Abstract
Dieting and the increased availability of highly palatable food are considered major contributing factors to the large incidence of eating disorders and obesity. This study was aimed at investigating the role of the cannabinoid (CB) system in a novel animal model of compulsive eating, based on a rapid palatable diet cycling protocol. Male Wistar rats were fed either continuously a regular chow diet (Chow/Chow, control group) or intermittently a regular chow diet for 2 days and a palatable, high-sucrose diet for 1 day (Chow/Palatable). Chow/Palatable rats showed spontaneous and progressively increasing hypophagia and body weight loss when fed the regular chow diet, and excessive food intake and body weight gain when fed the palatable diet. Diet cycled rats dramatically escalated the intake of the palatable diet during the first hour of renewed access (7.5 fold compared to controls), and after withdrawal they showed compulsive eating and heightened risk-taking behavior. The inverse agonist of the CB1 receptor, SR141716 reduced the excessive intake of palatable food with higher potency and the body weight with greater efficacy in Chow/Palatable rats, compared to controls. Moreover, SR141716 reduced compulsive eating and risk-taking behavior in Chow/Palatable rats. Finally, consistent with the behavioral and pharmacological observations, withdrawal from the palatable diet decreased the gene expression of the enzyme fatty acid amide hydrolase in the ventromedial hypothalamus while increasing that of CB1 receptors in the dorsal striatum in Chow/Palatable rats, compared to controls. These findings will help understand the role of the CB system in compulsive eating.
Keywords: compulsive eating, food intake, palatability, rimonabant, risk-taking behavior, SR141716
Introduction
The subjective evaluation of foods is an important determinant of their acceptability and an important evolutionary mechanism promoting the consumption of energy-dense foods in scarce environments (Birch, 1999). However, given the increased availability of calorie-dense, highly palatable foods in Western societies, this motivational mechanism is now largely contributing to the obesity epidemic and eating disorders (Hill et al, 2003; Mathes et al, 2009). Interestingly, as a compensatory cultural mechanism, many individuals attempt to control body weight by limiting themselves to perceived “safe” foods, which are generally less palatable than the “forbidden” ones (high-sugar or high-fat), from which they abstain. Dieting in turn increases craving for palatable food, promotes overeating and results in repeated, discrete alternations in food palatability across time (Polivy and Herman, 1985). This maladaptive pattern of food intake has raised the question whether obesity and eating disorders, like drug addiction, can be conceptualized as chronic relapsing conditions with alternating periods of abstinence (e.g., dieting) and relapse (compulsive overeating of palatable foods) (APA, 2000; Corwin and Grigson, 2009; Cottone et al, 2012).
In preclinical research, a variety of animal models have been designed to mimic the aberrant feeding pattern observed in humans as well as to study the underlying neurobiological mechanisms (Cifani et al, 2009; Corwin, Avena and Boggiano, 2011; Cottone et al, 2009a; Cottone et al, 2008b; Parylak et al, 2012). A successful procedure to induce excessive food intake in rodents is to provide limited access to highly palatable foods without the use of food restriction or food deprivation (Corwin and Buda-Levin, 2004). Highly rewarding foods, rich in fats and sugars, are offered ad libitum to experimental subjects intermittently within limited (brief: 1–2 hours (Corwin, 2004; Cottone, Wang, Park et al, 2012) or extended: 2 days (Cottone et al, 2009b)) periods of time, followed by a variable period of exposure (23 hours to 5 days) to a regular chow diet, which is typically undereaten by animals. Palatable diet withdrawal is critically important to induce overconsumption upon renewing its access, according to a classical deprivation effect (George et al, 2007; Maier et al, 2010; McBride and Li, 1998). Ultimately, cycles of palatable food access/withdrawal are essential to induce escalation and maintenance of excessive intake.
The first aim of this study was to characterize the behavioral effects of a novel experimental procedure of intermittent, but extended access to palatable food, which consisted of two-day access to a regular chow diet followed by one day of access to a highly palatable, sugary diet. Given the short cumulative length of regular chow and palatable accesses (3 days), this procedure would have the advantage of consisting of faster experimental cycling compared to other existing intermittent, extended access protocols (Cottone, Sabino, Roberto et al, 2009a; Cottone et al, 2008a). Since the cannabinoid (CB) system modulates feeding (DiPatrizio and Piomelli, 2012; Kunos, 2007; Matias, Bisogno and Di Marzo, 2006; Mechoulam et al, 2006) and is engaged by palatable diets (Bello et al, 2012; DiPatrizio and Simansky, 2008; Timofeeva et al, 2009), the second aim of our study was to investigate whether the consummatory and motivational outcomes of intermittent, extended access to highly palatable food were cannabinoid type-1 (CB1) receptor-dependent. For this purpose, in this animal model, we tested the effects of the CB1 receptor inverse agonist SR141716 on i) escalated excessive intake of palatable diet and ii) time spent and the amount of food eaten (modeling risk-taking behavior and compulsive eating, respectively) in an aversive, open compartment, where the palatable diet was offered, using a light/dark conflict test. Finally, the third aim of this study was to determine the effects of withdrawal from the intermittent, extended access to palatable diet on the gene expression of components of the endocannabinoid machinery, including the type-1 cannabinoid (CB1) receptor, and the enzymes involved in the synthesis and degradation of the two main endocannabinoids, anandamide (AEA) and 2-arachidonoylglycerol (2-AG) (N-acyl phosphatidylethanolamine phospholipase D (NAPE-PLD), diacylglycerol lipase α and β (DAGLα), fatty acid amide hydrolase (FAAH), and monoacylglycerol lipase (MAGL)) in brain areas involved in food intake and motivation.
Materials and Methods
Subjects
Male Wistar rats, weighing 180–230 g and 41–47 days old at arrival (Charles River, Wilmington, MA), were single housed in wire-topped, plastic cages (27 × 48 × 20 cm) on a 12 h reverse light cycle (lights off at 11:00 am), in an AAALAC-approved humidity- (60%) and temperature-controlled (22°C) vivarium. Rats had access to corn-based chow (Harlan Teklad LM-485 Diet 7012 (65% [kcal] carbohydrate, 13% fat, 21% protein, metabolizable energy 310 cal/100 g; Harlan, Indianapolis, IN) and water ad libitum at all times unless otherwise specified. Procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication number 85–23, revised 1996) and the Principles of Laboratory Animal Care and were approved by Boston University Medical Campus Institutional Animal Care and Use Committee (IACUC).
Drugs
The CB1 receptor inverse agonist SR141716 (rimonabant or 5-(4-Chlorophenyl)-1-(2,4-dichloro-phenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide) HCl was synthesized as reported previously (Kumar et al, 2008). SR141716 HCl was solubilized in an 18:1:1 mixture of saline:ethanol:cremophor and was administered intraperitoneally (0, 0.3, 1, 3 mg/kg, 1 ml/kg, −30 min pretreatment).
