Abstract
Uneven walking surfaces pose challenges to balance, especially in individuals with lower extremity amputation. The purpose of this study was to determine if lateral stability of persons with unilateral transtibial amputation (TTA) is compromised when walking on a loose rock surface. Thirteen TTA and 15 healthy controls walked over level ground and over a loose rock surface at four controlled speeds. Dependent measures, including medial-lateral center of mass (COM) motion, step width variability, lateral arm swing velocity, and mean and variability of the minimum margins of stability (MOSmin), were compared between subject groups and across conditions. TTA had greater average MOSmin than Control subjects (p = 0.018). TTA exhibited decreased MOSmin on their prosthetic limbs compared to their intact limbs (p = 0.036), while Control subjects did not exhibit side to side differences. Both groups increased MOSmin with increasing walking speed (p ≤ 0.001). There was no difference in the average MOSmin between walking surfaces (p = 0.724). However, the variability of MOSmin was greater on the rocks compared to level ground. Both subject groups increased step width, step width variability, COM range of motion and peak COM velocity when walking on the rock surface. TTA exhibited greater variability of both step width and MOSmin, which suggests that they made larger step-to-step, corrective responses, more often, to achieve the same average result.
Keywords: gait, amputation, irregular surface, lateral stability, step width variability
1. Introduction
Falls are common in individuals with lower-limb amputation, with more than half of individuals with unilateral lower-limb amputation falling each year [1, 2]. Environmental factors like uneven terrain contribute to instability [3] and increase the frequency of falling [4].
In particular, people with lower-limb amputation may have difficulty maintaining lateral stability during walking, as ankle control plays a dominant role in medial-lateral balance [5]. People with lower-limb amputation have limited ankle joint mobility on the prosthetic side, and they lack distal muscles and afferent feedback from their lower limb [6]. These deficits reduce their capacity to control center of pressure (CoP) position while standing on their prosthetic limb such that they must use predictive strategies while walking [6]. Individuals with lower limb amputation (LLA) are therefore assumed to be less stable than able bodied individuals and have greater difficulty walking across uneven surfaces. However, recent studies that have attempted to verify this assumption reported conflicting and sometimes counterintuitive results [3, 7, 8]. LLA were equally stable on uneven ground and level ground in one study [7], but exhibited increased stability on uneven ground in another [8]. Additionally, LLA were found to be equally stable [7], more unstable [3, 8], or more stable [3] than able-bodied controls, depending on what measure was used to quantify stability. Results for between-limb differences in LLA are likewise inconsistent and counterintuitive, suggesting increased stability on the prosthetic limb compared to intact limb [3] or no between-limb differences [7].
The discrepancies between studies may be due to differences in how ‘stability’ was quantified, differences in the complexity of the surfaces studied, differences in patients tested (vascular or traumatic), or the different adaptations different subjects used to walk on these surfaces. For instance, LLA walked slower than controls in one study [8], but not in another [7]. Curtze et al. found that persons with transtibial amputation (TTA) adjusted their gait by increasing the maximum velocity of the lateral component of arm-swing when walking on an irregular surface, while unimpaired subjects did not [7]. They suggested arm swing played a vital role in TTA’s ability to maintain lateral balance by helping redirect the center of mass (COM) back over the base of support.
This study therefore quantified the lateral stability margins of young adults with and without traumatic transtibial amputations while walking over level ground and over a loose rock surface at four controlled speeds. Stability was quantified by calculating minimum medial-lateral margins of stability (MOSmin) during walking. We hypothesized that: 1) TTA exhibit smaller MOSmin (i.e., would be more unstable) than Control subjects, 2) Both subject groups would exhibit smaller MOSmin when walking on the rock surface compared to level ground, 3) Subjects’ MOSmin would become smaller when walking at faster walking speeds, and 4) TTA would exhibit smaller MOSmin on their prosthetic limb due to lack of active ankle control, while control subjects would not exhibit side to side differences in MOSmin. Additionally, we measured the medial-lateral movement of the whole body center of mass (COM), step width, step width variability, and lateral velocity of arm swing to determine what, if any, specific adaptations subjects made to maintain their medial-lateral balance.
