Abstract
We evaluated the pharmacodynamic relationships between mycophenolic acid (MPA), the active metabolite of mycophenolate mofetil (MMF), and outcomes in 308 patients after nonmyeloablative hematopoietic cell transplant. Patients were conditioned with total body irradiation ± fludarabine, received grafts from HLA-matched related (N=132) or unrelated (N=176) donors, and received post-grafting immunosuppression with MMF and a calcineurin inhibitor. Total and unbound MPA pharmacokinetics were determined to day 25; maximum a posteriori Bayesian estimators were used to estimate total MPA concentration at steady state (Css). Rejection occurred in nine patients, eight of whom had a total MPA Css less than 3 μg/mL. In patients receiving a related donor graft, MPA Css was not associated with clinical outcomes. In patients receiving an unrelated donor graft, low total MPA Css was associated with increased grades 3–4 acute graft versus host disease (aGVHD) and increased non-relapse mortality, but not with day 28 T-cell chimerism, disease relapse, cytomegalovirus reactivation, or overall survival. We conclude that higher initial oral MMF doses and subsequent targeting of total MPA Css to greater than 2.96 μg/mL could lower grades 3–4 aGVHD and non-relapse mortality in patients receiving an unrelated donor graft.
INTRODUCTION
The development of nonmyeloablative allogeneic hematopoietic cell transplantation (HCT) conditioning regimens expanded the availability of this potentially curative procedure to patients who cannot tolerate the toxicity of myeloablative conditioning due to age or comorbidity.(1, 2) Postgrafting immunosuppression used with these regimens, which aims to facilitate allogeneic engraftment and control graft versus host disease (GVHD), often consists of a calcineurin inhibitor (CNI) and mycophenolate mofetil (MMF).
During the initial clinical trials of nonmyeloablative HCT, oral MMF was administered every 12 hours (Q12hr), as in solid organ transplant patients. Shortly thereafter, it was recognized that HCT recipients exhibit a shorter half-life of MMF’s primary metabolite, mycophenolic acid (MPA),(3, 4) compared to solid organ transplant recipients.(5) This finding was particularly important in nonmyeloablative HCT recipients, who rely on the balance between recipient and donor cells to ensure adequate immunosuppression of the recipient, optimal graft-versus-tumor (GVT) effect, and minimal GVHD.
Engraftment was adequately achieved in nonmyeloablative HCT recipients of a human leukocyte antigen (HLA)–matched related donor graft with Q12hr administration of oral MMF. Engraftment proved more challenging, however, for recipients of nonmyeloablative HCT with an HLA-matched unrelated donor graft;(3) this led to increasing the daily MMF dose by shortening the oral MMF dosing interval from Q12hr to every 8 hours (Q8hr).(6) Engraftment rates for patients with HLA-matched unrelated donors were further improved with the use of granulocyte colony-stimulating factor–mobilized peripheral blood mononuclear cell (G-PBMC) grafts instead of bone marrow grafts.(3) In patients undergoing nonmyeloablative conditioning with an unrelated donor graft, we have demonstrated that less frequent (Q12hr) MMF dosing and low total MPA area under the plasma concentration-time curve (AUC) are related to a higher risk of graft rejection.(6, 7) Low total MPA AUC is also related to low (<50%) donor chimerism, but no significant association was observed between total or unbound MPA AUC and acute GVHD (aGVHD) or relapse.(7)
Some HCT centers personalize MMF doses based on MPA pharmacokinetics using either trough concentration,(8, 9) AUC,(10) or Bayesian estimates of AUC.(11) Various investigators have reported a pharmacodynamic association between MPA and clinical outcomes in allogeneic HCT recipients (Supplemental Table 1).(7–9, 12–14) Many of these studies are, however, from small patient populations (< 75 patients) of adults (8, 12) or children.(9, 13) Studies in a homogenous group of HCT recipients with a sufficient number of patients are essential to elucidate any MPA pharmacodynamic associations. In the present study, we retrospectively analyzed data from two cohorts of patients receiving nonmyeloblative HCT from a related or unrelated donor; patients received postgrafting immunosuppression that included either Q12hr (N=167) or Q8hr (N=141) MMF and a CNI. Each cohort was analyzed for possible associations between MPA concentration at steady state (Css), defined as the AUC divided by the dosing interval, and graft rejection, day 28 T-cell chimerism, grades 2–4 aGVHD, relapse, non-relapse mortality (NRM), cytomegalovirus (CMV) reactivation, and overall survival.
MATERIALS AND METHODS
Patient characteristics and treatment plan
We retrospectively evaluated MPA pharmacodynamics in two separate cohorts of patients who received nonmyeloablative HCT with G-PBMC grafts to treat a variety of hematological malignancies between March 1998 and July 2006 at the Fred Hutchinson Cancer Research Center, Seattle, WA. Given the greater risk of graft rejection among bone marrow recipients compared with G-PBMC recipients receiving Q12hr MMF,(3) patients who received bone marrow as the source of stem cells were excluded. Written informed consent was obtained from all patients prior to participation in prospective treatment protocols, which included MPA pharmacokinetic sampling. Fifty-four percent of these ambulatory clinic patients participated in the MPA pharmacokinetic sampling. The Institutional Review Board at the Fred Hutchinson Cancer Research Center approved all study protocols, including this retrospective analysis, and an independent Data Safety Monitoring Board monitored safety in all prospective studies. Patient characteristics are summarized in Table 1.
Table 1.
Patients’ Characteristicsa
| Related | Donor Type Unrelated | All patients | |
|---|---|---|---|
| Total number | 132 | 176 | 308 |
| Sex, female/male (% female) | 58/74 (44%) | 59/117 (34%) | 117/191 (38%) |
| HCT-CIb | |||
| 0 | 43 (33%) | 44 (25%) | 87 (28%) |
| 1–2 | 35 (26%) | 50 (28%) | 85 (28%) |
| 3–4 | 37 (28%) | 57 (32%) | 94 (30%) |
| ≥5 | 15 (11%) | 24 (14%) | 39 (13%) |
| Not available | 2 (2%) | 1 (1%) | 3 (1%) |
| Recipients’ ages, yr | 54.5 (25.3 – 72.6) | 57.8 (9.2 – 74.5) | 55.9 (9.2 – 74.5) |
| Recipients’ age < 21 yr | 0 | 5 (2.8%) | 5 (1.6%) |
| CMV seropositive recipients | 85 (64%) | 105 (60%) | 190 (62%) |
| Kahl Disease risk(23) | |||
| Low | 30 (23%) | 44 (25%) | 74 (24%) |
| Standard | 66 (50%) | 80 (45%) | 146 (47%) |
| High | 36 (27%) | 52 (30%) | 88 (29%) |
| Female donor to male recipient | 42 (32%) | 40 (23%) | 82 (27%) |
| Donors’ age, yr | 53 (17 – 76) | 33 (18–59) | 42 (17–76) |
| HLA-matched graft | 130 (98%) | 142 (81%) | 272 (88%) |
| HLA-mismatched graft | 2 (2%) | 34 (19%) | 36 (12%) |
| Year of transplant | |||
| 1998 | 5 (4%) | 0 | 5 (2%) |
| 1999 | 21 (16%) | 0 | 21 (7%) |
| 2000 | 35 (26%) | 21 (12%) | 56 (18%) |
| 2001 | 23 (17%) | 19 (11%) | 42 (14%) |
| 2002 | 15 (11%) | 28 (16%) | 43 (14%) |
| 2003 | 0 | 25 (14%) | 25 (8%) |
| 2004 | 9 (7%) | 37 (21%) | 46 (15%) |
| 2005 | 14 (11%) | 27 (15%) | 41 (13%) |
| 2006 | 10 (8%) | 19 (11%) | 29 (9%) |
| Conditioning regimen | |||
| 2 Gy TBI | 30 (23%) | 1 (1%) | 31 (10%) |
| 2 Gy TBI + auto | 20 (15%) | 0 | 20 (7%) |
| 2 Gy TBI + FLU 90mg/m2 | 72 (55%) | 160 (91%) | 232 (75%) |
| 2 Gy TBI + FLU 90mg/m2+ auto | 8 (6%) | 13 (7%) | 21 (7%) |
| 3 Gy TBI + FLU 90mg/m2 | 2 (1%) | 2 (1%) | 4 (1%) |
| Post-grafting immunosuppression* | |||
| MMF Q8hr | 0 | 141 (80%) | 141 (46%) |
| MMF Q12hr | 132 (100%) | 35 (20%) | 167 (54%) |
| Cyclosporine+MMF | 104 (79%) | 147 (84%) | 251 (81%) |
| Tacrolimus+MMF | 28 (21%) | 29 (16%) | 57 (19%) |
Data shown as median (range) or as number (%); Abbreviations: autologous (auto); cytomegalovirus (CMV), fludarabine monophosphate (FLU), HCT comorbidity index (HCT-CI), human leukocyte antigen (HLA), mycophenolate mofetil (MMF), total body irradiation (TBI).
