Abstract
Aims
To determine the characteristics of the late Na current (INaL) and its arrhythmogenic potential in the progression of pressure-induced heart disease.
Methods and Results
Transverse aortic constriction (TAC) was used to induce pressure overload in mice. After one week the hearts developed isolated hypertrophy with preserved systolic contractility. In patch-clamp experiments both, INaL and the action potential duration (APD90) were unchanged.
In contrast, after five weeks animals developed heart failure with prolonged APDs and the slowed INaL decay time which could be normalized by addition of the INaL inhibitor ranolazine (Ran) or by the Ca/calmodulin-dependent protein kinase II (CaMKII) inhibitor AIP. Accordingly the APD90 could be significantly abbreviated by Ran, tetrodotoxin and the CaMKII inhibitor AIP. Isoproterenol increased the number of delayed afterdepolarizations (DAD) in myocytes from failing but not sham hearts. Application of either Ran or AIP prevented the occurrence of DADs. Moreover, the incidence of triggered activity was significantly increased in TAC myocytes and was largely prevented by Ran and AIP.
Western blot analyses indicate that increased CaMKII activity and a hyperphosphorylation of the Nav1.5 at the CaMKII phosphorylation site (Ser571) paralleled our functional observations five weeks after TAC surgery.
Conclusion
In pressure overload-induced heart failure a CaMKII-dependent augmentation of INaL plays a crucial role in the AP prolongation and generation of cellular arrhythmogenic triggers, which cannot yet be found in early and still compensated hypertrophy. Inhibition of INaL and CaMKII exert potent antiarrhythmic effects and might therefore be of potential therapeutic interest.
Keywords: Heart failure, hypertrophy, arrhythmias, INaL, CaMKII, ranolazine
Introduction
Patients with heart failure either die from pump failure or life threatening arrhythmias. Major and well accepted determinants of electrical remodelling in heart failure include prolongation of the cardiac action potential (AP) and an increased sarcoplasmatic reticulum (SR) Ca-leak occurring as spontaneous SR Ca-release events [1]. Both arrhythmic triggers are known to decrease the threshold of cardiac arrhythmias. A persistent Na current, also known as late Na current (INaL), has been discussed to be a potent contributor to the progression and complications of heart failure [2-10]. Under physiologic conditions Na channels open transiently and are quickly inactivated thereby generating the peak Na current (peak INa) which is responsible for the upstroke of the cardiac AP. However, some Na channels close and reopen or remain active, carrying INaL which persists throughout the whole AP. Although the amplitude of this current is very small compared to peak INa, the long persistence and its slow inactivation kinetics make this current substantial when elevated as it has been shown in pathological conditions like heart failure, ischemic metabolites, hypoxia, or pathological redox-signaling [6, 9, 11-13].
INaL is regarded as a relevant contributor to arrhythmias and its inhibition might be of antiarrhythmic potential (for review see [8, 14]). As INaL generates Na influx and thereby an inward current throughout the AP, it is expected to contribute to the prolongation of the cardiac AP making early afterdepolarizations (EADs) more likely to occur [7, 15]. Moreover, cellular Na cycling is tightly integrated with Ca homeostasis, as Na modulates the transport direction of the Na/Ca exchanger (NCX). Cellular Na overload as well as AP-prolongation stimulate the reverse mode NCX facilitating the efflux of Na in exchange for influx of Ca [1]. The net result is an elevated diastolic Ca concentration. Additionally, the cardiac ryanodine receptor (RyR2) was shown to be “leaky” in heart failure. Spontaneous SR Ca-release events, the so called Ca-sparks, occur [1, 16] and contribute to the elevation of diastolic Calevels. Ca, once released by spontaneously opening RyR2s, can be eliminated via the NCX, which possibly generates a depolarizing current (transient inward current, ITI) that gives rise to delayed afterdepolarizations (DADs) [7, 9, 17]. Thus, an elevation of diastolic [Ca]i and changes of the cardiac RyR2 open propensity are arrhythmogenic mechanisms in failing ventricular cardiomyocytes.
There is strong evidence that INaL is increased in animal models of heart failure as well as in human heart failure [5, 9, 11, 12, 15]. However, very little is known about the role and impact of INaL in isolated cardiac hypertrophy with preserved contractility as it commonly occurs in patients suffering from hypertensive heart disease as well as from aortic stenosis. Therefore one objective of the present study was to investigate changes in INaL in the course from pressure-induced hypertrophy with preserved contractility to heart failure.
Moreover, there is controversy regarding the quantitative contribution of an increased INaL to cause AP prolongation in heart failure, and even more so in hypertrophy. As INaL induces Na-dependent Ca-overload, the impact of INaL on DADs remains to be elucidated in a pathophysiologically relevant animal model in the absence of pharmacologic INaL inducers. Therefore, the second objective of our study was to explore the role of INaL as a contributor to AP prolongation and its consequences for generation of EADs or DADs in isolated hypertrophy with preserved contractility and in heart failure.
Furthermore, we were also interested in the underlying mechanism and possible pharmacological treatment.
Methods
An expanded Methods section is available in the Online Data Supplement.
The investigation conforms to the Guide for the Care and Use of Laboratory Animals (NIH publication No. 85–23, revised 1996) and was approved by a local ethics review board and by the Veterinary Institute of the Lower Saxony State Office for Consumer Protection and Food Safety (G10/220).
2.1. Transverse aortic constriction (TAC) and echocardiography
8 weeks old female C57/BL6J mice were anesthetized using intraperitoneal injections of ketamine and xylazine (100 mg/kg + 5 mg/kg) and pressure overload was induced by transversal aortic contstriction (27G needle). For analgesia (metamizole 1.33 mg/ml) was added to the drinking water 2 days before surgery and continued for 7 days after operation.
Transthoracic echocardiography was performed blinded using a Vevo2100 (VisualSonics, Toronto, Canada) system with a 30 MHz center frequency transducer. The animals were anesthetized with 3% isoflurane, and temperature-, respiration-, and ECG-controlled anesthesia was maintained with 1.5% isoflurane. Maximal left ventricular length (L), thicknesses of the septum, the posterior myocardial wall, the inner diameter of the left ventricle (LVEDD) and the area of the left ventricular cavity (Area) were measured according to standard procedures. The ejection fraction (EF) was calculated using the area-length method.
After completion of the experiments mice were killed in isofluran anaesthesia (5%) by cervical dislocation.
2.2. Cell isolation
The excised hearts were mounted on a Langendorff perfusion apparatus and were retrogradely perfused. Cardiomyocytes were isolated with liberase 1 (Roche diagnostics, Mannheim, Germany) and trypsin 0.6% digestion and were plated onto superfusion chambers. The glass inlays had been pretreated with laminin to allow cell adhesion and were then used for immediate measurements.
2.3. Patch-clamp experiments
Ruptured-patch whole-cell voltage- and current-clamp was used to measure action potentials and INaL as described previously [18, 19]. Measurements were performed at increasing stimulation frequencies to elicit Na currents or action potentials (APs). For Na current measurements myocytes were held at −120 mV and INaL was elicited using 250 ms depolarizing pulses to −20 mV. Each pulse was preceded by a 5 ms pre-pulse to +50 mV in order to optimize voltage control. The measured currents were normalized to the membrane capacitance. INa decay (first 200 ms) was fitted using a double exponential function y (t) = A1 exp (–t/τ1) + A2 exp (–t/τ2) + y0 as it was done previously [5, 18, 19].
