Abstract
Objectives
Elevated tissue levels of prostaglandin E2 (PGE2), produced by cyclooxygenase (COX) are an early event in colorectal cancer (CRC). Data suggest the efficacy of non-steroidal anti-inflammatory (NSAIDs) drugs, which inhibit COX activity, as cancer preventives; however, side effects of NSAIDs indicate unacceptable limitations. Ginger has been reported to have anti-inflammatory activities with significant CRC preventive potential. We investigated if consumption of 2.0 g ginger daily regulated the level of two key enzymes, which control PGE2 production, COX-1 and NAD+- dependent 15-hydroxyprostaglandin dehydrogenase (15-PGDH).
Methods
Thirty participants at normal and twenty participants at increased risk of CRC were randomized and given 2.0 g/day ginger or placebo for 28 days. Flexible sigmoidoscopy was used to obtain colon biopsies at baseline and end of the study. Tissue level of COX-1 and 15-PGDH were assessed using Western Blotting.
Results
After ginger consumption participants at increased risk of CRC, had significantly reduced colonic COX-1 protein level (23.8%± 41) compared to the placebo group (18.9%± 52; p=0.03). Protein levels of 15-PGDH in the colon were unchanged. In normal risk for CRC participants, neither protein levels of COX-1 nor 15-PGDH in the colon were altered by ginger consumption.
Conclusion
Ginger significantly lowered COX-1 protein expression in increased risk participants, but not in normal risk participants at normal for CRC. Ginger did not alter 15-PGDH protein expression in either increased or normal risk participants. Further investigation, in larger studies with a longer ginger intervention is needed to examine the ability of ginger to impact tissue level of prostaglandin.
Keywords: ginger root extract, cyclooxygenase, 15-hydroxyprostaglandin dehydrogenase, colon cancer risk, cancer risk reduction
Introduction
Colorectal cancer (CRC) is the third most prevalent and second most deadly cancer in the United States [1]. Anti-inflammatory drugs (NSAIDs) such as aspirin have been shown to reduce CRC risk potentially through reduction of prostaglandin E2 (PGE2) an inflammatory metabolite of arachidonic acid that is strongly linked to colon tumor initiation and progression[2–5]. Cyclooxygenase-1 & 2 (COX-1 &-2) is thought to be the rate limiting enzyme for PGE2 synthesis[6]. However, in normal colonic tissue COX-2 expression is very low, generally not measurable, and basal level of PGE2 is generated by COX-1 [7]. In addition to the production of PGE2, recent data has also suggested that PGE2 catabolism via the NAD+-dependent 15-hydroxyprostaglandin dehydrogenase (15-PGDH) enzyme is down-regulated in individuals with CRC [8]. As such, targeting enzymes, which both synthesize (COX-1 &-2) and catabolize (15-PGDH) PGE2 may be a more effective strategy for CRC prevention than simply inhibiting COX enzymes alone. While aspirin and other NSAIDs inhibit COX enzymes there is limited evidence that they effect 15-PGDH and the side effects of NSAIDs in the cardiovascular and gastrointestinal system have raised concerns for their daily prescription to an otherwise healthy populations[9, 10]. Therefore, alternative approaches such as utilizing natural nutritional components with low toxicity to affect COX/PGE2 signaling represent potential areas of investigation for the prevention of CRC.
Ginger root (Zingiber officinal Roscoe, Zingiberaceae) is a traditional herbal dietary substance, which has been used for gastrointestinal complaints for 1,000’s of years and is one of the most commonly consumed herbs in the United States [11, 12]. Investigation of ginger and ginger’s most abundant non-volatile pungent constituents the gingerols and shogaols have demonstrated cancer inhibitory activity, such as growth suppression of colon and lung cancer cells [13], tumor anti-antigenic potential [14]; and anti-inflammatory effects including inhibition of inducible COX-2 [15], inhibition of Nf-κB [16, 17] and inhibition of 5-lipoxygenase (5-LOX) [18, 19]. In vivo studies using animal models of colorectal inflammation and carcinogenesis have confirmed the anti-oxidative, anti-inflammatory and anti-carcinogenic activities of ginger preparations [20–23]. Additionally, a recent paper from our group showed that PGE2 was reduced by ginger intake in normal looking colon tissue biopsies from a population with normal risk of CRC [24]. However, there is no data thus far examining the impact of dietary ginger on COX-1 or 15-PGDH concentrations in human colon mucosa.
