Glaucoma is one of the most serious complications occurring after infantile cataract surgery. It is usually open-angle and can develop during the immediate postoperative period or years later. While early cataract surgery has been shown to be associated with improved visual outcomes,1,2 it has also been reported to increase the risk of developing glaucoma.3-5 A wide range of cumulative incidences of glaucoma has been reported following infantile cataract surgery.6-10 However, it is difficult to directly compare these studies because of their differing inclusion criteria and lengths of follow-up. Moreover, some studies used national registries8,9 or data from multiple institutions11,12 and as a result surgical techniques and follow-up examinations were not standardized. Furthermore, these studies differed in how they defined “glaucoma.” Some studies defined glaucoma solely on the basis of an elevated intraocular pressure (IOP),13-17 while other studies based the diagnosis on whether treatment had been initiated for glaucoma.4,8 Studies which based the diagnosis solely on elevated IOP may have over-diagnosed glaucoma, since there can be a lag between modestly elevated IOP and clinically significant optic disc deterioration or visual field abnormalities.18 In addition, the thicker corneas of aphakic eyes introduces a potential source of measurement error when using IOP alone as a criterion for diagnosing glaucoma.19-22
The aim of the current study is to report the long-term cumulative incidence of glaucoma in a cohort of children who all underwent congenital cataract surgery when <7months of age by the same surgeon (SRL) using a modern surgical technique with follow-up care provided by a pediatric ophthalmologist and a pediatric glaucoma specialist.
METHODS
This retrospective interventional consecutive case series was carried out with prospective approval from the Institutional Review Board at Emory University and in accordance with Health Insurance Portability and Accountability Act regulations. Informed consent was obtained from all study participants or their parents in the event the children were minors. In addition, assent was obtained from all minors who were ≥6 years of age. Patients for the study were identified by reviewing the surgical logs of one of the authors (SRL) and searching the Emory Clinic database for all patients who underwent unilateral or bilateral cataract surgery when <7 months of age between 1988 and 2010 by SRL. The main inclusion criteria were a child undergoing unilateral or bilateral cataract surgery when <7 months of age with a minimum follow-up of 3 years. Exclusion criteria included participation in the Infant Aphakia Treatment Study5,23 (a randomized clinical trial that is still ongoing) and syndromes associated with an increased risk of glaucoma.24,25 Data were extracted from the medical records and then entered on Case Report Forms. Data entered on the Case Report Forms included birth weight, type of cataract, date of cataract surgery, laterality, primary or secondary intraocular lens (IOL) implantation, refractive error, highest IOP (Tmax), the most recent central corneal thickness (CCT), cup/disc (C/D) ratio when diagnosed with glaucoma, axial length, visual field findings, date and type of glaucoma surgery, length of follow-up and glaucoma medications being taken, C/D ratio and visual acuity at the last follow-up examination. The Case Report Forms were then faxed and entered into a database.
SURGICAL PROCEDURE
All of the surgeries were performed using a limbal approach. After making two stab incisions, an infusion cannula was placed through one incision and a vitreous-cutting instrument though the other. The vitreous-cutting instrument was then used to create an anterior capsulectomy 5 mm or greater in diameter, to aspirate the lens nucleus and cortex, a posterior capsulectomy 4 mm or greater in diameter and to perform an anterior vitrectomy. If an IOL was implanted, the wound was enlarged and the IOL was implanted into the capsular bag. The incisions were then closed with absorbable sutures. Postoperatively, patients were treated with topical antibiotics and atropine for 1 week and corticosteroids for 4 weeks.
DIAGNOSIS OF GLAUCOMA AND GLAUCOMA SUSPECT
Glaucoma was defined as IOP >21 mmHg with one of the following anatomical changes: 1) corneal enlargement; 2) asymmetrical progressive myopic shift coupled with enlargement of the corneal diameter and/or axial length; and 3) increased optic nerve cupping defined as an increase of ≥ 0.2 in the cup-to-disc ratio or the use of a surgical procedure for IOP control. Glaucoma suspect was defined as either: 1) two consecutive IOP measurements on different dates >21 mmHg after topical corticosteroids had been discontinued without the anatomic changes listed above; or 2) the use of glaucoma medications to control IOP without the anatomic changes listed above. The date that these findings were first detected on clinical examination was defined as the onset date of glaucoma or glaucoma suspect.