For action potential recordings, low-resistance pipettes were used. Resting cell membrane potentials were similar in WT (−65±0.94 mV), TAC (compensated hypertrophy) (−64.86±0.63 mV) and in TAC (heart failure) (−64.94±0.77 mV) ventricular myocytes All patch-clamp experiments were conducted at room temperature.
2.4. Confocal microscopy
Cardiomyocytes were incubated with a Fluo-3 AM loading buffer. Experimental solution contained (mmol/L): NaCl 136, KCl 4, NaH2PO4 0.33, NaHCO3 4, CaCl2 2, MgCl2 1.6, HEPES 10, glucose 10 (pH 7.4, NaOH, room temperature) as well as 10−8 mol/L isoproterenol and the respective drugs. Cardiomyocytes were continuously superfused during experiments after washing out the loading buffer and any extracellular dye. Ca-spark measurements were performed with a laser scanning confocal microscope (LSM 5 Pascal, Zeiss, Jena, Germany) using a 40x oil-immersion objective. Fluo-3 was excited by an argon ion laser (488 nm) and emitted fluorescence was collected through a 505 nm long-pass emission filter. Fluorescence images were recorded in the line-scan mode with 512 pixels per line (width of each scanline: 38.4 μm) and a pixel time of 0.64 μs. One image consists of 10,000 unidirectional line scans, equating to a measurement period of 7.68 s. Experiments were conducted at resting conditions after loading the SR with Ca by repetitive field stimulation at 4 Hz. Ca-sparks were analyzed with the program SparkMaster for Image. The mean spark frequency of the respective cell (CaSpF) resulted from the number of sparks normalized to cell width and scan rate (100 μm−1*s−1). Spark size (CaSpS) was calculated as product of spark amplitude (F/F0), duration and width. From this, we inferred the average leak per cell by multiplication of CaSpS with CaSpF.
2.5. Drugs
10 μmol/L Ran ([+]N-(2,6-dimethylphenyl)-4-[2-hydroxy-3-(2-methoxyphenoxy)-propyl)-1-piperazine acetamide dihydrochloride]) was used because it is within the range of therapeutic plasma levels and inhibitory concentrations of 50% for inhibition of INaL (6 to 15 μmol/l), which does not significantly inhibit ICa, INa/Ca, or IKs [10]. Tetrodotoxin (TTX) selectively blocks INa and at low concentrations relatively selective for INaL [15]. Thus, we used TTX concentrations of 2 μmol/L. Autocamide-2-related Inhibitory Peptide (AIP, 1 μmol/L) was used to selectively inhibit Ca/calmodulin-dependent protein kinase II (CaMKII). Isoproterenol was used at a concentration of 10−8 mol/L.
2.6. Western blots
Myocardium was homogenized and protein concentration was determined by BCA assay (Pierce Biotechnology, Rockford, USA). Denatured tissue homogenates (on ice in 2% beta-mercaptoethanol) were subjected to Western blotting (5%, 8% or 12.5% SDS-polyacrylamide gels). The antibodies used are shown in the online supplement. Chemiluminescent detection was done with Immobilion Western Chemiluminescent HRP Substrate (Millipore, Billerica, USA).
2.7. Data analysis and statistics
All data are presented as mean ± S.E.M. Student’s t-test, 2-way or 1-way repeated measures ANOVA (RM-ANOVA) with post-hoc tests were used to test for significance. A two-sided P value of <0.05 was considered significant. Blinded procedures were performed in case of echocardiographic measurements due to the variability of this technique. All other experiments were performed in an unblinded manner.
Results
3.1 Mouse cardiac phenotype
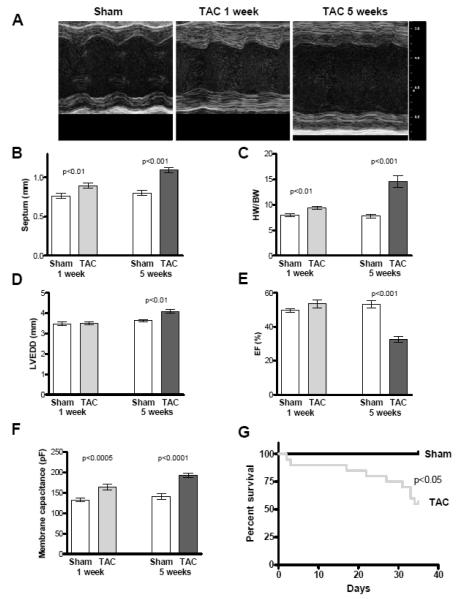
One week after TAC concentric hypertrophy was detectable, indicated by increases of septum width by 14% (sham, n=6, 0.76±0.04 mm vs. TAC, n=10, 0.87±0.05 mm; p<0.001, Figure 1B) and heart weight by 18% (HW/BW: sham 8.0±0.3 vs. TAC 9.4±0.4; p<0.001; Figure 1C). At this time point contractile function was still preserved (ejection fraction, EF: sham 49±3% vs. TAC 53±7%; p<0.16; Figure 1E).
Figure 1. Echocardiographic data.
Cardiac phenotype characteristics. A Representative echocardiographic recordings of sham and TAC mice 1 and 5 weeks after intervention (1 week: sham n=6, TAC n=10, 5 weeks: sham n=10, TAC n=15). B Mean values of septum width. C Mean values of heart weight / body weight ratio (HW/BW). D Mean values of ejection fraction (EF). E Mean values of left ventricular end-diastolic diameter (LVEDD). F Mean values of membrane capacitances. G Kaplan-Meier survival curve of sham and TAC animals which does not include mortalility within the first 24 hrs post-TAC surgery.
Five weeks after TAC surgery cardiac mass was further increased by 87% (HW/BW ratio: sham 7.8±0.9 vs. TAC 14.6±3.1; p<0.001; Figure 1C) and the animals showed signs of heart failure. Left ventricular EF was reduced to 33±7% in TAC compared to 53±6% in sham animals (p<0.001; Figure 1E). Dilatation as measured by an increased LVEDD by 12% was observed in TAC compared to sham hearts (4.1±0.3 mm vs. 3.6±0.2 mm p<0.001; Figure 1D).
Kaplan-Meier analysis of animal survival showed an increased mortality within the first days after surgery. This is followed by a stable phase with no further deaths until heart failure develops between weeks three-five after TAC intervention (Figure 1G). In this range animals were at higher risk to die and thus we investigated the role of the INaL at two time points, which are one and five weeks after surgery.