The purpose of the current study was to assess the effect of ingesting 2.0 g of ginger root extract daily for 28 days on COX-1 and 15-PGDH concentrations in normal appearing human colonic mucosa. Colonic COX-1 and 15-PGDH were examined in people at both normal risk and at increased risk for CRC.
Methods
Participants and Drug
We conducted two blinded parallel clinical trials where participants were randomized into two equal groups of either ginger or matching placebo (lactose) for 28 days. In the first study, which included those at normal risk for CRC, we randomized 33 adult participants (of whom 30 completed the study; 16 placebo and 14 ginger). We defined normal-risk as having: no history of familial colorectal cancer syndromes; and no first-degree relatives before the age of 60 diagnosed with colon cancer; and no adenomas >1 cm in size or those containing carcinoma in situ; and no history of CRC. The second study focused on people at high risk for CRC of which 21 were randomized and 20 completed the trial (10 ginger and 10 placebo). High risk for CRC was defined as having at least one of the following: a first-degree relative diagnosed with CRC before the age of 60; or prior CRC which must have been fully excised and either Duke’s A or B; or a history of a prior adenoma.
Other eligibility criteria for the normal risk participants have been previously published[24]. However, with the exception of the high risk and low risk definitions eligibility was identical between the two studies. In brief, in addition to the low or high risk determination participants needed to be 18 years or older in generally good health. Participants could also not have taken any corticosteroid, NSAID or aspirin during or within 14 days of starting the study nor could they have been eating any ginger supplements or foods that contain ginger. For both trials, study procedures were administered at the University of Michigan Clinical Research Unit (MCRU) after the participant gave written, informed consent. The study was approved by the University of Michigan Institutional Review Board.
Participants were asked to take eight 250 mg capsules of ginger extract daily for a total of 2.0 g/day. The ginger product used in both studies was manufactured by Pure Encapsulations® (Sudbury, MA). Details of formulation, quality control, and drug dispersal for the ginger extract has been described previously[24].
Randomization, Allocation and Blinding
In both studies participants were randomized equally to one of two groups: placebo or ginger extract (2.0 g). The randomization code was computer-generated by the study biostatistician and kept by the University of Michigan’s Investigational Drug Service (U of M IDS). The U of M IDS assigned the next available randomization number when told of the next eligible participant. Participants and study team members who worked with study data, assessed outcomes, or administered questionnaires were unaware of the treatment assignment.
Flexible Sigmoidoscopy and Tissue Process
Flexible sigmoidoscopies were done at baseline beforedrug treatment started and at day 28, as soonas possible to 24 hours after they ingested the final study drug. The participants were not prepared for the procedure withany enemas, however they were asked to evacuate their rectum within12 hours of the sigmoidoscopy. Four tissue samples were taken by the biopsy forceps and each biopsy specimen was taken approximately 2 to 5 cm from other biopsy sites in the distal sigmoid colonic mucosa.
Biopsy samples were frozen in liquid nitrogen at exactly 50seconds after the time the biopsy forceps were closed and then stored at −70°C until analysis. Two frozen colonic biopsy specimens approximately 5 mg each from the same participant and time point were combined and pulverized to a fine powder using a liquid-nitrogen-cooled mortar. Samples were then transferred to sealed microcentrifuge tubes, mixed with three volumes of ice-cold PBS buffer containing 0.1% BHT and 1mM EDTA and then homogenized by an Ultrasonic Processor (Misonix, Farmingdale, NJ) at 0 °C for 3 min. The protein concentration in the homogenate was determined by a Bradford protein assay (Bio-Rad, Hercules, CA).