Prior to 2008, IOP was measured in young children using a Tonopen (Reichert, Depew, New York), pneumotonometry (Reichert, Depew, New York), or a Perkins tonometer (Haag-Streit, Bern, Switzerland). In some cases, an examination-under-anesthesia was performed to measure IOP in uncooperative children. Goldmann applanation tonometry was generally used to measure the IOP in older children and some cooperative younger children. Since 2008, rebound tonometry (Icare, Helsinki, Finland) has been the preferred instrument for measuring IOP in young children at our institution.26 CCT was measured using a handheld pachymeter (DGH 55 Pachmate, DGH Technology, Exton, Pennsylvania).
All patients diagnosed with glaucoma or glaucoma suspect were treated by a pediatric glaucoma specialist.
STATISTICAL ANALYSIS
Descriptive statistics were calculated for person-level characteristics on the 37 patients and for eye-level characteristics on the 62 eyes. A Kaplan-Meier method was used to calculate the probability of glaucoma and glaucoma suspect, as well as the respective 95% confidence intervals, on a per-eye basis. Prior to conducting any inferential analyses, McNemar's test was used to check for independence in glaucoma diagnosis between the left and right eyes of patients. Since there was no significant dependence found (p=0.25), the eyes were treated independently. SAS 9.3 was used for all statistical analyses.
Visual acuity data were analyzed by converting to logMAR notation and calculating the median, which was then transformed back to the Snellen equivalent. The data were stratified by laterality. Values recorded as CUSM or CSM were excluded from the analyses (2 patients).
RESULTS
The records of 85 patients were reviewed who underwent unilateral or bilateral cataract surgery when <7 months of age between 1988 and 2010 by SRL. All of the eyes had IOP ≤21 mm Hg at the time of cataract surgery. Nine patients were excluded from the analysis because they were enrolled in the Infant Aphakia Treatment Study. Another 4 patients were excluded because they have syndromes known to be associated with an increased risk of glaucoma (Lowe syndrome (n=3)) and microphalmos, dermal aplasia and sclerocornea (MIDAS syndrome (n=1)). The remaining patients were either receiving their ophthalmic care elsewhere (n=34) or were unwilling to sign the informed consent (n=1). Thirty-seven patients and 62 eyes were enrolled in the study. Their median birth weight was 3480 grams (interquartile range (IQR), 3260-3748 grams). The patients underwent cataract surgery at a median age of 58 days (range, 5 to 210 days). Twenty-six of the cataracts were nuclear, 3 were total, 3 were posterior lentiglobus, 2 lamellar, 1 posterior polar, 1 persistent fetal vasculature, and 1 was not reported. The median follow-up was 7.9 years (range, 3.2 to 23.5 years). There were 11 patients (29.7%) who underwent unilateral cataract surgery and 26 (70.3%) who underwent bilateral cataract surgery. Six eyes underwent primary IOL implantation and 17 eyes secondary IOL implantation. All of the IOLs implanted were Acrysof IOLs (Models SA60AT, SN60AT, SN60WF, MN60AC, MA30AC)(Alcon surgical, Fort Worth, Texas). Three patients had Trisomy 21. After cataract surgery, none of the eyes underwent any other intraocular surgical procedures other than the implantation of a secondary IOL or glaucoma surgery.
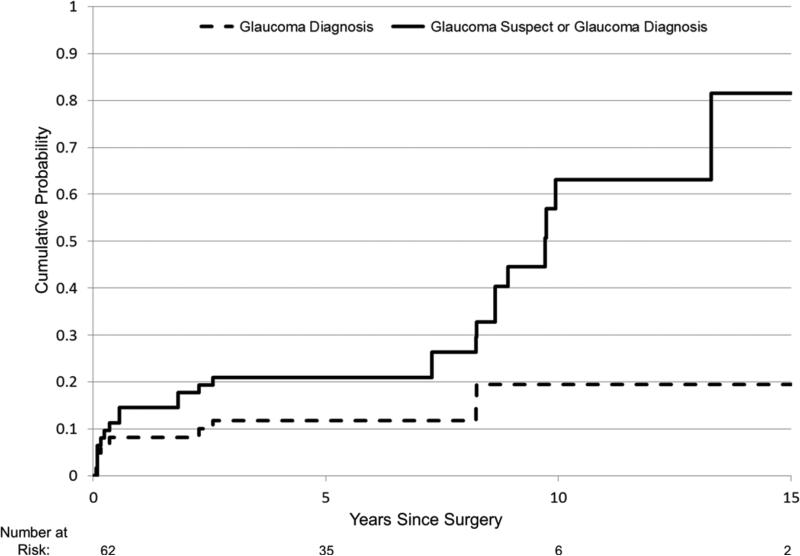
Nine eyes developed open-angle glaucoma (Table 1). No eyes developed angle-closure glaucoma. Three patients had bilateral and 3 patients had unilateral glaucoma. Only 1 of the 3 patients with unilateral glaucoma underwent cataract surgery in both eyes. Cataract surgery was performed at a median age of 5 weeks (range, 3-13 weeks) in these eyes. Glaucoma was diagnosed in the first postoperative year in 5 eyes, at the age of 2 years in 2 eyes, and at the age of 8 years in 2 eyes. The probability of an eye developing glaucoma was estimated to be 19.5% (95% CI: 10.0% - 36.1%) by 10 years after cataract surgery (Figure). We did not find a significant difference in the risk of developing glaucoma and the age at cataract surgery when stratified at ≤ vs. >6 weeks, ≤ vs. >2 months, and ≤ vs. >3 months. However, the power to find such a difference was low because of the limited number of patients in each of these age groups.