Development of a shortened, ad libitum palatable diet alternation
Figure 1 shows a schematic representation of the intermittent, extended access to a palatable diet procedure, which was modified from a previously described experimental protocol (Cottone, Sabino, Roberto et al, 2009a; Cottone, Sabino, Steardo et al, 2008a, 2009b; Iemolo et al, 2012). Briefly, after 1 week of acclimation, rats (n=20) were divided in two groups matched for food intake, body weight, and feed efficiency from a previous 3–4 days baseline period. One group was then provided a chow diet (“Chow”) ad libitum (Chow/Chow), whereas a second group was provided chow ad libitum for 2 days followed by 1 day of ad libitum access to a highly palatable, chocolate flavored, high-sucrose diet (“Palatable”; Chow/Palatable). The chow diet was the above described Harlan Teklad LM-485 Diet 7012. The palatable diet was a nutritionally complete, chocolate-flavored, high-sucrose (50% kcal), AIN-76A-based diet that is comparable in macronutrient proportions and energy density to the chow diet (chocolate-flavored Formula 5TUL: 66.7% [kcal] carbohydrate, 12.7% fat, 20.6% protein, metabolizable energy 344 cal/100 g; TestDiet, Richmond, IN; formulated as 45 mg precision food pellets to increase its preferredness). For brevity, the first 2 days (chow only) and the following 1 day (chow or palatable according to experimental group) are referred in all experiments as C and P phases. Diets were never concurrently available. Food intake and body weight were measured daily. Average food intake was calculated as the kcal intake in a certain phase divided by the number of days of that phase (2 for C phase, 1 for P phase). Average body weight change was calculated as the difference between the body weight at the end and at the beginning of a phase divided by the number of days of that phase (2 for C phase, 1 for P phase). Average and cumulative feed efficiency was calculated as mg body weight gained in a certain time interval (phase or cycle) divided by the kcal food intake in the same time interval. Cumulative food intake and cumulative body weight gain was calculated as the kcal food intake or body weight gained at the end of each cycle, since the beginning of the study. To evaluate the escalation of palatable diet intake, food intake was measured 1h after diet-switch at the beginning of each P phase. For the time course studies, food intake was measured at the beginning of either the C or the P phase (at the time of the diet switch), and then 1h, 3h, 6h, and 24h following the switch of the diet. As previously published (Cottone, Sabino, Steardo et al, 2008a, b, 2009b), the 5TUL Chocolate Diet (sugary Palatable diet) was uniformly preferred compared to the Harlan LM-485 chow diet.
Figure 1.
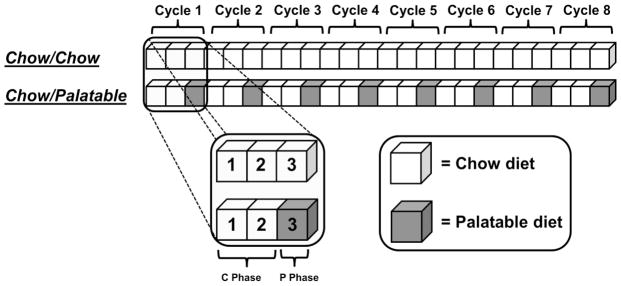
Schematic representation of the intermittent, extended access to a palatable diet procedure used in this study. Briefly, subjects were divided in two groups: a first group received a regula chow diet ad libitum daily (Chow/Chow), and a second group received two days of chow diet followed by a day of a highly palatable, chocolate flavored, high-sugary, diet (Chow/Palatable). For brevity, the first 2 days (chow only) and last day (chow or palatable per diet condition) of each cycle are referred to as C and P phases.
Effects of SR141716 on intake of palatable food and body weight
SR141716 (0, 0.3, 1, 3 mg/kg, i.p., −30 min) was administered at the onset of P phase in a within-subject Latin square design (n=11/group). Rats were then provided with a pre-weighed amount of food and intake was recorded 1h, 3h, 6h and 24h later. Body weights were recorded right before and 24h following drug administration.
Effects of SR141716 on risk-taking behavior and compulsive eating
The task was performed as previously described (Cottone, Wang, Park et al, 2012; Teegarden and Bale, 2007). Rats (n=44) were tested for 10 min in a light/dark rectangular box (50×100×35 cm) in which the aversive light compartment (50×70×35 cm) was illuminated by a 60 lux light. The dark side (50×30×35 cm) had an opaque cover and ~0 lux of light. A shallow, metal cup containing a pre-weighed amount of the same food received during the ad libitum diet alternation (Harlan Teklad LM-485 Diet 7012 for Chow/Chow rats or 45-mg chocolate pellets for Chow/Palatable rats) was positioned in the center of the light compartment. The two compartments were connected by an open doorway which allowed the subjects to move freely between the two. White noise was present. On the test day (beginning of P phase), following the 2 days (C phase) of withdrawal from palatable food, rats were pretreated with SR141716 (0 and 1 mg/kg, i.p.) 30-min before being placed into the light compartment, facing both the food cup and the doorway. The time spent in the open compartment and the amount of food eaten during the test were measured. The two dependent variables were then used to operationalize the constructs of ‘risk-taking behavior’ and ‘compulsive-like eating’, respectively. Because of rats’ innate fear for bright, aversive environments, the time spent exploring the light compartment of the light/dark box under normal, control conditions is minimal. An increased time spent in this compartment, as compared to control conditions, resulting from the presence of the highly palatable diet, was operationalized as ‘risk-taking behavior’ (Cottone, Wang, Park et al, 2012; Teegarden and Bale, 2007). Moreover, under normal, control conditions, eating behavior is typically suppressed when a rat faces adverse circumstances; a significant increase in food intake in spite of the adverse conditions, as compared to control conditions, was operationalized as a construct of ‘compulsive-like eating’ (Cottone, Wang, Park et al, 2012; Heyne et al, 2009; Johnson and Kenny, 2010). Water was not available during the 10-min test. At the end of the test, rats were removed from the light/dark box apparatus, returned to their home cages, and food was provided (chow or palatable diet, according to the experimental group).
Quantitative Real-Time PCR
The quantitative RT-PCR was performed as shown previously (Cottone, Wang, Park et al, 2012; Sabino et al, 2011). Chow/Chow and Chow/Palatable rats (n=7–12) were anesthetized and decapitated at the end of the C phase, before the putative renewed access to the highly palatable diet (same time point of pharmacological studies). Brains were quickly removed and coronally sliced in a brain matrix. Punches containing dorsal striatum (DS), nucleus accumbens (NAcc), lateral hypothalamus (LH) and ventromedial hypothalamus (VMH) were collected on an ice-cold stage. Total RNA was prepared from each brain punch using RNeasy mini kit (Qiagen, Valencia, CA) following the standard protocol for RNA extraction from animal tissues. Total RNA was prepared from each punch using the RNeasy lipid mini kit (Qiagen, Valencia, CA) as recommended for animal tissue. Total RNA was quantified by Nanodrop 1000 (Thermo Scientific, Wilmington, DE), and then reverse transcribed with QuantiTect Reverse Transcription Kit (Qiagen, Valencia, CA), which includes a DNA removal step. For quantitative real-time PCR, Roche Light Cycler 480 Master-plus SYBR Green mix (Roche Applied Science, Indianapolis, IN) was used. Reactions were carried out in a 10 μl volume using a 96-well plate Realplex2 machine (Eppendorf). The primers (0.5 μM final concentration) for CB 1 receptor, NAPE-PLD, DAGLα, FAAH, MAGL and the housekeeping gene cyclophilin A (CypA) were synthesized by SigmaAldrich (St. Louis, MO) with a standard desalting purification method. Fragments were amplified using a three-step RT-PCR protocol that included an initial 94 °C for 10min step to activate Taq polymerase, followed by 40 cycles of amplification. The sequences of the primers and the conditions are summarized in Table 1. Standard curves were constructed using purified and sequenced fragments. The results were analyzed by the second derivative methods were expressed in arbitrary units normalized by the expression levels of the reference gene, CypA. The standard curve and the samples were analyzed in duplicate. Gene-specific amplification was determined by melting curve analysis as one peak at the expected melting temperature and by agarose gel electrophoresis.