3.2 INaL in compensated hypertrophy and heart failure
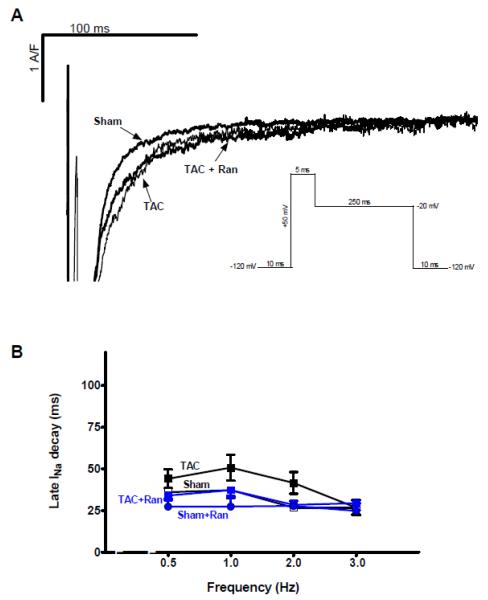
One week after TAC intervention cardiomyocytes did not show significant changes of INaL compared to sham myocytes (Figure 2A). At 2 Hz INaL decay in TAC myocytes was 41.6±6.5 vs. 26.7±1.8 ms (at 3 Hz: 26.7±4.5 vs. 26.8±4.2 ms) in cells from hearts of animals that underwent sham surgery (n=17 vs. 11, p=n.s., Figure 2B). Moreover, Ran had no significant effect on the small INaL of either groups (n=5 and 6, p=0.2). In contrast, at five weeks after TAC surgery, INaL was significantly increased as indicated by a slower decay time (Figure 3A). At 2 Hz INaL decay time was slowed to 96.4±12.6 ms compared to 35.4±4.1 ms in sham myocytes (p<0.05, n=26 vs.7, Figure 3B). Superfusion with Ran, TTX as well as AIP significantly reversed the TAC-induced effect on INaL decay time (51.8±5.3 ms (n=17), 47.1±6.3 ms (n=15) and 29.3±2.7 ms (n=18), p<0.05 vs. TAC). Again, Ran had no significant influence on the small INaL recorded at five weeks in sham myocytes compared to controls (sham+Ran 35.4±5.0 ms vs. control 35.4±4.1 ms, n=8 vs.7).
Figure 2. Effects of TAC on late INa after one week.
Measurements of INaL after one week of pressure overload. A Original recordings showing INaL in sham and TAC cells paced at 0.5 Hz in the presence of vehicle or ranolazine. B Mean values of INaL decay time in the corresponding groups (TAC 20, sham 14, TAC+Ran 7, sham+Ran 6 cells, no statistical differences between the groups using 2-way RM ANOVA).
Figure 3. Effects of TAC on late INa after 5 weeks.
Measurements of INaL after five weeks of TAC surgery. A Original tracings showing recorded INaL in sham and TAC cells in the presence of either vehicle, Ran, TTX, and AIP (in TAC) paced at 0.5 Hz. B Mean values of INaL decay time in the corresponding groups. There was a statistical difference between INaL decay time of TAC vs. sham (n=26 vs.7), TAC vs. TAC+Ran (n=17), TAC vs. TAC+TTX (n=16), TAC vs. TAC+AIP (n=18) vs. TAC. *=p<0.05 vs. sham #=p<0.05 vs. TAC
3.3 Role of INaL on action potential duration
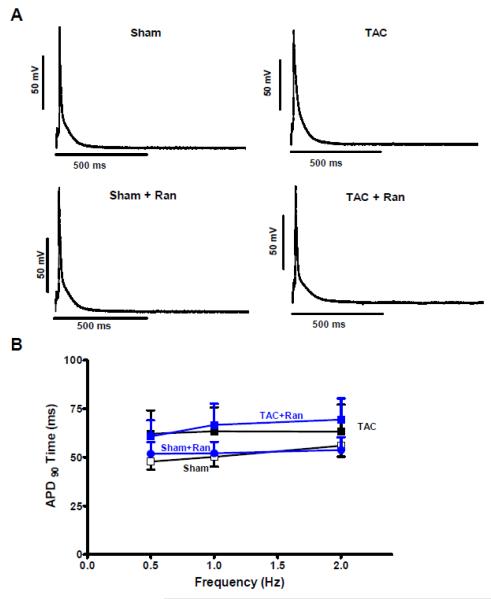
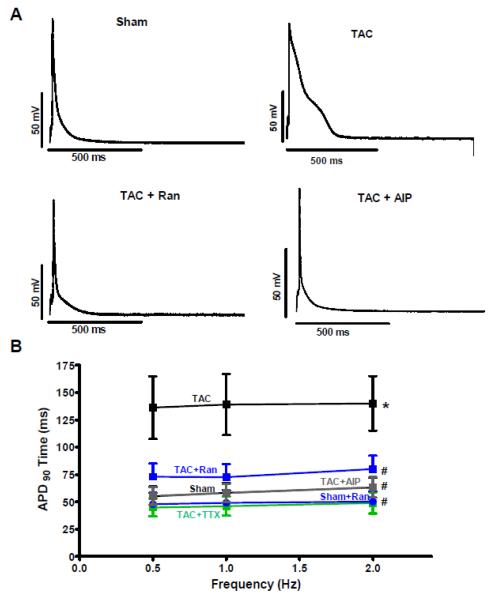
To determine the arrhythmogenic role of an increased INaL we measured APs in the groups stated above. In accordance to the INaL results we did not find significant prolongations of APs in myocytes isolated from animals one week after TAC surgery (Figure 4 A and B). However, five weeks post TAC surgery the AP prolongation became noticeable. This is illustrated in figure 5A where a representative original recording shows a markedly prolonged AP in a cell isolated from a TAC animal. APD90 at 2 Hz was increased to 140.0±24.9 ms compared to 63.1±9.1 ms in myocytes isolated from sham animals (n=15 vs. 8, RM-ANOVA p<0.05, Figure 5B). In these TAC myocytes with prolonged APD, Ran caused a significant abbreviation of APD90 to 80.0±12.5 ms (n=19) while it did not shorten APD of sham myocytes (n=8). Moreover, to confirm that these effects were really due to INaL inhibition we also performed experiments in the presence of TTX. As shown in figure 5B the APD90 could also be reduced to 49.0±9.8 ms in the presence of 2 μmol/L TTX compared to TAC control (RM-ANOVA p<0.05, n=10) and was not statistical different compared to that of Ran-treated cells (Figure 5B). Moreover, using AIP as a CaMKII-inhibitor APDs could be abbreviated to 63.4±9.3 ms in cardiomyocytes that were isolated from animals five weeks after TAC surgery (n=18, p<0.05).
Figure 4. Effects of TAC on action potentials after 1 week.
Measurements of APs after one week of TAC intervention. A Original tracings showing recorded APs in sham and TAC cells in the presence of vehicle or Ran paced at 0.5 Hz. B Mean values of APD (90%) in the corresponding groups (TAC 7, sham 12, TAC+Ran 19, sham+Ran 15 cells, no statistical differences between the groups using 2-way RM ANOVA).
Figure 5. Effects of TAC on action potentials after 5 week.