Table 1.
Clinical Findings of Glaucoma Eyes after Congenital Cataract Surgery
| Patient | Eye | Age Cataract Surgery (weeks) | Age Glaucoma Diagnosed (months) | Age Glaucoma Surgery (months) | Buphthalmos | Tmax (mm Hg) | CCT (μm) | Number of glaucoma medications at last exam | C/D Ratio glaucoma diagnosed | C/D Ratio at last exam |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | RT | 5 | 12 | 13 | Y | 37 | 575 | 0 | 0.1 | 0.1 |
| 11 | RT | 8 | 33 | 37 | Y | 36 | NA | 3 | 0.1 | 0.2 |
| 11 | LT | 8 | 30 | 29 | Y | 45 | NA | 3 | 0.6 | 0.8 |
| 12 | RT | 4 | 2 | 7 | Y | 38 | 832 | 3 | 0.5 | 0.8 |
| 12 | LT | 4 | 2 | 6 | Y | 39 | 801 | 3 | 0.5 | 0.7 |
| 13 | RT | 12 | 7 | 7 | Y | 42 | 557 | 0 | 0.4 | 0.3 |
| 14 | RT | 3 | 3 | 3 | Y | 35 | 528 | 0 | 0.6 | 0.1 |
| 15 | RT | 5 | 100 | 151 | N | 47 | 644 | 3 | 0.5 | 0.6 |
| 15 | LT | 4 | 100 | 133 | N | 51 | 667 | 3 | 0.5 | 0.55 |
RT= right; LT=left; Tmax=highest intraocular pressure measured; mm Hg=millimeters of mercury; CCT=central corneal thickness; μm=micrometers; C/D=cup/disc
Figure.
Figure Kaplan Meier curves showing cumulative probability of an eye developing glaucoma (dashed line) and glaucoma suspect or glaucoma (solid line) after congenital cataract surgery over time. The number of eyes at risk at each 5 year time interval are shown below the x axis.
All of the eyes diagnosed with glaucoma were initially treated with glaucoma medications. However, because of progressive buphthalmos or glaucomatous optic neuropathy coupled with uncontrolled IOP, all of these eyes subsequently underwent glaucoma surgery. At the time of glaucoma surgery, all of these eyes had microcystic cornea edema except for Patient 15 who was older when he presented with glaucoma. Three eyes underwent a suture trabeculotomy27 and 6 eyes had an Ahmed shunt implanted (New World Medical, Rancho Cucamonga, Calif). None of the eyes underwent any additional glaucoma surgeries. Six of the 9 eyes continue to take 3 medications to control IOP. One eye underwent primary IOL implantation at the age of 3 weeks and was diagnosed with glaucoma at age 3 months. None of the other eyes that developed glaucoma underwent primary IOL implantation. Tmax in these eyes was a median of 39 mm Hg (range, 35 to 51 mm Hg).
Sixteen eyes had elevated IOPs without progressive ocular enlargement or optic disc cupping and were diagnosed as glaucoma suspects (Table 2). These eyes underwent cataract surgery at a median age of 7.5 weeks (range, 1-15 weeks). They were diagnosed as a glaucoma suspect at a median age of 7.5 years (range, 1.5 to 10 years). The probability of an eye developing glaucoma or glaucoma suspect was estimated to be 63% (95% CI: 43.6% to 82.3%) by 10 years after congenital cataract surgery (Figure). Tmax in the glaucoma suspect eyes was a median of 31 mm Hg (range, 25 to 39 mm Hg). Glaucoma medications were started to lower the IOP in all of these eyes. Fourteen of these 16 eyes currently have their IOP controlled by taking a mean of 1.78 (range, 1-4) glaucoma medications each day. The other two eyes had their medications discontinued after being treated with 1-3 glaucoma medications daily for 5-6 years and their IOP has remained in the normal range. None of these 16 eyes had increased cupping of their optic discs and all visual fields have been normal. Four of the glaucoma suspect eyes underwent secondary IOL implantation; two eyes had ocular hypertension when secondary IOL implantation was performed and two eyes developed ocular hypertension 5 years later.