Table 1. qPCR Primer Sequences and Conditions of Reaction.
Primers and conditions used in qPCR for the detection of mRNA levels of cannabinoid receptor type 1, N-acyl-phosphatidylethanolamine phospholipase D, diacylglycerol lipase α, fatty acid amide hydrolase, monoacylglycerol lipase, and the housekeeping gene cyclophilin A in punches obtained from the brains of Chow/Chow and Chow/Palatable rats.
| Target gene | RGD symbol | Primers | Temperatures (°C) | Times (s) |
|---|---|---|---|---|
| Cyclophilin A (CypA) | Ppia | Primer A: TAT CTG CAC TGC CAA GAC TGA GTG Primer B: CTT CTT GCT GGT CTT GCC ATT CC |
95-58-72 | 20-15-10 |
| Cannabinoid receptor type 1 (CB1) | Cnr1 | Primer A: TGG GCA CCT TCA CGG TTC TG Primer B: GGA AGG CCT GCA TCG GAG ACT |
95-60-72 | 15-15-20 |
| Fatty acid amide hydrolase (FAAH) | Faah | Primer A: CAC TGT GAC CGC CGA GGA CGA TG Primer B: CAG CTG TTC CAC CTC CCG CAT GAA |
95-60-72 | 15-15-20 |
| Monoacylglycerol lipase (MAGL) | Mgll | Primer A: ACC TGG TCA ATG CGG ATG GAC Primer B: GTC ATA ACG GCC ACA GTG TTC CC |
95-58.7-72 | 15-10-10 |
| Diacylglycerol lipase α (DAGLα) | Dagla | Primer A: GCT CTT TGC CCT CGC TGC CTA TGG Primer B: GGG CGT GCA GGG CAG AGA CAA CAC |
95-60-72 | 15-15-20 |
| N-acyl-phosphatidylethanolamine phosphodiesterase (NAPE-PLD) | Napepld | Primer A: TGC TAG GGC CTT GGA ATC GGT TCT T Primer B: TCA GTT TCA CCG GCG GCT CTA AGT A |
95-58.1-72 | 15-10-15 |
Statistical analysis
Average daily food intake, average daily body weight change and average daily feed efficiency were analyzed using three-way mixed ANOVAs with Diet Schedule as a between-subjects factor, and Cycle and Phase as within-subject factors. First hour of palatable food consumption, cumulative food intake, cumulative body weight change and cumulative feed efficiency were analyzed using two-way ANOVAs with Diet Schedule as a between-subjects factor and Phase as a within-subject factor. Time course of food intake in C and P phase was analyzed using two-way ANOVAs with Diet Schedule as a between-subjects factor and Time as a within-subject factor. To analyze the time course of ingestion following SR141716 treatment, three-way ANOVAs on incremental food intake were performed, with Diet Schedule as a between-subjects factor and Treatment and Time as within-subject factors. The effects of SR141716 on the time spent in the open compartment and the amount of food eaten were analyzed using two-way ANOVAs, with Diet Schedule and Treatment as between-subjects factors. The effects of diet alternation on gene expression were analyzed using Student’s t-tests. Pair-wise effects were interpreted using Fisher LSD’s tests. The software/graphic packages used were Systat 12.0, SigmaPlot 11.0 (Systat Software Inc., Chicago, IL), InStat 3.0 (GraphPad, San Diego, CA) and Statistica 7.0 (Statsoft, Inc., Tulsa, OK).
Results
Effects of diet alternation on food intake, body weight and feed efficiency
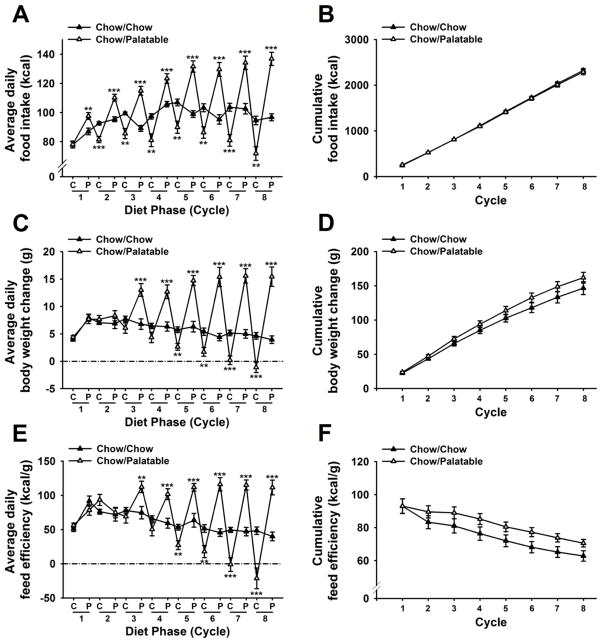
Figure 2A shows that alternating access to the chocolate-flavored, sugary, palatable diet progressively altered daily food intake of male rats in a diet-specific manner [Cycle × Diet Phase × Diet Schedule: F(7,126)=30.72, p<0.001]. Chow/Palatable rats were already overeating compared to control rats during the very first access to the palatable diet [t(18)= −3.22, p<0.01; Figure 1A, P phase of cycle 1], and upon returning to the regular chow diet, cycled rats showed a significant hypophagia during the following day compared to Chow/Chow rats [M±SEM: 94.1±1.4 kcal vs. 72.9±3.0 kcal; t(18)=6.43 p<0.001; Chow/Chow and Chow/Palatable rats, respectively, not shown] which lasted up to the completion of the 2 days of the C phase [t(18)=4.62, p<0.001; Figure 1A, C phase of cycle 2]. Therefore, Chow/Palatable rats spontaneously cycled their food intake, as function of the diet which was provided, and the magnitude of both chow diet hypophagia and palatable diet overeating progressively increased with further diet switches. The magnitude of the chow diet hypophagia in Chow/Palatable rats [M±SEM: 1398.1 kcal, −15.3% vs. Chow/Chow rats, sum of intakes during C phases across 8 cycles] was similar to the magnitude of the palatable diet overeating [M±SEM: 978.3 kcal, +19.2% vs. Chow/Chow rats, sum of intakes during P phases across 8 cycles], which resulted in a non-statistically significant difference in cumulative energy intake between the two groups, across the 8 cycles [Diet Schedule: F(1,18)=0.10, n.s.; Cycle × Diet Schedule: F(7,126)=0.37, n.s.; Figure 2B].
Figure 2.
Effects of intermittent, extended access to a palatable diet on (A) average daily food intake, (B) cumulative food intake, (C) average daily body weight change, (D) cumulative body weight change, (E) average daily feed efficiency, and (F) cumulative feed efficiency in male Wistar rats (n=20). Values of C phase represent the average of 2 days access to chow food. Data represent mean (±SEM). Symbols denote significant difference from Chow/Chow group **p<0.01, ***p<0.001.
