Abstract
Background
Few population-based studies have reported the prevalence of psoriatic disease.
Objective
We validated computerized diagnoses to estimate the prevalence of psoriasis and psoriatic arthritis.
Method
We identified adults with ≥1 ICD-9 diagnosis codes of 696.0 (psoriatic arthritis) or 696.1 (psoriasis) in clinical encounter data during 1996–2009, and used chart review to confirm the diagnoses in random samples of patients. We then used the best performing case-finding algorithms to estimate the point prevalence of psoriasis and psoriatic arthritis.
Results
The number of persons with a diagnosis for psoriasis (ICD-9 code 696.1) was 87,827. Chart review of a random sample of 101 cases with at least one dermatologist-rendered psoriasis code revealed a positive predictive value (PPV) of 90% (95% CI, 83–95) with sensitivity 88% (95% CI, 80–93). Psoriatic arthritis (code 696.0) was recorded for 5,187 patients, with the best performing algorithm requiring ≥2 diagnoses recorded by a rheumatologist or ≥1 diagnosis recorded by a rheumatologist together with ≥1 psoriasis diagnoses recorded by a dermatologist; the PPV was 80% (95% CI, 70–88) with sensitivity 73% (95% CI, 63–82). Among KPNC adults, the point prevalence of psoriasis, with or without psoriatic arthritis, was 939 (95% CI, 765–1142) per 100,000, and the overall prevalence of psoriatic arthritis, with or without psoriasis, was 68 (95% CI, 54–84) per 100,000.
Conclusion
Within an integrated health care delivery system, the use of computerized diagnoses rendered by relevant disease specialists is a valid method for identifying individuals with psoriatic disease.
Keywords: Psoriasis, Psoriatic Arthritis, Epidemiology, Incidence, Prevalence, Health Maintenance Organizations, Computerized Medical Information
INTRODUCTION
Psoriatic disease encompasses psoriasis, a chronic, multisystem inflammatory disorder involving the skin and nails, as well as psoriatic arthritis, which affects 5–20% of patients with psoriasis (1). Validated, population-based prevalence studies are critical to understanding psoriatic disease etiology, burden and temporal trends, yet only a small number of population-based studies have been reported in the United States (1–9). These studies have estimated the prevalence of psoriasis in adults at 1.2 – 4.8% and psoriatic arthritis at 0.15 – 0.25%. This variability could be due to differences in populations (ages, race/ethnicities), population sampling techniques, disease definitions (self-report vs. physician diagnosis), methods to validate the diagnosis (dermatologist vs. chart abstractor), and definitions of prevalence (point vs. lifetime).
We previously reported on case-finding and prevalence of psoriasis among pediatric members of Kaiser Permanente Southern California (7). The present report describes case-finding and prevalence of psoriasis and psoriatic arthritis among the racially and ethnically diverse adult members of Kaiser Permanente Northern California (KPNC), a large, integrated health-care delivery system whose population is similar to the general insured population in Northern California (10).
METHODS
Study population
KPNC is a pre-paid, comprehensive, integrated care organization that maintains computerized clinical data of all visits, procedures, pharmacy dispensed medications, and other medical goods and services to its 3.2 million members. These databases, comprising a variety of computerized information systems as well as an electronic medical record, provide the opportunity to build disease registries for efficient study of chronic diseases that otherwise cannot be easily identified in a stable and well-characterized population.
Referral to KPNC specialists in dermatology and rheumatology are often made by the primary care provider, although in some service areas, patient self-referral is also permitted. Physicians may refer patients to outside specialists when needed. Outside referrals are typically sent to nearby academic centers with claims captured by the KPNC information system. Outside referrals generally are made at the time of diagnosis for the purpose of obtaining a second opinion, during periods of extreme disease exacerbations that may be especially difficult to manage, or when access to a KPNC specialist is inadequate to meet the patient’s needs.
The present study, which was approved by the Kaiser Foundation Research Institute Institutional Review Board, included persons aged 18 years and older with ≥12 months of enrollment in KPNC between January 1, 1996 and December 31, 2009. At least 12 months of enrollment was required so that patients would have the opportunity to have a clinical visit with a dermatologist while they were a KPNC member. Preliminary cases included those with at least one International Classification of Diseases, Ninth Edition (ICD-9) diagnosis code of 696.0 (psoriatic arthritis) or 696.1 (psoriasis).
We obtained random samples of patients for case validation using three categories: psoriasis alone, psoriatic arthritis alone, and psoriasis with psoriatic arthritis. Based upon resource constraints of the study and the desire for similar confidence intervals around the positive predictive values of our validity estimates, we obtained random samples of 0.1% of patients with ≥1 ICD-9 code for psoriasis and no code for psoriatic arthritis, 1% of patients with ≥1 code for psoriatic arthritis alone, and 10% of patients with both codes. This was done by assigning random numbers from 0–1.000 and selecting all cases with numbers below 0.001, 0.010, and 0.10, respectively, for validation by chart review.