2. Methods
2.1 Subjects
Thirteen young adults (12 male, 1 female) with traumatic unilateral transtibial amputation participated. Their average age, height, leg length, and body mass were 28 ± 4 years, 1.81 ± 0.09 m, 0.93 ± 0.05 m and 88.6 ± 14.4 kg, respectively. All participants were screened to ensure they were free of orthopedic and neurological disorders to the intact side. Fifteen healthy young adults (12 male, 3 female) with a mean age of 22 ± 5 years, height of 1.71 ± 0.09 m, leg length of 0.89 ± 0.07 m and weight of 76.6 ± 11.6 kg also participated. All subjects provided written informed consent prior to participation.
2.2 Experimental Protocol
Kinematic data from 55 reflective markers were used to track full body kinematics at 120 Hz (Motion Analysis, Santa Rosa, CA) [9] while subjects walked over level ground and a loose rock surface (Figure 1A–B). Walking speeds were non-dimensionally scaled to leg length, l, according to , where Fn is the Froude number, and g is the gravitational constant [10]. Subjects walked at speeds corresponding to Fn of 0.06, 0.10, 0.16, and 0.23. A total of five left and five right strides with full kinematic data were analyzed for each speed, on each surface. One subject with TTA did not complete the fastest speed on the rock surface due to apprehension. All subjects wore their own athletic shoes during data collection.
Figure 1. Experimental Set-up.
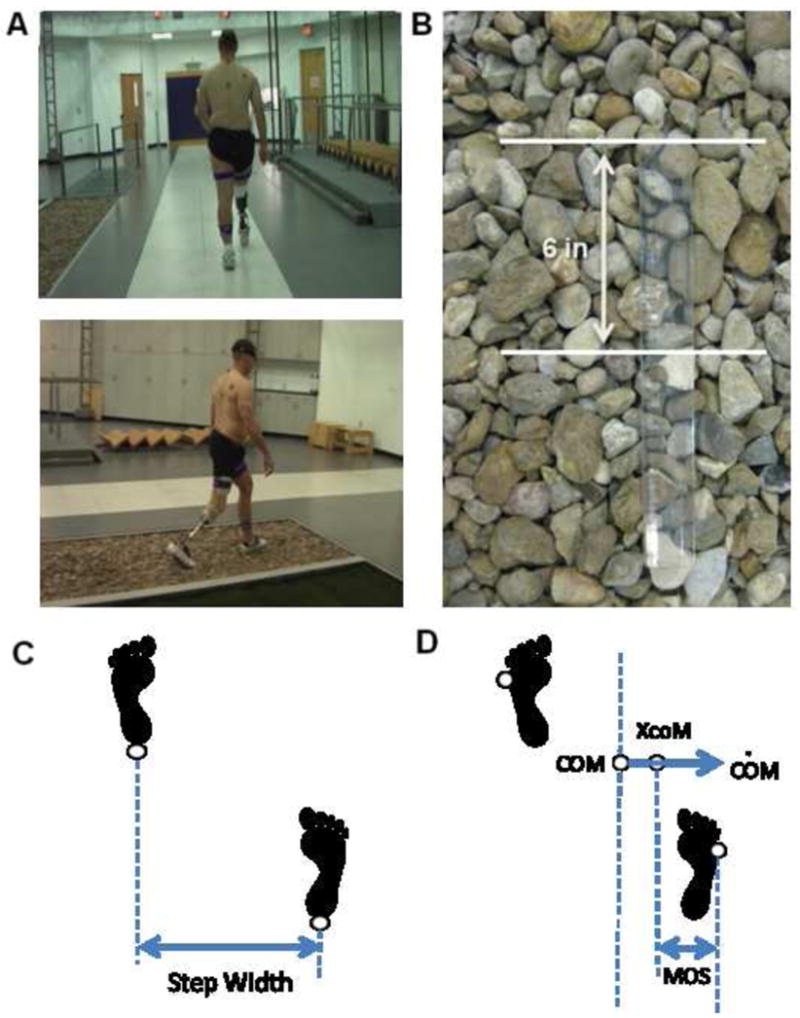
A) Subjects walked at four speeds across both level ground (top) and a rock surface (bottom). The rock surface was a 4.2-m long by 1.2-m wide pit filled with loose rocks. B) A close-up of the rocks surfaces is shown with a ruler to provide dimension. C) Step width was calculated as the medial-lateral distance between the heel markers at heel strike. D) The margin of stability (MOS) was the medial-lateral distance between the lateral border of the base of support (BOS), defined by the 5th metatarsal marker, and the vertical projection of the extrapolated center of mass (XcoM).