Measurements of APs in cells isolated from animals five weeks after TAC surgery. A Original tracings showing recorded action potentials in sham and TAC cells in the presence of vehicle, Ran, and AIP paced at 0.5 Hz. B Mean values of (APD90) in the corresponding groups. (TAC 15, sham 8, TAC+Ran 19, TAC+AIP 18, TAC+TTX 10 cells. *=p<0.05 vs. sham #=p<0.05 vs. TAC
3.4 Potential Arrhythmogenic triggers in pressure overload-induced heart failure
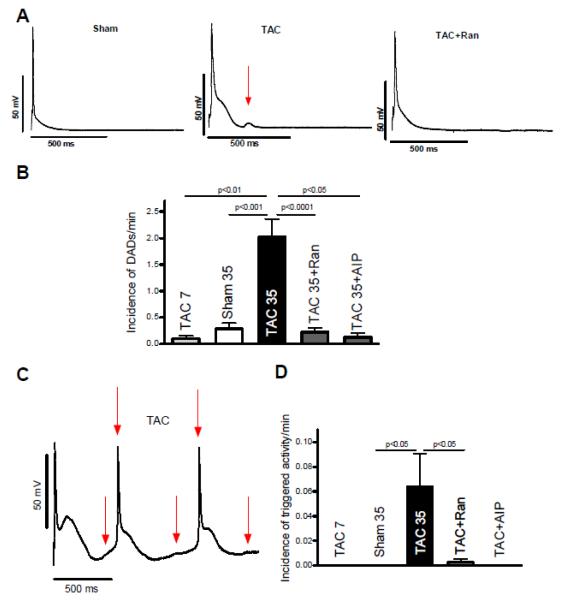
To further investigate the contribution of INaL on cellular arrhythmias we exposed cardiomyocytes isolated from animals five weeks after TAC surgery to 10−8 mol/L isoproterenol for 5 minutes. Interestingly, we did not observe clear EADs in the presence of isoproterenol. However, there was a large number of DADs (Figure 6A) with an incidence of 2.0±0.1/min in TAC compared to 0.3±0.1 DADs/min in sham cells (n=20 vs. 15, p<0.05, Figure 6B). This is important in so far that in our model DADs seem to be the major cellular arrhythmic triggers. In the presence of either Ran or AIP the incidence of DADs largely decreased to values of 0.2±0.1/min and 0.12±0.1(n=15 and 8, p<0.05, Figure 6A and B). Moreover, triggered activity never occurred in sham myocytes but was present in TAC cells with an incidence of 0.064±0.025/min (n=15 vs. 20, p<0.05 vs. sham, Figure 6C and D). When adding Ran to the bath solution triggered activity was suppressed to 0.003±0.003/min (p<0.05, n=16). Triggered activity did not occur in the presence of AIP in TAC myocytes (n=5, p<0.05 vs. TAC).
Figure 6. Arrhythmic triggers and SR Ca-leak.
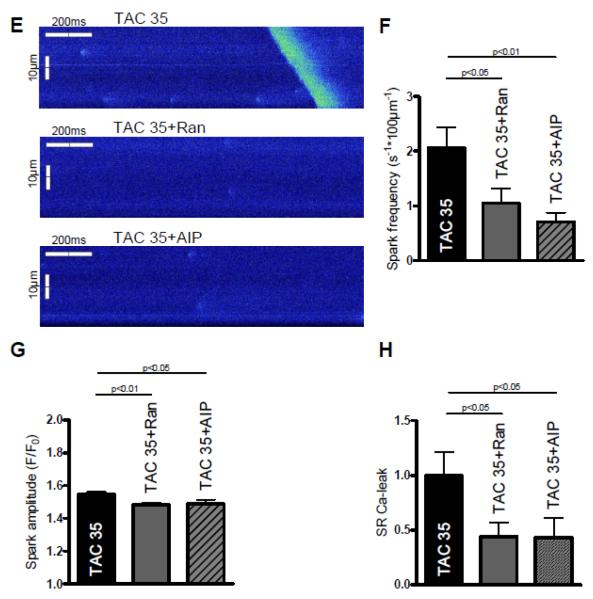
Measurements of APs five weeks after TAC surgery in the presence of 10−8 mol/L isoproterenol. A Original tracing of APs in the presence of isoproterenol. Red arrows indicate DADs. B Mean values of DAD-incidence in sham (n=15), TAC (n=20), Ran-treated (n=15) and AIP-treated TAC cells (n=5). C Original tracing of spontaneous APs in the presence of isoproterenol. Red arrows indicate triggered activity (unstimulated APs). D Mean values of triggered activity (meaning unstimulated APs). E Representative confocal line scans of cardiomyocytes under control conditions (above) as well as upon ranolazine (middle) and AIP-treatment (below) showing Ca-sparks and a diastolic Ca-wave (in control group). F Inhibition of INaL by ranolazine as well as CaMKII inhibition by AIP significantly decrease the frequency of diastolic Ca-sparks (n=30-38). G The amplitude of diastolic Ca-sparks is significantly reduced in the presence of ranolazine (n=80 vs. 128, control) as well as after CaMKII-inhibition with AIP (n=41). H The inhibitors yield a significant decrease of the resulting calculated SR Ca-leak (CaSpF − amplitude − duration − width, normalized to control group).
To further elucidate the underlying mechanism of DADs and triggered activity we performed measurements of spontaneous SR Ca-release events, the so called SR Ca-sparks. Figure 6F shows that the high SR Ca-spark frequency of cardiomyocytes isolated from animals five weeks after TAC surgery could be significantly suppressed from 2.1±0.4 to 1.1±0.3 s−1*100μm−1 upon Ran- and to 0.7±0.2 s−1*100μm−1 upon AIP-treatment (n=31 vs. 38 vs. 30, p<0.05). Accordingly, the calculated SR Ca-leak was largely decreased by either inhibition of INaL or CaMKII, which was mainly driven by a reduced SR Ca-spark frequency in the presence of the respective inhibitor (Figure 6H).
3.5 Changes in Na channel expression and regulation
It has been reported that INaL is modulated by the expression of different α- and β-Na channel subunits and by phosphorylation of the α1.5-isoform by CaMKIIδ [2, 8, 20], [21, 22].
At the stage of compensated hypertrophy (one week after TAC surgery) the expression of the neuronal Na channel Nav1.1 was slightly downregulated by 23% (p<0.05) while Nav1.6- was not significantly changed. The expression of the cardiac Na channel Nav1.5 was increased dramatically by 192% (p<0.05) and the beta1-subunit was upregulated by 60% (p<0.05). CaMKIIδ activity which was measured by phosphorylation at Thr-286 was increased by 45% one week after TAC surgery (p<0.05) while Nav1.5 phosphorylation at Ser571 was not significantly altered (Figure 7A-G).
Figure 7.
Protein analysis of CaMKIIδ and Na channel isoforms (n=6 per group and time point). A Exemplary western blots. B Mean values of Na channel isoform α1.1 normalised to GAPDH. C Mean values of Na channel isoform α1.5 normalised to GAPDH. D Mean values of Na channel isoform α1.6 normalised to GAPDH. E Mean values of beta1 subunit normalised to GAPDH. F Mean values of phosphorylated CaMKIIδ (at Thr-287) normalised to GAPDH. G Mean values of phosphorylated Nav1.5at Ser571 (CaMKII phosphorylation site) normalized to total Nav1.5.