Table 2.
Clinical Findings of Glaucoma Suspect Eyes after Congenital Cataract Surgery
| Patient | Eye | Age at Cataract Surgery (weeks) | Age Diagnosed Glaucoma Suspect (years) | Tmax (mm Hg) | CCT (μm) | Number of glaucoma medications at last exam | C/D ratio at last exam |
|---|---|---|---|---|---|---|---|
| 1 | LT | 6 | 1 1/2 | 25 | 636 | 0 | 0.1 |
| 2 | RT | 6 | 13 | 38 | NA | 1 | 0.1 |
| 2 | LT | 6 | 13 | 34 | NA | 1 | 0.1 |
| 3 | RT | 11 | 1 | 35 | 648 | 1 | 0.3 |
| 3 | LT | 11 | 3 1/2 | 38 | 626 | 1 | 0.3 |
| 4 | RT | 6 | 1/2 | 28 | 652 | 0 | 0.2 |
| 5 | RT | 15 | 7 1/2 | 32 | 634 | 3 | 0.4 |
| 5 | LT | 15 | 7 1/2 | 36 | 648 | 3 | 0.25 |
| 6 | RT | 13 | 9 | 28 | 632 | 1 | 0.65 |
| 6 | LT | 14 | 10 | 25 | 698 | 1 | 0.7 |
| 7 | RT | 1 | 7 1/2 | 28 | 723 | 1 | 0.55 |
| 7 | LT | 1 | 7 1/2 | 28 | 683 | 1 | 0.7 |
| 8 | RT | 4 | 2 | 28 | 710 | 2 | 0.3 |
| 8 | LT | 4 | 2 | 30 | 714 | 2 | 0.3 |
| 9 | RT | 12 | 9 | 38 | 737 | 4 | 0.25 |
| 10 | LT | 9 | 10 | 36 | 631 | 3 | 0.15 |
LT=left; RT=right; Tmax=highest intraocular pressure measured; mm Hg=millimeters of mercury; CCT=central corneal thickness; μm=micrometers; C/D=cup/disc
At the last follow-up examination, median visual acuities were similar in the normotensive and glaucoma suspect eyes that had undergone unilateral or bilateral cataract surgery (Table 3). While the median visual acuities were lower for eyes with glaucoma in both the unilateral and bilateral groups compared to normotensive and glaucoma suspect eyes, the sample sizes were too small to analyze these differences statistically.
Table 3.
Median logMAR and Snellen Equivalent Visual Acuities by Treatment Groups for Eyes after Congenital Cataract Surgery
| Unilateral | Bilateral | |
|---|---|---|
| Glaucoma | 0.85 20/142 (n=2) |
0.70 20/100 (n=7) |
| Glaucoma Suspect | 0.05 20/22 (n=2) |
0.30 20/40 (n=14) |
| Normotensive | 0.20 20/32 (n=18) |
0.30 20/40 (n=27) |
The CCT were lower for normotensive eyes (median, 599 μm; range, 460-969 μm, n=31) compared to eyes with glaucoma (median, 644 μm; range 528-832 μm; n=7) and glaucoma suspect (median, 650 μm; range, 626-723 μm, n=14), however the sample sizes were too small to perform a statistical comparison.
Discussion
We estimated that nearly two-thirds of the eyes in our series would develop glaucoma or become a glaucoma suspect by 10 years after congenital cataract surgery. While most of these eyes were only glaucoma suspects, it is likely that without medical therapy that some of the glaucoma suspect eyes would have developed glaucoma.18
We used the same definition of glaucoma as the Infant Aphakia Treatment Study which required that both ocular hypertension and progressive optic disc cupping or buphthalmos be present to diagnosis glaucoma.5 Many other studies reporting the probability of glaucoma following congenital cataract surgery have defined glaucoma on the basis of ocular hypertension alone.13-17 This is problematic since the Ocular Hypertension Treatment Study found that after a 5 year follow-up, only 9.5% of adult eyes with untreated ocular hypertension developed reproducible optic disc deterioration or visual field abnormalities.18 Thus is it likely that studies basing their definition of glaucoma on ocular hypertension alone have overestimated the risk of developing glaucoma. Egbert and coworkers6 used the same definition of glaucoma we used and reported a similar risk of developing glaucoma and glaucoma suspect after congenital cataract surgery (glaucoma, 19%; glaucoma and/or glaucoma suspect, 59%) after a 15 year follow-up. With a longer term follow-up, we presume that the incidence of glaucoma and glaucoma suspect will increase in the cohort of patients we studied.