2.3 Data Analysis
Kinematic and temporal spatial data were previously reported [11, 12]. Whole body center of mass (COM) was calculated as the weighted average of segment COMs based on anthropometric data [13]. Heel strikes were determined using a velocity-based detection algorithm [14] and verified by visual inspection. Step width was calculated based on the position of the heel markers at heel strike (Figure 1C).
Each subject’s dynamic stability was determined via a technique based on the inverted pendulum model of balance [15]. According to this theory, a person is dynamically stable when their extrapolated center of mass (XcoM) lies within the border of their base of support (BOS). XcoM was calculated as:
| (1) |
Where COM Z and CȮM Z were the position and velocity of the COM in the medial-lateral direction, and ωo was the eigenfrequency of a non-inverted pendulum with a pendulum length of 1.34 times trochanteric height [15]. At each instant in time, XcoM was used to define a margin of stability (MOS), proportional to the impulse needed to unbalance a subject, MOS=BOS − XcoM [15]. Without center of pressure data, the lateral boundary of the BOS was estimated from the position of the 5th metatarsal marker (Fig. 1D). MOSmin was calculated as the minimum value of MOS during the stance phase. Within-subject variability of step width and MOSmin were calculated as the standard deviation across all five cycles collected for each condition.
Upon visual inspection of the data, it was evident that subjects were not walking straight relative to the laboratory coordinate system. To look specifically at deviations away from the path of progression, we performed a rotation on the foot marker data and center of mass motion [16].
The COM of each arm was determined from the weighted averages of the COMs of the upper arm, lower arm, and hand. Lateral arm swing velocity was defined as the movement of the arm COM in the medial-lateral (z) direction with respect to the shoulder such that a positive value indicated movement of the arm toward the stance limb (i.e., for a right gait cycle, arm movement to the right was positive) (See Supplemental Material for additional details). For the arm swing velocity to effectively redirect the whole body COM, the peak velocity must occur prior to the change COM shift (ie. when COM velocity = 0). Thus peak lateral arm swing velocity was the maximum velocity achieved between 10% gait cycle and the time of COM shift (~ 35% gait cycle; Fig. 4, Supplemental Material).
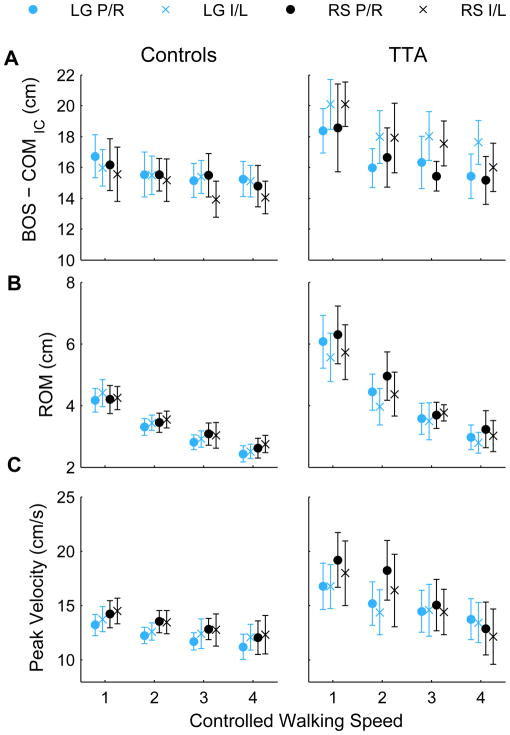
Figure 4. Medial-Lateral Center of Mass (COM) Motion.
A) The distance between the COM and base of support at initial contact, B) COM range of motion (ROM) during stance, and C) The peak velocity of COM during stance are shown for the rock surface (RS) and level ground (LG) at each of the four controlled walking speeds (shown from slowest to fastest). Average trajectories were computed for each subject across five walking trials for the prosthetic (‘P’) and intact (‘I’) limbs for patients with transtibial amputations (TTA) and right (‘R’) and left (‘L’) limbs for control subjects. Errorbars represent the 95% confidence intervals about the mean, across subjects.