Data collection
Data collection was accomplished during 2010. The period of observation began on the later of the member’s first enrollment date or January 1, 1996, and ended on the earlier of the disenrollment date or December 31, 2009. Relevant computerized medical information was obtained for all preliminary cases and included clinical and membership data recorded during 1996–2009, including information from the electronic medical record that was established during 2004–2006. Before the electronic medical record was established, the health plan maintained numerous computerized information systems including outpatient encounters, hospital diagnoses, laboratory results, pharmacy information, and diagnostic images, among others. These databases were used to provide clinical care and not to administer insurance claims. Outside claims are generated when patients are referred out of the plan, and these outside claims were accessed for the study.
Manual chart review by a trained abstractor was performed on random samples of preliminary cases obtained as described in the previous section. The primary purpose of the chart review was to confirm the diagnosis recorded in the computerized data and to obtain information on clinical characteristics of the disease. A single trained medical record abstractor with extensive experience ascertaining autoimmune disease cases reviewed the medical record. Study dermatologists and rheumatologists addressed any questions raised by the chart reviewer as needed to adjudicate cases. Data were accessed from the electronic medical record, computerized information systems, and paper-based medical records. The abstractor reviewed all outpatient clinic notes, hospital discharge summaries, laboratory results, radiology reports, and all other information in the medical record. All study records were readily accessible. Among the medical records selected for review, there were 6 cases (four with the diagnosis of psoriatic arthritis and two with the diagnosis of psoriasis) for which the outpatient clinic note corresponding to the date of the relevant computerized ICD-9 code was missing. The remainder of the information in the medical chart of those 6 patients did not confirm the relevant diagnosis and these patients were coded as not having any psoriatic disease.
Case definitions
Psoriasis is a clinical diagnosis based on the characteristic appearance of erythematous indurated plaques with silvery scale in common locations such as the scalp, elbows, knees, and intergluteal cleft. There are no special blood tests or diagnostic procedures although a skin biopsy occasionally may be used to rule out other disorders. For the purpose of the study, a diagnosis by a dermatologist written as text into the clinical progress note was considered confirmed psoriasis unless the diagnosis was described as a rule-out and was not subsequently confirmed. If the diagnosis was made by a provider other than a dermatologist, then we required supporting information, such as a phone conversation with a dermatologist or a statement that the diagnosis had been confirmed by an outside dermatologist, and the chart abstractor consulted with a study dermatologist before confirming the case. We also attempted to ascertain the percent body surface area involvement, but it was recorded for <5% of subjects and therefore was not tabulated.
Psoriatic arthritis often is diagnosed by ruling out other forms of inflammatory arthritis. No specific clinical, laboratory, or radiographic criteria exist for the diagnosis of psoriatic arthritis. Conditions to rule-out include rheumatoid arthritis (e.g., when rheumatoid factor was present at a significant titer), gout, and osteoarthritis. Radiographically, psoriatic arthritis is a unique blend of bone destruction and proliferation. Manifestations may include erosive arthritis giving rise to the classic “pencil-in-cup” deformity. For the purpose of the study, a positive rheumatoid factor was considered indicative of rheumatoid arthritis, especially with the concomitant presence of a positive test for antibodies that bind citrulline modified proteins (CCP). However, a negative rheumatoid factor did not rule out the diagnosis of rheumatoid arthritis. For patients who presented with joint disease before developing the cutaneous manifestations of psoriasis, and whose rheumatoid factor and CCP tests were negative, the concomitant presence of radiographic sacroileitis or spondylitis or a clinical history of enthesitis supported the diagnosis of psoriatic arthritis. If the diagnosis was made by a provider other than a rheumatologist, the chart abstractor consulted with a study rheumatologist who reviewed laboratory tests and radiology images and reports from the spine, sacroiliac joint, or pelvis. In addition, the reviewer considered the CASPAR criteria for psoriatic arthritis (11), which considered evidence of current psoriasis, a personal history of psoriasis, or a family history of psoriasis; typical psoriatic nail dystrophy including onycholysis, pitting, and hyperkeratosis observed on current physical examination; a negative test result for the presence of rheumatoid factor by any method except latex, either current dactylitis, defined as swelling of an entire digit, or a history of dactylitis recorded by a rheumatologist; and radiographic evidence of juxta-articular new bone formation appearing as ill-defined ossification near joint margins (but excluding osteophyte formation) on plain radiographs of the hand or foot.
Data analysis
Disease manifestations
We sought to obtain the following disease manifestations for psoriasis: type of psoriasis (scalp/plaque, guttate, pustular, inverse, and erythrodermic) and affected anatomic locations. For psoriatic arthritis, we sought to obtain a description of the arthritis (inflammatory joint disease, enthesopathy, or spine disease), the anatomic location of joint involvement, radiographic evidence of juxta-articular new bone formation, number of involved joints, involvement of the nails and presence of dactylitis.