The Nav1.1 was upregulated by 71% (p<0.05), whereas Nav1.6 was reduced by 29% (p<0.05) five weeks after TAC intervention. The Nav1.5, again, was upregulated by 57% (p<0.05) and the beta1-subunit showed an increase of 65% (p<0.05). Most importantly, the CaMKIIδ activity was elevated by 108% (p<0.05) and the phosphorylation of Nav1.5 at Ser571 was increased by 85% (p<0.05, Figure 7A-G) Comparing compensated hypertrophy (one week) and heart failure (five weeks after TAC surgery) the following differences were noted. 1) The Nav1.5 is even more upregulated one week after TAC compared to five weeks after TAC intervention (−70%, p<0.01); 2) The Nav1.1 expression changes from downregulated to upregulated (TAC 1 vs. 5 weeks: +185%, p<0.05); 3) CaMKIIδ activity shows a further increase (TAC 1 vs. 5 weeks, +40%, p<0.05) and 4) The Nav1.5 becomes hyperphosphorylated at the CaMKII-phosphorylation site Ser571 in hearts after five weeks but not after one week of TAC.
Discussion
The results of the present study show that 1) pressure overload-induced hypertrophy with preserved ejection fraction is neither associated with an increased INaL nor a significant prolongation of the APD. 2) In pressure overload-induced heart failure INaL is increased as indicated by a slowed decay time; 3) This increase in INaL contributes significantly to the prolonged APD as demonstrated by the effects of INaL inhibitors. 4) Failing cells developed DADs and triggered activity, which were likely caused by an increased SR Ca-leak under beta adrenergic stress. Cellular arrhythmogenic triggers could be largely prevented by either inhibition of INaL or CaMKII. 5) Slowed INaL decay time, APD prolongation and cellular arrhythmogenic triggers occurred in a CaMKII-dependent manner. 6) Western blot analyses showed an increased activity of CaMKII with a consecutive hyperphosphorylation of the Nav1.5 at Ser571 and an upregulation of the neuronal Na channel Nav1.1 in hearts after 5 weeks of TAC.
4.1 Animal model of hypertrophy and heart failure
Most of the work on INaL in combination with Ran as an inhibitor of this current has been done in models of myocardial ischemia or with pharmacological enhancers of INaL (e.g. ATX-II). We now provide data in a non-ischemic pressure overload-induced mouse model. One week after TAC surgery mice showed compensated hypertrophy with preserved systolic function and no increase in mortality, which indirectly indicates the absence of lethal arrhythmias.
Mortality increases with time during pressure overload condtions. This observation might be explained by the development of heart failure because EF markedly decreases five weeks after TAC intervention. The reason for the deaths might, however, also be lethal arrhythmias. To the best of our knowledge, telemetric recordings during the development of pressure overload-induced heart failure in mouse models have not been published so far. However, in-vivo electrophysiological measurements showed an increased rate of ventricular arrhythmias [23].
4.2 INaL and action potential duration in hypertrophy
Little is known about the INaL in pressure-induced hypertrophy, particularly in animals with preserved contractility. In hypertrophy and in the absence of heart failure (1 week of TAC) we did not find a significant increase of INaL. Likewise, these cells did not show prolonged APD, which is consistent with an unchanged INaL. Moreover, these animals had no increased risk to die suggesting that unchanged INaL and normal APD are associated with a smaller risk of sudden death. However, there are also reports of prolonged APs in pressure overload-induced heart disease [24]. Many studies did not differentiate between hypertrophy and heart failure which makes it difficult to interpret and to compare the results of these studies to the present report. Moreover, the severity of hypertrophy depends on the technique and the used protocol (e.g. needle size and time after TAC surgery). Thus, a direct comparison of results of different TAC studies is not straightforward. For example we also found a prolongation of the APD seven days after TAC operation in our previous work [24]. This difference might be explained by use of a different mouse line (FVBN previously vs. C57/BL6 here) and different age of investigated mice (12 vs. 8 weeks). The difference is also visible in the degree of hypertrophy (septum width: +18% previously vs. 14% here). This suggests that in this model the prolongation of the APD and the increased INaL are not present in compensated hypertrophy but might be one of the early changes during the development of higher degrees of hypertrophy and heart failure. In our previous work we did not investigate cardiomyocytes isolated from TAC-mice with heart failure. Moreover, INaL and its implication for APD prolongation and for cellular arrhythmogenic triggers were evaluated in the course of the current study. Finally, the findings of a crosstalk between INaL and CaMKII in our model of afterload-induced cardiac disease have not been described before.
4.3 Role of INaL in AP prolongation in heart failure
One abnormality most consistently reported in heart failure is the prolongation of the cardiac AP. This prolongation of AP may be caused by increases in depolarizing currents such as L-type Ca or NCX current as well as due to changes in Na transport proteins (e.g. Na channels, Na/K-ATPase and Na/H-exchanger). In addition, AP prolongation in heart failure is associated with a decrease in repolarizing K currents. However, the nature of the underlying ionic currents that are responsible for APD prolongation in heart failure remains a matter of debate. In fact, an increase in INaL of ventricular myocytes of failing hearts (including humans) has been reported [2, 4, 5, 9, 12, 15].
We now provide further evidence that INaL may prominently contribute to AP prolongation in pressure-induced heart failure. Our results clearly show that INaL decay slows in parallel to AP prolongation five weeks after TAC surgery. We further demonstrate the INaL-dependence of AP prolongation by performing experiments using inhibitors of INaL. Ran is known to preferentially inhibit the late component of INa vs. peak INa but is also known to inhibit IKr with an IC50 of ~11.5 μmol/L [25]. Thus to ascertain the role of INaL we performed experiments using TTX, as TTX specifically blocks Na channels with negligible effects on other ion current and transport systems. Both agents significantly reversed the slowed INaL decay time in parallel with a prominent abbreviation of the cardiac AP in myocytes isolated from animals five weeks after TAC surgery. Thus, it can be suggested that INaL is a major contributor to AP prolongation in pressure overload-induced heart failure.
Similar results were found by Maltsev and coworkers in myocytes isolated from human failing hearts [9]. In their experiments APD could be abbreviated up to ~75% by applying TTX to failing myocytes. Also two specific Na channel blockers (saxitonin and lidocaine) reduce APD in isolated cardiomyocytes from an ischemic dog heart failure model [26]. Similar results were reported by Undrovinas et al. using Ran in cells isolated from an ischemic heart failure model [27]. Moreover, a recent study also indicates that AP prolongation of myocytes from pressure overload-induced failing rat hearts could be attenuated by TTX [28]. Finally, several studies have consistently shown that pharmacological augmentation of INaL e.g. using ATX-II or ROS results in marked prolongation of the cardiac AP [6, 7, 29, 30], which could be blunted by application of Ran or TTX. Therefore, there is strong and growing evidence that elevation of INaL plays a major role in the prolongation of cardiac AP in the progression of pressure-induced heart failure.