It is likely that some of the glaucoma suspect eyes in our series would have developed glaucoma if their ocular hypertension had not been treated with glaucoma medications. We attempted to measure IOP at each follow-up examination so that ocular hypertension could be diagnosed and treated as soon as possible. Five eyes developed glaucoma during the first year of life and 4 eyes progressed from glaucoma suspect to glaucoma later in childhood despite receiving glaucoma medications. Egbert and coworkers6 reported that ocular hypertension was successfully treated in 75% of the eyes in their series. However, 5 eyes in their series (2 of whom were treated with glaucoma medications) developed glaucoma after a 10 year follow-up. Paradoxically, ocular hypertension resolved in two eyes in our series after long-term treatment with glaucoma medications. The IOP may have normalized in these two eyes secondary to maturation of the trabecular meshwork or resolution of subclinical inflammation. The normalization of IOP in these two eyes after long-term follow-up emphasizes the importance of critically assessing the need for glaucoma medications in glaucoma suspect eyes on a longitudinal basis.
We analyzed each eye separately in our study because we found no significant dependence between right and left eyes using the McNemar's test. In our series, there were several instances where the right and left eyes had different outcomes. Patient 1 developed glaucoma in her right eye and required a trabeculotomy to control IOP while her left eye remained a glaucoma suspect. Patient 10 was a glaucoma suspect in her left eye and required 3 glaucoma medications to control IOP in this eye whereas the IOP was never elevated in her right eye. In contrast, Egbert and coworkers6 analyzed patients rather than eyes after bilateral cataract surgery because they found a high correlation between the outcomes between the right and left eyes.
We chose to restrict our study to patients who were <7 months of age when they underwent cataract surgery because infants have been reported to be at higher risk of developing glaucoma after cataract surgery.4,28 This likely explains the high probability of glaucoma and glaucoma suspect developing in the eyes in our study. Haargaard and coworkers9 reported that after a 10 year follow-up, 32% of eyes that underwent cataract surgery when <9 months of age developed glaucoma compared to only 4% of children ≥9 months of age. Vishwanath and coworkers4 reported a 50% risk of developing glaucoma in one eye after bilateral lensectomies compared to a 15% risk if surgery was delayed beyond the first month of life after a 5 year follow-up. Mills and Robb29 reported that 13 of the 14 eyes in their series that developed open-angle glaucoma underwent cataract surgery when <1 year of age. In the Infant Aphakia Treatment Study, the odds of developing a glaucoma-related adverse event were 1.6 times higher for each month earlier that cataract surgery was performed.30 We did not find a significant difference in the risk of developing glaucoma when we stratified patients by age at cataract surgery, however all of the patients in our study had cataract surgery when 6 months of age or younger. While is not known why early cataract surgery predisposes eyes to developing glaucoma, it has been postulated that cataract surgery may interfere with the normal maturation of the trabecular meshwork.31
In our study, the same surgeon performed all of the cataract surgeries using a modern surgical technique. None of these eyes required additional intraocular procedures to treat postoperative complications other than glaucoma. In contrast, Chen and coworkers11 reported that 50% of the eyes in their series required additional intraocular procedures to treat postoperative complications such as pupillary membranes, retained lens material, posterior capsular opacification, and retinal detachment following cataract surgery. While primary IOL implantation is known to be associated with a higher risk of postoperative complications,32 only 1 patient in their series underwent primary implantation of an IOL. In the Infant Aphakia Treatment Study, 12% of eyes required one or more additional intraocular procedures after undergoing a lensectomy when <7 months of age.32 Rabiah14 reported that secondary membrane surgery increases the risk of glaucoma following pediatric cataract surgery. Since none of the eyes in our series underwent secondary membrane surgery, this cannot explain the high risk of glaucoma and glaucoma suspect in our series.