The conditioning regimen comprised a single fraction of 200 to 300 cGy total body irradiation (TBI) on day 0 with or without fludarabine (30 mg/m2/day intravenously) from day −4 to day −2 (cumulative dose 90 mg/m2).(3) In general, the post-grafting CNI was either cyclosporine or tacrolimus given through day +177. MMF was given at two different dose frequencies, either 15 mg/kg Q8hr or Q12hr. Adjusted ideal body weight(15) was used to determine MMF dosing, and all doses were rounded to the nearest 250mg. MMF doses were not adjusted based on MPA plasma concentrations, and patients were asked to take MMF at the same time daily. MMF treatment started on day 0 and, in general, continued until day 27 (related donor) or day 40 (unrelated donor) at which time the MMF dose was reduced by 10% per week in the absence of GVHD. The majority of donor grafts were matched for HLA-A, -B, -C, DRB1 at high resolution DNA typing and DQB1 by intermediate-resolution techniques, with the exception of two related and 34 unrelated donor grafts. Of the 36 patients with mismatched donor grafts, the two related and 22 of the 34 unrelated donor grafts had a 2 allele or antigen mismatch. The median follow-up among patients at the time of last contact was 3.09 years (range, 0.31 – 12.06 years).
Pharmacokinetic analysis
All patients had blood samples scheduled before the morning dose of MMF, and then at 1, 2, 4, 6, and 8 hours following the dose; in patients receiving Q12hr MMF, blood samples were also collected 10 hours after the morning dose. Blood samples were collected in ethylenediaminetetraacetic acid tubes, and total MPA plasma concentrations were quantified by reverse-phase high performance liquid chromatography (HPLC) with UV detection (adapted from Tsina et al.).(7, 16, 17) The dynamic range was 0.2 to 30 μg/mL and the interday coefficient of variation was less than 10%.(7)
Total MPA Css were evaluated on the following days: one Css on days 0 through 4, 246 Css on day 7 (includes days 5 through 9), 25 Css on days 10 through 18, 236 Css on day 21 (includes days 19 through 23), and 14 Css on days 24 through 25. Unbound MPA Css were collected on the following days: one Css on days 0 through 4, 178 Css on day 7 (includes days 5 through 9), 18 Css on days 10 through 18, 169 Css on day 21 (includes days 19 through 23), and 10 Css on days 22 through 25.
Estimation of total MPA AUCs was accomplished using maximum a posteriori probability (MAP) Bayesian estimation of pharmacokinetic parameters, incorporating a blend of individualized pharmacokinetic data and a population parameter prior. The MAP Bayesian method used individual patient 0–8 hour (Q8hr administration) or 0–12 hour (Q12hr administration) total MPA concentration-time data, together with a pharmacokinetic model and mean parameter values plus their variance (derived from a population pharmacokinetic analysis of 408 HCT recipients).(15) This integrated model describes the pharmacokinetics of total MPA AUC. The population prior parameters were not changed during this study. The estimated total MPA Css was calculated by dividing the Bayesian AUC estimate by the dosing interval,(7) specifically AUC/12 hour in the Q12hr MMF group and AUC/8 hour in the Q8hr MMF group. The Css term was used to compare the two groups of patients because of the varying administration schedules for oral MMF. The available MPA AUC data from day 0 to day 25 were used to calculate the average total MPA Css term for each patient.
The correlation between the total MPA trough concentration and MPA Css was evaluated as well. Because of the high interoccasion (i.e., within patient) variability of MPA absorption rate,(15) the predose MPA sample was not used as the trough concentration. The MPA trough concentration was defined as a concentration-time point collected between 7.5 and 8.5hr post-dose in patients receiving Q8hr MMF and between 9.5 and 10.5hr post-dose in patients receiving Q12hr MMF.
After total MPA concentrations were quantitated, the remaining plasma over one AUC was pooled together to estimate the unbound fraction of MPA. Pooling samples ensured sufficient volume to be above the assay’s limit of quantitation. Notably, the unbound fraction of MPA does not change over a total MPA concentration range of 1 to 60 μg/mL.(18) The unbound fraction of MPA was separated from the protein-bound MPA through equilibrium dialysis and quantitated as previously described.(7) Specifically, 200 μL of this pooled plasma was placed in a water-tight Teflon dialysis chamber separated by dialysis membranes (Spectrapor 4; 14k molecular weight cutoff; Spectrum Laboratories, Los Angeles, CA), then dialyzed for 2 hours against an equal volume of 10% phosphoric acid buffer (pH 7.2) in a water bath at 37°C. Following this, 120 μL dialysate was mixed with 500 μL acetonitrile; after vortexing and drying, samples were reconstituted in 20 mM phosphate buffer (pH 3.2). The total MPA and the fraction of MPA bound to plasma protein were quantitated, with the unbound drug percentage calculated as follows: unbound MPA = 100 × (1−bound MPA). The unbound Css were calculated by multiplying the unbound fraction of MPA by total Css.(7)
The MPA parameters evaluated for pharmacodynamic relationships were total MPA Css, unbound MPA Css, and total MPA trough concentration.
Toxicity
Neutropenia post-HCT was assessed only through day 28, because multiple potential confounding variables (e.g., viral infection or reactivation, corticosteroid therapy) could affect the neutrophil count after day 28. Neutropenia was evaluated by examining daily complete blood counts with differential and assessment of absolute neutrophil count (ANC). CMV reactivation was also evaluated, as it represents a significant consequence of immunosuppressed status; CMV serological status was assessed in each patient and donor prior to HCT. All patients underwent weekly testing to detect the CMV pp65 antigen for the first three months following HCT.