Chow/Palatable rats spontaneously cycled not only their food intake but also their body weight and feed efficiency [average daily body weight change: Cycle × Diet Phase × Diet Schedule: F(7,126)=22.54, p<0.001; average daily feed efficiency: Cycle × Diet Phase × Diet Schedule: F(7,126)=18.58, p<0.001; Figures 1C, and 1E]. Beginning from cycle 3, Chow/Palatable rats disproportionally gained more body weight than Chow/Chow rats during the P phases, resulting in a significantly increased local feed efficiency. The magnitude of these effects progressively amplified, cycle-by-cycle. Similarly, beginning from cycle 5, Chow/Palatable rats disproportionally gained less body weight than Chow/Chow rats during C phases, resulting in a decreased feed efficiency. These effects progressively intensified to the point that Chow/Palatable rats started losing absolute body weight during the C phase of cycle 8.
Although cumulative body weight gain did not differ between groups [Diet Schedule: F(1,18)=1.89, n.s.; Cycle × Diet Schedule: F(7,126)=1.55, n.s.; Figure 2E], Chow/Palatable rats showed an increased feed efficiency, compared to Chow/Chow rats [Diet Schedule: F(1,18)=2.36, n.s.; Cycle × Diet Schedule: F(7,126)=2.19, p<0.05; Figure 2F]. However, post hoc comparisons did not reveal any statistically significant effect at any of the time points analyzed.
Effects of diet alternation on escalation of palatable food consumption
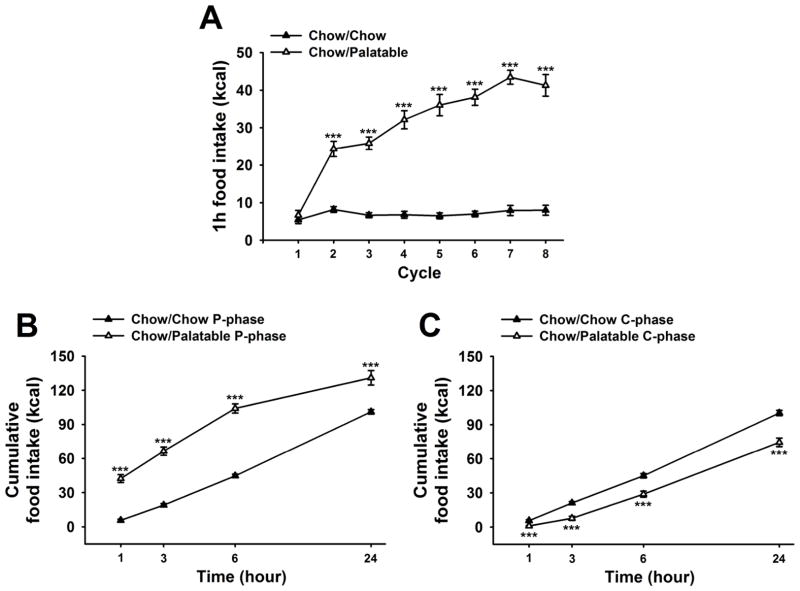
Figure 3A shows the effects of diet alternation on food intake during the first hour of access to the palatable diet. Chow/Palatable rats progressively and dramatically escalated the intake of the palatable diet during the first hour of access, as a function of the number of cycles [Cycle × Diet Schedule: F(7,126)=30.72, p<0.001]. The intake of diet-cycled rats became significantly higher than the intake of chow in control rats starting from the second palatable diet access; by the seventh access Chow/Palatable rats were able to consume approximately 5.5-fold the intake of Chow/Chow rats [M±SEM; 43.4±1.8 kcal. vs. 7.9±1.3 kcal., respectively; t(18)= −15.52, p<0.001].
Figure 3.
Effects of intermittent, extended access to a palatable diet on (A) food intake during the first hour of the P phase (when Chow/Palatable rats are fed the sugary, palatable diet), (B) time course food intake during P phase, and (C) time course food intake during C phase in male Wistar rats (n=20). Food intake was measured 1, 3, 6, and 24h. Data represent mean (±SEM). Symbols denote significant difference from Chow/Chow group ***p<0.001.
Effects of diet alternation on time course of food intake during P and C phases
As shown in Figure 3B, diet-cycled rat overeating during P phase was greatest towards the first hours following the switch to the palatable diet. Indeed, the degree to which Chow/Palatable rats overate compared to control Chow/Chow rats peaked at the first hour of access, and decreased during the remaining 23 hours [Diet Schedule: F(1,54)=66.6, p<0.001; Time × Diet Schedule: F(3,54)=23.02, p<0.001]. Their cumulative intake was 745.2±60.1% that of controls after 1h, 349.0±19.5% at 3h, 233.0±9.2% at 6h and 129.6±6.2% at 24h (M±SEM).
Analogously, the degree to which Chow/Palatable rats underate compared to control Chow/Chow rats bottomed at the first hour of access, and increased during the remaining 23 hours [cumulative intake was 18.2±7.4% that of controls after 1h, 36.4±8.7% at 3h, 64.0±5.9% at 6h and 74.3±3.8% at 24h (M±SEM), Figure 3C]. However, while a significant effect of the Diet Schedule was detected [F(1,54)=33.6, p<0.001], the Time × Diet Schedule interaction did not reach statistical significance [F(3,54)=1.46, n.s.].
Effects of SR141716 on food intake and body weight
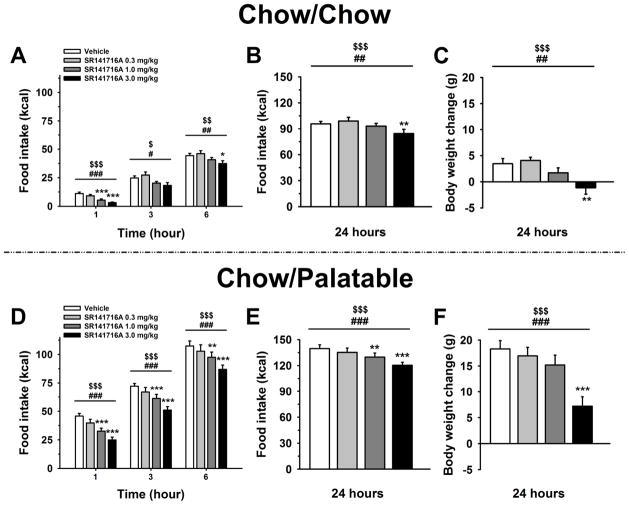
Pretreatment with the CB1 receptor inverse agonist SR141716 significantly decreased food intake [Dose: F(3,60)=16.91, p<0.001], but, as revealed by the significant Diet Schedule × Dose interaction, the anorectic effect was dissimilar between Chow/Chow and Chow/Palatable rats [F(3,60)=3.35, p<0.05]. As shown in Figure 4, although SR141716 maintained the same efficacy in both groups at the highest dose injected (3 mg/kg), the drug treatment more potently reduced the intake of the highly palatable diet in Chow/Palatable rats, than it reduced the consumption of the chow in control Chow/Chow rats. Indeed, pairwise comparisons showed that SR141716, at the dose of 1 mg/kg in Chow/Palatable rats, consistently decreased the intake of the palatable diet during the entire 24h observation period, compared to the vehicle condition, whereas the same dose only reduced intake in Chow/Chow rats during the first hour (Figures 4B, and 4F). SR141716 treatment also reduced body weight gain [Dose: F(3,60)=25.79, p<0.001], but again dissimilarly between Chow/Chow and Chow/Palatable rats [Diet Schedule × Dose: F(3,60)=3.91, p<0.05; Figures 4C, and 4F)]. Indeed, although drug treatment maintained the same potency in decreasing body weight gain in both the groups (lowest effective dose: 3 mg/kg), SR141716 reduced body weight more efficaciously in Chow/Palatable rats compared to control Chow/Chow rats. Indeed, the body weight loss induced by the 3 mg/kg dose was ~2.5-fold bigger in Chow/Palatable rats than in control Chow/Chow rats [M±SEM: 4.6±1.6 g vs. 11.1±1.3 g; t(20)=3.14 p<0.01; as difference from the respective vehicle condition in Chow/Chow and Chow/Palatable rats, respectively].