Validity of computerized data for identifying prevalent psoriasis and psoriatic arthritis
Case-finding algorithms were developed for use with all preliminary cases, including those that had not been selected into the random sample. Because some research studies are concerned with the co-occurrence of both psoriasis and psoriatic arthritis, we developed separate algorithms for psoriasis alone, psoriatic arthritis alone, and psoriasis with psoriatic arthritis. The variables that were examined for inclusion in the case-finding algorithms included the department where the diagnosis was recorded (dermatology, rheumatology, other) and the number of diagnoses recorded. We evaluated multiple possible case-finding algorithms, with >1 inpatient or outpatient visit to any department as the basis for comparison with all other algorithms. The sensitivity and positive predictive value (PPV) were determined for each of the case-finding algorithms under consideration.
The sensitivity was defined as the number of confirmed cases captured by the algorithm divided by the total number of confirmed cases with ≥1 relevant diagnosis code. The PPV was defined as the proportion of preliminary cases captured by the algorithm that were confirmed with the disease during chart review. We did not compute the specificity and negative predicted value; for uncommon diseases such as psoriasis, they exceed 99% and are not helpful for evaluating case-finding algorithms. The 95% confidence intervals (CI) were computed assuming Poisson distributions (12). All analyses were conducted using SAS version 9.13 (Cary, NC).
Estimation of the point prevalence
We applied the best case-finding algorithms we could define to estimate the prevalence of psoriasis and psoriatic arthritis in adult KPNC members. The best algorithm was defined as the one that provided the fewest number of falsely classified (false positive + false negative) cases. We corrected the estimates for the PPV by multiplying; we corrected for the sensitivity by dividing.
The age- and sex-specific point prevalence of psoriasis and psoriatic arthritis and their 95% CIs were calculated using as the denominator the number of males or females in each age group of KPNC members in each year. These were standardized using the direct method with the 2000 U.S. Census population providing weights (13,14). The age-and sex-standardized point prevalence was estimated on December 31, 2009; for this calculation, only persons who were health plan members on December 31, 2009 day were included. The 95% CIs for the sensitivity and PPV were computed assuming a Poisson distribution (12).
RESULTS
Validation of the Case-Finding Algorithms
The number of persons with at least one diagnosis code for psoriasis without psoriatic arthritis was 83,701 (Table 1). The average length of enrollment during 1996–2009 was 9.7 years. Seventy one percent of psoriasis diagnoses were made by a dermatologist. In comparing physician-rendered computerized psoriasis codes to the gold standard of chart review, the positive predictive value (PPV) of code 696.1 was 89% (95% CI, 79–95%) when recorded at least once by a dermatologist and 29% (95% CI, 12–52%) when recorded by non-dermatology providers. Requiring two diagnosis codes by a dermatologist increased the PPV of the case-finding algorithm to 95% (95% CI, 83–99%), but reduced the sensitivity to 63%, resulting in a larger number of incorrectly classified cases than when a single diagnosis code was used. We estimate the number of true cases of psoriasis without psoriatic arthritis to be 53,342. For psoriasis, false positive cases (n=7) included rule out diagnoses, inflammatory skin disorders that were subsequently diagnosed as other entities, inadequate documentation to classify psoriasis, and coding errors.
Table 1.
Sensitivity and positive predictive value (PPV) of various algorithms for confirming psoriasis (PsO) and psoriatic arthritis (PsA). Kaiser Permanente Autoimmune Disease Registry, Northern California, 1996–2009.*
| Disease codes and rendering physician | Chart Review** | ||||||
|---|---|---|---|---|---|---|---|
| Number in the population | Number reviewed* | No. of true positives | No. of false positives | Sensitivity (95% CI) | PPV (95% CI) | Estimated no. of true positives in the population | |
| PsO without PsA | |||||||
| ≥ 1 Any Physician | 83,701 | 87 | 65 | 22 | 100*** | 78 | |
| ≥ 1 Dermatology | 59,671 | 66 | 59 | 7 | 91 (80–96) | 89 (79– 95) | 53,342 |
| ≥ 2 Dermatology | 39,835 | 43 | 41 | 2 | 63 | 95 | |
| ≥ 1 Non-Dermatology | 24,022 | 21 | 6 | 15 | 9 | 29 | |
| PsA without PsO | |||||||
| ≥ 1 Any physician | 1,061 | 107 | 57 | 50 | 100*** | 53 | |
| ≥ 1 Rheumatology | 790 | 73 | 49 | 24 | 86 | 67 | |
| ≥ 2 Rheumatology | 605 | 54 | 44 | 10 | 77 (64–87) | 81 (68–90) | 493 |
| ≥ 1 Non-Rheumatology | 271 | 34 | 8 | 26 | 14 | 24 | |
| PsO with PsA | |||||||
| ≥ 1 Any physician | 4,126 | 50 | 32 | 18 | 100*** | 64 (49–77) | |
| ≥ 1 Dermatology AND ≥1 Rheumatology | 3,427 | 35 | 25 | 10 | 78 (60–90) | 71 (53–85) | 2,448 |
| Using the best performing algorithms**** | |||||||
| PsO with or without PsA | 63,098 | 101 | 91 | 10 | 88 (89–93) | 90 (83–95) | 55,790 |
| PsA with or without PsO | 4,032 | 89 | 71 | 18 | 73 (63–82) | 80 (70–88) | 2,941 |
The best performing algorithms are presented in bold.