Chimerism and graft rejection
On days 28, 56, and 84 after HCT, all patients’ peripheral blood samples were assessed for the percentage of donor CD3+ T-cells present. Flow cytometry was used to sort CD3+ cells and chimerism was measured using fluorescence in situ hybridization and polymerase chain reaction of polymorphic microsatellite regions for sex-mismatched and sex-matched grafts, respectively.(19) If donor CD3+ cells were less than or equal to 5% at any of the assessed time points after HCT, then the patient was noted to have graft rejection.
Acute GVHD, chronic GVHD, and disease relapse
Acute GVHD and chronic GVHD (cGVHD) were graded according to established criteria.(20–22) Hematological diseases were classified as low, standard, or high risk of relapse per the Kahl criteria to evaluate relapse rate in a consistent manner.(23) We defined disease relapse or disease progression as disease recurrence following complete remission or progression of persistent disease.
Statistical analysis
Graphical representation of the pharmacodynamic data are shown by the quartile of the total MPA Css, with the lower quartile (range: 0.61 to 1.76 μg/mL), interquartile range (1.77 to 2.96 μg/mL) and upper quartile (range: 2.97 to 4.6 μg/mL). Cumulative incidence curves for aGVHD were estimated using methods previously described.(24) Cox regression analysis was used to model the effect of MPA Css on time-to-event endpoints. Death and relapse were treated as competing risks for analysis of aGVHD and cGVHD. Relapse was treated as a competing risk for the analysis of nonrelapse mortality (NRM). The effects of MPA Css on hazard ratios (HRs) were expressed as the interquartile ranges for both related and unrelated donor grafts. Mean MPA Css were calculated up to day 25 and treated as fixed covariates. Cumulative mean MPA Css through day 25 was treated as a time-dependent covariate; that is, at each time the covariate represented the mean of all prior concentrations until the onset of GVHD or day 130, whichever occurred first. All reported p-values are two-sided, and those estimated from regression models are derived from the Wald test. No adjustments were made for multiple comparisons.
RESULTS
Pharmacokinetic results
We found considerable inter-individual variation of MPA plasma concentrations in this retrospective analysis of 308 patients from whom 522 MPA AUCs were available. The majority of this data (506 of 522 AUCs, 97%) were collected after oral MMF administration with the remainder (16 of 522, 3%) collected after IV MMF administration. The majority of patients had two MPA AUCs available (67% for total MPA and 43% for unbound MPA); this subgroup is described in detail in Table 2. Of the 522 AUCs, 376 (72%) had both total and unbound MPA AUCs available; the remainder (146, 28%) had only total MPA AUC. Graphical representation of the total MPA Css pharmacodynamic data are shown by the lower quartile (range: 0.61 to 1.76 μg/mL), interquartile range (1.77 to 2.96 μg/mL) and upper quartile (range: 2.97 to 4.6 μg/mL) (Figure 1 and Figure 2). Since the majority of patients had MPA Css data available on day 7 and day 21, the data from patients with an MPA Css on one of those days is described in Table 2. As expected, the total and unbound MPA Css increased with Q8hr administration compared to Q12hr administration. MMF dosed based on body weight led to considerable interpatient variability, expressed as mean ± standard deviation or fold-range (maximum/minimum). Specifically, with Q12hr administration, there was a 5 to 5.7-fold range for total MPA Css and 14.7 to 57.9-fold range for unbound MPA Css. Similarly, total MPA Css had a 6.3 to 11.4-fold range and unbound MPA Css had a 6 to 7.7-fold range with Q8hr administration. Recent data demonstrates that limited sampling schedules with Bayesian estimators can accurately estimate Css.(8, 15) The association of MPA pharmacokinetics with patient characteristics is reported in a separate population pharmacokinetic manuscript.(15) Notably, only the concomitant CNI, dosing weight, and albumin concentration – but not conditioning regimen - were associated with total MPA pharmacokinetics.(15) The effect of the concomitant CNI upon MPA Css is shown in Supplemental Figure 1, panels A & B.
Table 2.
Comparison of day 7 and day 21 plasma MPA pharmacokinetic data after MMF administrationa
| Day 7 | Day 21 | |||||||
|---|---|---|---|---|---|---|---|---|
| Q12hr | Q8hr | Q12hr | Q8hr | |||||
|
| ||||||||
| N | Mean ± SD (range) | N | Mean ± SD (range) | N | Mean ± SD (range) | N | Mean ± SD (range) | |
| Total MPA Cssb, μg/mL | 138b | 2.2 ±0.84 (0.95 – 5.4) | 108 | 2.9 ± 0.94 (1.1 – 6.9) | 130 | 2.1 ± 0.82 (0.94 – 4.7) | 106 | 3.0 ± 0.99 (0.61 – 7.0) |
| Unbound fraction, % | 108 | 0.94 ± 0.45 (0.43 – 4.3) | 70 | 1.1 ± 0.53 (0.61 – 4.3) | 99 | 0.96 ± 0.42 (0.11 – 3.5) | 70 | 1.1 ± 0.43 (0.59 – 3.0) |
| Unbound Css, ng/mL | 108 | 19.6 ± 10.4 (6.43 – 94.3) | 70 | 29.5 ± 12.8 (11.0 – 84.5) | 99 | 20.2 ± 11.4 (1.60 – 92.7) | 70 | 29.8 ± 12.7 (15.0 – 90.6) |
Abbreviations: concentration at steady state (Css), mycophenolate mofetil (MMF), mycophenolic acid (MPA), every 8 hours (Q8hr), every 12 hours (Q12hr);
Css was calculated as AUC0−τ divided by τ (with τ as the dosing interval). The Css term was used because of the varied administration schedules for oral MMF. The majority of participants had two MPA Css available and are presented in this table. Of the remaining patients (not in this table), 96 had one total MPA Css, two had three total MPA Css, 69 patients had no unbound MPA Css, 104 patients had one unbound Css, and two patients had three unbound Css (1%). Total MPA Css were evaluated on the following days: 1 Css on days 0 through +4, 246 Css on day+ 7 (includes days +5 through +9), 25 Css on days +10 through +18, 236 Css on day +21 (includes days +19 through +23), and 14 Css on days +24 through +25. Unbound MPA Css were collected on the following days: 1 Css on days 0 through +4, 178 Css on day+ 7 (includes days +5 through +9), 18 Css on days +10 through +18, 169 Css on day +21 (includes days +19 through +23), and 10 Css on days +22 through +25.
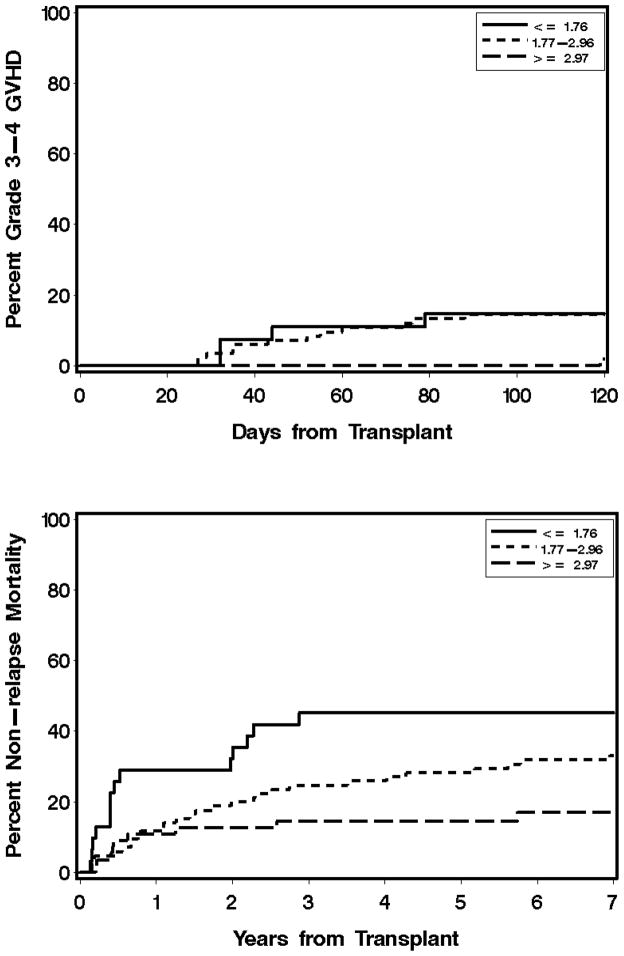
Figure 1. Association of total MPA Css with grades 3–4 acute GVHD (A) and non-relapse mortality (B) after day 25 in unrelated donor G-PBMC grafts.