The underlying mechanisms of INaL induction in the failing cardiomyocyte are still unclear. We now provide evidence that the prominent INaL in our mouse model of afterload-induced heart failure is CaMKII-dependent. The CaMKII inhibitor AIP significantly reversed the slowed INaL decay time und abbreviated APDs in cardiomyocytes isolated from animals five weeks after TAC surgery. Previous studies showed an elevated INaL in transgenic CaMKIIdelta mice, adenoviral CaMKII transfected rabbit cardiomyocytes, and guinea-pig ventricular myocytes in either the absence or presence of CaMKII that could be reversed by application of CaMKII inhibitors [2, 31]. Moreover, a recent study suggest that an increased INaL leads to activation of CaMKII, which in turn phosphorylates Nav1.5, further promoting INaL in genetically or pharmacologically modified mice cardiomyocytes [32]. Pharmacological inhibition of either CaMKII or INaL could ameliorate cardiac dysfunction caused by excessive Na influx. Thus, the present study confirms this finding and expands the knowledge in a clinically relevant model of afterload-induced heart failure.
4.4 INaL generates cellular arrhythmic triggers
Because afterdepolariations were rarely seen under basal conditions we increased cellular SR Ca stores using isoproterenol to unmask cellular arrhythmias. Experiments under these conditions may be of clinical relevance because the isoproterenol concentrations (eg, 10−8 mol/L) used in our study are equivalent to catecholamine levels found in human heart failure [33]. Here we show that isoproterenol prominently induced DADs without evidence for clear induced EADs although AP prolongation was present in these TAC cells. Nevertheless, occurrence of DADs was potent enough to cause typical triggered activity as indicated by repetitive unstimulated APs.
An enhanced INaL has been reported to increase [Na]i [18] as it occurs in heart failure [34]. The increased [Na]i leads to a subsequent Ca-overload due to the extrusion of Na via the reverse mode NCX and to a decreased Ca extrusion through NCX during its operation in the forward mode [3, 18]. This increase of diastolic Ca has been shown to produce spontaneous SR Ca-release events also known as Ca-sparks [1, 3]. Ca-sparks and waves are facilitated by leaky RyR22 in heart failure [16]. Ca-sparks are believed to participate as crucial events in the initiation and propagation of Ca-waves and the elimination of cytosolic Ca via the NCX generates a depolarizing current (transient inward current, ITI), which can give rise to DADs and which presumably underlies our observations [7, 9, 17].
Ran had strong antiarrhythmic effects by reducing the incidence of DADs and triggered activity in TAC myocytes most likely by attenuating the Na-induced Ca-overload resulting in a lower incidence of spontaneous SR Ca-release events as it was shown previously in failing cardiomyocytes [3, 7, 35]. Preliminary data from our group indicate that augmentation of INaL activates CaMKII which phosphorylates the RyR2 leading to increased SR Ca-sparks and DADs [35]. We therefore performed experiments using a CaMKII inhibitor and could show that AIP potently suppresses cellular triggers for arrhythmias as it was seen for Ran. Therefore, CaMKII seems to play an important role by mediating an INaL-induced leakiness of the cardiac RyR2 as demonstrated by a reduced Ca spark frequency in the presence of AIP. Ca-sparks and waves can give rise to DADs which in turn induce ITI causing triggered activity. Our present study thus demonstrates that inhibition of INaL either by applying Ran or AIP exerts potent antiarrhythmic effects on the cellular level.
4.5 Molecular basis of altered INaL in heart failure
Na channels are multi-protein complexes and their activity is both determined by the pore-forming alpha subunit and additional interacting partners [8]. In our model overexpression of Nav1.5 could not explain the slowed INaL decay time five weeks after TAC (~57%), because Nav1.5. was already increased after one week (~192%) which interestingly had no significant effects on INaL.
In-vivo knockdown of the beta1-subunit accelerates the decay time of INa [20] and an increased expression might therefore augment the INaL. Because the expression was increased at both time points a sole upregulation of the beta1-subunit does not explain an increased INaL. Normalization of the beta1-subunit to the alpha-subunit expression (Nav1.5) shows a decrease after one week and normalization after 5 weeks of TAC and this might contribute to the observed INaL-changes, especially the relative downregulation after one week could have prevented an increase in INaL. Neuronal isoforms of Na channels have been described to be upregulated in a rat TAC model before [28]. In our study Nav1.1 was upregulated whereas Nav1.6 was downregulated after 5 weeks of TAC surgery. Thus, a contribution of Nav1.1 to the augmented INaL could be an underlying mechanism, but this correlation remains highly speculative.
CaMKII can induce INaL by phosphorylation of Nav1.5 [2, 36]. CaMKII activity measured by phosphorylation at Thr-286 was increased after TAC surgery. This increase was much higher at five vs. one week post-TAC. Conclusively, INaL decay time was only significantly slowed five weeks after TAC surgery. Recently, different phosphorylation sites of the cardiac Na channels were described [21, 22].
In our study the CaMKII-dependent phosphorylation site of the Nav1.5 at Ser571 was significantly more phosphorylated in the late compared to the early time point after TAC intervention (Figure 6A and G), consistent with previous findings of increased phosphorylation at Ser571 in non-ischemic human heart failure [36]. Taken together with our functional findings, CaMKII seems to prominently mediate the augmentation of INaL in the progression of pressure overload-induced heart disease.
Conversely, increased cellular Ca has been shown to activate CaMKII which in turn induces INaL by phosphorylation of Na channels [2, 37], which might thus constitute a vicious circle. This pathway has been demonstrated by Yao and coworkers who showed that a Nav1.5-dependent increase in Na influx leads to activation of CaMKII, which phosphorylates Nav1.5, further promoting Na influx [32]. However, it should be also mentioned that there is one report showing that Ran directly influences RyR2 open probability, desensitizes Ca-dependent RyR2 activation and inhibits Ca oscillations which needs to be further investigated [38]. On the other hand ATX-II induces spontaneous diastolic Ca release from the SR and DADs which would rather support the indirect effect of Ran on RyR2 open probability via modulation of INaL [39,40]. Most importantly DADs can be also abolished by application of TTX which probably supports an INaL-dependent effect on DAD induction [7].
Taken together, activation of CaMKII and CaMKII-dependent augmentation of INaL via Na channel phosphorylation seems to be a major mechanism underlying the increase in INaL, APD prolongation, and induction of cellular arrhythmogenic triggers observed five weeks post-TAC.
4.6 Limitations
The difference in phenotype between animals after one vs. five weeks of TAC is a decrease in heart function, but also a further increase in hypertrophy (heart weight). Both changes occur at the same time and therefore low degree compensated hypertrophy and high degree decompensated hypertrophy but with heart failure might be the best definition of the phenotypes. INaL seems to be the major contributor to the AP prolongation at low frequencies where INaL is prominent e.g. 0.5-2 Hz at least in our model. However, higher frequencies were not investigated in this work. Possibly, other channels and currents might be involved in the AP prolongation at higher frequencies. Finally, all experiments were performed on a single cell level, making a direct translation of our results and conclusions regarding arrhythmias into an in-vivo situation difficult (e.g. no electrical cell to cell conduction). Therefore, because of the absence of data to indicate that ranolazine impacts global arrhythmia susceptibility in this model, the investigation is limited to describing the potential mechanisms by which late INaL contributes to cellular arrhythmia susceptibility during heart failure.