Figure 4.
Effects of pretreatment (−30 min) with the inverse agonist of the type-1 cannabinoid (CB1) receptor SR141716 (0, 0.3, 1, and 3 mg/kg, intraperitoneal) on 1, 3, 6 and 24h food intake and body weight change of Chow/Chow (A, B and C) and Chow/Palatable (D, E and F) male Wistar rats (n=22). Drug treatment was performed at the beginning of the P phase. Data represent mean (±SEM). Symbols denote significant difference from vehicle-treated rats *p<0.05, **p<0.01, ***p<0.001, linear trend dose effect $p<0.05, $$p<0.01, $$$p<0.001, main dose effect #p<0.05, ##p<0.01, ###p<0.001.
Effects of SR141716 on risk-taking behavior and compulsive eating
As shown in Figure 5A, the SR141716 effect on the time spent in the light, aversive compartment was selective for Chow/Palatable rats as drug treatment did not influence this measure in control Chow/Chow rats [Diet Schedule × Drug Treatment: F(1,40)=5.99, p<0.02]. As revealed by post hoc analysis, vehicle-treated Chow/Palatable rats spent more time in the aversive light compartment, where the palatable diet was positioned, compared to vehicle-treated Chow/Chow rats [p<0.02]. However, when Chow/Palatable rats were pretreated with 1 mg/kg of SR141716 (the lowest effective dose in the food intake experiment), the time spent there did not significantly differ from vehicle-treated Chow/Chow rats, and tended to differ from vehicle-treated Chow/Palatable rats [p=0.07].
Figure 5.
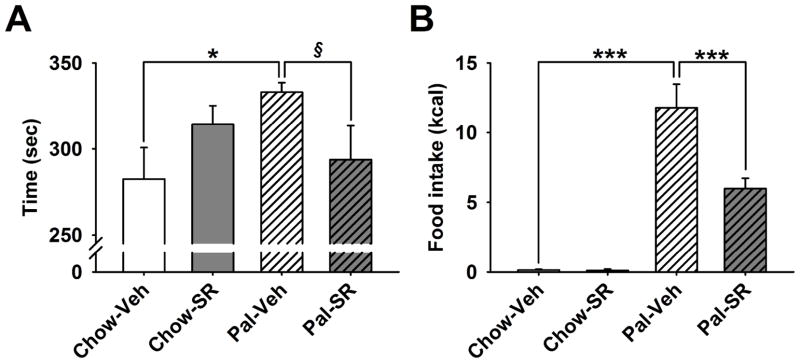
Effects of pretreatment (−30 min) with the inverse agonist of the type-1 cannabinoid (CB1) receptor SR141716 (0, 0.3, 1, and 3 mg/kg, intraperitoneal) on risk-taking behavior and compulsive eating in male Wistar rats (n=44). (A) Time spent in the open, aversive compartment; (B) food intake. Data represent mean (±SEM). Symbols denote significant difference from Chow/Palatable vehicle treated group *p<0.05, ***p<0.001. § denotes a trend (p=0.07).
Moreover, during the 10 minutes test, Chow/Palatable rats compulsively consumed ~70-fold the food consumed by control Chow/Chow rats, in spite of the aversive environmental conditions. This effect was potently reduced by SR141716 pretreatment [−49%, p<0.001; Diet Schedule × Drug Treatment: F(1,40)=9.37, p<0.01; Figure 5B]. SR141716 did not affect food consumption in Chow/Chow rats.
Quantitative Real-Time PCR
Chow/Palatable rats showed an increased gene expression of CB1 receptors in the DS, compared to Chow/Chow rats [t(11)=2.2, p<0.05; Figure 6A]. No differential effect in the mRNA expression of CB1 between groups was observed in the NAcc, LH and VMH [ts(12–13)<1.36, n.s.].
Figure 6.
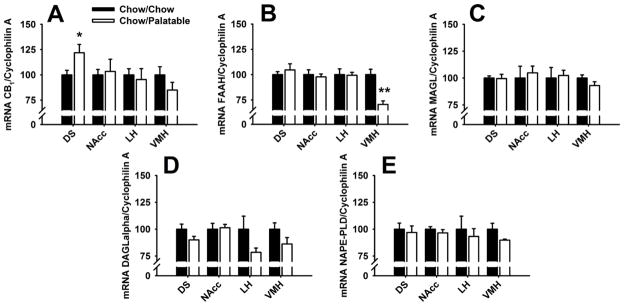
(A) cannabinoid receptor type 1 (CB1), (B) fatty acid amide hydrolase (FAAH), (C) monoacylglycerol lipase (MAGL), (D) diacylglycerol lipase α (DAGLα), and (E) N-acyl-phosphatidylethanolamine phospholipase D (NAPE-PLD) mRNA expression in the Dorsal Striatum (DS), Nucleus Accumbens (NAcc), Lateral Hypothalamus (LH), and Ventromedial Hypothalamus (VMH) of Chow/Chow and Chow/Palatable (n=7–12) rats. Data represent mean (±SEM) expressed as percent of DR group. Symbols denote significant difference from Chow/Chow group *p<0.05, ***p<0.001.
In addition, a decreased gene expression of the enzyme FAAH was observed in the VMH of Chow/Palatable rats as compared to Chow/Chow rats [t(9)=3.9, p<0.001; Figure 6B]. Chow/Chow and Chow/Palatable rats did not differ in the mRNA expression of FAAH in the NAcc, DS, and LH [ts(10–13)<0.63, n.s.].
Chow/Chow and Chow/Palatable rats did not differ in the expression of MAGL, DAGLα and NAPE-PLD in any of the brain areas analyzed [ts(9–14)<1.7, n.s.; Figures 6C, CD, and 6E].