MPA Css is the average of all values from day 0 through day 25. Total MPA Css values are lower quartile (0.61 to 1.76 μg/mL), interquartile range (1.77 to 2.96 μg/mL), and upper quartile (2.97 to 4.6 μg/mL).
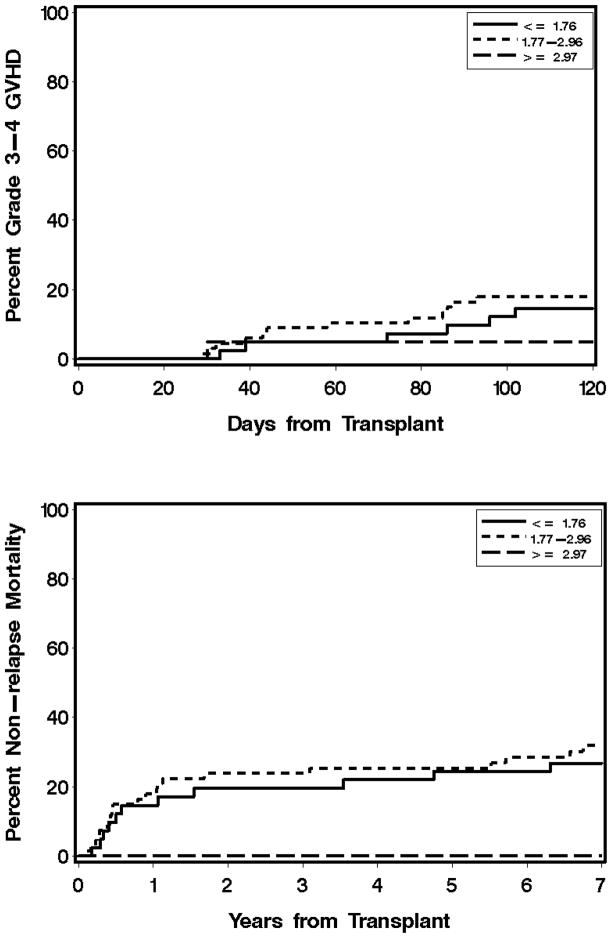
Figure 2. Lack of association of total MPA Css with grades 3–4 acute GVHD (A) and non-relapse mortality (B) after day 25 in related donor G-PBMC grafts.
MPA Css is the average of all values from days 0 through 25. Total MPA Css values are lower quartile (0.61 to 1.76 μg/mL), interquartile range (1.77 to 2.96 μg/mL), and upper quartile (2.97 to 4.6 μg/mL).
The association of MPA Css with the last concentration time point obtained from pharmacokinetic sampling for Css determination (Ctrough) was also evaluated. Prior studies have established poor association between these values (R2 ranging from 3%(4) to 49%,(4, 7, 14)) which was supported by our own data (Supplemental Figure 1, panels C & D). The total MPA Ctrough was not associated with clinical outcomes (Supplemental Table 3).
Neutropenia and CMV reactivation
Of the 308 patients, 232 (88 related, 144 unrelated) had an ANC nadir below 500/μL between days −7 and 28. Of those 232, 53 experienced their ANC nadir between days −7 and 7 and 179 between days 7 and 28. Among the 190 patients who were CMV seropositive before HCT, 130 (68%) experienced CMV reactivation. Specifically, 52 of 85 (61%) seropositive related and 78 of 105 (74%) unrelated experienced CMV reactivation. In CMV negative recipients with a CMV positive donor, CMV antigenemia was detected in four of twelve related and one of five unrelated donor graft recipients.
Donor chimerism and graft rejection
Nine of the 308 patients experienced rejection, all of whom received MMF with cyclosporine. A detailed description of the patients who rejected their grafts is reported in Table 3. One rejection occurred in a related donor graft recipient who was conditioned with TBI only; this patient received MMF Q12hr with total MPA Css of 4.31 μg/mL. The remaining eight patients who rejected their grafts received HLA-matched unrelated grafts after fludarabine/TBI conditioning. Of these eight patients, seven received an HLA-matched graft, six received Q12hr MMF and all had a total MPA Css < 3 μg/mL. Among the 36 patients who received an HLA-mismatched graft, there was one case of graft rejection. The average total MPA Css among the 36 patients receiving a mismatched graft was 2.49 μg/mL (range: 1.1–3.97).
Table 3.
Detailed description in patients who experienced graft rejection
| No. | Donor | Conditioning | Age (yr) | Diagnosis | CD34 dose | CD3 dose | HLA | CD3+ on day +28 | Week2 CSPb | MMFa
|
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MMF frequency | total MPA Css | Unbound MPA Css | ||||||||||
| 1 | Sibling | TBI | 53.8 | MM | 10.87 | 3.29 | Identical | 15 | 437 | Q12hr | 4.30 | NA |
| 2 | Unrelated | FLU/TBI | 60.1 | CML | 6.28 | 1.18 | A, B, C, DRB1, DQB1 | 15 | 443 | Q12hr | 1.15 | 20.65 |
| 3 | Unrelated | FLU/TBI | 45.3 | AML | 0.76 | 0.31 | A, B, C, DRB1, DQB1 | 9 | 502 | Q8hr | 2.47 | 34.88 |
| 4 | Unrelated | FLU/TBI | 40 | MDS | 1.48 | 1.47 | A, B, C, DRB1, DQB1 | 0 | 309 | Q12hr | 1.74 | 10.71 |
| 5 | Unrelated | FLU/TBI | 46.6 | NHL | 7.77 | 3.62 | A, B, C, DRB1, DQB1 | 59 | 576 | Q12hr | 2.42 | 19.24 |
| 6 | Sibling | FLU/TBI | 62.8 | NHL and MDS | 33.75 | 4.57 | Identical | 75 | 584 | Q12hr | 1.48 | 12.45 |
| 7 | Unrelated | FLU/TBI | 48.5 | CLL | 4.90 | 3.04 | A, B, C, DRB1, DQB1 | 1 | 339 | Q12hr | 1.95 | 14.18 |
| 8 | Unrelated | FLU/TBI | 38.6 | CML | 6.44 | 1.61 | A, B, C, DRB1, DQB1 | 40 | 350 | Q12hr | 1.68 | 17.99 |
| 9 | Unrelated | FLU/TBI | 60 | NHL | 4.08 | 1.25 | A, B, DRB1, DQB1; C antigen mistmatch | 30 | 459 | Q8hr | 2.32 | 20.28 |
Abbreviations: acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL), chronic myelogenous leukemia (CML), calcineurin inhibitor (CNI), cyclosporine (CSP), fludarabine monophosphate (FLU), human leukocyte antigen (HLA), myelodysplastic syndrome (MDS), multiple myeloma (MM), mycophenolate mofetil (MMF), mycophenolic acid (MPA), Non-Hodgkin’s lymphoma (NHL), total body irradiation (TBI).
