Abstract
Background
Clinical validation studies of the Healthcare Effectiveness Data and Information Set (HEDIS®) measures of inappropriate prescribing in the elderly are limited.
Objectives
The objective of this study was to examine associations of new exposure to High Risk Medication in Elderly (HRME) and drug-disease interaction (Rx-DIS) with mortality, hospital admission, and emergency care.
Methods
A retrospective database study was conducted examining new use of HRME and Rx-DIS in fiscal year 2006 (Oct 2005-Sep 2006; FY06), with index date being date of first HRME/Rx-DIS exposure, or first day of FY07 if no HRME/Rx-DIS exposure. Outcomes were assessed one year after index date. The participants are veterans who were ≥65 years old in FY06and received Veterans Health Administration (VA) care in FY05-06. A history of falls/hip fracture, chronic renal failure, and/or dementia per diagnosis codes defined the Rx-DIS subsample. The variables included a number of new unique HRME drug exposures and new unique Rx-DIS drug exposure (0, 1, >1) in FY06, and outcomes (i.e., 1-year mortality, hospital admission, and emergency care) up to one year after exposure. Descriptive statistics summarized variables for the overall HRME cohort and the Rx-DIS subset. Multivariable statistical analyses using Generalized Estimating Equations (GEE) models with a logit link accounted for nesting of patients within facilities. For these latter analyses, we controlled for demographic characteristics, chronic disease states, and indicators of disease burden the previous year (e.g., number of prescriptions, emergency/hospital care).
Results
Among the 1,807,404 veterans who met inclusion criteria, 5.2% had new HRME exposure. Of the 256,388 in the Rx-DIS cohort, 3.6% had new Rx-DIS exposure. Multivariable analyses found that HRME was significantly associated with mortality (1: adjusted odds ratio [AOR]=1.62, 95% CI 1.56-1.68; >1: AOR=1.80; 95% CI 1.45-2.23), hospital admission (1: AOR=2.31, 95% CI 2.22-2.40; >1: AOR=3.44, 95% CI 3.06-3.87) and emergency care (1: AOR=2.59, 95% CI 2.49-2.70; >1: AOR=4.18, 95% CI 3.71-4.71). Rx-DIS exposure was significantly associated with mortality (1: AOR=1.60, 95% CI 1.51-1.71; >1: AOR=2.00; 95% CI 1.38-2.91), hospital admission for one exposure (1: AOR=1.12, 95% CI 1.03-1.27; >1: AOR=1.18; 95% CI 0.71-1.95) and emergency care for two or more exposures (1: AOR=1.06; 95% CI 0.97-1.15; >1: AOR=2.0; 95% CI 1.35-3.10.
Conclusions
Analyses support the link between HRME/Rx-DIS exposure and clinically significant outcomes in older Veterans. Now is the time to begin incorporating input from both patients who receive these medications and providers who prescribe to develop approaches to reduce exposure to these agents.
1 Introduction
The goals of pharmacotherapy in older adults are to extend and improve quality of life, and treat and cure illness, however, exposure to potentially inappropriate medications can lead to considerable morbidity and mortality.1 Indeed, the Institute of Medicine has identified the recognition and prevention of drug-related problems in the elderly as a key priority for this decade.2, 3
Potentially inappropriate medications can be measured using either implicit or explicit measures. Implicit measures that require clinician judgment of appropriateness for each case are time-consuming and unrealistic to use in population-based studies.4 The most commonly used set of explicit criteria was first developed in 1991 by Dr. Mark Beers and updated twice.5-7 In 2006, using a subset of the 2003 Beers criteria, the National Committee on Quality Assurance (NCQA) created two measures to examine the quality of prescribing for older patients:8 a list of drugs to avoid (High Risk Medications in the Elderly [HRME]), and clinically relevant drug-disease interactions (Rx-DIS). These consensus-based quality measures included in the Healthcare Effectiveness Data and Information Set® (HEDIS®), but little information is available linking exposure based on these measures to important health outcomes/health services use.9
Prior studies examining prevalent use of the drugs included in the Beers Criteria (foundation of these HEDIS® measures), led to inconsistent results.9-13 We hypothesize that, because the majority of exposure represents chronic use14, 15, the source of these inconsistent findings may be due to survivor bias, where individuals who have adverse events discontinue the drug and those who tolerate the drug represent the vast majority of prevalent users.16 Thus, we sought to evaluate the clinical importance of HRME and Rx-DIS measures by assessing the association between new HRME and Rx-DIS exposure and the subsequent outcomes of emergency care, hospital admission and mortality using a cohort of community-dwelling elders receiving care in the Veterans Health Administration (VA).
2 Methods
2.1 Study Design, Setting, and Sample
This retrospective new-exposure design was conducted using VA national data. The cohort consisted of Veterans aged ≥65 years by October 1, 2005 (beginning Fiscal Year 2006 [FY06]) who received VA inpatient or outpatient care at least once each year in FY05 and FY06 (October 1, 2004 through September 30, 2006) and who were not in VA long-term care. Those with HRME or Rx-DIS exposure prior to FY06 (N=142,988) or who had subsequent new HRME or Rx-DIS exposure in FY07 (N=73,077) were excluded. This study was approved by Institutional Review Boards at the University of Texas Health Science Center at San Antonio and the Bedford VA.