Discussion
Consummatory and motivational outcomes of intermittent, extended access to a palatable diet
In the present study we developed a novel animal model of compulsive eating resulting from an intermittent, extended access to a sugary, highly palatable diet, which, compared to other existing models (Cottone, Sabino, Roberto et al, 2009a; Cottone, Sabino, Steardo et al, 2009b), is characterized by a considerably faster cycling protocol. Rats undergoing palatable diet alternation developed maladaptive feeding adaptations which were a function of the diet offered and were characterized by progressively increasing hypophagia of the less palatable, regular chow diet, and progressively increasing overeating of the sugary diet. Although palatable diet cycled rats showed large fluctuations of food intake, cumulative food intake of Chow/Palatable rats did not significantly differ from Chow/Chow group, since the magnitude of the hypophagia was comparable to the magnitude of the overeating. Palatable diet alternation also resulted in spontaneous fluctuations in body weight and feed efficiency. Interestingly, starting from the seventh palatable food withdrawal, cycled rats began undereating the regular chow diet at the point of spontaneously losing absolute body weight. Similarly to what was observed with food intake, cumulative body weight gain and feed efficiency were not affected by the intermittent, extended access to the palatable diet. Although palatable diet alternation did not influence body weight, we cannot exclude whether the two groups could still differ in fat mass. Future body composition analysis will be needed to specifically address this point. The alternating patterns of food intake, body weight gain and feed efficiency are analogous to the ones observed with the previously published protocol of intermittent, extended access to palatable food (Cottone, Sabino, Steardo et al, 2008a, 2009b), with the difference in that the same number of cycles in this study were completed in less than half of the time.
Importantly, intermittent, extended access to the highly palatable diet induced a dramatic escalation of food consumption during the first hour of access, as a function of the number of cycles. The escalation of palatable food intake during the first hour of access was an experience-dependent phenomenon as indicated by the strong Cycle × Diet Schedule interaction and by the excellent fit of intake to the sigmoidal associative learning function (min: 5.9±1.8, max: 44.2±3.0, ET50: 2.6±0.3, Hillslope: 2.2±0.6, r2=0.98, p<0.0001, M±SEM). During the first hour of renewed access to the palatable diet, cycled rats were able to consume up to ~7.5 fold the intake of control rats; the amount of food eaten by Chow/Palatable rats in 1 hour was equivalent to ~42% of the daily caloric intake of Chow/Chow rats. Escalation of a behavioral response similar to the one shown here has been also observed with intermittent, extended access to drugs of abuse like cocaine (Ahmed and Koob, 1998), heroin (Ahmed, Walker and Koob, 2000), and nicotine (George, Ghozland, Azar et al, 2007), as well as with short, intermittent access to palatable diets (Cottone, Sabino, Steardo et al, 2008b; Cottone, Wang, Park et al, 2012; Parylak, Cottone, Sabino et al, 2012). Given the very similar caloric density of the chow and the palatable diet, the excessive caloric intake reflected indeed an active binge-like eating of food. The observed excessive food intake in such a brief period of time was, therefore, consistent with a hedonic, rather than a homeostatic mechanism.
Withdrawal from intermittent, extended access to the palatable diet increased the motivation to compulsively eat the sugary diet while facing aversive conditions. Indeed, Chow/Palatable rats spent significantly more time in an aversive compartment and ate ~70 times more than control Chow/Chow rats when they were offered the palatable diet in an open, aversive compartment of a light/dark conflict box at the end of the 2 days of withdrawal of the C phase. The increased motivation for the palatable diet observed in the light/dark conflict test in Chow/Palatable rats’ compared to Chow/Chow rats was dependent on the diet history and not on the diet offered in the light compartment. Indeed, in performing a light/dark conflict test in Chow/Chow rats where we provided either the regular chow diet or the palatable in the aversive compartment we found that neither of the two groups ate the food and they spent a similar amount of time in the light compartment (p=1.00 and p=0.47, food intake and time in the light compartment, respectively, not shown). Overall these results suggest that withdrawal from intermittent, extended access to a highly palatable diet causes the emergence of compulsive eating and risk-taking behavior, similarly to what has been observed in alcohol and drug addiction and in certain forms of eating disorders and obesity (Cottone, Wang, Park et al, 2012; Heyne, Kiesselbach, Sahun et al, 2009; Hopf et al, 2010; Johnson and Kenny, 2010; Teegarden and Bale, 2007; Vanderschuren and Everitt, 2004).
Effects of SR141716 on excessive intake of highly palatable food, compulsive eating and risk-taking behavior
In this study we have found that the CB1 receptor inverse agonist, SR141716, reduced the excessive intake of the highly palatable diet in diet cycled rats more potently than it reduced the intake of chow in control rats. During the first hour of access to the highly palatable diet, where the largest difference between Chow/Chow and Chow/Palatable rats’ intake is observed, the 3 mg/kg dose of SR141716 reduced intake of the palatable diet by 43%. In addition, at the highest dose injected, the CB1 inverse agonist reduced with greater efficacy body weight in diet cycled rats compared to control chow-fed rats. These results are consistent with the hypothesis that CB1 receptor antagonism decreases the hedonic value of food and, therefore, preferentially decreases the intake of highly palatable diets (Arnone et al, 1997; Jarrett, Scantlebury and Parker, 2007; Mathes, Ferrara and Rowland, 2008; Simiand et al, 1998). On the other hand, our results may appear in contrast with recent evidence showing that the CB1 receptor antagonist SR147778 is less effective in reducing binge-like eating of a sweet fat diet compared to a control diet (Parylak, Cottone, Sabino et al, 2012). However, many differences between the two studies may account for different effects observed. Indeed, here we used an intermittent, extended access (24 hours) to a sugary diet and male rats were ad libitum fed for the entire diet alternation procedure, while in the Parylak’s study binge-like eating was induced by a highly limited (10 min) access to a sweet fat diet and female rats were exposed daily to a 2-hour food deprivation. In addition, contrarily to what observed here, binge-like eating rats showed increased body weight gain, feed efficiency, as well as adiposity (Parylak, Cottone, Sabino et al, 2012).
SR141716 injected at the lowest effective dose (1 mg/kg) in the dose-response study, reduced both risk-taking behavior and compulsive-like eating in the light/dark conflict test, without affecting behavior in control Chow/Chow rats. These observations suggest that CB1 receptors plays a role in the loss of control and in the compulsiveness associated with excessive intake of palatable food. More generally, our findings support the hypothesis that CB1 receptors mediate the rewarding properties of addictive substances and that its blockade represents a promising pharmacological tool to treat compulsive palatable food/drug seeking (Serrano and Parsons, 2011). Indeed, pharmacological blockade of CB1 receptor has been demonstrated to reduce compulsive seeking behavior for many drugs of abuse (Cippitelli et al, 2005; Cohen et al, 2005; De Vries et al, 2001; Fattore et al, 2003).
Quantitative RT-PCR analysis, performed during withdrawal from intermittent, extended access to a palatable diet (same time point of the dose-response and risk-taking behavior/compulsive eating experiments) revealed decreased mRNA levels of the anandamide degrading enzyme FAAH in the VMH and an increased gene expression of CB1 receptors in the DS in cycled rats. Both results point at an increased cannabinergic tone in the brain of Chow/Palatable rats, which is consistent with the preferential effects of SR141716 in reducing excessive intake of palatable food, compulsive eating and risk-taking behavior in food cycled rats. Indeed, the decreased expression of the enzyme FAAH in the VMH observed here suggests a reduced degradation mechanism and therefore increased levels of the endocannabinoid anandamide. The VMH is a key nucleus in the regulation of appetite (Suzuki et al, 2010), and the CB1 receptor mRNA within this area has been shown to be highly expressed compared to other hypothalamic nuclei (Mailleux and Vanderhaeghen, 1992). Very interestingly, microinfusion of anandamide in the VMH has been shown to induce overeating in pre-satiated rats (Jamshidi and Taylor, 2001). This effect was prevented by pretreatment with SR141716, suggesting a primary role for anandamide in the VMH in promoting appetite through the activation of CB1 receptors (Jamshidi and Taylor, 2001). Thus, we can hypothesize that withdrawal from intermittent, extended access to palatable food induced an increased anandamide tone in the VMH which may have contributed to the excessive intake observed upon renewing access to the palatable diet. Consistent with this hypothesis, SR141716 decreased palatable food intake with higher potency in cycled rats compared to control chow-fed rats.