Western blot Analysis
Proteins (30 μg) was separated by SDS-PAGE using a 10% resolving polyacrylamide gel and then transferred onto PVDF membrane. The membrane was incubated with COX-1 antibody (Invitrogen, Camarillo, CA), 15-PGDH antibody (Santa Cruz Biology, Inc., Santa Cruz, CA) and actin antibody (abcam, San Francisco, CA). The images were quantified by MCID software (InterFocus Imaging Ltd, Cambridge, England). Participates from either group were randomly selected onto the same gel, the absolute values of the protein expression were quantified using the darkness of the COX-1 or 15-PGDH bands divided by the darkness of Actin bands.
Statistics
Statistical analyses were conducted using PASW Statistics 18 (Somers, NY). Quantification of protein for COX-1 and 15-PGDH was normalized to actin. For each subject, the percent difference in COX-1 and 15-PGDH between baseline and day 28 within groups was calculated as 100(post-pre)/pre. A p-value of ≤ 0.05 was considered statistically significant. We did not control for batch variability as analysis controlling for gel-to-gel variation did not change results quantitatively (data not shown). We performed t-tests to compare the difference of protein expression of COX-1 or 15-PGDH at baseline between the two dietary groups and found no statistical difference across treatment groups. Because the studies were powered for the original endpoint of PGE2, we decided to conduct a post-hoc power analysis to determine our ability to detect significant changes in COX-1 and PGDH-15 enzyme. Based on our estimates of mean differences and associated variability for COX-1 in the high-risk group, a two-sample t-test with Satterthwaite’s approximation required 26 subjects per group in order to detect the observed effect size with 80% power and a 5% level of significance. The observed effect size for 15-PGDH was substantially smaller. A post-hoc power calculation similar to that for COX-1 yielded a requirement of 148 subjects per group.
Results
Participants
We screened 50 people for the normal risk study from January 2007 to June 2008; 42 people for the high-risk study from June 2009 thru January 2010. For the normal risk study we randomized 33 participants (17 to placebo and 16 to ginger) after excluding 17 people for either identified chronic health issues (n=14) or lack of further interest in the study (n=3). Of the 33 participants, one person (due to a GI complaint) from the placebo group and two (due to a headache complaint and one person lost to follow-up) from the ginger group did not complete the study.
In the people who were at increased risk for CRC we excluded 21, primarily because 13 of them were found not to be at increased risk for CRC, 2 of them had secondary cancers, 2 due to having lost interest in participating in the study, one because of a myocardial infarction between screening and baseline, one due to taking a supplement that would interfere with assessments of the study endpoints and one because of a suspected case of Lynch Syndrome. Of the 21 people randomized (10 to placebo and 11 to ginger) one participant in the ginger arm was later withdrawn after discovering that he was normal risk for CRC leaving 10 participants in the ginger and 10 in the placebo arm who completed the increased risk trial.
For both normal and increased risk participants there was no significant difference at baseline between the groups for any demographic or clinical characteristics (Table 1). The participants from the two trials were similar to each other with the exception of age. Participants in the high-risk study were on average older with a mean age of 51±12.9 (SD) while those in the normal risk study had a mean age of 33.5±11.5 (SD).
Table 1.