The mean CCT has been reported to increase during childhood. At age 1 year, the mean CCT in normal eyes is 553 μm in Caucasian children and 541 μm in African American children.15 By age 17 years, the mean CCT increases to 573 μm in Caucasian children and 551 μm in African American children. The CCT of the normotensive eyes in our study (median, 599 μm) were slightly greater than those reported in normal phakic children. The effect of CCT on the IOP measured in eyes after congenital cataract surgery is not known. The higher IOP in the glaucoma suspect eyes in our series (median, 650 μm) may be partially due to the increased median CCT in these eyes. Others have also reported thicker CCTs in eyes with ocular hypertension following congenital cataract surgery.19,22,33 The reason for thicker CCTs in these eyes is not known, but may be a consequence of trauma to the endothelium during cataract surgery.22
Only 6 eyes in our series underwent primary IOL implantation. One of these eyes developed glaucoma. This child underwent the primary implantation of an IOL at age 3 weeks. A large retrospective multi-centered study reported that glaucoma rarely develops in pediatric eyes following cataract surgery and IOL implantation.12 However, none of the eyes in this study underwent cataract surgery and IOL implantation during the first 12 months of life. Both Trivedi and coworkers17 and the Infant Aphakia Treatment Study5 have reported no statistically significant difference in the cumulative incidence of glaucoma after cataract surgery during infancy with or without IOL implantation. Wong and coworkers34 reported a higher incidence of glaucoma in eyes implanted with rigid polymethylmethacrylate (PMMA) compared to acylic IOLs. All of the patients in our series underwent the implantation of acrylic IOLs.
The median Snellen equivalent visual acuities were 20/40 or better for the treated eyes in both the unilateral and bilateral treatment groups. While the median visual acuities were worse for the treated eyes in the glaucoma groups, the sample sizes were too small to evaluate these differences statistically.
Our study had a number of shortcomings. First, not all eligible patients who underwent cataract surgery by SRL were included in this analysis. Most were excluded because they are now receiving their ophthalmic care elsewhere. It is possible that patients who continue to receive their care at Emory are different than patients who are followed elsewhere. Second, the median follow-up was only 7.9 years. It is likely that some of the glaucoma suspect eyes in our study will develop glaucoma with a longer follow-up. Chen and coworkers11 have reported that 24% of patients who develop glaucoma after congenital cataract surgery do so after the age of 6 years. In some eyes, glaucoma does not develop until adulthood. Finally, IOP can be difficult to measure in young children and it is possible that some elevated IOPs were spurious. To avoid this possibility, examinations-under-anesthesia were performed if there was a concern that the IOP measured in an office setting was unreliable. Furthermore, all elevated IOPs measured by a pediatric ophthalmologist were independently confirmed by a pediatric glaucoma specialist.
The strength of our study was that all of the cataract surgeries were performed by the same surgeon and the patients with glaucoma suspect and glaucoma were all managed by a pediatric glaucoma specialist. In addition, we had a relatively long-term follow-up of these patients (median, 7.9 years).
In conclusion, careful monitoring of eyes for glaucoma following early congenital cataract surgery is important because of the high risk of these eyes developing glaucoma. Early diagnosis and treatment of eyes with elevated IOP may delay the onset of glaucoma in these eyes.
Acknowledgments
a. Supported by National Institutes of Health Core Grant EY06360 and Research to Prevent Blindness, Inc, New York, New York
c. Involved in design of study (SRL, AP, ADB); analysis and interpretation (SRL, AP, HMS, ML, ADB); writing the article (SRL, ML, ADB); critical revision of the article (SRL, HMS, ML, ADB); final approval of the article (SRL, AP, HMS, ML, ADB); data collection (SRL, AP, ADB); provision of patients (SRL, ADB); statistical expertise (HMS, ML); and literature search (SRL, ADB).
Biography


Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure:
b. Financial Disclosures: SRL (none), AP (none), MJL (none), ADB (Merck, travel support, honorarium).