In addition, we observed an increased gene expression of CB1 receptors in the DS of rats withdrawn from the palatable diet. The DS is a brain area heavily involved in reward processing and decision-making, and it integrates sensorimotor, cognitive, and motivational/emotional information (Balleine, Delgado and Hikosaka, 2007). CB1 receptors, which are heavily expressed in this area (Pettit et al, 1998), play an important role in modulation of reward, and their stimulation has been demonstrated to increase dopamine release (Szabo, Muller and Koch, 1999). Therefore, it can be hypothesized that the increased expression of CB1 receptors in the DS of palatable food withdrawn rats would determine a dopamine overflow, which may in turn be responsible for the heightened motivation for the sugary diet even in adverse conditions.
In summary our behavioral, pharmacological and molecular observations give important insights into the neurobiology of compulsive eating and will be important to develop novel pharmacological treatments.
Acknowledgments
We thank Stephen St Cyr and Patrick De Souza for technical assistance and Rahul Rao for editorial assistance. This publication was made possible by grant numbers DA023680, DA030425, MH091945, MH093650 and AA016731 from the National Institute on Drug Abuse (NIDA), the National Institute of Mental Health (NIMH) and the National Institute on Alcohol Abuse and Alcoholism (NIAAA), by the Peter Paul Career Development Professorship (P.C.) and by Boston University’s Undergraduate Research Opportunities Program (UROP). This research was also supported by the NIH Intramural Research Programs of the National Institute on Drug Abuse, and the National Institute of Alcohol Abuse and Alcoholism, NIH, DHHS. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure/Conflict of interest
The authors declare no conflict of interest
Authors’ contribution
R.D., V.S. and P.C. were responsible for the study concept and design; R.D. and M.V. performed behavioral experiments; X.W. performed quantitative RT-PCR; K.C.R. provided critical reagents; R.D., M.V. and P.C. performed data analysis; R.D., V.S. and P.C. drafted the manuscript. All authors provided critical revision of the manuscript for important intellectual content and approved final version for publication.
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282(5387):298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22(4):413–421. doi: 10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; 2000. Text Revision. [Google Scholar]
- Arnone M, Maruani J, Chaperon F, Thiebot MH, Poncelet M, Soubrie P, Le Fur G. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 1997;132(1):104–106. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27(31):8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello NT, Coughlin JW, Redgrave GW, Ladenheim EE, Moran TH, Guarda AS. Dietary conditions and highly palatable food access alter rat cannabinoid receptor expression and binding density. Physiol Behav. 2012;105(3):720–726. doi: 10.1016/j.physbeh.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch LL. Development of food preferences. AnnuRev Nutr. 1999;19:41–62. doi: 10.1146/annurev.nutr.19.1.41. [DOI] [PubMed] [Google Scholar]
- Cifani C, Polidori C, Melotto S, Ciccocioppo R, Massi M. A preclinical model of binge eating elicited by yo-yo dieting and stressful exposure to food: effect of sibutramine, fluoxetine, topiramate, and midazolam. Psychopharmacology (Berl) 2009;204(1):113–125. doi: 10.1007/s00213-008-1442-y. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Bilbao A, Hansson AC, del Arco I, Sommer W, Heilig M, Massi M, Bermudez-Silva FJ, Navarro M, Ciccocioppo R, de Fonseca FR. Cannabinoid CB1 receptor antagonism reduces conditioned reinstatement of ethanol-seeking behavior in rats. Eur J Neurosci. 2005;21(8):2243–2251. doi: 10.1111/j.1460-9568.2005.04056.x. [DOI] [PubMed] [Google Scholar]
- Cohen C, Perrault G, Griebel G, Soubrie P. Nicotine-associated cues maintain nicotine-seeking behavior in rats several weeks after nicotine withdrawal: reversal by the cannabinoid (CB1) receptor antagonist, rimonabant (SR141716) Neuropsychopharmacology. 2005;30(1):145–155. doi: 10.1038/sj.npp.1300541. [DOI] [PubMed] [Google Scholar]
- Corwin RL. Binge-type eating induced by limited access in rats does not require energy restriction on the previous day. Appetite. 2004;42(2):139–142. doi: 10.1016/j.appet.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Avena NM, Boggiano MM. Feeding and reward: perspectives from three rat models of binge eating. Physiol Behav. 2011;104(1):87–97. doi: 10.1016/j.physbeh.2011.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin RL, Buda-Levin A. Behavioral models of binge-type eating. Physiol Behav. 2004;82(1):123–130. doi: 10.1016/j.physbeh.2004.04.036. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Grigson PS. Symposium overview--Food addiction: fact or fiction? J Nutr. 2009;139(3):617–619. doi: 10.3945/jn.108.097691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Roberto M, Bajo M, Pockros L, Frihauf JB, Fekete EM, Steardo L, Rice KC, Grigoriadis DE, Conti B, Koob GF, Zorrilla EP. CRF system recruitment mediates dark side of compulsive eating. Proc Natl Acad Sci U S A. 2009a;106(47):20016–20020. doi: 10.1073/pnas.0908789106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, Zorrilla EP. Intermittent access to preferred food reduces the reinforcing efficacy of chow in rats. Am J Physiol Regul Integr Comp Physiol. 2008a;295(4):R1066–1076. doi: 10.1152/ajpregu.90309.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, Zorrilla EP. Opioid-dependent anticipatory negative contrast and binge-like eating in rats with limited access to highly preferred food. Neuropsychopharmacology. 2008b;33(3):524–535. doi: 10.1038/sj.npp.1301430. [DOI] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, Zorrilla EP. Consummatory, anxiety-related and metabolic adaptations in female rats with alternating access to preferred food. Psychoneuroendocrinology. 2009b;34(1):38–49. doi: 10.1016/j.psyneuen.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Wang X, Park JW, Valenza M, Blasio A, Kwak J, Iyer MR, Steardo L, Rice KC, Hayashi T, Sabino V. Antagonism of sigma-1 receptors blocks compulsive-like eating. Neuropsychopharmacology. 2012;37(12):2593–2604. doi: 10.1038/npp.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries TJ, Shaham Y, Homberg JR, Crombag H, Schuurman K, Dieben J, Vanderschuren LJ, Schoffelmeer AN. A cannabinoid mechanism in relapse to cocaine seeking. Nat Med. 2001;7(10):1151–1154. doi: 10.1038/nm1001-1151. [DOI] [PubMed] [Google Scholar]