A random sample of approximately 0.1% of patients with psoriasis codes alone, 1% of patients with psoriasis and psoriatic arthritis codes, and 10% of patients with psoriatic arthritis codes alone was selected, by assigning random numbers from 0–1.000 and selecting all cases with numbers below 0.001, 0.010, and 0.10, respectively, for validation by chart review.
By definition.
For PsO, ≥1 PsO diagnosis from dermatology. For PsA, ≥2 PsA diagnosis from rheumatology or ≥1 PsA diagnosis from rheumatology AND ≥1 PsO diagnosis from dermatology.
Psoriatic arthritis without psoriasis was recorded for 1,061 patients. The average length of enrollment during 1996–2009 was 8.9 years. The PPV of code 696.0 was 67% (95% CI, 55–77%) when recorded at least once by a rheumatologist and 24% (95% CI, 11–42%) when recorded by a non-rheumatology provider, with 86% of correctly classified cases being recorded by a rheumatologist. Requiring two diagnosis codes by a rheumatologist increased the PPV to 81% (95% CI, 68–90%) while decreasing the sensitivity to 77% (95% CI, 64–87%), yielding a better performing algorithm for identifying psoriatic arthritis. We estimate the number of true positives in the population to be 493. For psoriatic arthritis, false positive cases were primarily ‘rule-outs’ with final diagnoses of gout, small joint arthritis, rheumatoid arthritis, Sjogren’s disease, and polymyalgia rheumatica.
Persons with diagnoses for both psoriasis and psoriatic arthritis numbered 4,126, with an average length of enrollment during 1996–2009 of 10.2 years. Of these, 3,427 (83%) had ≥1 psoriasis diagnosis recorded by a dermatologist as well as ≥1 psoriatic arthritis diagnosis recorded by a rheumatologist; the PPV of this algorithm for identifying patients meeting both psoriasis and psoriatic arthritis case definitions was 71% (95% CI, 53–85) with sensitivity 78% (95% CI, 60–90%). Among the 10 patients with a dermatologist-recorded diagnosis of psoriasis and a rheumatologist-recorded diagnosis of psoriatic arthritis who underwent chart review and were found not to meet both case definitions, 7 met the study definition for psoriasis alone, 2 met the definition for psoriatic arthritis alone, and 1 had neither psoriasis nor psoriatic arthritis. Based on our definition, we estimate the number of true positives in the population with psoriasis and psoriatic arthritis to be 2,448.
Finally, we identified the best-performing algorithms to examine psoriasis without or without psoriatric arthritis. Requiring ≥1 psoriasis diagnosis recorded by a dermatologist revealed a PPV of 90% (95% CI, 83–95) with sensitivity 88% (95% CI, 80–93). Similarly, for psoriatic arthritis with or without psoriasis, requiring ≥2 diagnoses for psoriatic arthritis recorded by a rheumatologist, or ≥1 diagnosis recorded by a rheumatologist together with ≥1 diagnosis for psoriasis recorded by a dermatologist, revealed a PPV of 80% (95% CI, 70–88) with sensitivity 73% (95% CI, 63–82).
Point Prevalence
Among adults, the point prevalence of psoriasis alone, psoriatic arthritis alone, and psoriasis with psoriatic arthritis, standardized to the age and sex distribution of the 2000 U.S. Census, were 883 (95% CI, 876–890), 12.6 (95% CI, 11.6–13.7), and 55.8 (95% CI, 54.0–57.6) per 100,000 persons on December 31, 2009. The overall point prevalence of psoriasis, with or without psoriatic arthritis, was 939 (95% CI, 765–1142) per 100,000, with 6% of these cases being complicated by psoriatic arthritis. The overall prevalence of psoriatic arthritis, with or without psoriasis, was 68.4 (95% CI, 54–84) per 100,000, with 82% of these cases having clinical documentation supporting concurrent psoriasis. The point prevalence of psoriasis increased with age (Figure 1), while psoriatic arthritis prevalence increased to about 60–69 years, after which the prevalence leveled off. These point prevalences did not differ by gender.
Figure 1. Age- and sex-specific point prevalence1 per 100,000 persons (shown on Y-axis) of psoriasis with or without psoriatic arthritis (N=55,770) and psoriatic arthritis with or without psoriasis (N=2,941). Men and women aged ≥18 years. Kaiser Permanente Northern California, December 31, 2009.
