1+ associates with the fission yeast spindle pole body and is essential for viability. J Cell Biol. 1995;129:1033–47. doi: 10.1083/jcb.129.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim DU, Hayles J, Kim D, Wood V, Park HO, Won M, et al. Analysis of a genome-wide set of gene deletions in the fission yeast Schizosaccharomyces pombe. Nat Biotechnol. 2010;28:617–23. doi: 10.1038/nbt.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burgess SM, Kleckner N. Collisions between yeast chromosomal loci in vivo are governed by three layers of organization. Genes Dev. 1999;13:1871–83. doi: 10.1101/gad.13.14.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wollman R, Cytrynbaum EN, Jones JT, Meyer T, Scholey JM, Mogilner A. Efficient chromosome capture requires a bias in the ‘search-and-capture’ process during mitotic-spindle assembly. Curr Biol. 2005;15:828–32. doi: 10.1016/j.cub.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 47.Grishchuk EL, Spiridonov IS, McIntosh JR. Mitotic chromosome biorientation in fission yeast is enhanced by dynein and a minus-end-directed, kinesin-like protein. Mol Biol Cell. 2007;18:2216–25. doi: 10.1091/mbc.E06-11-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yao J, He X. Kinetochore assembly: building a molecular machine that drives chromosome movement. Mol Biosyst. 2008;4:987–92. doi: 10.1039/b719627j. [DOI] [PubMed] [Google Scholar]
- 49.Buttrick GJ, Millar JB. Ringing the changes: emerging roles for DASH at the kinetochore-microtubule Interface. Chromosome Res. 2011;19:393–407. doi: 10.1007/s10577-011-9185-8. [DOI] [PubMed] [Google Scholar]
- 50.Kitajima TS, Ohsugi M, Ellenberg J. Complete kinetochore tracking reveals error-prone homologous chromosome biorientation in mammalian oocytes. Cell. 2011;146:568–81. doi: 10.1016/j.cell.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 51.Magidson V, O’Connell CB, Lončarek J, Paul R, Mogilner A, Khodjakov A. The spatial arrangement of chromosomes during prometaphase facilitates spindle assembly. Cell. 2011;146:555–67. doi: 10.1016/j.cell.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang D, Vjestica A, Oliferenko S. The cortical ER network limits the permissive zone for actomyosin ring assembly. Curr Biol. 2010;20:1029–34. doi: 10.1016/j.cub.2010.04.017. [DOI] [PubMed] [Google Scholar]