- DiPatrizio NV, Piomelli D. The thrifty lipids: endocannabinoids and the neural control of energy conservation. Trends Neurosci. 2012;35(7):403–411. doi: 10.1016/j.tins.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPatrizio NV, Simansky KJ. Activating parabrachial cannabinoid CB1 receptors selectively stimulates feeding of palatable foods in rats. J Neurosci. 2008;28(39):9702–9709. doi: 10.1523/JNEUROSCI.1171-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Cossu G, Deiana S, Fratta W. Cannabinoid mechanism in reinstatement of heroin-seeking after a long period of abstinence in rats. Eur J Neurosci. 2003;17(8):1723–1726. doi: 10.1046/j.1460-9568.2003.02607.x. [DOI] [PubMed] [Google Scholar]
- George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, O’Dell LE, Richardson HN, Koob GF. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc Natl Acad Sci U S A. 2007;104(43):17198–17203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyne A, Kiesselbach C, Sahun I, McDonald J, Gaiffi M, Dierssen M, Wolffgramm J. An animal model of compulsive food-taking behaviour. Addict Biol. 2009;14(4):373–383. doi: 10.1111/j.1369-1600.2009.00175.x. [DOI] [PubMed] [Google Scholar]
- Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science. 2003;299(5608):853–855. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- Hopf FW, Chang SJ, Sparta DR, Bowers MS, Bonci A. Motivation for alcohol becomes resistant to quinine adulteration after 3 to 4 months of intermittent alcohol self-administration. Alcohol Clin Exp Res. 2010;34(9):1565–1573. doi: 10.1111/j.1530-0277.2010.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iemolo A, Valenza M, Tozier L, Knapp CM, Kornetsky C, Steardo L, Sabino V, Cottone P. Withdrawal from chronic, intermittent access to a highly palatable food induces depressive-like behavior in compulsive eating rats. Behav Pharmacol. 2012;23(5–6):593–602. doi: 10.1097/FBP.0b013e328357697f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamshidi N, Taylor DA. Anandamide administration into the ventromedial hypothalamus stimulates appetite in rats. Br J Pharmacol. 2001;134(6):1151–1154. doi: 10.1038/sj.bjp.0704379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett MM, Scantlebury J, Parker LA. Effect of delta9-tetrahydrocannabinol on quinine palatability and AM251 on sucrose and quinine palatability using the taste reactivity test. Physiol Behav. 2007;90(2–3):425–430. doi: 10.1016/j.physbeh.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13(5):635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar AA, Singh SG, Venkate AC, Lalit W. An Improved process for the preparation of rimonabant. 200862480. Patent publication WO. 2008:A2.
- Kunos G. Understanding metabolic homeostasis and imbalance: what is the role of the endocannabinoid system? Am J Med. 2007;120(9 Suppl 1):S18–24. doi: 10.1016/j.amjmed.2007.06.007. discussion S24. [DOI] [PubMed] [Google Scholar]
- Maier EY, Ahrens AM, Ma ST, Schallert T, Duvauchelle CL. Cocaine deprivation effect: cue abstinence over weekends boosts anticipatory 50-kHz ultrasonic vocalizations in rats. Behav Brain Res. 2010;214(1):75–79. doi: 10.1016/j.bbr.2010.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailleux P, Vanderhaeghen JJ. Distribution of neuronal cannabinoid receptor in the adult rat brain: a comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience. 1992;48(3):655–668. doi: 10.1016/0306-4522(92)90409-u. [DOI] [PubMed] [Google Scholar]
- Mathes CM, Ferrara M, Rowland NE. Cannabinoid-1 receptor antagonists reduce caloric intake by decreasing palatable diet selection in a novel dessert protocol in female rats. Am J Physiol Regul Integr Comp Physiol. 2008;295(1):R67–75. doi: 10.1152/ajpregu.00150.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathes WF, Brownley KA, Mo X, Bulik CM. The biology of binge eating. Appetite. 2009;52(3):545–553. doi: 10.1016/j.appet.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matias I, Bisogno T, Di Marzo V. Endogenous cannabinoids in the brain and peripheral tissues: regulation of their levels and control of food intake. Int J Obes (Lond) 2006;30(Suppl 1):S7–S12. doi: 10.1038/sj.ijo.0803271. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Li TK. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12(4):339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Berry EM, Avraham Y, Di Marzo V, Fride E. Endocannabinoids, feeding and suckling--from our perspective. Int J Obes (Lond) 2006;30(Suppl 1):S24–28. doi: 10.1038/sj.ijo.0803274. [DOI] [PubMed] [Google Scholar]
- Parylak SL, Cottone P, Sabino V, Rice KC, Zorrilla EP. Effects of CB1 and CRF1 receptor antagonists on binge-like eating in rats with limited access to a sweet fat diet: lack of withdrawal-like responses. Physiol Behav. 2012;107(2):231–242. doi: 10.1016/j.physbeh.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit DA, Harrison MP, Olson JM, Spencer RF, Cabral GA. Immunohistochemical localization of the neural cannabinoid receptor in rat brain. J Neurosci Res. 1998;51(3):391–402. doi: 10.1002/(SICI)1097-4547(19980201)51:3<391::AID-JNR12>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Polivy J, Herman CP. Dieting and binging. A causal analysis. AmPsychol. 1985;40(2):193–201. doi: 10.1037//0003-066x.40.2.193. [DOI] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Blasio A, Iyer MR, Steardo L, Rice KC, Conti B, Koob GF, Zorrilla EP. Activation of sigma-receptors induces binge-like drinking in Sardinian alcohol-preferring rats. Neuropsychopharmacology. 2011;36(6):1207–1218. doi: 10.1038/npp.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano A, Parsons LH. Endocannabinoid influence in drug reinforcement, dependence and addiction-related behaviors. Pharmacol Ther. 2011;132(3):215–241. doi: 10.1016/j.pharmthera.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simiand J, Keane M, Keane PE, Soubrie P. SR 141716, a CB1 cannabinoid receptor antagonist, selectively reduces sweet food intake in marmoset. Behav Pharmacol. 1998;9(2):179–181. [PubMed] [Google Scholar]
- Suzuki K, Simpson KA, Minnion JS, Shillito JC, Bloom SR. The role of gut hormones and the hypothalamus in appetite regulation. Endocr J. 2010;57(5):359–372. doi: 10.1507/endocrj.k10e-077. [DOI] [PubMed] [Google Scholar]
- Szabo B, Muller T, Koch H. Effects of cannabinoids on dopamine release in the corpus striatum and the nucleus accumbens in vitro. J Neurochem. 1999;73(3):1084–1089. doi: 10.1046/j.1471-4159.1999.0731084.x. [DOI] [PubMed] [Google Scholar]
- Teegarden SL, Bale TL. Decreases in Dietary Preference Produce Increased Emotionality and Risk for Dietary Relapse. Biol Psychiatry. 2007;61(9):1021–1029. doi: 10.1016/j.biopsych.2006.09.032. [DOI] [PubMed] [Google Scholar]
- Timofeeva E, Baraboi ED, Poulin AM, Richard D. Palatable high-energy diet decreases the expression of cannabinoid type 1 receptor messenger RNA in specific brain regions in the rat. J Neuroendocrinol. 2009;21(12):982–992. doi: 10.1111/j.1365-2826.2009.01921.x. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305(5686):1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]