Rejections only occurred in patients receiving CSP.
Among 308 patients, the majority (234, 76%) had a total MPA Css less than 3 μg/mL. Eight of 234 patients (3.4%) with a low total Css (< 3 μg/mL) rejected their G-PBMC grafts, while one of the 74 (1.3%) patients whose Css was above 3 μg/mL had graft rejection (P=0.36), as described above. Among the 234 patients with total MPA Css less than 3 μg/mL, 147 (62.8%) were in the Q12hr MMF group and 87 (37.2%) were in the Q8hr MMF group. Of the 141 patients receiving Q8hr MMF, a minority (38%) had an average total MPA Css greater than 3 μg/mL.
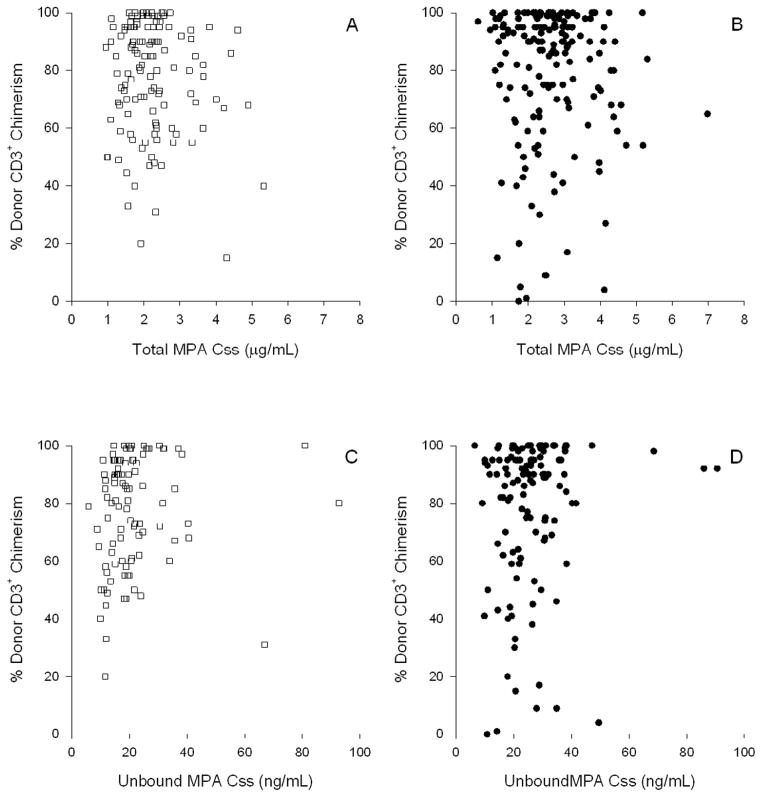
Other studies in the setting of nonmyeloablative HCT have found that low donor T-cell chimerism levels are predictive of graft rejection.(3, 25, 26) Considering this, we evaluated whether total or unbound MPA Css was associated with the subsequent degree of day 28 donor T-cell chimerism. After adjusting for disease risk, HLA mismatch, mean week 2 CNI concentration,(27) year of transplant,(27) and female donor to male recipient, there was no statistically significant association between mean total MPA Css and day 28 donor T-cell chimerism of 50% or lower for patients receiving related (odds ratio (OR) 0.83, 95% CI 0.38–1.82, P=0.63) or unrelated grafts (OR 1.39, 95% CI 0.82–2.37, P=0.23). Among the 32 patients with donor T-cell chimerism of 50% or lower, the average total MPA Css ranged from 1.1 to 5.3 μg/mL, and average unbound Css (available in 27 patients) ranged from 9.82 to 66.8 ng/mL. Graphical representation of this data is presented in Figure 3.
Figure 3. Lack of association between MPA Css, both total (A, B) and unbound (C, D), with day 28 donor T-cell chimerism in related (A, C) and unrelated (B,D) donor PSBC grafts.
MPA Css is the average of all values from days 0 through 25.
Graft-versus-host disease
The median onset of aGVHD was day 35, with 162 patients experiencing aGVHD on or after day 25. Of these, 142 (55 related, 87 unrelated) patients had grades 2–4 aGVHD and 33 (18 related, 15 unrelated) had grades 3–4 aGVHD. One hundred eleven patients with either no or grade 1 aGVHD had a mean day 7 total MPA Css of 2.64 μg/mL compared with a mean of 2.44 μg/mL among the 190 patients who developed grades 2–4 aGVHD beyond day 7. Of those patients with unbound MPA Css available, the mean unbound MPA Css for the 111 patients with either no or grade 1 aGVHD was 24.33 ng/mL, compared with 24.35 ng/mL for the 190 patients who developed grades 2–4 aGVHD. There was no statistically significant association between total MPA Css and subsequent grades 3–4 GVHD for patients receiving related grafts (HR 1.50, 95% CI 0.62–3.65, P=0.37), as shown in Figure 2a. There was, however, a statistically significant association for those receiving grafts from unrelated donors (HR 0.54, 95% CI 0.29–1.01, P=0.05; Figure 1a). Grades 2–4 aGVHD and cGVHD were not associated with total MPA Css (Table 4) or unbound MPA Css (Supplemental Table 2). As reflected in Figure 1a, patients in the upper quartile—specifically, those with a total MPA Css > 2.96 μg/mL—had the lowest risk of grades 3–4 aGVHD.
Table 4.
Effect of increasing mean total MPA Css as fixed covariate on clinical outcomes occurring after day 25a
| Donor Type | ||||
|---|---|---|---|---|
| Related (N=132) | Unrelated (N=176) | |||
| OR/HR (95% CI) | P-value | OR/HR (95% CI) | P-value | |
| Day 28 T cell chimerism < 50% | 0.83 (0.38–1.82) | 0.63 | 1.39 (0.82–2.37) | 0.23 |
| Acute GVHD 2–4 | 0.86 (0.56–1.32) | 0.48 | 0.79 (0.59–1.07) | 0.13 |
| Acute GVHD 3–4 | 1.50 (0.62–3.65) | 0.37 | 0.54 (0.29–1.01) | 0.05 |
| Chronic GVHD | 0.68 (0.47–0.99) | 0.04 | 0.97 (0.75–1.27) | 0.84 |
| Relapse | 1.07 (0.74–1.55) | 0.73 | 1.06 (0.76–1.47) | 0.75 |
| Neutrophil nadir | 1.53 (0.90–2.61) | 0.12 | 0.87 (0.62–1.24) | 0.45 |
| CMV reactivation | 1.00 (0.63–1.59) | 0.99 | 0.88 (0.64–1.19) | 0.40 |
| Non-relapse mortality | 0.68 (0.35–1.34) | 0.27 | 0.58 (0.39–0.86) | 0.007 |
| Overall mortality | 1.01 (0.72–1.43) | 0.96 | 0.78 (0.61–1.01) | 0.06 |
MPA Css fit as continuous variable (truncated at 97.5th percentile to avoid outliers); HR per unit of MPA Css (μg/mL), adjusted for Kahl disease risk, antigen/2-allele mismatch, mean CNI concentration during week 2, year of transplant, female donor to male patient, tacrolimus prophylaxis, and fludarbine in conditioning (related donors only). Mean CNI week 2 fit as continuous variable, rescaled as standard deviation units to account for difference between cyclosporine and tacrolimus concentrations.