2.4 Statistics
Subject demographics (age, height, and weight) between control and amputee groups were compared using t-tests. Dependent measures were first compared using mixed design repeated measures ANOVAs with walking surface (Rock/Level), walking speed (1–4), and limb (TTA: prosthetic/intact, Control: right/left) as within-subjects factors and subject group (TTA/Control) as a between subject factor. Repeated measures ANOVAs where then performed on each group separately where there were significant main effects or interactions with ‘Group’. Height and weight were initially included as potential covariates. However, since neither was significant for any comparison, they were subsequently dropped. Statistical analyses were performed using SPSS v19 (SPSS Inc., Chicago, IL).
3. Results
TTA were, on average, 6 yrs older (p = 0.001), 9 cm taller (p = 0.012), and 11.1 kg heavier (p = 0.029) than control subjects. The two groups had similar leg lengths (p = 0.22), and thus walked at similar dimensionless speeds (Supplemental Material).
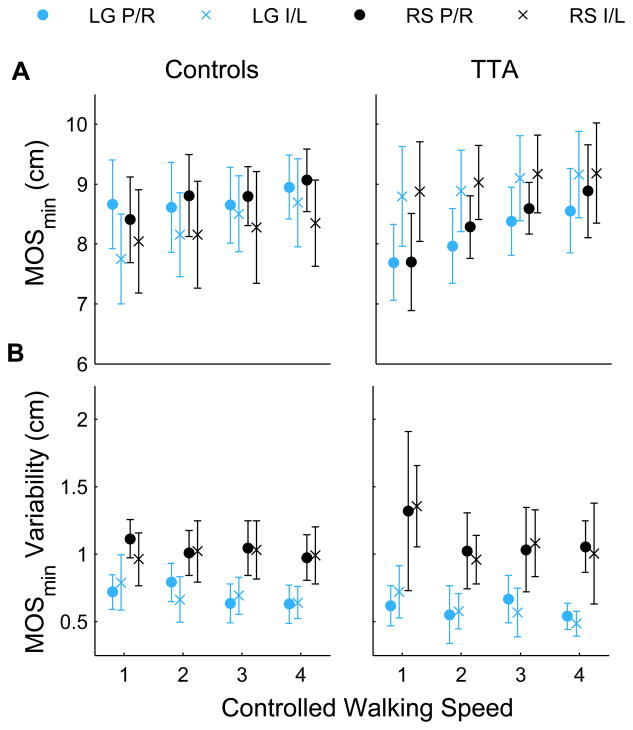
TTA exhibited a greater average MOSmin than control subjects (pGroup = 0.018; Fig. 2A). MOSmin increased with walking speed (pSpd < 0.001), but was not sensitive to differences in walking surface (pSur = 0.724). TTA exhibited smaller MOSmin on their prosthetic limb compared to intact limb (pLimb = 0.036), while Controls exhibited no significant between-limb differences (pLimb = 0.070; pGroup × Limb = 0.018). For TTA, the difference between the limbs decreased with increasing walking speed (pSpd×Limb = 0.021).
Figure 2. Margin of Stability.
Minimum margins of stability, MOSmin, and the within subject variability of MOSmin are shown for the control and TTA subjects. Data is shown for the rock surface (‘RS’) and level ground (‘LG’) at each of the four controlled walking speeds (shown from slowest to fastest). ‘×’ represent the right leg for the controls and the intact limb for the amputees. ‘○’ represent the left side for the controls and prosthetic limb for the amputees. Error bars represent ± 95 % confidence intervals about the mean.
There were no between-group differences in variability of MOSmin (pGroup = 0.907; Fig. 2B). There was a significant overall main effect for walking speed (pSpd = 0.011). Analyzing the groups separately, the decrease in MOSmin variability with increasing walking speed was significant in TTA (pSpd = 0.027) but not Control (pSpd = 0.545) subjects. For both groups, MOSmin variability was greater on the rock surface than level ground (pSur < 0.001).
There were no between-group differences in average step width (pGroup = 0.222; Fig. 3A). TTA exhibited greater step width variability than control subjects (pGroup < 0.001; Fig. 3B). Both groups increased step width (pSur = 0.017) and step width variability (pSur < 0.001) on the rock surface compared to level ground. TTA exhibited a greater difference in step width variability between walking surfaces than Control subjects (pGroup × Surface = 0.014; Fig. 3B). TTA also had greater step width variability when stepping onto their intact limb (pLimb = 0.001). Control subjects exhibited no between-limb differences (pLimb = 1.0; pGroup × Limb < 0.001).