2.2 Data Sources
We created an analytic dataset by combining national VA outpatient medication dispensing information from Pharmacy Benefits Management (PBM) Services, Medical SAS® Dataset files and the VA Vital Status Mini file. The VA PBM prescription data included the start date, generic drug name, and day supply for each drug dispensed.17 Medical SAS® Datasets contained International Classification of Diseases-9 (ICD-9) Clinical Modification codes for inpatient and outpatient diagnoses.18, 19 The VA Vital Status Mini file included date of death (98% sensitivity when validated against the National Death Index).20, 21
2.3 Outcome Measures
We created three dichotomous dependent variables during the study period: mortality (Vital Status files), emergency care (VA stop code pair 102-101 in outpatient data), and VA hospital admission (date of admission using inpatient data). Outcomes were examined from initial exposure to one year after the index date which was defined as exposure for HRME and Rx-DIS respectively; for those without HRME or Rx-DIS, these outcomes were assessed in FY07.
2.4 Primary Independent Variables
2.4.1 Exposure to High Risk Medications in the Elderly
We identified new use of any HRME drug using the generic drug name in the VA PBM data. Specific medications included in this measure are included in Electronic Supplementary Material 1: HEDIS HRME measures. Those with no HRME exposure in FY05, and HRME exposure in FY06 were classified as having new HRME exposure, with the index date as the day of prescription. Those without HRME prescription were classified as no exposure, with October 1, 2005 as the index date.
2.4.2 Exposure to Drug-Disease Interactions
To create the Rx-DIS variable, we first identified a subset of the cohort with falls/hip fracture, dementia or chronic renal failure (CRF; includes only a portion of those with chronic kidney disease) using the ICD-9 codes algorithm developed and tested by NCQA.22
Next we identified the specific drugs/classes that could exacerbate these disease/conditions based on the NCQA algorithms: a) falls/fractures: tricyclic antidepressants, conventional or atypical antipsychotics, and specific sleep agents (e.g., zolpidem); b) dementia: highly anticholinergic agents (i.e., gastrointestinal antispasmodics, skeletal muscle relaxants) and tricyclic antidepressants; c) CRF: nonsteroidal anti-inflammatory drugs (NSAID).23 Those with no Rx-DIS exposure in FY05 but with Rx-DIS exposure in FY06 were classified as new Rx-DIS exposure, with date of first prescription as the index date. For those without Rx-DIS exposure, the index date was October 1, 2005.
For the both independent variables we further identified new exposure as none, one or two or more (distinct drug) exposures to examine the possibility of a “dose response” relationship.
2.5 Covariates
We controlled for a number of demographic and clinical characteristics that could potentially confound an association between HRME or Rx-DIS24 and our three outcomes.11, 25, 26 These variables were selected for inclusion in this analysis since they were identified as being associated with HRME and Rx-DIS in our prior research.14, 15
2.5.1 Demographic Characteristics
We identified patient demographic characteristics (age, sex, race/ethnicity) using data fields from FY04-FY06. With the exception of race/ethnicity, these demographic characteristics are well documented and complete in the medical record. Missing data on race/ethnicity is common in VA outpatient data; however, multiple years of data help mitigate this problem. Categories of race/ethnicity were white, black, other (Native American or Asian), Hispanic (of any race), and missing. Finally, our prior studies have found that income under the VA poverty limit is associated with exposure to HRME and Rx-DIS, so we included an indicator for poverty based on the VA means test.18, 19
2.5.2 Clinical Characteristics
Clinical characteristics included a number of measures that directly indicate disease burden and several variables that are associated with high disease burden. Previous studies indicate that individuals with higher disease burden as defined by more medications, more physical comorbidities and psychiatric conditions are at risk for adverse outcomes.2, 27-29 We first counted the number of unique medications each individual received during FY05, which includes medications prescribed for “as needed” use. We also used ICD-9-CM codes found in VA inpatient and outpatient data (FY04-05) to identify individuals with physical and psychiatric conditions using the Selim comorbidity indices,30, 31 developed as a measure of disease burden in research studies involving Veterans. For physical conditions (Selim Physical), we counted the number of chronic disease states from 30 possible conditions (CKD is included in this count, but not dementia or falls/fractures). The Selim Psychiatric index includes a count of 6 mental health conditions: schizophrenia, bipolar, depressive, substance use, anxiety and post-traumatic stress disorders. In order to facilitate interpretation of findings we created categorical variables. Based on the empirical distribution and our prior research, we classified medications as 0-5, 6-8, 9-11 and 12 or more. For physical conditions, we identified individuals with zero to one, two to three, four to five and six or more chronic conditions; we identified individuals with zero, one, and two or more psychiatric conditions.
We also included prior healthcare utilization that may indicate disease burden. Our prior research indicates that older Veterans who receive geriatric care have been found to have higher disease burden and increased risk of HRME and Rx-DIS; therefore, we identified individuals who received geriatric care (outpatient or inpatient) in FY05. 8 We also identified individuals who had at least one incidence of emergency care or hospital admission in FY05 as indicators of disease burden.
Finally, while primary care may reduce need for emergency care and hospital admission for ambulatory care-sensitive conditions, it may also be an indicator of disease burden; 32 thus we counted the number of primary care visits in FY05. Based on the empirical distribution, we classified patients as having zero to one, two to four and five or more primary care visits to ease interpretation of findings.