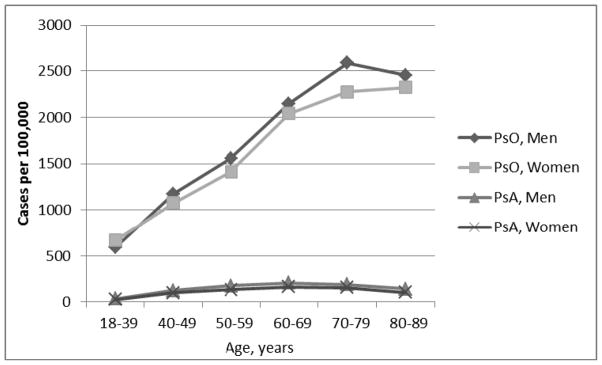
1The prevalence proportions are corrected for the sensitivity and positive predictive value of the validated case-finding algorithms.
PsO = psoriasis with or without psoriatic arthritis.
PsA = psoriasis with or without psoriasis.
Summary of Demographic and Disease Manifestations of Chart-review Confirmed Cases
There were 154 persons confirmed on chart review with psoriatic disease, 65 with psoriasis alone, 57 with psoriatic arthritis, and 32 with both diseases. About half of the patients were female, and the majority were <60 years of age at first clinical record of psoriatic disease. With regard to race/ethnicity, the majority of reviewed cases were Caucasian (63% with psoriasis alone, 74% with psoriatic arthritis alone, and 69% with both), although Hispanics (5–11%) and Asians (5–12%) were also represented. Among non-Caucasians, the majority was multiracial/other/unknown (15–16%) and only one individual (psoriasis only) was African-American. The type of psoriasis was not recorded for about two-thirds of the subjects, among those for whom it was recorded, plaque psoriasis predominated (28%). The most common location of psoriasis was the scalp. A rheumatoid factor test was performed in about 60% of psoriatic arthritis patients, and was negative among all cases. Among psoriatic arthritis patients, the most common location of joint involvement was the hands/wrists/fingers. Fingernail involvement was noted in the chart of 11% of patients with psoriasis, 32% of patients with psoriatic arthritis alone, and 56% of patients with both manifestations. Seventy-six percent of patients with psoriatic arthritis alone (n = 43) and 87% of patients with both psoriasis and psoriatic arthritis (n = 28) underwent radiographic examination, with only a single case (psoriatic arthritis alone) having evidence of juxta-articular new bone formation.
DISCUSSION
We developed and validated case-finding algorithms and estimated the prevalence of psoriasis and psoriatic arthritis among adult members of KPNC for the period 1996 through 2009. The overall prevalence of psoriasis, with or without psoriatic arthritis, was 939 (95% CI, 765–1142) per 100,000, with 6% of these cases having concomitant clinical documentation of psoriatic arthritis. The overall prevalence of psoriatic arthritis, with or without psoriasis, was 68.4 (95% CI, 54–84) per 100,000, with 82% of these cases having concomitant clinical documentation of current psoriasis.
A recent systematic review by Parisi and colleages (2012) detailed past studies of the global incidence and prevalence of psoriasis in children and adults. (15) In the United States, a study comparable to ours that used electronic healthcare claims data from two medical insurance databases reported a period prevalence of 0.91% (95% 0.90–0.92), strikingly similar to our rate of 0.94, though no case validations were reported in that study (16). Another study of psoriasis prevalence conducted in the United States was based in individuals self-selecting for a skin cancer screening program; dermatologist-rendered diagnosis provided prevalence estimates ranging from 1.2% to 3.4% (4). Other estimates were based on self-reported information from population-based surveys (8–9), while another was based in African Americans (6). These studies have reported psoriasis prevalence estimates in adults ranging from 0.91 – 3.15% (4, 6, 8–9, 16).
In the United Kingdom, psoriasis patients registered with general practitioners in the General Practice Research Database (1987–2002) and in The Health Improvement Network (2003 – 2009) provided prevalence estimates of 1.5% and 1.9%, respectively (17,18). The computer-based diagnosis of psoriasis was confirmed in 82% (N=3994) of patients in the GPRD, using manual review of the electronic medical record as the gold standard.(19) The present study is similar to the GPRD study in that we ascertained psoriatic disease using validated algorithms with computerized physician-rendered diagnoses in a large, diverse population. Icen and colleagues (2008) also evaluated the validity of computerized diagnostic codes in the population-based setting of Olmsted County, Minnesota, where all diagnoses and procedures are indexed in an electronic database (20). The medical record of 2556 adults with diagnostic codes consistent with psoriasis (1976–1979) were reviewed manually to validate the diagnosis. Based on medical record review, 1458 (57%) subjects were confirmed with psoriasis, with 81% were confirmed by a dermatologist. The PPV of ICD9 code 696.1 was 69% (95% CI: 67%, 71%). The study we report here required a dermatologist-rendered diagnosis, and observed a higher PPV of 90% (with 85% CI, 83–95).