4.7 Conclusions
The progression of pressure overload-induced heart failure is paralleled by changes of INaL and APD. In our model, hypertrophy with preserved systolic contractility was not associated with either an effect on INaL decay time or a prolongation of the APD. However, when hearts developed LV pump failure INaL increased along with AP prolongation. Inhibition of INaL as well as CaMKII could largely counteract the AP prolongation in heart failure indicating a significant contribution of INaL and CaMKII (likely via INaL) on AP prolongation in the failing heart. A CaMKII-enhanced INaL appears to play a crucial role in the development of cellular arrhythmias associated with the progression of pressure overload-induced heart failure. Inhibition of INaL and CaMKII in order to suppress arrhythmias in heart failure merits further investigation.
Supplementary Material
Highlights.
INaL is enhanced in heart failure but not in compensated hypertrophy
Increased INaL causes action potential prolongation in heart failure
These changes trigger cellular arrhythmias which are prevented by INaL and CaMKII inhibition
CaMKII regulates INaL and thereby cellular arrhythmias via phosphorylation of the Nav1.5
Acknowledgements
We gratefully acknowledge the expert assistance of T. Schulte, S. Wollborn, and T. Sowa.
Funding This work was supported by grants from the Deutsche Forschungsgemeinschaft through SFB 1002 (K.T., G.H., L.S.M., S.S.), Heisenberg program Ma1982/4-2 (L.S.M.), and GRK 1816 RP3 (L.S.M., S.W.), Deutsches Zentrum für Herz-Kreislauf-Forschung (L.S.M., G.H.), Fondation Leducq (L.S.M., P.J.M), Deutsche Gesellschaft für Kardiologie (C.M.S.), German Heart Foundation/German Foundation of Heart Research, Research Program, Faculty of Medicine of the University (S.S.), Saving Tiny Hearts Society, NIH (P.J.M.: HL084583, HL083422; T.J.H.: HL096805, HL114893), and AHA Established Investigator Award (P.J.M.).
Footnotes
Conflict of interests Dr. Maier receives research grants from Gilead and is involved in clinical trials with Gilead and Menarini, companies that distribute ranolazine. Dr. Sossalla and Dr. Maier receive speaker’s honoraria from Berlin-Chemie.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. 2nd ed Kluwer Academic Publishers; Dordrecht, Netherlands: 2001. [Google Scholar]
- [2].Wagner S, Dybkova N, Rasenack EC, Jacobshagen C, Fabritz L, Kirchhof P, et al. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest. 2006;116:3127–38. doi: 10.1172/JCI26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Undrovinas NA, Maltsev VA, Belardinelli L, Sabbah HN, Undrovinas A. Late sodium current contributes to diastolic cell Ca2+ accumulation in chronic heart failure. J Physiol Sci. 2010;60:245–57. doi: 10.1007/s12576-010-0092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Undrovinas AI, Maltsev VA, Sabbah HN. Repolarization abnormalities in cardiomyocytes of dogs with chronic heart failure: role of sustained inward current. Cell Mol Life Sci. 1999;55:494–505. doi: 10.1007/s000180050306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sossalla S, Maurer U, Schotola H, Hartmann N, Didié M, Zimmermann W, et al. Diastolic dysfunction and arrhythmias caused by overexpression of CaMKIIδC can be reversed by inhibition of late Na+ current. Basic Res Cardiol. 2011;106:263–72. doi: 10.1007/s00395-010-0136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Song Y, Shryock JC, Wagner S, Maier LS, Belardinelli L. Blocking late sodium current reduces hydrogen peroxide-induced arrhythmogenic activity and contractile dysfunction. J Pharmacol Exp Ther. 2006;318:214–22. doi: 10.1124/jpet.106.101832. [DOI] [PubMed] [Google Scholar]
- [7].Song Y, Shryock JC, Belardinelli L. An increase of late sodium current induces delayed afterdepolarizations and sustained triggered activity in atrial myocytes. Am J Physiol Heart Circ Physiol. 2008;294:H2031–9. doi: 10.1152/ajpheart.01357.2007. [DOI] [PubMed] [Google Scholar]
- [8].Maltsev VA, Undrovinas A. Late sodium current in failing heart: friend or foe? Prog Biophys Mol Biol. 2008;96:421–51. doi: 10.1016/j.pbiomolbio.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Maltsev VA, Sabbah HN, Higgins RS, Silverman N, Lesch M, Undrovinas AI. Novel, ultraslow inactivating sodium current in human ventricular cardiomyocytes. Circulation. 1998;98:2545–52. doi: 10.1161/01.cir.98.23.2545. [DOI] [PubMed] [Google Scholar]
- [10].Belardinelli L, Shryock JC, Fraser H. Inhibition of the late sodium current as a potential cardioprotective principle: effects of the late sodium current inhibitor ranolazine. Heart. 2006;92 Suppl 4:iv6–iv14. doi: 10.1136/hrt.2005.078790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Despa S, Islam MA, Weber CR, Pogwizd SM, Bers DM. Intracellular Na+ concentration is elevated in heart failure but Na+/K+ pump function is unchanged. Circulation. 2002;105:2543–8. doi: 10.1161/01.cir.0000016701.85760.97. [DOI] [PubMed] [Google Scholar]
- [12].Valdivia CR, Chu WW, Pu J, Foell JD, Haworth RA, Wolff MR, et al. Increased late sodium current in myocytes from a canine heart failure model and from failing human heart. J Mol Cellular Cardiol. 2005;38:475–83. doi: 10.1016/j.yjmcc.2004.12.012. [DOI] [PubMed] [Google Scholar]
- [13].Ju YK, Saint DA, Gage PW. Hypoxia increases persistent sodium current in rat ventricular myocytes. J Physiol. 1996;497:337–47. doi: 10.1113/jphysiol.1996.sp021772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sossalla S, Maier LS. Role of ranolazine in angina, heart failure, arrhythmias, and diabetes. Pharmacology and Therapeutics. 2012;133:311–22. doi: 10.1016/j.pharmthera.2011.11.003. [DOI] [PubMed] [Google Scholar]
- [15].Maltsev VA, Silverman N, Sabbah HN, Undrovinas AI. Chronic heart failure slows late sodium current in human and canine ventricular myocytes: implications for repolarization variability. Eur J Heart Fail. 2007;9:219–27. doi: 10.1016/j.ejheart.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sossalla S, Fluschnik N, Schotola H, Ort KR, Neef S, Schulte T, et al. Inhibition of elevated Ca2+/calmodulin-dependent protein kinase II improves contractility in human failing myocardium. Circ Res. 2010;107:1150–61. doi: 10.1161/CIRCRESAHA.110.220418. [DOI] [PubMed] [Google Scholar]
- [17].Lederer WJ, Tsien RW. Transient inward current underlying arrhythmogenic effects of cardiotonic steroids in Purkinje fibres. J Physiol. 1976;263:73–100. doi: 10.1113/jphysiol.1976.sp011622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sossalla S, Wagner S, Rasenack EC, Ruff H, Weber SL, Schondube FA, et al. Ranolazine improves diastolic dysfunction in isolated myocardium from failing human hearts-role of late sodium current and intracellular ion accumulation. J Mol Cellular Cardiol. 2008;45:32–43. doi: 10.1016/j.yjmcc.2008.03.006. [DOI] [PubMed] [Google Scholar]