Relapse
One hundred thirty-four patients relapsed; 63 had related donors and 71 had unrelated donors. We found no association between mean total MPA Css and relapse hazard for patients with related (HR 1.07, 95% CI 0.74–1.55, P=0.73) or unrelated donors (HR 1.06, 95% CI 0.76–1.47, P=0.75). There was no association between unbound MPA Css and relapse for patients with related (HR 1.06, 95% CI 0.77–1.44, P=0.73) or unrelated donors (HR 1.14, 95% CI 0.87–1.50, P=0.34).
Non-relapse and overall mortality
Ninety patients died of NRM. Of these, 35 had a related donor and 55 had an unrelated donor. Among recipients of a related donor graft, we found no association between total MPA Css and risk of NRM (HR 0.68, 95% CI 0.35–1.34, P=0.27, Figure 2b). For recipients of an unrelated donor graft, there was a lower risk of NRM associated with total MPA Css (HR 0.58, 95% CI 0.39–0.86, P=0.007). Figure 1b shows that those patients with a total MPA Css > 2.96 μg/mL had the lowest non-relapse mortality.
We found that there was no statistically significant relationship between total MPA Css or unbound MPA Css and overall mortality. Patients receiving a related graft had a hazard of overall mortality of 1.01 (95% CI 0.72–1.43, P=0.96). Those receiving an unrelated graft had a slightly lower hazard of overall mortality; it was not, however, statistically significant (HR 0.78, 95% CI 0.61–1.01, P=0.06).
DISCUSSION
In this analysis, we evaluated MPA pharmacodynamics in 308 consecutive patients who were given nonmyeloablative conditioning before receiving allogeneic grafts to treat hematological malignancies. To our knowledge, this is the largest analysis of MPA pharmacodynamics in HCT patients to date (Supplemental Table 1). Among nonmyeloablative HCT recipients with a related donor graft, MPA Css was not associated with clinical outcomes (Table 4, Supplemental Table 2). Among patients given nonmyeloablative HCT with an unrelated donor graft, low MPA Css predicted the severity of aGVHD (Figure 1a), which is consistent with the findings of other investigators.(12, 14) Low total MPA Css also predicted high non-relapse mortality, potentially due to a higher risk of severe GVHD. Since total MPA Css was not associated with CMV reactivation or neutropenia (Table 4), oversuppression of the immune system is not apparent. There were, however, few patients who did not experience CMV reactivation, so it may not be possible to observe any adverse effects from elevated MPA Css.
The initial challenge of reliable engraftment for recipients of a nonmyeloablative HCT from an unrelated donor has been overcome by shortening the MMF dosing interval from Q12hr to Q8hr(6) and using G-PBMC grafts.(3) Low total MPA Css has previously been related to low (<50%) T-cell donor chimerism measured on days 28, 56, and 84.(7) The optimal day 28 donor T-cell chimerism is 50–90% in nonmyeloablative HCT recipients, with chimerism >40% associated with lower rejection risk.(28) Donor chimerism >50% is associated with higher complete remission rates through the GVT effect, which involves the immunoreactivity of donor cells against recipient cells. Donor chimerism <90% is associated with lower rates of grades 2–4 GVHD.(26, 28) In contrast to our previous study,(7) T-cell chimerism was not associated with total MPA Css (Figure 3). In this analysis, only day 28 T-cell chimerism was evaluated because all subsequent T-cell chimerism values occur after MMF has typically been discontinued. Thus, the day 28 T-cell chimerism is the only timepoint at which the MMF dose could be personalized to a target MPA Css. Additional studies regarding the association of MPA Css with chimerism are needed, as optimizing day 28 donor T-cell chimerism could lower rates of graft rejection and GVHD while maximizing the GVT effect.(29)
Nevertheless, other endpoints regarding the effectiveness of MMF are associated with total MPA Css in unrelated donor grafts. Low total MPA Css is associated with a higher risk of grades 3–4 aGVHD (Figure 1a). We hypothesize that personalizing the postgrafting MMF dose to achieve a target total MPA Css could lower severe aGVHD and NRM in patients receiving nonmyeloablative conditioning and an unrelated donor graft. Notably, other strategies—such as shortening the MMF dosing interval(6) or prolonged MMF administration (to day 180) and shortened cyclosporine treatment—have not decreased the incidence of GVHD among recipients of unrelated G-PBMC grafts given nonmyeloablative conditioning.(30) To achieve higher MPA Css, postgrafting MMF doses should be adjusted for the covariates associated with MPA clearance identified in our recent population pharmacokinetic model.(15) In a study of 408 patients, we found that total MPA clearance, adjusted for body weight, is increased in patients receiving cyclosporine as the concomitant CNI and in patients with lower albumin concentrations.(15) Supplemental Figure 1 (A&B) clearly shows that total MPA Css is lower in those patients who received concomitant cyclosporine compared to those receiving tacrolimus. These data support the recent findings by DeWinter et al. that higher MPA clearance in HCT recipients is due to higher predose cyclosporine concentrations and lower albumin concentrations than in renal transplant recipients.(31) All patients who experienced rejection received concomitant cyclosporine (Table 3). Notably, MPA pharmacokinetic parameters are not associated with the graft source.(15) Thus, the apparently different pharmacodynamic associations of total MPA Css between related and unrelated donors (Table 4) is not due to pharmacokinetic differences.
In addition to characterizing the covariates associated with MPA pharmacokinetics, population pharmacokinetic models can be used in conjunction with limited sampling schedules (LSS) to estimate an individual’s MPA Css without difficult, frequent, and invasive pharmacokinetic sampling. The creation of a population pharmacokinetic model and LSS can greatly facilitate the identification of pharmacodynamic relationships.(32) Our study team has observed an association between reduced total MPA Css and higher aGVHD and NRM following nonmyeloablative conditioning with an unrelated donor graft. This is consistent with the findings of Jacobson et al., who reported correlations between low total MPA trough concentrations and higher rates of graft failure and between low unbound MPA AUC and more frequent aGVHD in patients receiving different conditioning and grafts than our population.(14) In multi-center studies with an adequately sized HCT patient population receiving a homogenous post-grafting immunosuppressive regimen, this LSS can be used to identify MPA pharmacodynamics associated with clinical outcomes.
Furthermore, a population pharmacokinetic model and LSS would facilitate personalized MMF dosing to a target MPA Css; these approaches have been used with busulfan(33) and cyclophosphamide(34) in HCT recipients. In the setting of renal transplant, MMF dose personalization using MPA pharmacokinetics suggested lower rejection rates and gastrointestinal toxicity.(35, 36) Two of the three studies used population pharmacokinetic-based LSS to personalize oral MMF doses, reflecting acceptance of these tools in the solid organ transplant community. The target exposures are achieved with higher oral MMF doses and the clinical benefit of higher initial doses of oral MMF in renal transplant patients is being investigated.(36) In nonmyeloablative HCT with an unrelated donor graft, similar prospective studies are needed in which initial oral MMF doses are increased based on covariates associated with total MPA pharmacokinetics. Shortening the administration interval to every 6 hours apparently increased toxicity but did not improve efficacy in a phase I/II study of MMF with cyclosporine as acute GVHD prophylaxis in high-dose conditioning recipients.(4) Therefore, increasing the initial dose while maintaining the administration interval at 8 hours seems prudent. Notably, initial oral MMF doses would need to be ~33% higher with concomitant cyclosporine to achieve a similar total MPA Css to that achieved with oral MMF with concomitant tacrolimus, since cyclosporine coadministration increases total MPA clearance by 33.8%.(15) Also, increased total MPA clearance is associated with decreasing albumin concentration; thus, those patients with low albumin would need higher initial oral MMF doses. Further studies, in which oral MMF doses are personalized using a population pharmacokinetic-based LSS, are also needed.