A population-based study in Taiwan using ICD-9 codes rendered in 2006 observed a lower prevalence of 235 per 100,000 (21). However, the prevalence of psoriasis shows ethnic and geographic variation, and is less prevalent in persons of Asian descent as compared to persons of European descent (22), which may partially explain the differences in our findings. Among German workers, a full-body skin examination by a dermatologist (2004–2009) estimated the prevalence of psoriasis at 2% (23, 24). In a study of the diagnosis of psoriatic disease (combined psoriasis and psoriatic arthritis) as rendered by general practitioners using a representative sample of the general population in South Italy, the estimated prevalence of psoriatic disease (939 per 100,000) was nearly identical to our estimate (25).
The modest differences in psoriasis prevalence across these studies may represent genuine differences in susceptibility across populations, including, for example, differences in race/ethnicity, with the KPNC population having a higher population of Hispanic whites and blacks who generally have a lower prevalence of psoriasis compared to non-Hispanic whites. (10) These differences in prevalence may also be related to several other factors: (1) differences in demographics, including age, of the source population; (2) differences in geography, with psoriasis prevalence being lower in sunny climates, (22); (3) methodological differences in case definition, (i.e., relying on self-report vs. physician-rendered diagnoses, rendering of diagnoses by specialists compared with general practitioners, and rendering of diagnosis in electronic medical record vs. insurance claims data); and (4) and definitions of prevalence (point vs. lifetime). (15)
With respect to the prevalence of psoriatic arthritis, which we estimated at 68 per 100,000, others have reported levels ranging from 20 to 420 per 100,000 world-wide (25–34). In the United States, a population-based cohort of subjects in Olmsted County, Minnesota aged ≥18 years who fulfilled CASPAR criteria between 1970–1999 reported a point prevalence of 158 per 100,000 (95% CI 132, 185) (34). One possible explanation for the difference in our prevalence estimates is the difference in study populations. The population of Olmstead County, Minnesota is primarily white, non-Hispanic (35), a subgroup with known higher rates of psoriatic arthritis (22), whereas the population of KPNC has much more racial and ethnic diversity (10). U.S.-based studies validating computerized algorithms for psoriasis patients have been lacking, and our findings help address this gap in the literature.
Key strengths of this study include the size and diversity of the population, the availability of detailed paper and electronic medical records and electronic databases, and the use of chart abstraction to validate the diagnoses. The KPNC adult membership is generally representative of the broader community and is very similar to that large insured population with regard to sociodemographic and health characteristics (including obesity rates), as well as the general population in Northern California. However, the KPNC population has significantly lower percentages of adults in the lower (but not upper) ranges of income and educational attainment (10).
One of the limitations of this study is that the prevalence estimates do not include patients who did not seek medical attention for their disease. Thus, the study may have under-ascertained mild disease, particularly among those who were enrolled in the health plan for a short time, although the study was restricted to members with at least 12 months of enrollment. Another limitation was that the treating physician often did not record characteristics of psoriatic disease such as percent body surface area and nail involvement. Chart review has inherent limitations compared with other potential gold standards, such as prospective population surveys, although the latter are far more costly. Finally, we did not test more complicated algorithms, such as excluding cases if they had diagnoses that could mimic psoriatic disease (such as a gout or rheumatoid arthritis when examining psoriatic arthritis, and eczema when examining psoriasis).
The validated case-finding algorithms presented here should be useful to others who use large computerized databases to conducted research into psoriasis and psoriatic arthritis. Identification of an algorithm for use with computerized data enables Efficient identification of cases with psoriasis and psoriatic arthritis, and will be useful for advancing understanding of these diseases, including the frequency of occurrence, medical service utilization, long-term outcomes, and the safety of drug therapies.
Acknowledgments
This research was supported by grants from the National Institute of Allergy and Infectious Diseases (NIAID 1RC1AI086107-01). Dr. Harrold was supported by K23AR053856 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Curtis receives salary support from the NIH (AR053351) and AHRQ (R01 R01HS018517).
Abbreviations
- ICD-9
International Classification of Diseases, 9th revision
- KPNC
Kaiser Permanente Northern California
- PPV
positive predictive value
Footnotes
Conflicts of Interest: Dr. Herrinton has research contracts with Centocor, Genentech, and Proctor and Gamble; Dr. Asgari with Genentech and Valeant; and Dr. Wu with Abbott Laboratories, Amgen, and Pfizer. Dr. Harrold is an epidemiologic consultant for the Consortium of Rheumatology Researchers of North America (CORRONA). Dr. Gelfand receives grants to the trustees of the University of Pennsylvania from Amgen, Abbott, and Novartis, and is a consultant for Amgen, Abbott, Pfizer, Merck, and CentocorJanssen. Dr. Curtis receives consulting fees/honoraria and research support from Roche/Genentech, UCB/Centocor, CORRONA, Amgen, Pfizer, BMS, Crescendo, and Abbott.
