Abstract
CD1d-restricted invariant Natural Killer T (iNKT) cells boost humoral immunity to T-dependent Ags that are co-administered with the CD1d-binding glycolipid Ag α-galactosylceramide (α-GC). Observations that mice lacking iNKT cells have decaying Ab responses following vaccination has led to the hypothesis that iNKT cells express plasma cell (PC) survival factors that sustain specific Ab titers. Bone marrow (BM) chimeric mice in which the entire hematopoetic compartment or iNKT cells selectively lacked BAFF, APRIL, or both BAFF and APRIL were created and immunized with NP-KLH adsorbed to Alum or mixed with α-GC. In comparison to BAFF- or APRIL-sufficient BM chimeras, absence of hematopoetic compartment- and iNKT-derived BAFF and APRIL was associated with rapidly decaying Ab titers and reduced PC numbers. The iNKT cell-derived BAFF or APRIL assumed a greater role in PC survival when α-GC was used as the adjuvant for immunization. These results show that iNKT-derived BAFF and APRIL each contribute to survival of PCs induced by immunization. This study sheds new light on the mechanisms through which iNKT cells impact humoral immunity and may inform design of vaccines that incorporate glycolipid adjuvants.
Keywords: CD1d, B cell, cytokine, NKT cell, plasma cell
INTRODUCTION
Invariant Natural Killer T cells (iNKT) are CD1d-restricted T cells that express an invariant T cell Ag receptor (TCR) α-chain (Vα14 in mice, Vα24 in humans) and have limited β-chain usage (1). There are at least two major modes of iNKT activation. APCs expressing CD1d in complex with foreign glycolipids activate the semi-variant TCR on iNKT cells leading to cellular activation (2). Bacterial LPS transactivates iNKT cells via IL-12-secreting DCs expressing CD1d/self-glycolipid (3).
Marine sponge-derived α-galactosylceramide (α-GC) is the most extensively studied foreign CD1d-binding glycolipid (2). Use of α-GC as an adjuvant for vaccination with foreign protein Ags (Ags) demonstrated that iNKT cells are regulators of adaptive immunity. Tumor-specific cytotoxic T cell responses (4–6) as well as Ag, toxin and virus-specific Ab responses (7–10) are enhanced by α-GC activation of iNKT cells.
α-GC-activated iNKT cells have several effects on T-dependent humoral immunity, enhancing primary and secondary Ab titers, affinity maturation, memory B cell generation, and induction of long-lived PCs (LLPC) (7, 9–12). The absence of iNKT cells (in CD1d−/− or Jα18−/− mice) is associated with non-responsiveness to α-GC (7, 9, 11–15). Adoptive transfer and BM chimera approaches have shown that B cell CD1d is required for the adjuvant effect of α-GC on T-dependent Ab and germinal center responses (14, 16, 17). iNKT-mediated B cell help for Ab responses may, in turn, be mediated by cognate and/or non-cognate mechanisms, depending on whether B cells are specific for T-independent lipid Ag or T-dependent protein Ag (17–20).
To date, the contribution of iNKT cells to the induction, rather than the maintenance, of humoral immunity has received the bulk of attention. Nonetheless, two key observations prompted the study described herein. Ab titers generated in response to vaccination decayed more rapidly in iNKT cell-deficient Jα18−/− mice than in C57Bl/6 (B6) wild-type controls (12). iNKT activation at the time of vaccination led to durable Ag-specific PC responses (11). This suggests that iNKT cells contribute to the maintenance of Ag-specific PCs and do so in a manner dependent on PC survival factors.
The TNF-family members, B cell activating factor of the TNF family (BAFF,) and a proliferation inducing ligand (APRIL), are important factors for survival of peripheral B cells and/or PCs (21). BAFF and APRIL are expressed by neutrophils, monocytes, macrophages, DCs, and, activated T cells and B cells (22). BAFF binds three receptors: BR3 (BAFF receptor 3); BCMA (B-Cell maturation Ag); and TACI (transmembrane activator and calcium modulator and cyclophilin ligand interactor), while APRIL binds BCMA and TACI (21).
Deficiency of either BAFF or BR-3 leads to a diminished pool of mature peripheral B cells (22). BCMA and APRIL are each critical to the establishment and/or survival of BM-resident PCs (23). BAFF and APRIL are not essential for memory B cell survival (24).
BAFF- and APRIL-encoding mRNA have been detected following microarray analysis of murine iNKT cells (25, 26) (NCBI GEO database, accession numbers GSM156736 and GSE15907). We therefore tested the hypothesis that iNKT-derived BAFF and/or APRIL regulate PC survival. To achieve this goal, BM chimeric mice were generated in which the entire hematopoietic system or iNKT cells selectively lacked expression of BAFF, APRIL, or both. We show that iNKT-derived BAFF and APRIL are individually dispensable for induction and maintenance of α-GC-enhanced Ab responses. If both BAFF and APRIL are absent, then induction of Ab responses is intact, but the titers decay more rapidly than in wild-type controls or in mice singly-deficient for BAFF or APRIL. We have therefore uncovered part of the mechanism by which iNKT cells impact the maintenance of humoral immunity.
MATERIALS AND METHODS
Mice
Female B6 mice (CD45.2- and CD45.1-congenic) were purchased from the National Cancer Institute (Bethesda, MD). Jα18−/− mice have a gene deletion in the TCR locus preventing rearrangement of Type I NKT cell α-GC-reactive TCR (27), and were kindly provided by Dr. Kronenberg (La Jolla Institute for Allergy and Immunology, La Jolla, CA). Jα18−/− mice were maintained at OUHSC. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at OUHSC. BAFF−/− (28), APRIL−/− and BAFF−/−/APRIL−/− (double knockout, DKO) mice on a homogenous B6 (CD45.2) genetic background (at least 9 backcrosses) were maintained at the University of Southern California (Los Angeles, CA) with IACUC approval. Long bones and spleens were harvested and shipped to OUHSC overnight in ice-cold media. Cells were then prepared and used for experiments. Vα14 TCR–transgenic mice were purchased from Jackson Laboratories (Bar Harbor, ME).
Reagents
HRP-conjugated anti-IgG1 was from Southern Biotechnology (Birmingham, AL). Fluorochrome-conjugated monoclonal antibodies (mAbs) were from BD Biosciences (San Jose, CA). FcγR-blocking mAb (2.4G2) was from Bio-X-Cell (West Lebanon, NH). CD1d tetramers loaded with α-GC analog PBS57 were provided by the NIAID Tetramer Facility (Emory University, Atlanta, GA). Recombinant BAFF and APRIL, BAFF- and APRIL-specific Ab and blocking peptides and α-GC, were from Axxora Inc. (San Diego, CA). Imject-Alum (Alum) was from Pierce (Rockford, IL). iNKT hybridoma cells (NKT.3c3) were kindly provided by Dr. Mitchell Kronenberg (LIAI, LA Jolla, CA).
SDS-PAGE and Immunoblotting
Cell lysates were subjected to SDS-PAGE and immuno-blotting using standard procedures. ECL Plus reagent (GE Healthcare, Piscataway, NJ) was used to detect membrane-bound Ab and imaged using a FluorChem Q imager (Alpha Innotech, Santa Clara, CA).
BM Chimeras
Six week-old female B6 CD45.1 mice were irradiated (700 Rad) and rested for 18 h before a second irradiation (500 Rad). After a further 4 h, 106 CD45.2-expressing donor BM cells were transferred i.v. to the recipients. The single donor chimeras consisted of BM from: B6, BAFF−/−, APRIL−/− or DKO mice. The mixed donor chimeras consisted of the following 50:50 mixtures: Jα18−/−/B6−/−; Jα18−/−/BAFF−/−; Jα18−/−/APRIL−/− or Jα18−/−/DKO−/−. Recipients were housed for 12 weeks before confirming engraftment and commencing experiments.
Isolation of splenocytes, BM, and iNKT cells
Splenocytes and BM cells were obtained as described previously (11). Cells were enumerated using a Nexcelom cell counter (Lawrence, MA). The iNKT cells were isolated by magnetic sorting using PE-B220-based negative selection followed by APC-CD1d-tetramer-based positive selection. This was done according to manufacturer’s instructions (Miltenyi Life Technologies, Grand Island, NY). Purity and yield were measured using cell counting and flow cytometry.