Baseline Characteristics {Mean (SD) Δ}
| Characteristics | Normal risk group | High risk group | ||
|---|---|---|---|---|
|
| ||||
| Placebo (n=17) | Ginger (n=16) | Placebo (n=10) | Ginger (n=10) | |
| Age | 33.4 ±10.1 | 34.5 ±13.1 | 50.8±14.6 | 51.1 ±11.7 |
| Sex, No. (%) | ||||
| Men | 9 (52.9) | 7 (43.8) | 3 (30.0) | 4 (40.0) |
| Women | 8 (47.1) | 9 (56.2) | 7 (70.0) | 6 (60.0) |
| Ethnicity, No. (%) | 1 (5.9) | 0 (0) | ||
| Race, No. (%) | ||||
| White | 11 (64.7) | 10 (62.5) | 7 (70.0) | 8 (80.0) |
| Other† | 6 (35.3) | 6 (37.5) | 3 (30.0) | 2 (20.0) |
| Reason for High Risk for CRC* | ||||
| 1st Degree Relative with CRC | 6 (60.0) | 4 (40.0) | ||
| Prior Adenoma | 6 (60.0) | 6 (60.0) | ||
| Prior CRC | 0 (0.0) | 1 (10.0) | ||
SD = ± standard deviation
African American, Asian, Pacific Islander, American Indian/Alaskan Native
1st degree relative must have had a diagnosis of colorectal cancer before the age of 60; Prior colorectal cancer must have been fully excised and either Duke’s A or B; Values add up to >100% due to participants having several reasons for being at high risk for colorectal cancer.
Cyclooxygenase-1
We found that there was no significant difference (p=0.06) in COX-1 protein expression between the ginger and the placebo groups for the normal risk participants (Table 2 and Fig 1). However, our results did indicate that for participants with increased risk of CRC, the protein expression of COX-1 in the colon biopsies was significantly inhibited (p=0.03) by consumption of ginger root extract after 28 days of intervention compared to the placebo group (Table 2 and Fig 2). In the increased risk subjects colonic COX-1 protein expression was decreased by 23.8% ± 42% from pre-intervention to post-intervention in the ginger arm and increased COX-1 protein expression (18.9% ± 52%) in the placebo arm.
Table 2.
Protein Levels in Colonic Biopsies in Participants at Normal/High Risk for Colorectal Cancer {Mean (SD)a}
| Normal CRC risk group | Placebo, n =16 | Ginger, n =14 | |||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Protein Normalized to Actin | Baseline | 28-day follow-up | Mean % Change (SD) BTWN Baseline and Week 4b | Baseline | 28-day follow-up | Mean % Change (SD) BTWN Baseline and Week 4b | p-valuec |
| COX-1 | 0.56 (0.17) | 0.64 (0.24) | 16.02 (37.50) | 0.52 (0.23) | 0.52 (0.26) | −3.66 (24.87) | 0.06 |
| 15-PGDH | 0.33 (0.22) | 0.23 (0.12) | 8.62 (65.01) | 0.29 (0.27) | 0.26 (0.26) | 3.18 (58.19) | 0.83 |
| High CRC risk group | Placebo, n =10 | Ginger, n =10 | |||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Protein Normalized to Actin | Baseline | 28-day follow-up | Mean % Change (SD) BTWN Baseline and Week 4b | Baseline | 28-day follow-up | Mean % Change (SD) BTWN Baseline and Week 4b | p-valuec |
| COX-1 | 1.23 (1.19) | 1.32 (1.30) | 18.86 (52.21) | 2.30 (2.32) | 1.46 (1.53) | −23.82 (41.76) | 0.03 |
| 15-PGDH | 0.40 (0.32) | 0.37 (0.41) | 0.58 (45.37) | 0.49 (0.52) | 0.37 (0.31) | −12.81 (32.29) | 0.49 |
SD = ± standard deviation
Mean percent change between baseline and week 4 is calculated as average of (protein expression at time 2/protein expression at time 1) per participant then minus 100%.
Independent t-test
Figure 1.