Figure 3. Step Width Mean and Variability.
The average and within-subject variability of step width (SW) are shown for the control subjects and subjects with transtibial amputations (TTA). For TTA subjects, this was the prosthetic (‘P’) and intact (‘I’), while for controls this was right (‘R’) and left (‘L’). Subjects walked across a rock surface (RS; ‘×’) and over level ground (LG; ‘○’) at four controlled speeds (shown from slowest to fastest). Error bars represent ± 95 % confidence intervals about the mean.
At initial contact, TTA exhibited a greater distance between the lateral border of their base of support (BOS) and their COM than Controls (pGroup = 0.008; Fig 4A). There was a greater distance between the COM and BOS when TTA stepped on their intact limb, than their prosthetic limb (pLimb < 0.001). Both groups showed a significant decrease in distance between their COM and BOS with increased walking speed (pSpd ≤ 0.001). There were no differences between walking surfaces (pSur = 0.137) or between limbs of control subjects (PLimb = 0.178).
TTA displayed increased medial-lateral center of mass motion (COMM-L) during stance compared to Control subjects (Fig. 4B; pGroup < 0.001). COM motion decreased with increasing walking speed for both groups (pSpd < 0.001). There were significant Group × Limb (p = 0.002) and Group × Speed (p < 0.001) interactions. At the slower speeds, TTA had increased COM motion on their prosthetic limb compared to their intact limb (pLimb = 0.019, pLimb×Spd =0.023). There was no effect of walking surface on COM motion in either group (pSur > 0.165).
Peak COM velocity was greater in TTA than Control subjects (Fig 4C; pGroup = 0.006). Control subjects had greater peak velocity on the rock surface compared to level ground (pSur = 0.005), while TTA did not (pSur = 0.323). Peak COM velocity decreased with increasing walking speed for both groups (pSpd < 0.001). This decrease was greater on the rock surface than the level surface for TTA (pSpd×Sur = 0.003).
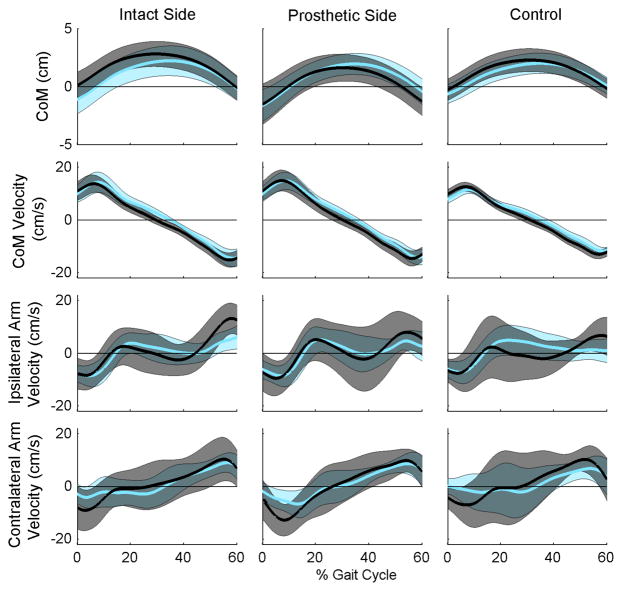
Both the magnitude and timing of peak lateral arm swing velocity were highly variable across subjects (Fig. 5). In many trials there was no clear velocity peak. The maximum lateral arm swing velocity prior to redirection of the COM was not different between TTA and control subjects for either the ipsilateral (pGroup = 0.766) or contralateral (pGroup = 0.574) arms. There were no main effects for surface (pSur > 0.135) or limb (pLimb > 0.075) and no significant interactions with ‘Group.’
Figure 5. Center of Mass Movement and Arm Swing Velocity.
Lines represent the average behavior across subjects during walking overground (cyan) and on the rock surface (black) at controlled speed 3. Bands represent the standard deviation across subjects. Control data is shown for the right side only. Positive values are toward the stance limb, Left) Intact, Middle) Prosthetic, and Right) Right limb of control subjects.