References Cited
- 1.Schön MP, Boehncke WH. Psoriasis. N Engl J Med. 2005;352(18):1899–912. doi: 10.1056/NEJMra041320. [DOI] [PubMed] [Google Scholar]
- 2.Koo J. Population-based epidemiologic study of psoriasis with emphasis on quality of life assessment. Dermatol Clin. 1996;14(3):485–96. doi: 10.1016/s0733-8635(05)70376-4. [DOI] [PubMed] [Google Scholar]
- 3.Gelfand JM, Gladman DD, Mease PJ, Smith N, Margolis DJ, Nijsten T, Stern RS, Feldman SR, Rolstad T. Epidemiology of psoriatic arthritis in the population of the United States. J Am Acad Dermatol. 2005;53(4):573. doi: 10.1016/j.jaad.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 4.Lima XT, Minnillo R, Spencer JM, Kimball AB. Psoriasis prevalence among the 2009 AAD National Melanoma/Skin Cancer Screening Program participants. J Eur Acad Dermatol Venereol. 2012 Apr 4; doi: 10.1111/j.1468-3083.2012.04531. [DOI] [PubMed] [Google Scholar]
- 5.Wilson FC, Icen M, Crowson CS, McEvoy MT, Gabriel SE, Kremers HM. Time trends in epidemiology and characteristics of psoriatic arthritis over 3 decades: a population-based study. J Rheumatol. 2009;36(2):361–7. doi: 10.3899/jrheum.080691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gelfand JM, Stern RS, Nijsten T, Feldman SR, Thomas J, Kist J, Rolstad T, Margolis DJ. The prevalence of psoriasis in African Americans: results from a population-based study. J Am Acad Dermatol. 2005;52(1):23–6. doi: 10.1016/j.jaad.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 7.Wu JJ, Black MH, Smith N, Porter AH, Jacobsen SJ, Koebnick C. Low prevalence of psoriasis among children and adolescents in a large multiethnic cohort in southern California. J Am Acad Dermatol. 2011;65(5):957–64. doi: 10.1016/j.jaad.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Stern RS, Nijsten T, Feldman SR, Margolis DJ, Rolstad T. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004 Mar;9(2):136–9. doi: 10.1046/j.1087-0024.2003.09102.x. [DOI] [PubMed] [Google Scholar]
- 9.Kurd SK, Gelfand JM. The prevalence of previously diagnosed and undiagnosed psoriasis in US adults: results from NHANES 2003–2004. J Am Acad Dermatol. 2009;60(2):218–24. doi: 10.1016/j.jaad.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon NP. Internal Division of Research report. Oakland, CA: Kaiser Permanente Division of Research; Jan, 2012. [Accessed September 16, 2012]. Similarity of the Adult Kaiser Permanente Membership in Northern California to the Insured and General Population in Northern California: Statistics from the 2009 California Health Interview Survey. Available at: http://www.dor.kaiser.org/external/chis_non_kp_2009. [Google Scholar]
- 11.Taylor WJ, Helliwell PS. Development of diagnostic criteria for psoriatic arthritis: methods and process. Curr Rheumatol Rep. 2004 Aug;6(4):299–305. doi: 10.1007/s11926-004-0042-z. Review. [DOI] [PubMed] [Google Scholar]
- 12.Garwood F. Fiducial Limits for the Poisson Distribution. Biometrica. 1936;28:437–442. [Google Scholar]
- 13.U.S. Census Bureau. National Level Census Data for the United States: 2000. published April 2, 2001, http://www.census.gov/population/cen2000/phc-t1/tab01.txt.
- 14.Kleinbaum DG, Kupper LL, Morgenstern H. Epidemiologic research: Principles and quantitative methods. New York: Van Nostrand Reinhold; 1982. p. 98. [Google Scholar]
- 15.Parisi R, Symmons DP, Griffiths CE, Ashcroft DM. Global Epidemiology of Psoriasis: A Systematic Review of Incidence and Prevalence. J Invest Dermatol. 2012 Sep 27; doi: 10.1038/jid.2012.339. [DOI] [PubMed] [Google Scholar]
- 16.Robinson D, Jr, Hackett M, Wong J, Kimball AB, Cohen R, Bala M IMID Study Group. Co-occurrence and comorbidities in patients with immune-mediated inflammatory disorders: an exploration using US healthcare claims data, 2001–2002. Curr Med Res Opin. 2006 May;22(5):989–1000. doi: 10.1185/030079906X104641. [DOI] [PubMed] [Google Scholar]
- 17.Gelfand JM, Weinstein R, Porter SB, Neimann AL, Berlin JA, Margolis DJ. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141(12):1537–41. doi: 10.1001/archderm.141.12.1537. [DOI] [PubMed] [Google Scholar]
- 18.Seminara NM, Abuabara K, Shin DB, Langan SM, Kimmel SE, Margolis D, Troxel AB, Gelfand JM. Validity of The Health Improvement Network (THIN) for the study of psoriasis. Br J Dermatol. 2011;164(3):602–9. doi: 10.1111/j.1365-2133.2010.10134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huerta C, Rivero E, Rodríguez LA. Incidence and risk factors for psoriasis in the general population. Arch Dermatol. 2007 Dec;143(12):1559–65. doi: 10.1001/archderm.143.12.1559. [DOI] [PubMed] [Google Scholar]