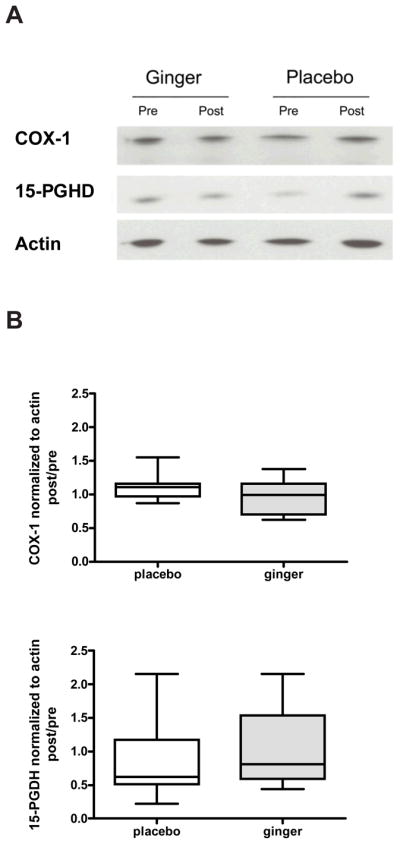
COX-1 and 15-PGDH protein expressions in colon biopsies from healthy participants. Representative immunoblots of COX-1, 15-PGDH and actin protein level are shown (A). The quantification of the immunoblots is shown for COX-1 (top panel) and 15-PGDH (bottom panel). Values are expressed as ratio of post/pretreatment for COX-1or 15-PGDH protein/actin protein for each participant with normal risk of CRC. Results are expressed as mean ± SEM (n = 14 in ginger group and n = 16 in placebo group).
Figure 2.
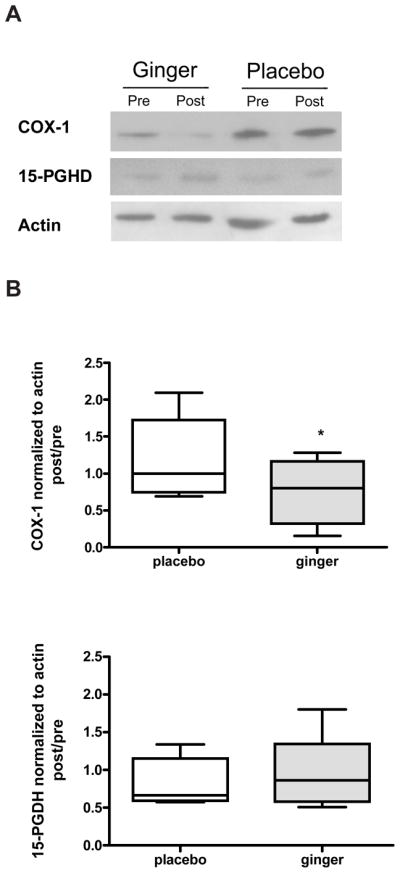
COX-1 and 15-PGDH protein expressions in colon biopsies from high CRC participants. Representative immunoblots of COX-1, 15-PGDH and actin protein level are shown (A). The quantification of the immunoblots is shown for COX-1 (top panel) and 15-PGDH (bottom panel). Values are expressed as ratio of post/pretreatment for COX-1or 15-PGDH protein/actin protein for each participant with high risk of CRC. Results are expressed as mean ± SEM (n = 10 in both placebo and ginger groups).
15-hydroxyprostaglandin
Our results did not indicate any significant difference in changes of colonic 15-PGDH protein levels between the ginger and placebo group after 28 days of ginger supplementation (Table 2 and Figs 1 & 2). This was true for both the normal and increased risk for CRC participants (p=0.83 and 0.49, respectively).
Discussion
We found that after short-term ginger root extract intervention that basal COX-1 protein expression in normal appearing colonic mucosa was significantly decreased (p=0.03) by 23.8 % in the ginger group compared to an 18.9% increase in the placebo group in participants at increased risk for CRC. In contrast, in participants at normal risk for CRC we saw no significant difference (p=0.06) between groups with the ginger group experiencing a very slight decrease of 3.7% and the placebo group having an increase of 16.0%. There was also, no significant change in basal 15-PGDH protein expression in either normal (p=0.83) or increased risk (p=0.49) for CRC participants regardless if they received ginger or placebo.