4. Discussion
This study compared medial-lateral center of mass control in persons with unilateral transtibial amputations (TTA) and able-bodied control subjects. We tested four hypotheses, only one of which was supported. Contrary to expectations, TTA exhibited greater margins of stability than able-bodied control subjects (Fig. 2A), indicating they were laterally more stable despite their lack of active ankle control. Both groups were also laterally more stable when walking at faster speeds, but equally stable on the different surfaces. The one hypothesis that was supported was that TTA exhibited smaller MOSmin (i.e., decreased stability) on their prosthetic limbs, while control subjects did not exhibit side-to-side differences in lateral stability.
4.1 Comparison across speeds
At the faster walking speeds, both groups exhibited decreased COM-to-BOS distance (Fig. 4A), medial-lateral COM range of motion (Fig. 4B), and peak COM velocity (Fig. 4C). When combined, these changes lead to increased mean medial-lateral MOSmin in both groups when they walked at faster speeds (Fig. 2A). Additionally, TTA also decreased variability (SD) of their medial-lateral MOSmin (Fig. 2B). The changes in medial-lateral COM range of motion (Fig. 4B) were consistent with previous work [17]. Hof et al. [18] also reported a trend (although not significant) toward increased medial-lateral MOSmin at faster walking speeds. While these findings suggest these subjects were laterally more stable at faster speeds, this does not necessarily imply they were “safer” in terms of risk of falling in general. Indeed, in the elderly, although slower walking speeds increase the risk of indoor falls, faster walking speeds increase the risk of outdoor falls [19–21], where irregular terrains abound. Elderly subjects who walk faster are also more likely to trip and fall [22], primarily due to the need for much faster response times [23]. The increase medial-lateral MOSmin observed at faster walking speeds here are more likely an adaptation these subjects adopt to mitigate any increased risk of falling, similar to the way the TTA also exhibited larger medial-lateral MOSmin than Controls (Fig. 2A), in spite of the fact that these patients have a higher risk of falling [1–4].
4.2 Comparison between surfaces
Mean MOSmin were not different between surfaces for either group (Fig. 2). This result, while seemingly counterintuitive, agrees with previous work in healthy individuals walking on irregular and compliant surfaces [7, 24], and TTA walking on an irregular surface [7]. There are several possible reasons for these results. First, the different surfaces used may not be difficult enough to challenge balance in these patients. This seems unlikely, however, especially as the loose rocks were not only irregular, but they also slide and/or sink underneath the feet. This likely requires subjects to make active adjustments to control lateral CoP position.
Another possibility is that subjects adapted their walking patterns to maintain a constant MOSmin in spite of any challenge surface provides. TTA increased their average step width and step width variability (Figs. 3A & 3B). Control subjects increased their COM velocity (Fig. 4C) and step width variability (Fig 3C). Neither the TTA nor Control subjects increased their peak arm swing velocity, however. Due to relative timing differences, arm swing velocity is not likely to be employed as a strategy to minimize the MOS. The minimum MOS typically occurred around 10% of the gait cycle, far sooner than any movement of the COM.
Finally, the average MOSmin may not be the most appropriate or sensitive measure of subtle challenges in walking stability. The goal during walking may not be to maximize MOSmin, but rather to maintain a sufficient MOSmin as to not lose balance. In this sense, the variability of MOSmin may reveal how well controlled this parameter is under the different conditions. Variability of MOSmin was greater when walking on the rock surface in both subject groups. Similarly, healthy individuals had similar average MOSmin but greater variability when walking on compliant foam compared to level ground [24]. Another possibility is that individuals do not attempt to control either the mean or variability of MOSmin, but rather how quickly they can correct a negative MOSmin [25]. The current experimental set-up did not allow us to quantify this since we could only record a few consecutive steps on each pass through the rock surface.
4.3 Comparison between limbs
Controls did not exhibit any side-to-side differences. TTA, on the other hand, exhibited differences between their intact and prosthetic limb for several dependent measures (Figs. 2–5). TTA had greater step width variability when stepping onto their intact limb. They also had greater COM range of motion when standing on their prosthetic limb, perhaps due to an inability to stabilize the CoP under the foot without ankle muscle control. Additionally, when TTA stepped onto their prosthetic limb, there was a reduced distance between the lateral border of the BOS and the COM. This likely contributed to the reduced MOSmin on their prosthetic compared to their intact limb, as it would require them to increase their COM velocity on the intact side to maintain similar MOSmin on both limbs.