Flow Cytometry
Cells were incubated at 4°C or room temperature at a density of 107 cells/ml in RPMI plus 10% FCS with 2.4G2 mAb at a final concentration of 20 μg/ml. Fluorochrome-conjugated mAbs were added at a 2–10 μg/ml final concentration as appropriate or with APC-conjugated CD1d tetramer at a 4 μg/ml final concentration. After 1 h, unbound mAb or tetramer was removed by washing and centrifugation. Cells were fixed with 1% w/v para-formaldehyde and analyzed using Becton-Dickinson FACSCalibur and BD-Accuri cytometers (Palo Alto, CA). Forward and side scatter gating was used to select live splenocytes and bone marrow cells. The iNKT cell and mature B cell populations were then determined by anti-TCRβ/CD1d-tetramer and anti-B220/anti-CD93 staining respectively.
Cytokine Assays
Two hundred microliters of splenocytes at a density of 107 cells/ml were added to round-bottom sterile microtiter plates and followed by addition of media or media containing α-GC at a final concentration of 50, 250, or 500 ng/ml. Plates were incubated under 5 % CO2 for 6–48 h before collection of supernatants which were stored at −80°C until required. IL-4 and IFNγ concentrations were measured using multi-cytokine assay kits (Millipore, Bedford, MA) in conjunction with Bio-Plex instrumentation (Bio-Rad, Mountain View, CA). BAFF concentrations were measured by ELISA using a commercially-available kit (R&D Systems, Minneapolis, MN).
Immunization schedule
As described previously(10–12, 29) a single s.c. immunization consisting of 10 μg NP-KLH in 200 μl sterile-endotoxin-free PBS, NP-KLH mixed with 100 μl PBS and 100 μl Alum, or NP-KLH mixed with 4 μg of α-GC was administered over both flanks. Mice were bled on d 28 and then boosted s.c. with 10 μg of NP-KLH in PBS and bled on d 35, 61, and 91. At the end of the experiment, mice were euthanized in order to obtain BM.
Serum Ig measurement
Mice were bled and serum samples were prepared and stored at −20 °C until required. Endpoint NP-specific Ig titers (greatest serum dilution at which reactivity with Ag could not be detected) were then determined by ELISA as described previously (11). Ab titers were plotted using GraphPad Prism 5.0 software.
ELISPOT
ELISPOTS were performed as described previously (11). Spots, corresponding to Ab secreting cells were enumerated using a KS ELISPOT 4.10 plate reader (Carl Zeiss, Inc. Thornwood, NY).
Statistics
GraphPad Prism 5 software (La Jolla, CA) was used for statistical analysis. A non-parametric Mann-Whitney U test was used to assess experiments with two experimental groups (or multiple groups with independent matched controls) which provide an exact p value. Multiple experimental groups were assessed by one-way ANOVA with Dunn’s post-test. Comparisons of pairs of groups in the Dunn’s post-test indicate a p value within a range. Three, two, and one asterisks (***, **, and *) denote p values of <0.001, 0.001 – 0.01, and 0.01 – 0.05 respectively.
RESULTS
Characterization of iNKT cells in BAFF−/−, APRIL−/−, and DKO mice
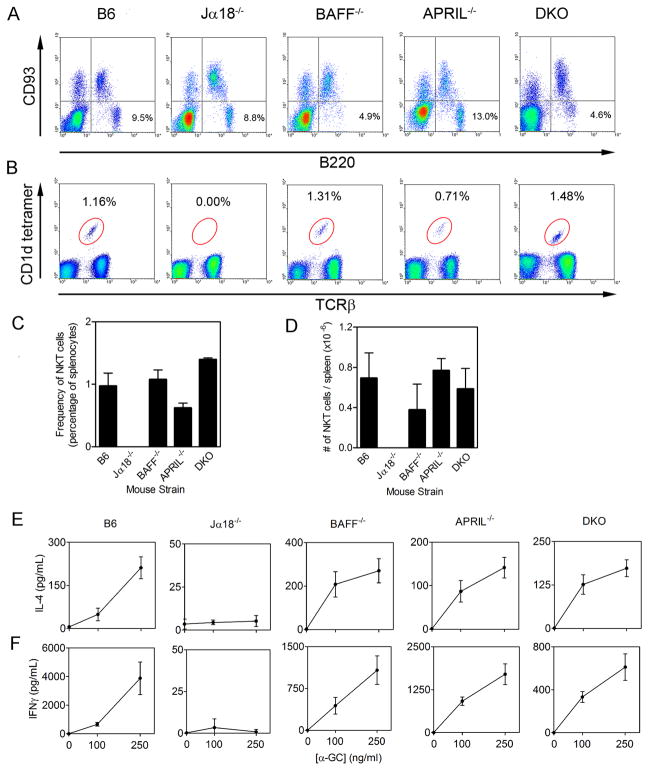
BAFF−/− mice are well recognized to have defects in the development and survival of mature B cells, while APRIL−/− mice have normal B cell maturation (22). B220+/CD93− mature B cells in the BM of B6, Jα18 BAFF−/−, APRIL−/−, and DKO−/− mice was analyzed by flow cytometry (Fig 1A). B6 and Jα18−/− mice had comparable frequencies of mature B cells, whereas BAFF−/− mice had fewer mature B−/− cells. APRIL mice had normal B cell frequencies while DKO mice had a low frequency of mature B cells, similar to the BAFF−/− mice.
Figure 1. B cells and NKT cells in donor mice.
BM cells and splenocytes were harvested from the donor B6, Jα18−/−, BAFF−/−, APRIL−/− and DKO mice (n = 3–5 per group). (A) BM cells were analyzed by flow cytometry for B220+ versus CD93− mature B cells. (B) Splenocytes were analyzed by flow cytometry for TCRβ+ versus CD1d tetramer+ cells. (C) Shows mean ± SD frequency and (D) shows absolute numbers ± SD of NKT cells. (E–F) Splenocytes from B6, Jα18−/−, BAFF−/−, APRIL−/−, and DKO mice were stimulated in vitro with α-GC and incubated for 48 h before collecting supernatants and measuring IL-4 (E) and IFNγ (F) by ELISA. Data show mean ± SD for triplicate samples. Data shown in A–F are representative of two independent experiments.
Splenic iNKT cells were analyzed by flow cytometry and found to be intact across all the strains except Jα18−/− mice (Fig 1B–D). Similarly, the functional capacity of iNKT cells in the BAFF−/−, APRIL−/−, and DKO mice was intact as demonstrated by secretion of IL-4 and IFNγ in response to in vitro stimulation of splenocytes with α-GC (Fig 1E–F). Differences in number of iNKT cells were expected because variances in B cells affect the relative proportion of iNKT cells in the mixed splenocytes and therefore affect the absolute amounts of IL-4 and IFNγ produced.
Absence of BAFF and APRIL in the hematopoietic compartment is associated with rapid decline of specific Ab titers
Single-donor BM chimeras were generated whereby the hematopoietic compartment was able to express both BAFF and APRIL (B6 chimera), selectively lacked BAFF (BAFF−/− chimera) or APRIL (APRIL−/− chimera), or lacked both BAFF and APRIL (DKO chimera) (Fig 2). This was achieved by engrafting irradiated CD45.1-expressing mice with CD45.2-expressing donor BM cells. BM B220+/CD93− mature B cells were restored comparably in all chimeras (Fig 2A). NKT cells were comparably restored in all chimeras except the DKO which had a higher splenic iNKT frequency (Fig 2B). Total numbers of spleen and BM cells recovered from each chimera were similar, thus B cell and NKT cell frequency was reflective of total cell numbers. CD45.2+ donor-derived cells constituted >95% of the reconstituted lymphocytes in the spleen consistent with our previous work (data not shown) (15, 29). CD4+ T cells, CD8+ T cells and DCs were also comparably restored (data not shown).
Figure 2. Re-constitution of immune cell compartments in BM chimeras lacking BAFF and APRIL in the hematopoetic compartment.