2.6 Statistical Analysis
Descriptive statistics were used to summarize variables for the overall HRME cohort and the Rx-DIS subset. With the exception of race, missing data was extremely rare. Because our prior work indicated that those without race/ethnicity identified in the VA data tend to be healthier, seen less frequently in the VA and white, we included those individuals as unclassified, to avoid skewing our findings toward the population with high disease burden. We then calculated the unadjusted odds ratios using logistic regression analyses followed by multivariable statistical analyses using Generalized Estimating Equations (GEE) models with a logit link to account for nesting of patients within facilities. These models examined the association of new HRME exposure with all-cause mortality, emergency care, and hospital admission.33, 34 A similar approach was used for separate analyses for Rx-DIS and these outcomes. Theoretically meaningful interactions (e.g., geriatric care and HRME/Rx-DIS exposure) were included; only significant interactions were included in the final model. Effects were reported as adjusted odds ratios (AOR) with 95% confidence intervals (95% CI).
We also conducted secondary analyses using propensity score methods to assess the extent to which the relationship between HRME/Rx-DIS exposure predicted adverse outcomes, above and beyond characteristics associated with individual propensity to have HRME/Rx-DIS exposure. Propensity for HRME and Rx-DIS exposure (separate scores) was calculated using logistic regression analysis (covariates described above predicting each exposure), and included as a predictor in logistic regression models predicting each outcome with the propensity score and the exposure variable.35 Comparison of the AOR allows an assessment of the contribution of individual and exposure variables for each outcome. Statistical analyses were performed using SAS® software (version 9.2; Cary, NC) and IBM SPSS® statistics for Windows (version 20; Armonk, NY).
3 Results
Overall, 1,807,404 older veterans met inclusion criteria. Our study sample (see Table 1) was predominantly male, white and the majority had ≥2 comorbidities (70% and 90% for the total sample and the Rx-DIS subsample, respectively) and reported income under the poverty limit (60% and 70%). The mean day supply for HEDIS® medications (HRME and Rx-DIS) was 89.2 days (SD=106.0; range 1 to 365). During the one-year study period, 81,003 (4.6%) died, 87,118 (4.9%) had one or more hospital admissions, and 244,106 (13.5%) received emergency care.
Table I. Descriptive statistics for those with and without HRME/Rx-DIS exposure.
| Variable | HRME Exposure FY06 (n=94,648) |
No HRME Exposure FY06 (n=1712756) |
Rx-DIS Exposure FY06 (n=9101) |
No Rx-DIS Exposure FY06 (n=247487) |
|---|---|---|---|---|
| Demographics | % | % | % | % |
| Age | ||||
| 65-74 years | 51.4 | 45.0 | 30.6 | 29.2 |
| 75-84 years | 41.4 | 46.1 | 54.4 | 55.2 |
| ≥85 years | 7.3 | 8.9 | 15.0 | 15.6 |
| Women | 2.7 | 1.6 | 2.1 | 1.8 |
| Race/ethnicity | ||||
| White | 71.2 | 65.7 | 69.1 | 68.3 |
| African American | 9.4 | 6.1 | 12.2 | 10.5 |
| Hispanic | 5.3 | 3.0 | 7.1 | 4.1 |
| Other | 1.4 | 1.3 | 1.5 | 1.4 |
| Unknown | 12.6 | 24.0 | 10.1 | 15.8 |
| Under poverty limit | 75.6 | 57.7 | 79.5 | 70.3 |
| Clinical characteristics FY05 | ||||
| Count of medications | ||||
| 0-5 | 26.5 | 49.9 | 17.8 | 26.7 |
| 6-8 | 24.2 | 25.9 | 23.5 | 26.1 |
| 9-11 | 20.4 | 13.8 | 21.8 | 20.8 |
| ≥12 | 28.9 | 10.5 | 36.9 | 26.4 |
| Physical comorbidities FY05 | ||||
| 0-1 | 17.6 | 29.0 | 7.4 | 9.5 |
| 2-3 | 42.7 | 42.2 | 28.2 | 33.4 |
| 4-5 | 26.9 | 18.2 | 31.1 | 30.9 |
| ≥6 | 13.3 | 5.1 | 33.3 | 26.2 |
| Psychiatric comorbidities FY05 | ||||
| 0 | 78.4 | 87.8 | 62.3 | 74.8 |
| 1 | 15.9 | 9.9 | 25.6 | 18.7 |
| ≥2 | 5.7 | 2.4 | 12.2 | 6.6 |
| 1 or more emergency visits FY05 | 31.1 | 13.0 | 39.3 | 26.4 |
| 1 or more hospital admissions FY05 | 12.9 | 5.1 | 20.5 | 14.4 |
| 1 or more geriatric care visits FY05 | 2.5 | 2.4 | 7.9 | 6.3 |
| Primary care visits FY05 | ||||
| 0-1 | 14.1 | 27.4 | 11.7 | 16.9 |
| 2-4 | 51.7 | 55.9 | 45.2 | 51.2 |
| ≥5 | 34.2 | 16.7 | 43.0 | 31.9 |
| Outcomes up to one year after index date | ||||
| Hospital admission | 16.0 | 4.2 | 4.6 | 3.1 |
| Emergency care | 38.6 | 12.1 | 6.2 | 3.5 |
| Death | 9.1 | 4.2 | 15.6 | 10.0 |
HRME = High risk medication in the elderly
Rx-DIS=Drug disease interaction
FY = Fiscal Year.