Our prior work in the same group of normal risk for CRC participants showed that a 2.0 g dose of ginger root extract given for 28 days was able to significantly reduce PGE2 concentrations in normal appearing colonic mucosa compared to placebo[22]. Similar to our findings, previous evidence determined that ginger extract or key ginger constituents reduced COX-2 mRNA expression activity in vitro, which in turn resulted in a decrease of PGE2 production[15, 25, 26]. Although in several studies, COX-2 was reported to be responsible for the anti-inflammatory effect of ginger; the expression of COX-2 was so low in normal colon tissue that other mechanisms could be involved in controlling basal PGE2 levels. Thus, it appears that COX-1 is the most likely candidate for the synthesis of basal levels of PGE2 in healthy colon mucosa. Also, COX-1 inhibition is a probable target of the ginger root extract for reducing PGE2 concentrations in healthy appearing colon mucosa[24]. As a consequence, we have hypothesized that the reduction of colonic PGE2 in our ginger trial could be due to the decrease of COX-1 protein expression reported upon here. However, we were unable to interrogate PGE synthase, an enzyme, which is responsible for the final stage of synthesis for PGE2. As such, decreased PGE2 concentrations in healthy colon tissue could be due to the inhibition of either or both COX-1 and PGE synthase by ginger root extract. On the other hand, since COX-1 is a constitutively expressed protein with function of producing eicosanoids that can be pro-inflammatory and anti-inflammatory; one would not expect a substantial change in healthy people. Therefore, a mild reduction of COX-1 would potentially decrease the production of PGE2 and in the meantime maintain the basal levels of eicosanoids that are necessary for healthy function of colonic mucosa.
This study had several limitations. This includes a relatively small sample size, which was powered based on our primary endpoint, PGE2; a short intervention period; and a fairly large amount of variability for 15-PGDH. Our samples sizes were relatively small and intervention period short as this study was intended to be a pilot for a larger human trial. Thus, it is possible that extended ginger consumption and more participants might provide additional power to detect the effects of dietary ginger root intake on the prostaglandin pathway. In addition, we analyzed factors (data not shown), which may have potentially interfered with the study outcome, such as age, gender, BMI and other characteristics of the participants. However, no additional factors were identified which were associated with the changes in COX-1 and 15-PGDH protein expression in either normal risk or high CRC risk participants. It is still possible that elements, which were not taken into consideration in our studies, may be responsible for changes in enzyme protein expression.
As indicated, there were considerable variations in changes of 15-PGDH protein expression in both groups. This is in contrast to COX-1 protein expression where little variability was observed. The large variability in 15-PGDH protein expression implies that responders and non-responders were included in a relatively small group of participants. Despite this, whether ginger is capable of altering 15-PGDH protein expression in a sub-set of people requires further research in a larger study sample.
In addition, it is important to note that the Western Blotting assay for the two studies was run separately, and at different times. As such, we can only compare the data within the study not across the two studies. Despite, not being able to compare the protein levels across the two studies; we are able to compare the Post/Pre ratio of COX-1 or 15 PGDH protein expression between the placebo and ginger group within studies as both Post and Pre samples from one individual were included on one gel.
Conclusion
In conclusion, we conducted two human trials using placebo/ginger root extract supplementation for 28 days. Our results indicate that ginger extract was able to reduced basal COX-1 protein expression in colon biopsies from participants with increased risk of CRC, indicating that ginger may potentially decrease human colonic PGE2 synthesis from arachidonic acid, especially in people with high risk of CRC. Whether ginger can be used a cancer preventive agent for colorectal cancer in healthy population still requires further investigation with bigger groups of participants and for longer intervention periods.
Acknowledgments
Source of Funding: This publication was made possible in part by Grant Number P30 CA047904, P30 CA 48592, CA130810 (GI SPORE) and K07CA102592, K24CA80846 from the National Cancer Institute (NCI) and University of Michigan Clinical Research Center UL1RR024986, and the Kutsche Family Memorial Endowment.