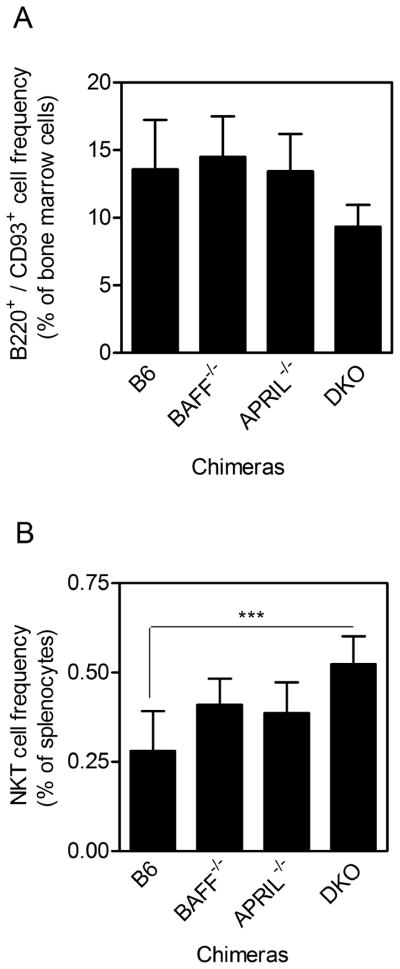
(A) BM cells were harvested from reconstituted chimeras and analyzed by flow cytometry. Graph depicts B220- and CD93- expressing cells as a percentage of total BM-derived cells. (B) Splenocytes were harvested from reconstituted chimeras and analyzed by flow cytometry. Graph depicts CD45.2+ anti-TCRβ- and CD1d tetramer-binding cells as a percentage of total splenocytes. Total BM cell and splenocyte recovery was comparable across all groups (not depicted). Data show mean ± S.D. values for analyses using 3–5 mice per group. Significantly higher iNKT frequency in the DKO than in the B6 splenocytes is indicated.
Prior to immunization with NP-KLH/Alum or NP-KLH/α-GC, anti-NP IgG1 titers could not be detected in the serum of the single chimeras (not depicted).
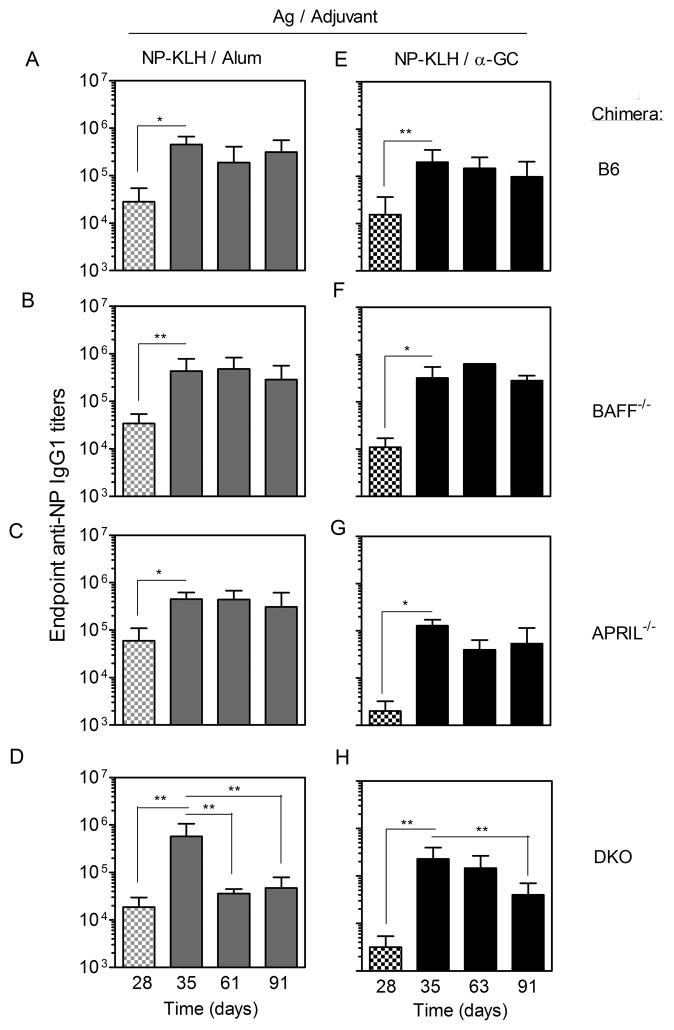
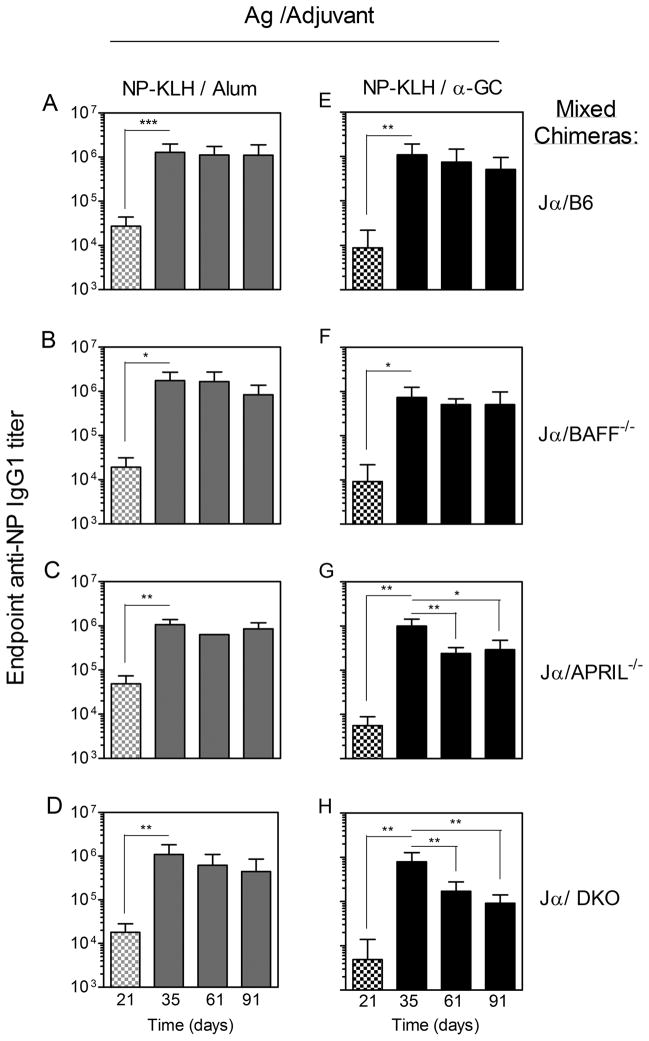
Following immunization of the single chimeras with NP-KLH/Alum, anti-NP IgG1 titers were detectable after 28 d (Fig 3A–D). When a booster vaccine was given consisting of NP-KLH alone, the titers were further increased. This is consistent with published reports that BAFF and APRIL are individually (30) and collectively (24) dispensable for Ab recall responses. Comparable anti-NP IgG1 titers were typically observed for the single chimeras on d 35 (7 d after booster vaccine administration) (Fig 3A–D). The large Ab titers in the absence of BAFF were consistent with a previous demonstration that radiation-resistant stromal cells provide sufficient BAFF to support engraftment and function of BAFF−/− donor cells (31). In the B6, BAFF−/−, and APRIL−/− chimeras, the endpoint titers displayed a non-significant decline over the next 56 d (d 91 after initial immunization) (Fig 3A–C). In contrast, the DKO chimeras showed a dramatic and significant (93.7%) decline in endpoint IgG1 titer by d 61 (Fig 3D). The decline in IgG1 titers in the DKO chimeras was not compensated by increases in other IgG subclasses including IgG2b and IgG2c, which had much lower titers than IgG1 (data not shown).
Figure 3. Immunization-induced Ab titers in chimeras lacking BAFF and APRIL in the hematopoetic compartment.