References
- 1.Birch EE, Stager DR. The critical period for surgical treatment of dense congenital unilateral cataract. Invest Ophthalmol Vis Sci. 1996 Jul;37(8):1532–1538. [PubMed] [Google Scholar]
- 2.Lambert SR, Lynn MJ, Reeves R, Plager DA, Buckley EG, Wilson ME. Is there a latent period for the surgical treatment of children with dense bilateral congenital cataracts? J AAPOS. 2006 Feb;10(1):30–36. doi: 10.1016/j.jaapos.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Magnusson G, Abrahamsson M, Sjostrand J. Glaucoma following congenital cataract surgery: an 18-year longitudinal follow-up. Acta Ophthalmol Scand. 2000 Feb;78(1):65–70. doi: 10.1034/j.1600-0420.2000.078001065.x. [DOI] [PubMed] [Google Scholar]
- 4.Vishwanath M, Cheong-Leen R, Taylor D, Russell-Eggitt I, Rahi J. Is early surgery for congenital cataract a risk factor for glaucoma? Br J Ophthalmol. 2004 Jul;88(7):905–910. doi: 10.1136/bjo.2003.040378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beck AD, Freedman SF, Lynn MJ, Bothun E, Neely DE, Lambert SR. Glaucoma-related adverse events in the Infant Aphakia Treatment Study: 1-year results. Arch Ophthalmol. 2012 Mar;130(3):300–305. doi: 10.1001/archophthalmol.2011.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egbert JE, Christiansen SP, Wright MM, Young TL, Summers CG. The natural history of glaucoma and ocular hypertension after pediatric cataract surgery. J AAPOS. 2006 Feb;10(1):54–57. doi: 10.1016/j.jaapos.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 7.McClatchey SK, Parks MM. Myopic shift after cataract removal in childhood. J Pediatr Ophthalmol Strabismus. 1997 Mar-Apr;34(2):88–95. doi: 10.3928/0191-3913-19970301-07. [DOI] [PubMed] [Google Scholar]
- 8.Chak M, Rahi JS. Incidence of and factors associated with glaucoma after surgery for congenital cataract: findings from the British Congenital Cataract Study. Ophthalmology. 2008 Jun;115(6):1013–1018. e1012. doi: 10.1016/j.ophtha.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Haargaard B, Ritz C, Oudin A, et al. Risk of glaucoma after pediatric cataract surgery. Invest Ophthalmol Vis Sci. 2008 May;49(5):1791–1796. doi: 10.1167/iovs.07-1156. [DOI] [PubMed] [Google Scholar]
- 10.Autrata R, Rehurek J, Vodickova K. Visual results after primary intraocular lens implantation or contact lens correction for aphakia in the first year of age. Ophthalmologica. 2005 Mar-Apr;219(2):72–79. doi: 10.1159/000083264. [DOI] [PubMed] [Google Scholar]
- 11.Chen TC, Walton DS, Bhatia LS. Aphakic glaucoma after congenital cataract surgery. Arch Ophthalmol. 2004 Dec;122(12):1819–1825. doi: 10.1001/archopht.122.12.1819. [DOI] [PubMed] [Google Scholar]
- 12.Asrani S, Freedman S, Hasselblad V, et al. Does primary intraocular lens implantation prevent “aphakic” glaucoma in children? J AAPOS. 2000 Feb;4(1):33–39. doi: 10.1016/s1091-8531(00)90009-0. [DOI] [PubMed] [Google Scholar]
- 13.Swamy BN, Billson F, Martin F, et al. Secondary glaucoma after paediatric cataract surgery. Br J Ophthalmol. 2007 Dec;91(12):1627–1630. doi: 10.1136/bjo.2007.117887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rabiah PK. Frequency and predictors of glaucoma after pediatric cataract surgery. Am J Ophthalmol. 2004 Jan;137(1):30–37. doi: 10.1016/s0002-9394(03)00871-7. [DOI] [PubMed] [Google Scholar]
- 15.Bradfield YS, Melia BM, Repka MX, et al. Central corneal thickness in children. Arch Ophthalmol. 2011 Sep;129(9):1132–1138. doi: 10.1001/archophthalmol.2011.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simon JW, Mehta N, Simmons ST, Catalano RA, Lininger LL. Glaucoma after pediatric lensectomy/vitrectomy. Ophthalmology. 1991 May;98(5):670–674. doi: 10.1016/s0161-6420(91)32235-8. [DOI] [PubMed] [Google Scholar]
- 17.Trivedi RH, Wilson ME, Jr., Golub RL. Incidence and risk factors for glaucoma after pediatric cataract surgery with and without intraocular lens implantation. J AAPOS. 2006 Apr;10(2):117–123. doi: 10.1016/j.jaapos.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002 Jun;120(6):701–713. doi: 10.1001/archopht.120.6.701. discussion 829-730. [DOI] [PubMed] [Google Scholar]