Pure Encapsulations® (Sudbury, MA) generously donated the ginger extract.
Footnotes
Conflict of Interests: None Declared
Contributor Information
Yan Jiang, Email: yanjian@med.umich.edu.
D. Kim Turgeon, Email: kturgeon@med.umich.edu.
Benjamin D. Wright, Email: bdwright@med.umich.edu.
Elkhansa Sidahmed, Email: sidahmed@med.umich.edu.
Mack T. Ruffin, Email: mruffin@med.umich.edu.
Dean E. Brenner, Email: dbrenner@med.umich.edu.
Ananda Sen, Email: anandas@med.umich.edu.
Suzanna M. Zick, Email: szick@med.umich.edu.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Arber N, Eagle CJ, Spicak J, Racz I, Dite P, Hajer J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355:885–895. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 3.Baron J, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891–899. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 4.Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Solomon SD, Kim K, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873–884. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 5.Juarranz M, Calle-Puron ME, Gonzalez-Navarro A, Regidor-Poyatos E, Soriano T, Martinez-Hernandez D, et al. Physical exercise, use of Plantago ovata and aspirin, and reduced risk of colon cancer. Eur J Cancer Prev. 2002;11:465–472. doi: 10.1097/00008469-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Song I, Ball TM, Smith WL. Different suicide inactivation processes for the peroxidase and cyclooxygenase activities of prostaglandin endoperoxide H synthase-1. Biochem Biophys Res Commun. 2001;289:869–875. doi: 10.1006/bbrc.2001.6071. [DOI] [PubMed] [Google Scholar]
- 7.Singer I, Kawka DW, Schloemann S, Tessner T, Riehl T, Stenson WF. Cyclooxygenase 2 is induced in colonic epithelial cells in inflammatory bowel disease. Gastroenterology. 1998;115:297–306. doi: 10.1016/s0016-5085(98)70196-9. [DOI] [PubMed] [Google Scholar]
- 8.Backlund MG, Mann JR, Holla VR, Buchanan FG, Tai HH, Musiek ES, et al. 15-Hydroxyprostaglandin dehydrogenase is down-regulated in colorectal cancer. J Biol Chem. 2005;280:3217–3223. doi: 10.1074/jbc.M411221200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solomon SD, McMurray JJ, Pfeffer MA, Wittes J, Fowler R, Finn P, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 10.James MW, Hawkey CJ. Assessment of non-steroidal anti-inflammatory drug (NSAID) damage in the human gastrointestinal tract. Br J Clin Pharmacol. 2003;56:146–155. doi: 10.1046/j.1365-2125.2003.01934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Afzal M, Al-Hadidi D, Menon M, Pesek J, Dhami MS. Ginger: an ethnomedical, chemical and pharmacological review. Drug Metabol Drug Interact. 2001;18:159–190. doi: 10.1515/dmdi.2001.18.3-4.159. [DOI] [PubMed] [Google Scholar]
- 12.Cavaliere C, Lynch P, Ellen M, Blumenthal M. Herbal supplement sales rise in all channels in 2009. HerbalGram. 2010;86:62–65. [Google Scholar]
- 13.Sang S, Hong J, Wu H, Liu J, Yang CS, Pan MH, et al. Increased growth inhibitory effects on human cancer cells and anti-inflammatory potency of shogaols from Zingiber officinale relative to gingerols. J Agric Food Chem. 2009;57:10645–10650. doi: 10.1021/jf9027443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown AC, Shah C, Liu J, Pham JT, Zhang JG, Jadus MR. Ginger’s (Zingiber officinale Roscoe) inhibition of rat colonic adenocarcinoma cells proliferation and angiogenesis in vitro. Phytother Res. 2009;23:640–645. doi: 10.1002/ptr.2677. [DOI] [PubMed] [Google Scholar]