4.4 Limitations
The TTA studied here were otherwise healthy and very active. Therefore, these results may not extrapolate all populations of TTA. Additionally, it was not possible to measure center of pressure when subjects walked on the rock surface. We therefore used the position of the 5th metatarsal marker to estimate the lateral border of the base of support on both surfaces. It is possible the subject groups differed in how their CoP moved underneath the foot during stance. For instance, Hof et al. [18] found that patients with transfemoral amputations were able to move their CoP away from the XcoM during stance, when standing on their intact limb, but not on their prosthetic side. With the current experimental paradigm, such differences were not detectable.
5. Conclusion
Patients with transtibial amputations and healthy control subjects altered their gait when walking on a rock surface by increasing their base of support and altering their foot placement. Both subject groups thus maintained similar average lateral MOSmin on level ground and a more challenging loose rock surface. Patients with transtibial amputations made different adjustments on their intact and prosthetic sides. Despite adjusting foot position and center of mass movement, they had decreased lateral MOSmin on their prosthetic side compared to their intact limb. TTA had greater average MOSmin than healthy controls. TTA did exhibit greater variability of both step width and MOSmin, which suggests they made larger step-to-step corrective responses, more often, to achieve the same average result.
Supplementary Material
Research Highlights.
Uneven walking surfaces disrupt balance in those with lower extremity amputation.
Patients with unilateral transtibial amputations walked on a loose rock surface.
Both patients and controls increased base of support and altered foot placement.
Both groups maintained similar lateral margins of stability on both surfaces.
Patients made larger step corrections, more often, to maintain lateral stability.
Acknowledgments
Partial support was provided by the Military Amputee Research Program (to JMW), a US Army Medical Specialty Corps Long-Term Health Education Training Fellowship (to SJS), and NIH grant 1-R01-HD059844 (to JBD). We would like to thank Emily Sinitski and Jordan Sturdy for their help with data processing.
Footnotes
The views expressed herein are those of the authors and do not reflect the official policy or position of Brooke Army Medical Center, the U.S. Army Medical Department, the U.S. Army Office of the Surgeon General, the Department of the Army, Department of Defense or the U.S. Government.
Conflict of Interest Statement:
The authors have no conflict of interest with this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kulkarni J, Toole C, Hirons R, Wright S, Morris J. Falls in patients with lower limb amputations: prevalence and contributing factors. Physiother. 1996;82:130–136. [Google Scholar]
- 2.Miller WC, Speechley M, Deathe B. The prevalence and risk factors of falling and fear of falling among lower extremity amputees. Arch Phys Med Rehabil. 2001;82:1031–1037. doi: 10.1053/apmr.2001.24295. [DOI] [PubMed] [Google Scholar]
- 3.Kendell C, Lemaire ED, Dudek NL, Kofman J. Indicators of dynamic stability in transtibial prosthesis users. Gait Posture. 2010;31:375–379. doi: 10.1016/j.gaitpost.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Ülger Ö, Topuz S, Bayramlar K, Erbahçeci F, Sener G. Risk factors, frequency, and causes of falling in geriatric persons who has had a limb removed by amputation. Top Ger Rehabil. 2010;26:156–163. [Google Scholar]
- 5.Winter DA, Prince F, Frank JS, Powell C, Zabjek KF. Unified theory regarding A/P and M/L balance in quiet stance. J Neurophysiol. 1996;75:2334–2343. doi: 10.1152/jn.1996.75.6.2334. [DOI] [PubMed] [Google Scholar]
- 6.Viton JM, Mouchnino L, Mille ML, Cincera M, Delarque A, Pedotti A, Bardot A, Massion J. Equilibrium and movement control strategies in trans-tibial amputees. Prosthet Orthot Int. 2000;24:108–116. doi: 10.1080/03093640008726533. [DOI] [PubMed] [Google Scholar]