- 19.Lim Z, Muir KW, Duncan L, Freedman SF. Acquired central corneal thickness increase following removal of childhood cataracts. Am J Ophthalmol. 2011 Mar;151(3):434–441. e431. doi: 10.1016/j.ajo.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 20.Lupinacci AP, da Silva Jordao ML, Massa G, Arieta CE, Costa VP. Central corneal thickness in children with congenital cataract and children with surgical aphakia: a case-control study. Br J Ophthalmol. 2009 Mar;93(3):337–341. doi: 10.1136/bjo.2008.137596. [DOI] [PubMed] [Google Scholar]
- 21.Muir KW, Duncan L, Enyedi LB, Wallace DK, Freedman SF. Central corneal thickness: congenital cataracts and aphakia. Am J Ophthalmol. 2007 Oct;144(4):502–506. doi: 10.1016/j.ajo.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Simsek T, Mutluay AH, Elgin U, Gursel R, Batman A. Glaucoma and increased central corneal thickness in aphakic and pseudophakic patients after congenital cataract surgery. Br J Ophthalmol. 2006 Sep;90(9):1103–1106. doi: 10.1136/bjo.2006.096370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lambert SR, Buckley EG, Drews-Botsch C, et al. A randomized clinical trial comparing contact lens with intraocular lens correction of monocular aphakia during infancy: grating acuity and adverse events at age 1 year. Arch Ophthalmol. 2010 Jul;128(7):810–818. doi: 10.1001/archophthalmol.2010.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walton DS, Katsavounidou G, Lowe CU. Glaucoma with the oculocerebrorenal syndrome of Lowe. J Glaucoma. 2005 Jun;14(3):181–185. doi: 10.1097/01.ijg.0000158850.07732.05. [DOI] [PubMed] [Google Scholar]
- 25.Cape CJ, Zaidman GW, Beck AD, Kaufman AH. Phenotypic variation in ophthalmic manifestations of MIDAS syndrome (microphthalmia, dermal aplasia, and sclerocornea). Arch Ophthalmol. 2004 Jul;122(7):1070–1074. doi: 10.1001/archopht.122.7.1070. [DOI] [PubMed] [Google Scholar]
- 26.Lambert SR, Melia M, Buffenn AN, Chiang MF, Simpson JL, Yang MB. Ophthalmic technology assessment: Rebound tonometry in children. Ophthalmology. doi: 10.1016/j.ophtha.2012.09.058. Available online 9 Feb 2013. [DOI] [PubMed] [Google Scholar]
- 27.Beck AD, Lynch MG. 360 degrees trabeculotomy for primary congenital glaucoma. Arch Ophthalmol. 1995 Sep;113(9):1200–1202. doi: 10.1001/archopht.1995.01100090126034. [DOI] [PubMed] [Google Scholar]
- 28.Lundvall A, Zetterstrom C. Complications after early surgery for congenital cataracts. Acta Ophthalmol Scand. 1999 Dec;77(6):677–680. doi: 10.1034/j.1600-0420.1999.770614.x. [DOI] [PubMed] [Google Scholar]
- 29.Mills MD, Robb RM. Glaucoma following childhood cataract surgery. J Pediatr Ophthalmol Strabismus. 1994 Nov-Dec;31(6):355–360. doi: 10.3928/0191-3913-19941101-03. discussion 361. [DOI] [PubMed] [Google Scholar]
- 30.Cole SR, Beck RW, Moke PS, et al. The Amblyopia Treatment Index. J AAPOS. 2001 Aug;5(4):250–254. doi: 10.1067/mpa.2001.117097. [DOI] [PubMed] [Google Scholar]
- 31.Kuhli-Hattenbach C, Luchtenberg M, Kohnen T, Hattenbach LO. Risk factors for complications after congenital cataract surgery without intraocular lens implantation in the first 18 months of life. Am J Ophthalmol. 2008 Jul;146(1):1–7. doi: 10.1016/j.ajo.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 32.Plager DA, Lynn MJ, Buckley EG, Wilson ME, Lambert SR. Complications, adverse events, and additional intraocular surgery 1 year after cataract surgery in the infant aphakia treatment study. Ophthalmology. 2011 Dec;118(12):2330–2334. doi: 10.1016/j.ophtha.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyahara S, Amino K, Tanihara H. Glaucoma secondary to pars plana lensectomy for congenital cataract. Graefes Arch Clin Exp Ophthalmol. 2002 Mar;240(3):176–179. doi: 10.1007/s00417-001-0419-x. [DOI] [PubMed] [Google Scholar]
- 34.Wong IB, Sukthankar VD, Cortina-Borja M, Nischal KK. Incidence of early-onset glaucoma after infant cataract extraction with and without intraocular lens implantation. Br J Ophthalmol. 2009 Sep;93(9):1200–1203. doi: 10.1136/bjo.2008.155200. [DOI] [PubMed] [Google Scholar]