The only pharmacodynamic association between total MPA Css and outcomes was observed in patients receiving an unrelated donor graft. In patients receiving a related donor graft, future studies should seek to evaluate additional biomarkers, such as inosine monophosphate dehydrogenase (IMPDH) activity. MPA selectively and reversibly inhibits IMPDH activity, which has been associated with rejection in renal transplant patients.(37) The feasibility of evaluating IMPDH activity in HCT recipients has been recently established.(38) IMPDH activity may be an important biomarker, as it is influenced by MPA concentrations and an individual patient’s sensitivity to IMPDH inhibition by MPA.
In conclusion, we found that low total MPA Css was associated with an increased risk of grades 3–4 aGVHD and NRM in recipients of unrelated donor grafts after nonmyeloablative conditioning. These finding were not observed in patients receiving a related donor graft. Studies are needed to identify alternative biomarkers—such as IMPDH activity—in these patients. Future prospective trials should address the clinical benefit of higher initial oral MMF doses – personalized based on covariates – and/or personalizing oral MMF doses to a target MPA Css above 2.96 μg/mL to lower aGVHD and NRM rates in unrelated donor patients receiving nonmyeloablative conditioning.
Supplementary Material
Acknowledgments
The authors are very grateful to the patients who participated in this study. In addition, the authors wish to thank the following staff for their invaluable help in making this work possible: Brian Phillips, Linda Risler, and Michelle Bouvier. The authors also wish to thank all physicians, nurses, and support personnel for their care of patients on this study.
Footnotes
Financial disclosure statement: Dr. Li is an employee of Amgen, Inc. This work is supported in part by grants: HL91744, CA18029, CA78902, HL36444, HL093294, 1TL1RR025016. The authors report no additional disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mineishi S. Overcoming the age barrier in hematopoietic stem cell transplantation: progress, but still a long way to go. Jama. 2011;306:1918–1920. doi: 10.1001/jama.2011.1612. [DOI] [PubMed] [Google Scholar]
- 2.Sorror ML, Sandmaier BM, Storer BE, et al. Long-term outcomes among older patients following nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation for advanced hematologic malignancies. Jama. 2011;306:1874–1883. doi: 10.1001/jama.2011.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maris MB, Niederwieser D, Sandmaier BM, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood. 2003;102:2021–2030. doi: 10.1182/blood-2003-02-0482. [DOI] [PubMed] [Google Scholar]
- 4.Nash RA, Johnston L, Parker P, et al. A phase I/II study of mycophenolate mofetil in combination with cyclosporine for prophylaxis of acute graft-versus-host disease after myeloablative conditioning and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2005;11:495–505. doi: 10.1016/j.bbmt.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of mycophenolate in solid organ transplant recipients. Clin Pharmacokinet. 2007;46:13–58. doi: 10.2165/00003088-200746010-00002. [DOI] [PubMed] [Google Scholar]
- 6.Maris MB, Sandmaier BM, Storer BE, et al. Unrelated donor granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cell transplantation after nonmyeloablative conditioning: the effect of postgrafting mycophenolate mofetil dosing. Biol Blood Marrow Transplant. 2006;12:454–465. doi: 10.1016/j.bbmt.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 7.Giaccone L, McCune JS, Maris MB, et al. Pharmacodynamics of mycophenolate mofetil after nonmyeloablative conditioning and unrelated donor hematopoietic cell transplantation. Blood. 2005;106:4381–4388. doi: 10.1182/blood-2005-06-2217. Epub 2005 Sep 4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Royer B, Larosa F, Legrand F, et al. Pharmacokinetics of mycophenolic acid administered 3 times daily after hematopoietic stem cell transplantation with reduced-intensity regimen. Biol Blood Marrow Transplant. 2009;15:1134–1139. doi: 10.1016/j.bbmt.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Osunkwo I, Bessmertny O, Harrison L, et al. A pilot study of tacrolimus and mycophenolate mofetil graft-versus-host disease prophylaxis in childhood and adolescent allogeneic stem cell transplant recipients. Biol Blood Marrow Transplant. 2004;10:246–258. doi: 10.1016/j.bbmt.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Haentzschel I, Freiberg-Richter J, Platzbecker U, et al. Targeting mycophenolate mofetil for graft-versus-host disease prophylaxis after allogeneic blood stem cell transplantation. Bone Marrow Transplant. 2008;42:113–120. doi: 10.1038/bmt.2008.85. [DOI] [PubMed] [Google Scholar]
- 11.Saint-Marcoux F, Guigonis V, Decramer S, et al. Development of a Bayesian estimator for the therapeutic drug monitoring of mycophenolate mofetil in children with idiopathic nephrotic syndrome. Pharmacol Res. 2011;63:423–431. doi: 10.1016/j.phrs.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Jenke A, Renner U, Richte M, et al. Pharmacokinetics of intravenous mycophenolate mofetil after allogeneic blood stem cell transplantation. Clin Transplant. 2001;15:176–184. doi: 10.1034/j.1399-0012.2001.150306.x. [DOI] [PubMed] [Google Scholar]
- 13.Bhatia M, Militano O, Jin Z, et al. An age-dependent pharmacokinetic study of intravenous and oral mycophenolate mofetil in combination with tacrolimus for GVHD prophylaxis in pediatric allogeneic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2010;16:333–343. doi: 10.1016/j.bbmt.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson P, Rogosheske J, Barker JN, et al. Relationship of mycophenolic acid exposure to clinical outcome after hematopoietic cell transplantation. Clin Pharmacol Ther. 2005;78:486–500. doi: 10.1016/j.clpt.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Mager DE, Bemer MJ, Sandmaier BM, Maloney D, McCune JS. Population Pharmacokinetics and Dose Optimization of Mycophenolic Acid in HCT Recipients Receiving Oral Mycophenolate Mofetil. Journal of Clinical Pharmacology. doi: 10.1002/jcph.14. Epub 2013 Feb http://onlinelibrary.wiley.com/doi/10.1002/jcph.14/abstract. [DOI] [PMC free article] [PubMed]
- 16.Tsina I, Chu F, Hama K, et al. Manual and automated (robotic) high-performance liquid chromatography methods for the determination of mycophenolic acid and its glucuronide conjugate in human plasma. J Chromatogr B Biomed Appl. 1996;675:119–129. doi: 10.1016/0378-4347(95)00343-6. [DOI] [PubMed] [Google Scholar]
- 17.Renner UD, Thiede C, Bornhauser M, Ehninger G, Thiede HM. Determination of mycophenolic acid and mycophenolate mofetil by high-performance liquid chromatography using postcolumn derivatization. Analytical chemistry. 2001;73:41–46. doi: 10.1021/ac0006730. [DOI] [PubMed] [Google Scholar]
- 18.Nowak I, Shaw LM. Mycophenolic acid binding to human serum albumin: characterization and relation to pharmacodynamics. Clin Chem. 1995;41:1011–1017. [PubMed] [Google Scholar]