All comparisons between exposed and unexposed statistically significant p <.001
3.1 HRME Exposure
In FY 06, 94,684 (5.2%) had new HRME exposure; 94% (n=89,052) of those had exposure to one HRME drug. The most common HRME groups prescribed were antihistamines (1.7%), skeletal muscle relaxants (1.5%), opioids (0.8%), and gastrointestinal antispasmodics (0.4%). Unadjusted odds ratios for HRME exposure are provided in Figure 1 for mortality, Figure 2 for hospital admission, and Figure 3 for emergency care.
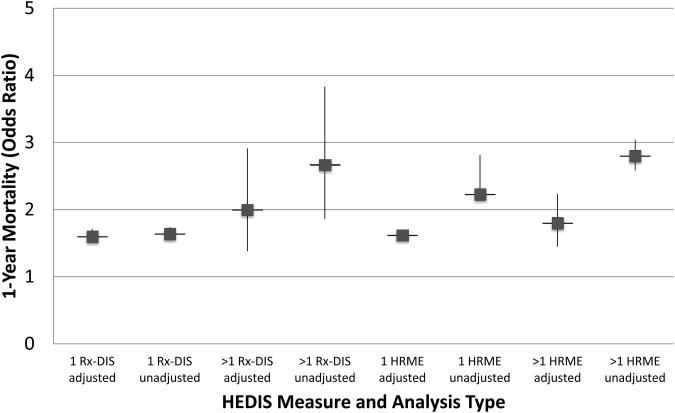
Figure 1. HEDIS measures: Adjusted and unadjusted odds ratios for 1-year mortality.
Cohort for high risk medications in the elderly (HRME) analysis N=1,807,404. Cohort for drug disease interaction analysis (Rx-DIS) N=256,388
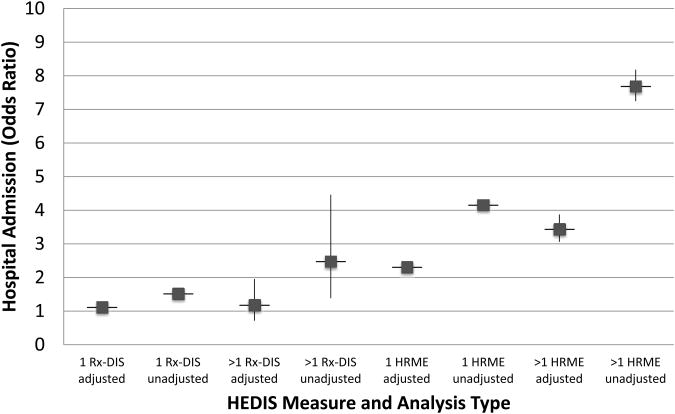
Figure 2. HEDIS measures: Adjusted and unadjusted odds ratios for hospital admission.
Cohort for high risk medications in the elderly (HRME) analysis N=1,807,404. Cohort for drug disease interaction analysis (Rx-DIS) N=256,388
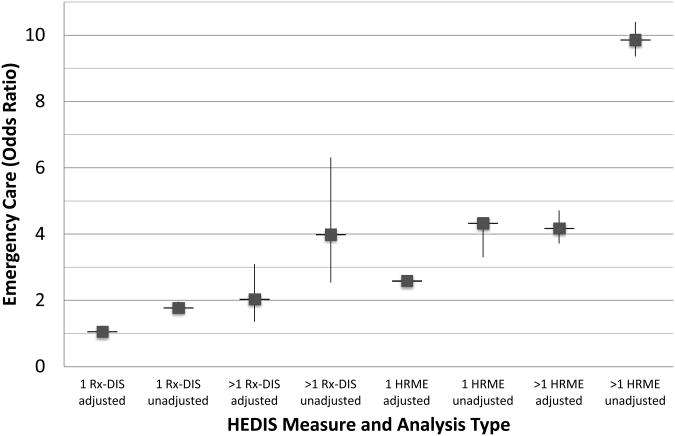
Figure 3. HEDIS measures: Adjusted and unadjusted odds ratios for emergency care.
Cohort for high risk medications in the elderly (HRME) analysis N=1,807,404. Cohort for drug disease interaction analysis (Rx-DIS) N=256,388
Figure 1 shows results from the GEE models for all-cause 1-year mortality and unadjusted odds ratios for HRME exposure; the results for the full model are available as Electronic Supplementary Material 2: full models HRME. New exposure to 1 or >1 HRMEs was significantly associated with 1-year mortality, adjusting for covariates (1: AOR=1.62, 95% CI 1.56-1.68; >1: AOR=1.80; 95% CI 1.45-2.23). Figures 2 and 3 illustrate that after controlling for demographics, health status and access to health care, new exposure to 1 or >1 HRMEs was also significantly associated with all-cause hospital admission (1: AOR=2.31, 95% CI 2.22-2.40; >1: AOR=3.44, 95% CI 3.06-3.87) and emergency care (1: AOR=2.59, 95% CI 2.49-2.70; >1: AOR=4.18, 95% CI 3.71-4.71), respectively. The significantly larger AOR's for higher levels of HRME for emergency care and hospital admission support a dose-dependent relationship for these outcomes. Full model results are available as Electronic Supplementary Material 2: full models HRME. The geriatric care by HRME exposure variable indicated a consistent relationship for individuals with HRME exposure regardless of geriatric care, and was thus removed from the final model.