- 20.Icen M, Crowson CS, McEvoy MT, Gabriel SE, Maradit Kremers H. Potential misclassification of patients with psoriasis in electronic databases. J Am Acad Dermatol. 2008 Dec;59(6):981–5. doi: 10.1016/j.jaad.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai TF, Wang TS, Hung ST, Tsai PI, Schenkel B, Zhang M, Tang CH. Epidemiology and comorbidities of psoriasis patients in a national database in Taiwan. J Dermatol Sci. 2011;63(1):40–6. doi: 10.1016/j.jdermsci.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Chandran V, Raychaudhuri SP. Geoepidemiology and environmental factors of psoriasis and psoriatic arthritis. J Autoimmun. 2010;34(3):J314–21. doi: 10.1016/j.jaut.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Augustin M, Herberger K, Hintzen S, Heigel H, Franzke N, Schäfer I. Prevalence of skin lesions and need for treatment in a cohort of 90 880 workers. Br J Dermatol. 2011;165(4):865–73. doi: 10.1111/j.1365-2133.2011.10436.x. [DOI] [PubMed] [Google Scholar]
- 24.Schaefer I, Rustenbach SJ, Zimmer L, Augustin M. Prevalence of skin diseases in a cohort of 48,665 employees in Germany. Dermatology. 2008;217(2):169–72. doi: 10.1159/000136656. [DOI] [PubMed] [Google Scholar]
- 25.Sardu C, Cocco E, Mereu A, Massa R, Cuccu A, Marrosu MG, Contu P. Population based study of 12 autoimmune diseases in Sardinia, Italy: prevalence and comorbidity. PLoS One. 2012;7(3):e32487. doi: 10.1371/journal.pone.0032487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li YH, Wang SY, Pan SS, Li C, Wang HY, Li ZG. Epidemiology of eight common rheumatic diseases in China: a large-scale cross-sectional survey in Beijing. Rheumatology (Oxford) 2012;51(4):721–9. doi: 10.1093/rheumatology/ker370. [DOI] [PubMed] [Google Scholar]
- 27.Pedersen OB, Svendsen AJ, Ejstrup L, Skytthe A, Junker P. The occurrence of psoriatic arthritis in Denmark. Ann Rheum Dis. 2008;67(10):1422–6. doi: 10.1136/ard.2007.082172. [DOI] [PubMed] [Google Scholar]
- 28.Hanova P, Pavelka K, Holcatova I, Pikhart H. Incidence and prevalence of psoriatic arthritis, ankylosing spondylitis, and reactive arthritis in the first descriptive population-based study in the Czech Republic. Scand J Rheumatol. 2010;39(4):310–7. doi: 10.3109/03009740903544212. [DOI] [PubMed] [Google Scholar]
- 29.Soriano ER, Rosa J, Velozo E, Schpilberg M, Imamura PM, Diaz J, Catoggio LJ. Incidence and prevalence of psoriatic arthritis in Buenos Aires, Argentina: a 6-year health management organization-based study. Rheumatology (Oxford) 2011;50(4):729–34. doi: 10.1093/rheumatology/keq369. [DOI] [PubMed] [Google Scholar]
- 30.Nossent JC, Gran JT. Epidemiological and clinical characteristics of psoriatic arthritis in northern Norway. Scand J Rheumatol. 2009;38(4):251–5. doi: 10.1080/03009740802609558. [DOI] [PubMed] [Google Scholar]
- 31.Yang Q, Qu L, Tian H, Hu Y, Peng J, Yu X, Yu C, Pei Z, Wang G, Shi B, Zhang F, Zhang Y, Zhang F. Prevalence and characteristics of psoriatic arthritis in Chinese patients with psoriasis. J Eur Acad Dermatol Venereol. 2011;25(12):1409–14. doi: 10.1111/j.1468-3083.2011.03985.x. [DOI] [PubMed] [Google Scholar]
- 32.Catanoso M, Pipitone N, Salvarani C. Epidemiology of psoriatic arthritis. Reumatismo. 2012 Jun 5;64(2):66–70. doi: 10.4081/reumatismo.2012.66. [DOI] [PubMed] [Google Scholar]
- 33.Ibrahim G, Waxman R, Helliwell PS. The prevalence of psoriatic arthritis in people with psoriasis. Arthritis Rheum. 2009;61(10):1373–8.30. doi: 10.1002/art.24608. [DOI] [PubMed] [Google Scholar]; Radtke MA, Reich K, Blome C, Rustenbach S, Augustin M. Prevalence and clinical features of psoriatic arthritis and joint complaints in 2009 patients with psoriasis: results of a German national survey. J Eur Acad Dermatol Venereol. 2009;23(6):683–91. doi: 10.1111/j.1468-3083.2009.03159.x. [DOI] [PubMed] [Google Scholar]
- 34.Gladman DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005 Mar;64(Suppl 2):ii14–7. doi: 10.1136/ard.2004.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. [Accessed September 26, 2012];Minnesota demographics. http://www.minnesota-demographics.com/olmsted-county-demographics.