- [19].Sossalla S, Kallmeyer B, Wagner S, Mazur M, Maurer U, Toischer K, et al. Altered Na+ currents in atrial fibrillation effects of ranolazine on arrhythmias and contractility in human atrial myocardium. J Am Coll Cardiol. 2010;55:2330–42. doi: 10.1016/j.jacc.2009.12.055. [DOI] [PubMed] [Google Scholar]
- [20].Mishra S, Undrovinas NA, Maltsev VA, Reznikov V, Sabbah HN, Undrovinas A. Post-transcriptional silencing of SCN1B and SCN2B genes modulates late sodium current in cardiac myocytes from normal dogs and dogs with chronic heart failure. Am J Physiol Heart Circ Physiol. 2011;301:H1596–605. doi: 10.1152/ajpheart.00948.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hund TJ, Koval OM, Li J, Wright PJ, Qian L, Snyder JS, et al. A beta(IV)- spectrin/CaMKII signaling complex is essential for membrane excitability in mice. J Clin Invest. 2010;120:3508–19. doi: 10.1172/JCI43621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ashpole NM, Herren AW, Ginsburg KS, Brogan JD, Johnson DE, Cummins TR, et al. Ca2+/calmodulin-dependent protein kinase II (CaMKII) regulates cardiac sodium channel NaV1.5 gating by multiple phosphorylation sites. J Biol Chem. 2012;287:19856–69. doi: 10.1074/jbc.M111.322537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Boulaksil M, Winckels SK, Engelen MA, Stein M, van Veen TA, Jansen JA, et al. Heterogeneous Connexin43 distribution in heart failure is associated with dispersed conduction and enhanced susceptibility to ventricular arrhythmias. Eur J Heart Fail. 2010;12:913–21. doi: 10.1093/eurjhf/hfq092. [DOI] [PubMed] [Google Scholar]
- [24].Toischer K, Rokita AG, Unsold B, Zhu W, Kararigas G, Sossalla S, et al. Differential cardiac remodeling in preload versus afterload. Circulation. 2010;122:993–1003. doi: 10.1161/CIRCULATIONAHA.110.943431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Antzelevitch C, Belardinelli L, Zygmunt AC, Burashnikov A, DiDiego JM, Fish JM, et al. Electrophysiological effects of ranolazine, a novel antianginal agent with antiarrhythmic properties. Circulation. 2004;110:904–10. doi: 10.1161/01.CIR.0000139333.83620.5D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Maltsev VA, Undrovinas AI. Relationship between steady-state activation and availability of cardiac sodium channel: evidence of uncoupling. Cell Mol Life Sci. 1998;54:148–51. doi: 10.1007/s000180050135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Undrovinas AI, Belardinelli L, Undrovinas NA, Sabbah HN. Ranolazine improves abnormal repolarization and contraction in left ventricular myocytes of dogs with heart failure by inhibiting late sodium current. J Cardiovasc Electrophysiol. 2006;17(Suppl 1):S169–S77. doi: 10.1111/j.1540-8167.2006.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Xi Y, Wu G, Yang L, Han K, Du Y, Wang T, et al. Increased late sodium currents are related to transcription of neuronal isoforms in a pressure-overload model. Eur J Heart Fail. 2009;11:749–57. doi: 10.1093/eurjhf/hfp092. [DOI] [PubMed] [Google Scholar]
- [29].Wu L, Shryock JC, Song Y, Belardinelli L. An increase in late sodium current potentiates the proarrhythmic activities of low-risk QT-prolonging drugs in female rabbit hearts. J Pharmacol Exp Ther. 2006;316:718–26. doi: 10.1124/jpet.105.094862. [DOI] [PubMed] [Google Scholar]
- [30].Wu L, Rajamani S, Shryock JC, Li H, Ruskin J, Antzelevitch C, et al. Augmentation of late sodium current unmasks the proarrhythmic effects of amiodarone. Cardiovasc Res. 2008;77:481–8. doi: 10.1093/cvr/cvm069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Aiba T, Hesketh GG, Liu T, Carlisle R, Villa-Abrille MC, O’Rourke B, et al. Na+ channel regulation by Ca2+/calmodulin and Ca2+/calmodulin-dependent protein kinase II in guinea-pig ventricular myocytes. Cardiovasc Res. 2010;85:454–63. doi: 10.1093/cvr/cvp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yao L, Fan P, Jiang Z, Viatchenko-Karpinski S, Wu Y, Kornyeyev D, et al. Nav1.5- dependent persistent Na+ influx activates CaMKII in rat ventricular myocytes and N1325S mice. Am J Physiol Cell Physiol. 2011;301:C577–86. doi: 10.1152/ajpcell.00125.2011. [DOI] [PubMed] [Google Scholar]
- [33].Klensch H. The basal noradrenaline level in human peripheral venous blood. Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;290:218–24. [PubMed] [Google Scholar]
- [34].Pieske B, Maier LS, Piacentino V, 3rd, Weisser J, Hasenfuss G, Houser S. Rate dependence of [Na+]i and contractility in nonfailing and failing human myocardium. Circulation. 2002;106:447–53. doi: 10.1161/01.cir.0000023042.50192.f4. [DOI] [PubMed] [Google Scholar]
- [35].Mallwitz A, Sag CM, Sossalla ST, Hartmann N, Steuer N, Sowa T, et al. CaMKII-dependent SR Ca leak contributes to proarrhythmogenic effects of increased late Na current in isolated cardiac myocytes. Clin Res Cardiol. 2011;(Suppl 1) [Google Scholar]
- [36].Koval OM, Snyder JS, Wolf RM, Pavlovicz RE, Glynn P, Curran J, et al. Ca2+/calmodulin-dependent protein kinase II-based regulation of voltage-gated Na+ channel in cardiac disease. Circulation. 2012;126:2084–94. doi: 10.1161/CIRCULATIONAHA.112.105320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ma J, Luo A, Wu L, Wan W, Zhang P, Ren Z, et al. Calmodulin kinase II and protein kinase C mediate the effect of increased intracellular calcium to augment late sodium current in rabbit ventricular myocytes. Am J Physiol Cell Physiol. 2011;15:C1141–51. doi: 10.1152/ajpcell.00374.2011. [DOI] [PubMed] [Google Scholar]
- [38].Parikh A, Mantravadi R, Kozhevnikov D, Roche MA, Ye Y, Owen LJ, et al. Ranolazine stabilizes cardiac ryanodine receptors: a novel mechanism for the suppression of early afterdepolarization and torsades de pointes in long QT type 2. Heart Rhythm. 2012;9:953–60. doi: 10.1016/j.hrthm.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wasserstrom JA, Sharma R, O’Toole MJ, Zheng J, Kelly JE, Shryock J, et al. Ranolazine antagonizes the effects of increased late sodium current on intracellular calcium cycling in rat isolated intact heart. J Pharmacol Exp Ther. 2009;331:382–91. doi: 10.1124/jpet.109.156471. [DOI] [PubMed] [Google Scholar]
- [40].Song Y, Shryock JC, Wu L, Belardinelli L. Antagonism by ranolazine of the pro-arrhythmic effects of increasing late INa in guinea pig ventricular myocytes. J Cardiovasc Pharmacol. 2004;44:192–9. doi: 10.1097/00005344-200408000-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.