Results of propensity score adjusted analyses indicated that the impact of HRME exposure was similar to regression models and, while statistically significant, was small compared to patient characteristics associated with HRME exposure (e.g., AOR for HRME exposure and mortality: [1: AOR=1.71, 95% CI 1.67-1.76; >1: AOR=1.81; 95% CI 1.66-1.98]; AOR for HRME propensity [(AOR=88.24, 95% CI 78.84-98.77)]
3.2 Rx-DIS Exposure
Table 1 also shows the characteristics of those 256,388 older veterans who had dementia (N=102,332; 39.6%), falls/fracture (n=42,738; 16.8%) and/or CRF (N=135,828; 53.0%) in FY06. Among these individuals, 3.7% (n=9,101) had new Rx-DIS exposure; 98% of those had a single type of Rx-DIS exposure (n=8,935). The most common type of Rx-DIS exposure was anticholinergic medications. One year after the index date, 26,202 (10.2%) died, 12,895 (5.0%) received hospital admission, and 9,219 (3.6%) received emergency care. Unadjusted odds ratios for one and more than one Rx-DIS are provided for 1-year mortality (Figure 1), hospital admission (Figure 2), and emergency care (Figure 3).
After controlling for potential confounders, we found exposure to 1 or >1 Rx-DIS was associated with 1-year mortality (1: AOR=1.60, 95% CI 1.51-1.71; >1: AOR=2.00; 95% CI 1.38-2.91; see Figure 1). Figure 2 shows that, after controlling for confounders, one but not two or more new Rx-DIS exposures were associated with hospital admission (1: AOR=1.12, 95% CI 1.03-1.27; >1: AOR=1.18; 95% CI 0.71-1.95). Adjusting for the same set of covariates, GEE models for all-cause emergency care found that Rx-DIS exposures were associated with emergency care (1: AOR=1.06; 95% CI 0.97-1.15; >1: AOR=2.0; 95% CI 1.35-3.10; see Figure 3). Full model results are available as Electronic Supplementary Material 3: full models Rx-DIS. The geriatric care by Rx-DIS exposure variable indicated a consistent relationship for individuals with Rx-DIS exposure regardless of geriatric care, and was thus removed from the final model.
Results of propensity score adjusted analyses indicated that results were similar, despite the significant effect of the propensity for Rx-DIS exposure (e.g., AOR for Rx-DIS exposure and mortality: [1: AOR=1.38, 95% CI 1.19-1.60; >1: AOR=2.89; 95% CI 1.79-4.66]; AOR for Rx-DIS propensity [(AOR=3.49, 95% CI 2.40-5.08)].
4 Discussion
Our study demonstrated that new use of drugs included in the NCQA's HRME measure was associated with a greater than two-fold increased risk for emergency care and/or hospital admission. Moreover, there was a suggestion that there was a dose-response relationship with higher numbers of HRMEs being related to greater risk of acute care. Exposure to HRME and Rx-DIS was also consistently associated with increased mortality risk. Thus, our findings provide support for the validity of these HEDIS® quality measures in an older Veteran population.
Our study findings for the association with hospital admission are consistent with findings from Albert et al. who found that prevalent HRME use over a three-year period came with a nearly two-fold increased risk of hospital admission in a retiree cohort receiving employer-based drug benefits.36 The difference in the magnitude of our point estimate versus that seen by Albert et al. may be explained by Albert's inclusion of prevalent exposure since those who took HRME drugs in the past and discontinued them due to adverse effects would bias the association with health services use towards the null.36 Our new-user design (one third the number of individuals with exposure compared to prevalent use) minimizes this potential misclassification bias and strengthens our results.
While adverse drug events (ADEs) are one possible mechanism for these findings, two recently published studies that examined individual cases of drug-related hospital admissions in older adults found few admissions that could be attributed to drugs (1-4%) that are part of the HRME list.37, 38 It is possible, though, that taking a medication from the HRME list serves as a proxy for other types of prescribing problems as measured by implicit measures such as the Medication Appropriateness Index (MAI).39 This is an important consideration since potentially inappropriate prescribing defined by the MAI has been shown to be related to a host of poor clinical outcomes including poor blood pressure control and quality of life, and hospital admission.28, 40-43 Future research should examine the possibility that inappropriate prescribing based on HEDIS® criteria may also be associated with other measures of quality related to chronic conditions.
It was also interesting to note that exposure to either new HRME drugs or Rx-DIS increased the risk of one-year mortality by approximately 60%. This finding is consistent with some but not all previous studies using the overall Beers drugs-to-avoid list.24, 26, 44 While some observed mortality effects may be due to confounding by disease severity, we believe this is unlikely given that we controlled for medical and psychiatric comorbidities, polypharmacy and prior emergency care or hospital admission which are among the most important risk factors for mortality in older adults.45 Moreover, we also used a propensity score approach that led to the same results. Thus, it is possible that the HEDIS® HRME is more sensitive to issues related to mortality than the broader list of drugs to avoid.