Sera were collected from the (A–D) NP-KLH/Alum-immunized and (E–H) NP-KLH/α-GC-immunized chimeras on the days indicated. Samples were analyzed by ELISA and endpoint anti-NP IgG1 titers determined. Data shows mean endpoint titer ±SD. Checkered bars indicate sera collected before the booster vaccine, whereas solid bars represent sera collected after the booster. Titers in pre-bleed sera were below 1/200 (not depicted). Numbers of mice per group were: (A, D, E, H) n = 7–11, (B, C, F, G) n = 3 – 5. Statistically significant differences between experimental groups were determined by one-way ANOVA and Dunn’s post-test.
Following immunization of the single chimeras with NP-KLH/α-GC, all groups had measurable primary IgG1 titers before administration of the booster vaccine on d 28 (Fig 3E–H). APRIL−/− and DKO chimeras, but not BAFF−/− chimeras had lower titers than the B6 controls, suggesting a contribution of APRIL to α-GC-driven primary Ab responses (p=0.01–0.05). All groups had a similar anti-NP IgG1 titer on d 35 (Fig 3 E–H), showing that BAFF and APRIL were individually and collectively dispensable for α-GC-driven Ab recall responses. The endpoint titers modestly declined over the subsequent 56 d in the B6, BAFF−/−, and APRIL−/− chimeras but the differences were not significant (Fig 3E–F). In DKO chimeras, however, the IgG1 titers showed a dramatic (82.5%) and statistically significant decline by d 91 (Fig 3H).
To establish that a decline in Ab titer was attributable to lack of PC persistence, BM cells were assessed at the end of the experiment for cells secreting NP-specific IgG1. The number of NP-specific PCs at d 95 in the DKO chimeras immunized with NP-KLH/Alum (Fig 4A) or NP-KLH/α-GC (Fig 4B), was lower than in the B6 chimeras. ELISPOT analysis was confined to the BM because very few splenic PCs can be detected 3 months after immunization (11).
Figure 4. Reduced PC numbers in chimeras lacking BAFF and APRIL in the hematopoetic compartment.
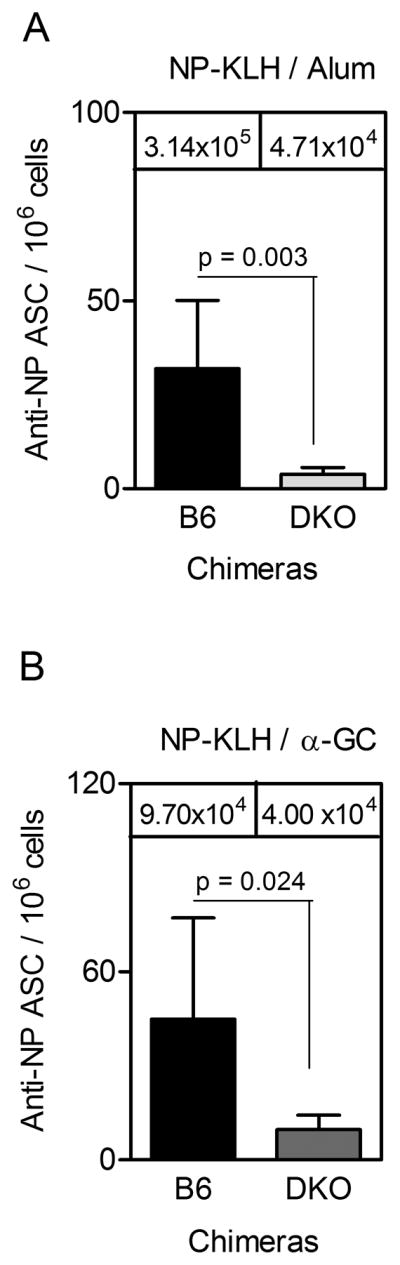
BM cells were collected on d 95 post-immunization from (A) NP-KLH/Alum-immunized chimeras and (B) NP-KLH/α-GC-immunized chimeras. Samples were then assessed by ELISPOT. Each bar shows the mean ± SD number of anti-NP IgG1 secreting cells per million total BM cells. Mean IgG1 titers at day 91 are shown for reference. For B6 and DKO chimeras, 7 and 6 mice per group were used respectively. Statistical significance was assessed using a two-tailed Mann Whitney t-test.
These results collectively show that the maintenance of immunization-induced PCs and serum Ab titers were highly dependent on the expression of BAFF and APRIL whether Alum or α-GC was used as the adjuvant.
BAFF and APRIL expression by iNKT cells
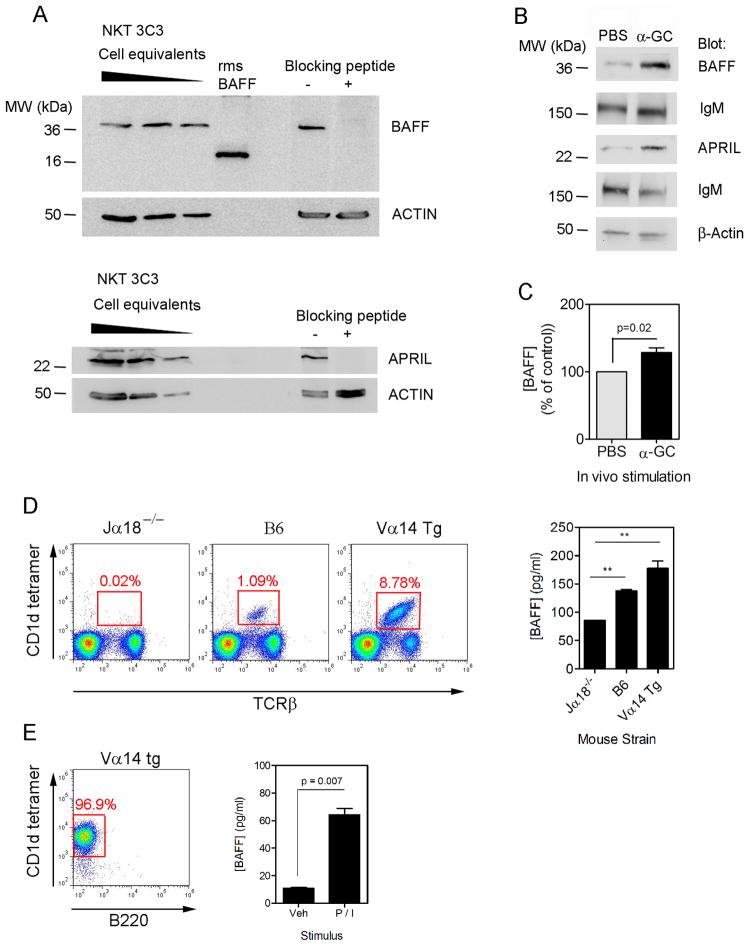
Expression of BAFF and APRIL before and after iNKT activation was examined (Fig 5). Mouse hybridoma iNKT cell (3C3) lysates were analyzed by SDS-PAGE and immuno-blotting revealed constitutive expression of BAFF and APRIL (Fig 5A). Blocking peptides confirmed the specificity of the Abs used.
Figure 5. Expression of BAFF and APRIL by splenic iNKT cells.
(A) NKT.3c3 hybridoma cell lysates (2.4, 1.2 and 0.6 × 106 cell equivalents per lane) were resolved by SDS-PAGE and transferred to nitrocellulose membranes before immunoblotting for BAFF (upper panels) and APRIL (lower panels). Recombinant murine soluble BAFF (rmsBAFF) served as a positive control for BAFF expression. Membranes were also prepared and incubated with anti-BAFF or anti-APRIL alone or in combination with a 100-fold molar excess of blocking peptide. (B) B6 mice were immunized i.p. with PBS or PBS containing α-GC. After 16 h, splenocytes were harvested and lysates prepared before immuno-blotting as indicated. (C) B6 mice were immunized i.p. with α-GC or PBS vehicle before collecting splenocytes, culturing for 6 h and measuring BAFF by ELISA. Data shown mean ± S.D. (n=6). Data are pooled from two experiments. (D) Splenocytes were obtained from Jα18−/−, B6, and Vα14 Tg mice and analyzed by flow cytometry (dot plots) or cultured before collecting supernatants and measuring BAFF concentrations by ELISA (bar graph). Values in bar graph represent mean ± SD for triplicate samples and are representative of two independent experiments. (E) Dot plot shows isolated iNKT cells and graph shows mean ± SD concentrations of secreted BAFF (for duplicate samples) following culture of cells with media or media containing PMA/ionomycin. Data are representative of three independent experiments.