- 7.Curtze C, Hof AL, Postema K, Otten B. Over rough and smooth: Amputee gait on an irregular surface. Gait Posture. 2011;33:292–296. doi: 10.1016/j.gaitpost.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 8.Lamoth CJ, Ainsworth E, Polomski W, Houdijk H. Variability and stability analysis of walking of transfemoral amputees. Med Eng Phys. 2010;32:1009–1014. doi: 10.1016/j.medengphy.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Wilken JM, Rodriguez KM, Brawner M, Darter BJ. Reliability and Minimal Detectible Change values for gait kinematics and kinetics in healthy adults. Gait Posture. 2012;35:301–307. doi: 10.1016/j.gaitpost.2011.09.105. [DOI] [PubMed] [Google Scholar]
- 10.Vaughan CL, O’Malley MJ. Froude and the contribution of naval architecture to our understanding of bipedal locomotion. Gait Posture. 2005;21:350–362. doi: 10.1016/j.gaitpost.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Gates DH, Wilken JM, Scott SJ, Sinitski EH, Dingwell JB. Kinematic adaptations when walking over an uneven rock surface. Gait Posture. 2012;35:36–42. doi: 10.1016/j.gaitpost.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gates DH, Dingwell JB, Scott SJ, Sinitski EH, Wilken JM. Gait characteristics of individuals with transtibial amputation walking on a destabilizing rock surface. Gait Posture. 2012;36:33–39. doi: 10.1016/j.gaitpost.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dempster WT, Gabel WC, Felts WJ. The anthropometry of the manual work space for the seated subject. Am J Phys Anthropol. 1959;17:289–317. doi: 10.1002/ajpa.1330170405. [DOI] [PubMed] [Google Scholar]
- 14.Zeni JA, Jr, Richards JG, Higginson JS. Two simple methods for determining gait events during treadmill and overground walking using kinematic data. Gait Posture. 2008;27:710–714. doi: 10.1016/j.gaitpost.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hof AL, Gazendam MG, Sinke WE. The condition for dynamic stability. J Biomech. 2005;38:1–8. doi: 10.1016/j.jbiomech.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 16.Courtine G, Schieppati M. Human walking along a curved path. I. Body trajectory, segment orientation and the effect of vision. Eur J Neurosci. 2003;18:177–190. doi: 10.1046/j.1460-9568.2003.02736.x. [DOI] [PubMed] [Google Scholar]
- 17.Orendurff MS, Segal AD, Klute GK, Berge JS, Rohr ES, Kadel NJ. The effect of walking speed on center of mass displacement. J Rehabil Res Dev. 2004;41:829–834. doi: 10.1682/jrrd.2003.10.0150. [DOI] [PubMed] [Google Scholar]
- 18.Hof AL, van Bockel RM, Schoppen T, Postema K. Control of lateral balance in walking. Experimental findings in normal subjects and above-knee amputees. Gait Posture. 2007;25:250–258. doi: 10.1016/j.gaitpost.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Bergland A, Jarnlo GB, Laake K. Predictors of falls in the elderly by location. Aging Clin Exp Res. 2003;15:43–50. doi: 10.1007/BF03324479. [DOI] [PubMed] [Google Scholar]
- 20.Quach L, Galica AM, Jones RN, Procter-Gray E, Manor B, Hannan MT, Lipsitz LA. The nnonlinear relationship between gait speed and falls: The maintenance of balance, independent living, intellect, and zest in the elderly of Boston study. J Am Ger Soc. 2011;59:1069–1073. doi: 10.1111/j.1532-5415.2011.03408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelsey JL, Procter-Gray E, Hannan MT, Li W. Heterogeneity of falls among older adults: Implications for public health prevention. Am J Public Health. 2012;102:2149–2156. doi: 10.2105/AJPH.2012.300677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pavol MJ, Owings TM, Foley KT, Grabiner MD. Gait characteristics as risk factors for falling from trips induced in older adults. J Gerontol A Biol Sci Med Sci. 1999;54A:M583–M590. doi: 10.1093/gerona/54.11.m583. [DOI] [PubMed] [Google Scholar]
- 23.van den Bogert AJ, Pavol MJ, Grabiner MD. Response time is more important than walking speed for the ability of older adults to avoid a fall after a trip. J Biomech. 2002;35:199–205. doi: 10.1016/s0021-9290(01)00198-1. [DOI] [PubMed] [Google Scholar]
- 24.MacLellan MJ, Patla AE. Adaptations of walking pattern on a compliant surface to regulate dynamic stability. Exp Brain Res. 2006;173:521–530. doi: 10.1007/s00221-006-0399-5. [DOI] [PubMed] [Google Scholar]
- 25.McAndrew PM, Wilken JM, Dingwell JB. Dynamic stability of human walking in visually and mechanically destabilizing environments. J Biomech. 2011;44:644–649. doi: 10.1016/j.jbiomech.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.