While our findings for emergency care were consistent with prior work for both single and multiple HRME exposures (all AOR>2), it was somewhat surprising that the risk of hospital admission was only shown to be increased by 12% in those with a single Rx-DIS exposure, and not at all (roughly 18% but not statistically significant) in those with two or more exposures. This modest risk is inconsistent with three studies that showed, even when controlling for drugs to avoid in the elderly, measures for drug-disease interactions were independently associated with increased risk of adverse drug events, 25, 42, 46, 47 and functional status problems.11, 25 What might account for this discrepancy? Possibly Rx-DIS could be resolved in the emergency department and not result in hospital admission. Alternatively, those with the most severe complications may have died, accounting for the strong relationship between Rx-DIS and mortality. In addition, the HEDIS® Rx-DIS measure examined drugs that interact with only three conditions as opposed to the 12 conditions in the recently updated Beers criteria recently developed by an expert panel convened by the American Geriatric Society.48 Moreover, that updated list of drugs to avoid is significantly expanded beyond the earlier Beers list used to develop the current HEDIS® measure.
It is also possible that we underestimated conditions included in the Rx-DIS measure, particularly falls. The rates we found for falls/fractures are likely an underestimate as well given that injurious falls that would lead to emergency care/hospitalization represent only a small number (5-15%) of falls that occur in community dwelling adults yearly.49
Our study also may have underestimated the true rate of NSAID use as VA PBM data does not account for NSAIDs purchased over-the-counter. On the other hand, for impoverished and high-disability VA patients, copayments are waived making over-the-counter NSAIDs more expensive.
While our study has a number of strengths including the use of a large national sample, a longitudinal new user design and careful consideration of medication use and health outcomes in an integrated health care system, several additional limitations exist. First, our data do not include variables that are not available in the national VA data sets. We did not have access to care received from Medicare which leads to incomplete ascertainment of emergency care or hospital admission, unlike VA mortality data which has been found to be very accurate.21 Individuals with HRME or Rx-DIS tend to have more chronic disease14, 15 and therefore may be more likely to receive hospital admission or emergency care in a Medicare setting than individuals without such exposure. Because that would bias toward the null hypothesis, we believe our results are conservative. Moreover, for certain conditions such as falls and fractures, we could not control for functional status or other unmeasured variables as potential confounders as this data is not consistently collected in the electronic medical record. We did however, control for a number of important physical and psychiatric comorbidities that are important risk factors for serious falls/fractures and prior emergency care. We found that adjusted odds ratios were generally lower than unadjusted odds ratios, indicating that controlling for those variables associated with HRME and Rx-DIS exposure attenuated the relationship between exposure and outcome. Moreover, analyses controlling for propensity to have HRME/Rx-DIS exposure were similar to those using regression analysis,49 but it was interesting that the impact of propensity to have HRME exposure had much stronger adjusted odds ratios than propensity to have Rx-DIS exposure, suggesting that the inclusion criteria for Rx-DIS led to a more homogeneous sample.
Second, inclusion of individuals who received one or more inpatient or outpatient visits in the VA during FY05 and FY06may have the effect of including individuals who are relatively healthy (and thus less similar to those with HRME or Rx-DIS exposure), or who receive non-VA care. However, analysis restricted to those with two or more visits each year revealed the same results as those from the overall cohort analyses.
Moreover, the analyses did not account for time on medication. Many HRME/Rx-DIS are used “as needed,” making day supply an uncertain marker of exposure, with the likelihood of gaps in use, regardless of the number of days received. Similarly, adverse drug events may occur on day 1 or day 360. This may, however, be an option for future research. Finally, given that the sample was mostly older males and VA patients, it is not clear how well this information generalizes to older women and non-VA males.50
4.1 Conclusions
Our data indicate that HRME/Rx-DIS exposure is associated with clinically important adverse outcomes in the elderly. While a newer Beers list is being evaluated for additions to the current HEDIS measures, it is time to begin incorporating input from both patients who receive these medications and providers who prescribe to develop approaches to reduce exposure to these agents.
Supplementary Material
Acknowledgments
This study was funded by VA Health Services Research and Development Service, IIR 06-062 (Dr. Pugh PI). The funding agency had no role in data collection, analysis, or manuscript development. No conflicts reported for any co-authors. We also acknowledge assistance with manuscript preparation by Jeffrey Tabares, Margaret Wells, and Kathleen Franklin. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. The authors acknowledge and appreciate support from the South Texas Veterans Healthcare System/Audie L. Murphy Division and the VERDICT research program, and from the Central Texas Veterans Health Care System, Center for Applied Health Research. Data from this paper was presented at the VA Health Services Research and Development Annual Research Meeting, National Harbor, MD. July, 2012.