Splenocytes were collected from B6 mice after injection with PBS or PBS/α-GC. Splenic cell lysates were prepared and analyzed for BAFF and APRIL expression (Fig 5B). Constitutive expression was detected and α-GC treatment led to increased expression relative to two loading controls (IgM and β-actin). B6 mice were also immunized with PBS or α-GC before collecting splenocytes for culture and measuring secreted BAFF by ELISA. BAFF concentrations were modestly (~30%), but significantly higher in splenocyte cultures from α-GC-treated mice than from PBS-treated mice (Fig 5C).
Unfortunately, Abs claimed to detect BAFF or APRIL by flow cytometry were not specific, as were reagents to detect APRIL by ELISA. Detection of secreted BAFF in B6 mice also proved difficult, due to low iNKT numbers, rather than reagent specificity. We therefore compared iNKT numbers in Jα18−/−, B6, and Vα14 TCR Tg mice (Fig 5D). As expected iNKT cells were near absent in Jα18−/− splenocytes (0.02%, detectable in B6 splenocytes (1.09%), but accounted for 8.78% of Vα14 TCR Tg splenocytes. Culturing splenocytes from these mouse strains revealed that constitutive BAFF secretion correlated with the NKT frequency. Treatment with α-GC in vitro did not increase BAFF secretion (data not shown). Importantly, BAFF secretion by iNKT cells isolated from Vα14 TCR Tg mice increased following stimulation with PMA/ionomycin (Fig 5E). The data collectively show that splenic iNKT cells could increase BAFF expression following activation.
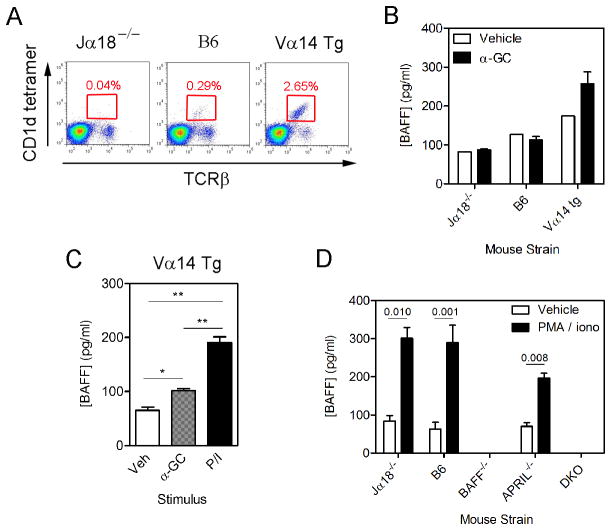
We then obtained bone marrow cells from Jα18−/−, B6, and Vα14 TCR Tg mice and examined iNKT frequency (Fig 6A). The iNKT cells were near absent in samples from the Jα18−/− mice (0.04%) and detectable in samples from the B6 mice (0.29%). The iNKT cells accounted for 2.65% of the cells in samples from the Vα14 TCR Tg mice. BM cells were then cultured with vehicle, α-GC, or PMA/ionomycin (Fig 6B, C). Treatment in vitro did not increase BAFF secretion in the BM cells from Jα18−/− or B6 mice (Fig 6B). Constitutive BAFF secretion was highest in the cultures from Vα14 TCR Tg mice (Fig 6B). Treatment in vivo also did not induce BAFF secretion from the B6 BM cells (data not shown).
Figure 6. Expression of BAFF by bone marrow iNKT cells.
(A) Shows frequency of bone marrow iNKT cells in Jα18−/−, B6, and Vα14 Tg mice. (B–D) Bone marrow cells were cultured in triplicate as indicated before collection of supernatants and measurement of BAFF concentrations by ELISA. (B) Cells from Jα18−/−, B6, and Vα14 Tg mice were treated for 6 hr with vehicle or α-GC (C) Cells from Vα14 Tg mice were treated as in (B) except that PMA/ionomycin treatment was included. (B) and (C) are representative of three independent experiments that produced similar results. (D) Bone marrow cells from strains depicted were treated with vehicle or PMA/ionomycin. Data in B–D show mean ± SD BAFF concentration for triplicate samples. Data in (B) and (C) are representative of three independent experiments. (D) is from a single experiment using duplicate samples.
In contrast, treatment in vitro with α-GC led to a modest, but significant increase in BAFF secretion by cells derived from the Vα14 TCR Tg mice (Fig 6B, C). As compared to α-GC treatment, a larger response was evident when PMA/ionomycin was used for treatment (Fig 6C, D). The PMA/ionomycin treatment was also used to demonstrate specificity of the reagents by culturing BM cells from the BAFF−/−, APRIL−/−, and DKO−/− donor mice (Fig 6D). As expected, BAFF was not secreted by cells from BAFF−/− or DKO donor mice. BAFF secretion by the APRIL−/− mice appeared lower than in the B6 or Jα18−/− BM cells, but ANOVA comparison of all PMA/ionomycin-stimulated groups did not detect significant differences.
BM NKT cells were isolated from Vα14 Tg mice by FACS and cultured with or without PMA/ionomycin, BAFF secretion was not detected (data not shown). The data therefore suggest that BM iNKT cells do not express BAFF directly, but potentiate its production by other cell types in the bone marrow, since higher NKT cell numbers were associated with more BAFF secretion.
Absence of iNKT cell-derived BAFF and APRIL is associated with rapid decline of specific Ab titers
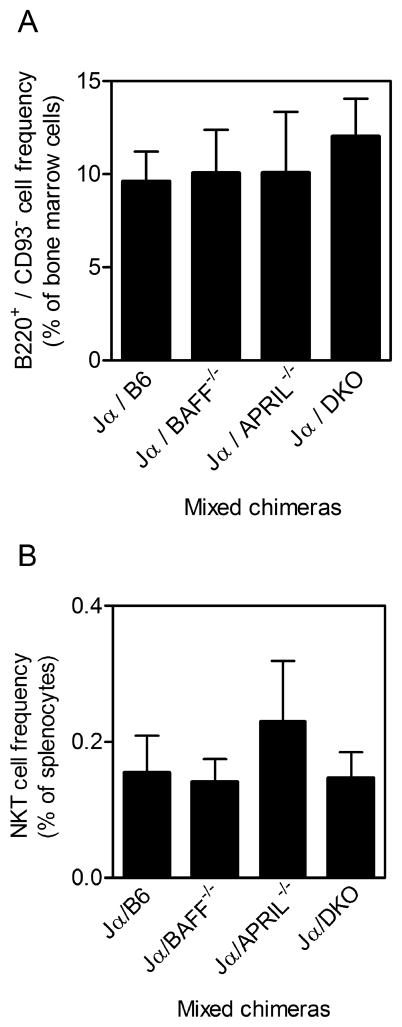
Mixed-donor BM chimeras were generated in which iNKT cells were able to express both BAFF and APRIL (Jα18−/−/B6), selectively lacked BAFF (Jα18−/−/BAFF−/−) or APRIL (Jα18−/−/APRIL−/−), or lacked both BAFF and APRIL (Jα18−/−/DKO). This was achieved by engrafting irradiated CD45.1-expressing mice with a 50:50 mix of CD45.2-expressing Jα18−/− and either (i) B6, (ii) BAFF−/−, (iii) APRIL−/−, or (iv) DKO donor cells. As demonstrated in our previous studies using mixed chimeras (15, 29, 32), major immune cell types including B cells, CD4+ T cells, CD8+ T cells, and DCs were reconstituted equally across experimental groups (data not shown). BM and spleen showed comparable restoration of B220+/CD93− mature B cells (Fig 7A) and iNKT cells respectively (Fig 7B). Furthermore, >95% of reconstituted cells, including iNKT cells, were donor-derived CD45.2+ cells (data not shown). Therefore, hematopoietic compartment-derived BAFF could not be produced by iNKT cells in the Jα18−/−/BAFF−/− chimeras. Similarly, iNKT cells could not produce APRIL in Jα18−/−/APRIL−/− chimeras nor could they produce BAFF or APRIL in Jα18−/−/DKO chimeras.