- 19.Bryant E, Martin PJ. Documentation of Engraftment and Characterization of Chimerism Following Hematopoietic Cell Transplantation. In: Thomas ED, Blume KG, Forman SJ, editors. Hematopoietic Cell Transplantation. Malden, MA: Blackwell Science, Inc; 1999. pp. 197–206. [Google Scholar]
- 20.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 21.Sullivan KM, Agura E, Anasetti C, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991;28:250–259. [PubMed] [Google Scholar]
- 22.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Kahl C, Storer BE, Sandmaier BM, et al. Relapse risk in patients with malignant diseases given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood. 2007;110:2744–2748. doi: 10.1182/blood-2007-03-078592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Statistics in medicine. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 25.McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–3400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 26.Baron F, Baker JE, Storb R, et al. Kinetics of engraftment in patients with hematologic malignancies given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood. 2004;104:2254–2262. doi: 10.1182/blood-2004-04-1506. [DOI] [PubMed] [Google Scholar]
- 27.Ram R, Storer B, Mielcarek M, et al. Association between calcineurin inhibitor blood concentrations and outcomes after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:414–422. doi: 10.1016/j.bbmt.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baron F, Little MT, Storb R. Kinetics of engraftment following allogeneic hematopoietic cell transplantation with reduced-intensity or nonmyeloablative conditioning. Blood Rev. 2005;19:153–164. doi: 10.1016/j.blre.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Deeg HJ, Maris MB, Scott BL, Warren EH. Optimization of allogeneic transplant conditioning: not the time for dogma. Leukemia. 2006;20:1701–1705. doi: 10.1038/sj.leu.2404327. [DOI] [PubMed] [Google Scholar]
- 30.Baron F, Sandmaier BM, Storer BE, et al. Extended Mycophenolate Mofetil and Shortened Cyclosporine Failed to Reduce Graft-versus-Host Disease after Unrelated Hematopoietic Cell Transplantation with Nonmyeloablative Conditioning. Biol Blood Marrow Transplant. 2007;13:1041–1048. doi: 10.1016/j.bbmt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Winter BC, Mathot RA, Sombogaard F, et al. Differences in clearance of mycophenolic acid among renal transplant recipients, hematopoietic stem cell transplant recipients, and patients with autoimmune disease. Ther Drug Monit. 2010;32:606–614. doi: 10.1097/FTD.0b013e3181efd715. [DOI] [PubMed] [Google Scholar]
- 32.Li H, Mager DE, Bemer MJ, et al. A Limited Sampling Schedule to Estimate Mycophenolic Acid Area Under the Concentration-Time Curve in Hematopoietic Cell Transplantation Recipients. J Clin Pharmacol. 2011;52:1654–1664. doi: 10.1177/0091270011429567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bleyzac N, Souillet G, Magron P, et al. Improved clinical outcome of paediatric bone marrow recipients using a test dose and Bayesian pharmacokinetic individualization of busulfan dosage regimens. Bone Marrow Transplant. 2001;28:743–751. doi: 10.1038/sj.bmt.1703207. [DOI] [PubMed] [Google Scholar]
- 34.McCune JS, Batchelder A, Guthrie KA, et al. Personalized Dosing of Cyclophosphamide in the Total Body Irradiation-Cyclophosphamide Conditioning Regimen: A Phase II Trial in Patients With Hematologic Malignancy. Clin Pharmacol Ther. 2009;85:615–622. doi: 10.1038/clpt.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Gelder T, Hilbrands LB, Vanrenterghem Y, et al. A randomized double-blind, multicenter plasma concentration controlled study of the safety and efficacy of oral mycophenolate mofetil for the prevention of acute rejection after kidney transplantation. Transplantation. 1999;68:261–266. doi: 10.1097/00007890-199907270-00018. [DOI] [PubMed] [Google Scholar]
- 36.Le Meur Y, Buchler M, Thierry A, et al. Individualized mycophenolate mofetil dosing based on drug exposure significantly improves patient outcomes after renal transplantation. Am J Transplant. 2007;7:2496–2503. doi: 10.1111/j.1600-6143.2007.01983.x. [DOI] [PubMed] [Google Scholar]
- 37.Glander P, Hambach P, Braun KP, et al. Pre-transplant inosine monophosphate dehydrogenase activity is associated with clinical outcome after renal transplantation. Am J Transplant. 2004;4:2045–2051. doi: 10.1111/j.1600-6143.2004.00617.x. [DOI] [PubMed] [Google Scholar]
- 38.Laverdiere I, Caron P, Couture F, Guillemette C, Levesque E. Liquid chromatography-coupled tandem mass spectrometry based assay to evaluate inosine-5′-monophosphate dehydrogenase activity in peripheral blood mononuclear cells from stem cell transplant recipients. Analytical chemistry. 2012;84:216–223. doi: 10.1021/ac202404y. [DOI] [PubMed] [Google Scholar]
- 39.Kiehl MG, Shipkova M, Basara N, et al. Mycophenolate mofetil in stem cell transplant patients in relation to plasma level of active metabolite. Clin Biochem. 2000;33:203–208. doi: 10.1016/s0009-9120(00)00053-9. [DOI] [PubMed] [Google Scholar]
- 40.Furlong T, Martin P, Flowers ME, et al. Therapy with mycophenolate mofetil for refractory acute and chronic GVHD. Bone Marrow Transplant. 2009;44:739–748. doi: 10.1038/bmt.2009.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacobson PA, Huang J, Wu J, et al. Mycophenolate pharmacokinetics and association with response to acute graft-versus-host disease treatment from the Blood and Marrow Transplant Clinical Trials Network. Biol Blood Marrow Transplant. 2010;16:421–429. doi: 10.1016/j.bbmt.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hiwarkar P, Shaw BE, Tredger JM, et al. Mycophenolic acid trough level monitoring: relevance in acute and chronic graft versus host disease and its relation with albumin. Clin Transplant. 2011;25:222–227. doi: 10.1111/j.1399-0012.2010.01226.x. [DOI] [PubMed] [Google Scholar]
- 43.Bornhauser M, Schuler U, Porksen G, et al. Mycophenolate mofetil and cyclosporine as graft-versus-host disease prophylaxis after allogeneic blood stem cell transplantation. Transplantation. 1999;67:499–504. doi: 10.1097/00007890-199902270-00001. [DOI] [PubMed] [Google Scholar]
- 44.Basara N, Blau WI, Kiehl MG, et al. Mycophenolate mofetil for the prophylaxis of acute GVHD in HLA-mismatched bone marrow transplant patients. Clin Transplant. 2000;14:121–126. doi: 10.1034/j.1399-0012.2000.140204.x. [DOI] [PubMed] [Google Scholar]
- 45.Saint-Marcoux F, Royer B, Debord J, et al. Pharmacokinetic modelling and development of Bayesian estimators for therapeutic drug monitoring of mycophenolate mofetil in reduced-intensity haematopoietic stem cell transplantation. Clin Pharmacokinet. 2009;48:667–675. doi: 10.2165/11317140-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnston L, Florek M, Armstrong R, et al. Sirolimus and mycophenolate mofetil as GVHD prophylaxis in myeloablative, matched-related donor hematopoietic cell transplantation. Bone Marrow Transplant. 2012;47:581–588. doi: 10.1038/bmt.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.