References
- 1.Steinman MA, Hanlon JT. Managing medications in clinically complex elders: “There's got to be a happy medium”. JAMA. 2010;304(14):1592–601. doi: 10.1001/jama.2010.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Institute of Medicine To err is human: building a safer health system. Washington, DC: National Academies Press; 1999. [PubMed] [Google Scholar]
- 3.Wenger NS, Young RT. Quality indicators for continuity and coordination of care in vulnerable elders. J Am Geriatr Soc. 2007;55(s2):S285–S92. doi: 10.1111/j.1532-5415.2007.01334.x. [DOI] [PubMed] [Google Scholar]
- 4.Brook RH, McGlynn EA, Shekelle PG. Defining and measuring quality of care: a perspective from US researchers. Qual Assur Health Care. 2000;12(4):281–95. doi: 10.1093/intqhc/12.4.281. [DOI] [PubMed] [Google Scholar]
- 5.Beers MH, Ouslander JG, Rollingher I, et al. Explicit criteria for determining inappropriate medication use in nursing home residents. Arch Intern Med. 1991;151(9):1825–32. [PubMed] [Google Scholar]
- 6.Beers MH. Explicit criteria for determining potentially inappropriate medication use by the elderly. Arch Intern Med. 1997;157(14):1531–6. [PubMed] [Google Scholar]
- 7.Fick DM, Cooper JW, Wade WE, et al. Updating the Beers Criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med. 2003;163(22):2716–24. doi: 10.1001/archinte.163.22.2716. [DOI] [PubMed] [Google Scholar]
- 8.Pugh MJ, Hanlon JT, Zeber JE, et al. Assessing potentially inappropriate prescribing in the elderly Veterans Affairs population using the HEDIS® 2006 quality measure. J Manag Care Pharm. 2006;12(7):537–45. doi: 10.18553/jmcp.2006.12.7.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albert SM, Colombi A, Hanlon J. Potentially inappropriate medications and risk of hospitalization in retirees. Drugs Aging. 2010;27(5):407–15. doi: 10.2165/11315990-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pugh MJ, Palmer RF, Parchman ML, Mortensen E, Markides K, Espino DV. Association of suboptimal prescribing and change in lower extremity physical function over time. Gerontology. 2007;53(6):445–53. doi: 10.1159/000119460. [DOI] [PubMed] [Google Scholar]
- 11.Fillenbaum GG, Hanlon JT, Landerman LR, Artz MB, O'Connor H, Dowd B, et al. Impact of inappropriate drug use on health services utilization among representative older community-dwelling residents. Am J Geriatr Pharmacother. 2004;2(2):92–101. doi: 10.1016/s1543-5946(04)90014-1. [DOI] [PubMed] [Google Scholar]
- 12.Hanlon JT, Artz MB, Pieper CF, Lindblad CI, Sloane RJ, Ruby CM, et al. Inappropriate medication use among frail elderly inpatients. Ann Pharmacother. 2004;38(1):9–14. doi: 10.1345/aph.1D313. [DOI] [PubMed] [Google Scholar]
- 13.Fu AZ, Liu GG, Christensen DB. Inappropriate medication use and health outcomes in the elderly. J Am Geriatr Soc. 2004;52(11):1934–9. doi: 10.1111/j.1532-5415.2004.52522.x. [DOI] [PubMed] [Google Scholar]
- 14.Pugh MJ, Hanlon JT, Wang C-P, Semla T, Burk M, Amuan ME, et al. Trends in use of high-risk medications for older veterans: 2004 to 2006. J Am Geriatr Soc. 2011;59(10):1891–8. doi: 10.1111/j.1532-5415.2011.03559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pugh MJ, Starner CI, Amuan ME, Berlowitz DR, Horton M, Marcum ZA, et al. Exposure to potentially harmful drug–disease interactions in older community-dwelling veterans based on the Healthcare Effectiveness Data and Information Set quality measure: Who is at risk? J Am Geriatr Soc. 2011;59(9):1673–8. doi: 10.1111/j.1532-5415.2011.03524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915–20. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 17.VA Information Resource Center. VIReC research user guide: VHA pharmacy prescription data. Washington, DC: VA Information Resource Center (VIReC); 2008. [Accessed September 2008]. [online]; Available from URL: http://www.virec.research.va.gov/References/RUG/RUG.htm. [Google Scholar]
- 18.VA Information Resource Center. Medical SAS® inpatient dataset FY2009: VIReC research user guide. [Accessed February 2011];Hines (IL): VIReC. 2011 [online]; Available from URL: http://www.virec.research.va.gov/DataSourcesName/Medical-SAS-Datasets/MedSAS-Inpt-RUG/MedSAS-RUG-Inpt.htm.
- 19.VA Information Resource Center. VHA Medical SAS® outpatient datasets and inpatient encounters dataset FY2009: VIReC Research User Guide. Washington, DC: VA Information Resource Center; 2011. [Accessed April 2011]. [online]; Available from URL: http://www.virec.research.va.gov/DataSourcesName/Medical-SAS-Datasets/MedSAS-Outpt-RUG/MedSAS-RUG-Outpt.htm. [Google Scholar]
- 20.Arnold N, Sohn MW, Maynard C, et al. VIReC Technical Report 2: VA-NDI mortality data merge project. Hines (IL): VA Information Resource Center; 2006. [Google Scholar]
- 21.Sohn M, Arnold N, Maynard C, et al. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2. doi: 10.1186/1478-7954-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Committee on Quality Assurance. Proposed changes to existing measure for HEDIS® 2012: potentially harmful drug-disease interactions in the elderly. (DDE) Washington, DC: National Committee for Quality Assurance; 2011. [Google Scholar]
- 23.National Committee on Quality Assurance. HEDIS® 2008 NDC Lists. Washington, DC: 2011. [Accessed February 4, 2011]. [online]; Available from URL: http://www.NCQA.org/tabid/598/Default.aspx. [Google Scholar]