Figure 7. Re-constitution of immune cell compartments in BM chimeras lacking BAFF and APRIL in the iNKT compartment.
(A) BM cells were harvested from reconstituted chimeras and analyzed by flow cytometry. Graph depicts B220- and CD93- expressing cells as a percentage of total BM-derived cells. (B) Splenocytes were harvested from reconstituted chimeras and analyzed by flow cytometry. Graph depicts CD45.2+ anti-TCRβ- and CD1d tetramer-binding cells as a percentage of total splenocytes. Total BM cell and splenocyte recovery was comparable across all groups (not depicted). Data show mean ± S.D. values for analyses using 3–5 mice per group.
As expected, each of the mixed chimeras immunized with NP-KLH/Alum generated primary anti-NP IgG1 titers that were significantly boosted by administration of NP-KLH alone. Consequently, 35 d following immunization with NP-KLH/Alum (Fig 8A–D), each of the mixed chimeras had similar NP-specific IgG1 titers. Again, IgG1 was the dominant Ab subclass produced (data not shown). Sera were collected from each chimera on d 61 and 91 after immunization and titers compared to the ‘starting’ IgG1 titers on d 35. In the NP-KLH/Alum-immunized chimeras, the IgG1 titers were sustained in all groups (Fig 8A–D). These results indicated that NKT-derived BAFF and APRIL did not play a major role in persistence of Ab responses following NP-KLH/Alum immunization.
Figure 8. Immunization-induced Ab titers in chimeras lacking NKT-derived BAFF and APRIL.
Sera were collected from the (A–D) NP-KLH/Alum-immunized and (E–H) NP-KLH/α-GC-immunized chimeras on the ds indicated. Samples were analyzed by ELISA and endpoint anti-NP IgG1 titers determined. Data shows mean endpoint titer ±SD. Checkered bars indicate sera collected before the booster vaccine, whereas solid bars represent sera collected after the booster. Titers in pre-bleed sera were below 1/200 (not depicted). Numbers of mice per group were: (A, D, E, H) n = 7–11, (B, C, F, G) n = 4 – 6. Statistically significant differences between experimental groups were determined by one-way ANOVA and Dunn’s post-test.
Endpoint anti-NP IgG1 titers were also monitored in chimeras immunized with NP-KLH/α-GC (Fig 8E – H). In the Jα18−/−/B6 and the Jα18−/−/BAFF−/− groups, the primary IgG1 titers were boosted by immunization with NP-KLH alone on d 28 and did not exhibit significant decreases from d 35 to d 91 (Fig 8E, F), In contrast, in the Jα18−/−/APRIL−/− and the Jα18−/−/DKO groups, also boosted on d 28, titers significantly declined between d 35 and d91 by 71% and 88.6% respectively (Fig 8H).
To confirm that the reduction in serum Ab titers was due to declining PC numbers, ELISPOT analyses were performed on d 95. The numbers of PCs in the BM in the Jα18−/−/B6 and Jα18−/−/DKO groups were compared. Following NP-KLH/Alum immunization, PC numbers were significantly lower in the Jα18−/−/DKO group than in the Jα18−/−/B6 controls (Fig 9A). A similar result was observed for the NP-KLH/α-GC-immunized mixed chimeras (Fig 9B), but PC numbers were dramatically (and significantly) lower in the Jα18−/−/DKO group than in the Jα18−/−/B6 controls. Collectively these results indicate that iNKT-derived BAFF and APRIL contribute substantially to the maintenance of PC frequency and Ab titers following immunization with Ag mixed with α-GC, and perhaps following immunization with Ag adsorbed to Alum.
Figure 9. Reduced PC numbers in chimeras lacking BAFF and APRIL in the iNKT compartment.
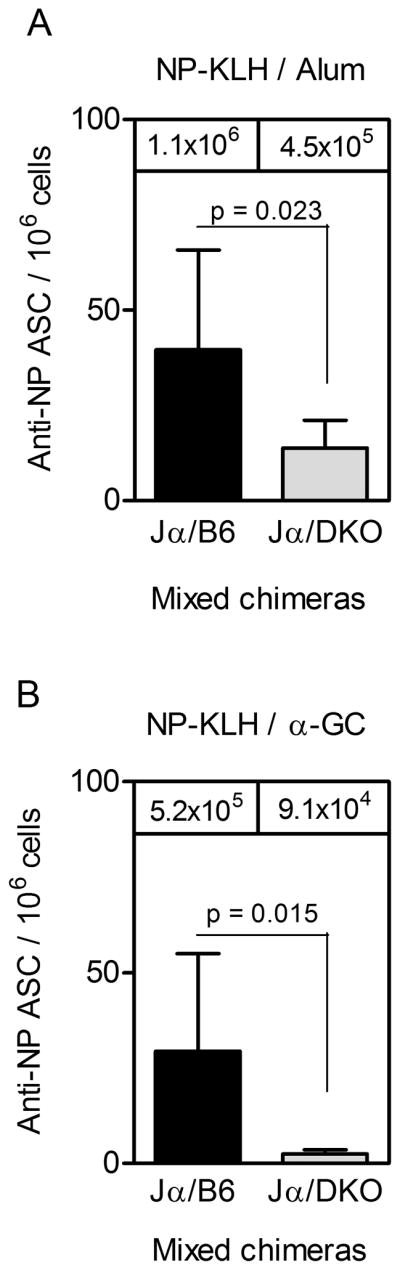
BM cells were collected on d 95 post-immunization from (A) NP-KLH/Alum-immunized chimeras and (B) NP-KLH/α-GC-immunized chimeras. Samples were then assessed by ELISPOT. Each bar shows the mean ± SD number of anti-NP IgG1 secreting cells per million total BM cells. Mean IgG1 titers at day 91 are shown for reference. For Jα18−/−/B6 and Jα18−/−/DKO chimeras, 11 and 7 mice per group were used respectively. Statistical significance was assessed using a two-tailed Mann Whitney t-test.
DISCUSSION
We report for the first time that iNKT cell-derived and -dependent BAFF and APRIL contribute to the maintenance of immunization-induced Ag-specific PCs. BAFF and APRIL derived from BM stromal cells as well as myeloid and lymphoid cells contribute to maintenance of PCs in the BM (31, 33). Co-immunization of mice with T-dependent Ag plus α-GC led to induction of LLPCs (11). In the absence of iNKT cells, Jα18−/− mice were unable to sustain long term-Ab titers following immunization with Ag plus α-GC (7). We therefore hypothesized that iNKT-derived BAFF and APRIL could account for iNKT-induced LLPCs.
Single BM chimeras were generated where the reconstituted hematopoietic compartment was competent for BAFF and APRIL expression, selectively lacked BAFF, selectively lacked APRIL, or lacked both BAFF and APRIL. When BAFF and APRIL were both absent, Ab titers and PCs were not sustained regardless of whether Alum or α-GC was used as the adjuvant. This observation is important because it reveals that the mechanisms by which iNKT cells contribute to PC survival are BAFF- and APRIL-dependent, regardless of their cellular source.
Mixed chimeras were generated in which iNKT cells lacked the ability to secrete BAFF, APRIL, or both BAFF and APRIL. In these mixed chimeras, iNKT-derived BAFF and APRIL, individually, were redundant for PC survival following immunization with Ag/Alum. In the absence of iNKT-derived BAFF and APRIL, the results were less clear. Ab titers did trend downwards but the differences between the Jα18−/−/DKO group and the Jα18−/−/B6 controls were not statistically significant. However, ELISPOT analysis of BM cells revealed significant differences between groups. Statistical versus biological significance aside, there could be interesting explanations for the apparent discrepancy. For example, there could be a residual population of PCs in spleen supported by a non-iNKT source of BAFF and/or APRIL that helps to maintain Ab titers. Alternatively, the few PCs remaining in BM could secrete more Ab per cell and help to sustain Ab titers.