- 15.van Breemen RB, Tao Y, Li W. Cyclooxygenase-2 inhibitors in ginger (Zingiber officinale) Fitoterapia. 2011;82:38–43. doi: 10.1016/j.fitote.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SO, Chun KS, Kundu JK, Surh YJ. Inhibitory effects of [6]-gingerol on PMA-induced COX-2 expression and activation of NF-kappaB and p38 MAPK in mouse skin. Biofactors. 2004;21:27–31. doi: 10.1002/biof.552210107. [DOI] [PubMed] [Google Scholar]
- 17.Aktan F, Henness S, Tran VH, Duke CC, Roufogalis BD, Ammit AJ. Gingerol metabolite and a synthetic analogue Capsarol inhibit macrophage NF-kappaB-mediated iNOS gene expression and enzyme activity. Planta Med. 2006;72:727–734. doi: 10.1055/s-2006-931588. [DOI] [PubMed] [Google Scholar]
- 18.Flynn DL, Rafferty MF, Boctor AM. Inhibition of human neutrophil 5-lipoxygenase activity by gingerdione, shogaol, capsaicin and related pungent compounds. Prostaglandins Leukot Med. 1986;24:195–198. doi: 10.1016/0262-1746(86)90126-5. [DOI] [PubMed] [Google Scholar]
- 19.Kiuchi F, Iwakami S, Shibuya M, Hanaoka F, Sankawa U. Inhibition of prostaglandin and leukotriene biosynthesis by gingerols and diarylheptanoids. Chem Pharm Bull (Tokyo) 1992;40:387–391. doi: 10.1248/cpb.40.387. [DOI] [PubMed] [Google Scholar]
- 20.Ko JK, Leung CC. Ginger extract and polaprezinc exert gastroprotective actions by anti-oxidant and growth factor modulating effects in rats. J Gastroenterol Hepatol. 2010;25:1861–1868. doi: 10.1111/j.1440-1746.2010.06347.x. [DOI] [PubMed] [Google Scholar]
- 21.Manju V, Nalini N. Chemopreventive efficacy of ginger, a naturally occurring anticarcinogen during the initiation, post-initiation stages of 1,2 dimethylhydrazine-induced colon cancer. Clin Chim Acta. 2005;358:60–67. doi: 10.1016/j.cccn.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 22.Yoshimi N, Wang A, Morishita Y, Tanaka T, Sugie S, Kawai K, et al. Modifying effects of fungal and herb metabolites on azoxymethane-induced intestinal carcinogenesis in rats. Jpn J Cancer Res. 1992;83:1273–1278. doi: 10.1111/j.1349-7006.1992.tb02758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manju V, Nalini N. Effect of ginger on bacterial enzymes in 1,2-dimethylhydrazine induced experimental colon carcinogenesis. Eur J Cancer Prev. 2006;15:377–383. doi: 10.1097/00008469-200610000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Zick SM, Turgeon DK, Vareed SK, Ruffin MT, Litzinger AJ, Wright BD, et al. Phase II study of the effects of ginger root extract on eicosanoids in colon mucosa in people at normal risk for colorectal cancer. Cancer Prev Res (Phila) 2011;4:1929–1937. doi: 10.1158/1940-6207.CAPR-11-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jolad SD, Lantz RC, Chen GJ, Bates RB, Timmermann BN. Commercially processed dry ginger (Zingiber officinale): composition and effects on LPS-stimulated PGE2 production. Phytochemistry. 2005;66:1614–1635. doi: 10.1016/j.phytochem.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Tjendraputra E, Tran VH, Liu-Brennan D, Roufogalis BD, Duke CC. Effect of ginger constituents and synthetic analogues on cyclooxygenase-2 enzyme in intact cells. Bioorg Chem. 2001;29:156–163. doi: 10.1006/bioo.2001.1208. [DOI] [PubMed] [Google Scholar]