- 24.Fick DM, Mion LC, Beers MH, et al. Health outcomes associated with potentially inappropriate medication use in older adults. Res Nurs Health. 2008;31(1):42–51. doi: 10.1002/nur.20232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanlon JT, Fillenbaum GG, Kuchibhatla M, et al. Impact of inappropriate drug use on mortality and functional status in representative community dwelling elders. Med care. 2002;40(2):166–76. doi: 10.1097/00005650-200202000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Spinewine A, Schmader KE, Barber N, et al. Appropriate prescribing in elderly people: how well can it be measured and optimised? Lancet. 2007;370(9582):173–84. doi: 10.1016/S0140-6736(07)61091-5. [DOI] [PubMed] [Google Scholar]
- 27.Budnitz DS, Lovegrove MC, Shehab N, et al. Emergency hospitalizations for adverse drug events in older americans. N Engl J Med. 2011;365(21):2002–12. doi: 10.1056/NEJMsa1103053. [DOI] [PubMed] [Google Scholar]
- 28.Hellstrom LM, Bondesson A, Hoglund P, et al. Impact of the Lund Integrated Medicines Management (LIMM) model on medication appropriateness and drug-related hospital revisits. Eur J Clin Pharmacol. 2011;67(7):741–52. doi: 10.1007/s00228-010-0982-3. [DOI] [PubMed] [Google Scholar]
- 29.Aspden P, Wolcott J, Bootman JL, Cronenwett LR, editors. Preventing medication errors: Quality Chasm Series. Washington, D.C.: National Academies Press; 2007. [Google Scholar]
- 30.Selim AJ, Berlowitz DR, Ren XS, et al. The comorbidity index. In: Davies M, editor. Measuring and managing health care quality. New York (NY): Aspen Publishers; 2002. pp. 91–4. [Google Scholar]
- 31.Selim A, Fincke G, Ren X, et al. Comorbidity assessments based on patient report: results from the Veterans Health Study. J Ambul Care Manage. 2004;27(3):281–95. doi: 10.1097/00004479-200407000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Bindman AB, Grumbach K, Osmond D, et al. Preventable hospitalizations and access to health care. JAMA. 1995;274(4):305–11. [PubMed] [Google Scholar]
- 33.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–30. [PubMed] [Google Scholar]
- 34.Liang KY, Zeger SL. Longitudinal data-analysis using generalized linear-models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 35.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Albert SM, Colombi A, Hanlon J. Potentially inappropriate medications and risk of hospitalization in retirees: analysis of a US retiree health claims database. Drugs Aging. 2010;27(5):407–15. doi: 10.2165/11315990-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Budnitz DS, Shehab N, Kegler SR, et al. Medication use leading to emergency department visits for adverse drug events in older adults. Ann Intern Med. 2007;147(11):755–65. doi: 10.7326/0003-4819-147-11-200712040-00006. [DOI] [PubMed] [Google Scholar]
- 38.Marcum ZA, Amuan ME, Hanlon JT, et al. Prevalence of unplanned hospitalizations caused by adverse drug reactions in older veterans. J Am Geriatr Soc. 2012;60(1):34–41. doi: 10.1111/j.1532-5415.2011.03772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lund BCSM, Chrischilles EA, Kaboli PJ. Beers criteria as a proxy for inappropriate prescribing of other medications among older adults. Ann Pharmacother. 2011;45(11):1363–70. doi: 10.1345/aph.1Q361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmader KE, Hanlon JT, Landsman PB, et al. Inappropriate prescribing and health outcomes in elderly veteran outpatients. Ann Pharmacother. 1997;31(5):529–33. doi: 10.1177/106002809703100501. [DOI] [PubMed] [Google Scholar]
- 41.Olsson IN, Runnamo R, Engfeldt P. Medication quality and quality of life in the elderly, a cohort study. Health Qual Life Outcomes. 2011;9:95. doi: 10.1186/1477-7525-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lund BC, Carnahan RM, Egge JA, et al. Inappropriate prescribing predicts adverse drug events in older adults. Ann Pharmacother. 2010;44(6):957–63. doi: 10.1345/aph.1m657. [DOI] [PubMed] [Google Scholar]
- 43.Hanlon JT, Wang X, Handler SM, et al. Potentially inappropriate prescribing of primarily renally cleared medications for older veterans affairs nursing home patients. J Am Med Dir Assoc. 2011;12(5):377–83. doi: 10.1016/j.jamda.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chirn-Bin C, Ding-Cheng C. Comparison of published explicit criteria for potentially inappropriate medications in older adults. Drugs Aging. 2010;27(12):947–57. doi: 10.2165/11584850-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 45.Zekry D, Loures Valle BH, Graf C, et al. Prospective comparison of 6 comorbidity indices as predictors of 1-year post-hospital discharge institutionalization, readmission, and mortality in elderly individuals. J Am Med Dir Assoc. 2012;13(3):272–8. doi: 10.1016/j.jamda.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 46.Chrischilles EA, VanGilder R, Wright K, et al. Inappropriate medication use as a risk factor for self-reported adverse drug effects in older adults. J Am Geriatr Soc. 2009;57(6):1000–6. doi: 10.1111/j.1532-5415.2009.02269.x. [DOI] [PubMed] [Google Scholar]
- 47.Hanlon JT, Schmader KE. How important are drug–drug interactions to the health of older adults? Am J Geriatr Pharmacother. 2011;9(6):361–3. doi: 10.1016/j.amjopharm.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 48.The American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616–31. doi: 10.1111/j.1532-5415.2012.03923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Centers for Disease Control and Prevention (CDC) The state of aging and health in America 2007. Whitehouse Station (NJ): The Merck Company Foundation; 2007. [Google Scholar]
- 50.Morgan R, Teal C, Reddy S, et al. Measurement in Veterans Affairs health services research: Veterans as a special population. Health Serv Res. 2005;40(5 Pt 2):1573–83. doi: 10.1111/j.1475-6773.2005.00448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.