In contrast, when iNKT cells were unable to express APRIL alone or both BAFF and APRIL, Ag-specific Ab titers were not sustained following immunization with Ag/α-GC. The difference in decline of Ab titers did not differ significantly between APRIL−/− iNKT cells and DKO iNKT cells. This suggests that iNKT-derived APRIL assumes a measurable role in maintaining Ab titers following iNKT activation with α-GC. This was not surprising as the BCMA receptor binds APRIL with a higher affinity than it binds BAFF, indicating that the APRIL-BCMA axis is favored over the BAFF-BCMA axis (21). In addition, APRIL but not BAFF can bind proteoglycans such as syndecans expressed on PCs (34), resulting in increased interaction of APRIL with PCs.
It is possible that the relative contribution of iNKT-derived or -regulated BAFF and APRIL may depend on the immunizing Ag and the specific α-GC adjuvant being used. There are at least 100 variants on the α-GC structure, some chemically synthesized, others derived from pathogenic bacteria. It is possible that during natural infection, the relative production of BAFF and APRIL by iNKT cells is determined by the CD1d/glycolipid presented, and the avidity and affinity of TCR interaction which may influence the outcome of TCR signaling (35).
Possible limitations imposed by the experimental system were considered. Engraftment of irradiated recipient mice with a 50/50 mix of two different BM types had an inbuilt assumption that the two donor cell types would engraft and expand equally. This was difficult to test directly because both donor sources were CD45.2-expressing and because BAFF−/− or APRIL−/− donors on a CD45.1 background were not available. However, we observed previously that reconstitution of irradiated CD45.2 mice with a 50/50 mix of Jα18−/− BM and B6 (CD45.1-expressing) BM led to engraftment such that around 65% of the reconstituted cells were presented by Jα18−/−-derived cells (15, 29). The engraftment ratio was highly consistent across several individual mice. Arguably, disruption to the hematopoietic compartment-derived BAFF or APRIL is minimal because fewer non-iNKT cells were BAFF−/− or APRIL−/− than predicted. Furthermore, the intact and sustained Ab responses in the Ag/Alum-immunized mixed chimeras provided evidence against a global deficiency of BAFF or APRIL. Attempts were made to engraft with an 80/20 mixture of BM donor cells, but iNKT cells failed to reconstitute (data not shown).
We were able to discern that activation of splenic and BM iNKT cells led to increased BAFF expression, and that splenic, but not BM iNKT cells could express BAFF directly. Importantly, constitutive expression of BAFF correlated with NKT numbers (by comparing Jα18−/− to B6 to Vα14) thus demonstrating the potential for iNKT cells to regulate the production of survival signals by other cell types.
Our data raise the question of how, where, and when Ag-specific PCs are exposed to iNKT-derived BAFF and APRIL, and why it should matter, given that BAFF from the BM stroma can adequately support PCs in the absence of hematopoetic BAFF (31). Since splenic iNKT cells can secrete BAFF directly, then perhaps they provide survival signals to Ag-experienced B cells or plasmablasts before migration to the BM or further differentiation into LLPC (33). This idea is supported by reports that iNKT cells can interact directly with CD1d+ B cells (36, 37), and that BAFF and APRIL can act in an autocrine manner (38). Furthermore, as spleen has limited niches for PC survival (40), local iNKT cells may provide survival signals to PCs thereby contributing to sustained Ab titers.
Since we did not detect BAFF expression by BM iNKT cells, this suggests that PC survival is largely governed by stromal- or myeloid cell-derived BAFF. However, the total amount of BAFF produced by BM cultures correlated with the number of iNKT cells suggesting that they could help to coordinate BAFF and APRIL expression by other cells types.
Different tissue microenvironments support the existence of different functional subsets of NKT cells (39). iNKT cells in the spleen and the BM could therefore represent different functional sub-sets. It is also possible that BAFF-secreting iNKT cells could circulate to and from the BM providing opportunities for direct interaction with PCs.
Our studies have revealed a new aspect of iNKT function and of the role of BAFF and APRIL in vaccination-induced humoral immunity. Determining how, when, and where iNKT-derived (and regulated) BAFF and APRIL influence PCs is an active focus of our current investigations that may illuminate possible mechanisms of action in patients with abnormal humoral immune responses.
Acknowledgments
This research was funded by NIH RO1 grants: AI078993 to M.L.L. and AR050193 to W.S.
We acknowledge generous support from the NIAID Tetramer Facility (Emory University, Atlanta, GA) for supplying CD1d tetramers. We thank Amy M. Johnson for management and genotyping of the Vα14 TCR Tg colony. We thank Jacob Keeling for assistance with BAFF detection.
Footnotes
AUTHOR CONTRIBUTIONS/DISCLOSURES
H.B.S. designed and performed experiments, analyzed data and wrote the paper. S.K.J., T.S.D., P.R. and G.A.L. performed experiments and analyzed data. W.S. contributed novel reagents, analyzed data and wrote the paper. M.L.L. designed and directed the project, performed experiments, analyzed data, and wrote the paper. The authors declare no competing financial interests.
References
- 1.Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annual review of immunology. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- 2.Venkataswamy MM, Porcelli SA. Lipid and glycolipid antigens of CD1d-restricted natural killer T cells. Seminars in immunology. 22:68–78. doi: 10.1016/j.smim.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brigl M, Tatituri RV, Watts GF, Bhowruth V, Leadbetter EA, Barton N, Cohen NR, Hsu FF, Besra GS, Brenner MB. Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. The Journal of experimental medicine. 2011;208:1163–1177. doi: 10.1084/jem.20102555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujii S, Shimizu K, Kronenberg M, Steinman RM. Prolonged IFN-gamma-producing NKT response induced with alpha-galactosylceramide-loaded DCs. Nature immunology. 2002;3:867–874. doi: 10.1038/ni827. [DOI] [PubMed] [Google Scholar]
- 5.Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. The Journal of experimental medicine. 2003;198:267–279. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hermans IF, Silk JD, Gileadi U, Salio M, Mathew B, Ritter G, Schmidt R, Harris AL, Old L, Cerundolo V. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J Immunol. 2003;171:5140–5147. doi: 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]
- 7.Galli G, Pittoni P, Tonti E, Malzone C, Uematsu Y, Tortoli M, Maione D, Volpini G, Finco O, Nuti S, Tavarini S, Dellabona P, Rappuoli R, Casorati G, Abrignani S. Invariant NKT cells sustain specific B cell responses and memory. Proc Natl Acad Sci U S A. 2007;104:3984–3989. doi: 10.1073/pnas.0700191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ko SY, Ko HJ, Chang WS, Park SH, Kweon MN, Kang CY. alpha-Galactosylceramide can act as a nasal vaccine adjuvant inducing protective immune responses against viral infection and tumor. J Immunol. 2005;175:3309–3317. doi: 10.4049/jimmunol.175.5.3309. [DOI] [PubMed] [Google Scholar]
- 9.Lang GA, Exley MA, Lang ML. The CD1d-binding glycolipid alpha-galactosylceramide enhances humoral immunity to T-dependent and T-independent antigen in a CD1d-dependent manner. Immunology. 2006;119:116–125. doi: 10.1111/j.1365-2567.2006.02413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devera TS, Aye LM, Lang GA, Joshi SK, Ballard JD, Lang ML. CD1d-dependent B-cell help by NK-like T cells leads to enhanced and sustained production of Bacillus anthracis lethal toxin-neutralizing antibodies. Infection and immunity. 2010;78:1610–1617. doi: 10.1128/IAI.00002-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devera TS, Shah HB, Lang GA, Lang ML. Glycolipid-activated NKT cells support the induction of persistent plasma cell responses and antibody titers. European journal of immunology. 2008;38:1001–1011. doi: 10.1002/eji.200738000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lang GA, Johnson AM, Devera TS, Joshi SK, Lang ML. Reduction of CD1d expression in vivo minimally affects NKT-enhanced antibody production but boosts B-cell memory. International immunology. 2011;23:251–260. doi: 10.1093/intimm/dxq477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devera TS, Aye LM, Lang GA, Joshi SK, Ballard JD, Lang ML. CD1d-dependent B-cell help by NK-like T cells leads to enhanced and sustained production of Bacillus anthracis lethal toxin-neutralizing antibodies. Infection and immunity. 78:1610–1617. doi: 10.1128/IAI.00002-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang GA, Devera TS, Lang ML. Requirement for CD1d expression by B cells to stimulate NKT cell-enhanced antibody production. Blood. 2008;111:2158–2162. doi: 10.1182/blood-2007-10-117309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devera TS, Joshi SK, Aye LM, Lang GA, Ballard JD, Lang ML. Regulation of anthrax toxin-specific antibody titers by natural killer T cell-derived IL-4 and IFNgamma. PloS one. 2011;6:e23817. doi: 10.1371/journal.pone.0023817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tonti E, Fedeli M, Napolitano A, Iannacone M, von Andrian UH, Guidotti LG, Abrignani S, Casorati G, Dellabona P. Follicular Helper NKT Cells Induce Limited B Cell Responses and Germinal Center Formation in the Absence of CD4+ T Cell Help. J Immunol. 2012;188:3217–3222. doi: 10.4049/jimmunol.1103501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barral P, Sanchez-Nino MD, van Rooijen N, Cerundolo V, Batista FD. The location of splenic NKT cells favours their rapid activation by blood-borne antigen. The EMBO journal. 2012;31:2378–2390. doi: 10.1038/emboj.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tonti E, Galli G, Malzone C, Abrignani S, Casorati G, Dellabona P. NKT-cell help to B lymphocytes can occur independently of cognate interaction. Blood. 2009;113:370–376. doi: 10.1182/blood-2008-06-166249. [DOI] [PubMed] [Google Scholar]
- 19.Tonti E, Fedeli M, Napolitano A, Iannacone M, von Andrian UH, Guidotti LG, Abrignani S, Casorati G, Dellabona P. Follicular helper NKT cells induce limited B cell responses and germinal center formation in the absence of CD4(+) T cell help. J Immunol. 2012;188:3217–3222. doi: 10.4049/jimmunol.1103501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King IL, Fortier A, Tighe M, Dibble J, Watts GF, Veerapen N, Haberman AM, Besra GS, Mohrs M, Brenner MB, Leadbetter EA. Invariant natural killer T cells direct B cell responses to cognate lipid antigen in an IL-21-dependent manner. Nature immunology. 2012;13:44–50. doi: 10.1038/ni.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bossen C, Schneider P. BAFF, APRIL and their receptors: structure, function and signaling. Seminars in immunology. 2006;18:263–275. doi: 10.1016/j.smim.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Mackay F, Schneider P, Rennert P, Browning J. BAFF AND APRIL: a tutorial on B cell survival. Annu Rev Immunol. 2003;21:231–264. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 23.O’Connor BP, V, Raman S, Erickson LD, Cook WJ, Weaver LK, Ahonen C, Lin LL, Mantchev GT, Bram RJ, Noelle RJ. BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med. 2004;199:91–98. doi: 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benson MJ, Dillon SR, Castigli E, Geha RS, Xu S, Lam KP, Noelle RJ. Cutting edge: the dependence of plasma cells and independence of memory B cells on BAFF and APRIL. J Immunol. 2008;180:3655–3659. doi: 10.4049/jimmunol.180.6.3655. [DOI] [PubMed] [Google Scholar]
- 25.Niemeyer M, Darmoise A, Mollenkopf HJ, Hahnke K, Hurwitz R, Besra GS, Schaible UE, Kaufmann SH. Natural killer T-cell characterization through gene expression profiling: an account of versatility bridging T helper type 1 (Th1), Th2 and Th17 immune responses. Immunology. 2008;123:45–56. doi: 10.1111/j.1365-2567.2007.02701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen NR, Brennan PJ, Shay T, Watts GF, Brigl M, Kang J, Brenner MB, Monach P, Shinton SA, Hardy RR, Jianu R, Koller D, Collins J, Gazit R, Garrison BS, Rossi DJ, Narayan K, Sylvia K, Fletcher A, Elpek K, Bellemare-Pelletier A, Malhotra D, Turley S, Best AJ, Knell J, Goldrath A, Jojic V, Regev A, Cohen N, Brennan P, Brenner M, Kreslavsky T, Bezman NA, Sun JC, Kim CC, Lanier LL, Miller J, Brown B, Merad M, Gautier EL, Jakubzick C, Randolph GJ, Kim F, Rao TN, Wagers A, Heng T, Painter M, Ericson J, Davis S, Ergun A, Mingueneau M, Mathis D, Benoist C. Shared and distinct transcriptional programs underlie the hybrid nature of iNKT cells. Nature immunology. 2013;14:90–99. doi: 10.1038/ni.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, Kaneko Y, Koseki H, Kanno M, Taniguchi M. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 28.Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, Frew E, Scott ML. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 29.Shah HB, Joshi SK, Lang ML. CD40L-null NKT cells provide B cell help for specific antibody responses. Vaccine. 2011;29:9132–9136. doi: 10.1016/j.vaccine.2011.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scholz JL, Crowley JE, Tomayko MM, Steinel N, O’Neill PJ, Quinn WJ, 3rd, Goenka R, Miller JP, Cho YH, Long V, Ward C, Migone TS, Shlomchik MJ, Cancro MP. BLyS inhibition eliminates primary B cells but leaves natural and acquired humoral immunity intact. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:15517–15522. doi: 10.1073/pnas.0807841105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gorelik L, Gilbride K, Dobles M, Kalled SL, Zandman D, Scott ML. Normal B cell homeostasis requires B cell activation factor production by radiation-resistant cells. J Exp Med. 2003;198:937–945. doi: 10.1084/jem.20030789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joshi SK, Lang GA, Devera TS, Johnson AM, Kovats S, Lang ML. Differential contribution of dendritic cell CD1d to NKT cell-enhanced humoral immunity and CD8+ T cell activation. Journal of leukocyte biology. 2012 doi: 10.1189/jlb.1111559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belnoue E, Pihlgren M, McGaha TL, Tougne C, Rochat AF, Bossen C, Schneider P, Huard B, Lambert PH, Siegrist CA. APRIL is critical for plasmablast survival in the bone marrow and poorly expressed by early-life bone marrow stromal cells. Blood. 2008;111:2755–2764. doi: 10.1182/blood-2007-09-110858. [DOI] [PubMed] [Google Scholar]
- 34.Huard B, McKee T, Bosshard C, Durual S, Matthes T, Myit S, Donze O, Frossard C, Chizzolini C, Favre C, Zubler R, Guyot JP, Schneider P, Roosnek E. APRIL secreted by neutrophils binds to heparan sulfate proteoglycans to create plasma cell niches in human mucosa. The Journal of clinical investigation. 2008;118:2887–2895. doi: 10.1172/JCI33760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Venkataswamy MM, Porcelli SA. Lipid and glycolipid antigens of CD1d-restricted natural killer T cells. Seminars in immunology. 2010;22:68–78. doi: 10.1016/j.smim.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang PP, Barral P, Fitch J, Pratama A, Ma CS, Kallies A, Hogan JJ, Cerundolo V, Tangye SG, Bittman R, Nutt SL, Brink R, Godfrey DI, Batista FD, Vinuesa CG. Identification of Bcl-6-dependent follicular helper NKT cells that provide cognate help for B cell responses. Nature immunology. 2011;13:35–43. doi: 10.1038/ni.2166. [DOI] [PubMed] [Google Scholar]
- 37.Lang ML. How do natural killer T cells help B cells? Expert review of vaccines. 2009;8:1109–1121. doi: 10.1586/erv.09.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mackay F, Tangye SG. The role of the BAFF/APRIL system in B cell homeostasis and lymphoid cancers. Current opinion in pharmacology. 2004;4:347–354. doi: 10.1016/j.coph.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 39.Eberl G, Lees R, Smiley ST, Taniguchi M, Grusby MJ, MacDonald HR. Tissue-specific segregation of CD1d-dependent and CD1d-independent NK T cells. J Immunol. 1999;162:6410–6419. [PubMed] [Google Scholar]
- 40.Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dorner T, Hiepe F. Competence and competition: the challenge of becoming a long-lived plasma cell. Nature reviews. 2006;6:741–750. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]