Abstract
Purpose
The purpose of the study was to assess the value of reinforcing diabetes self-management for improving glycemia and self-care among adults with type 2 diabetes who had at least three hours of prior diabetes education.
Methods
In this randomized controlled trial, 134 participants (75% White, 51% female, 59±9 years old, 13±8 years with diabetes, A1C=8.4±1.2%) were randomized to either a group map-based program (Intervention) or group education on cholesterol and blood pressure (Control). Participants were assessed for A1C levels, diabetes self-care behaviors (3-day pedometer readings, 6-minute walk test, blood glucose checks, frequency of self-care), and psychosocial factors (distress, frustration, quality of life) at baseline, 3, 6 and 12 months post-intervention and health literacy at baseline.
Results
Groups did not differ on baseline characteristics including A1C levels, health literacy, or self-care; however, the Intervention group had more years of education than Controls. Intervention arm participants modestly improved A1C levels at 3 months post-intervention but did not maintain that improvement at 6 and 12 months while Control patients did not improve A1C levels at any time during follow-up. Importantly, frequency of self-reported self-care, diabetes quality of life, diabetes-related distress and frustration with diabetes self-care improved in both groups over time.
Conclusions
Reinforcing self-care with diabetes education for patients who have not met glycemic targets helps improve A1C and could be considered a necessary component of ongoing diabetes care. The best method to accomplish reinforcement needs to be established.
Keywords: Type 2 diabetes, diabetes education intervention, diabetes self-care
Type 2 diabetes is a chronic condition that requires patients to follow specific recommendations and prescriptions for the rest of their lives. Self-care recommendations include regular physical activity1,2, frequent blood glucose monitoring accompanied by appropriate actions based on results3, following meal plans 4, taking preventative measures and clinic attendance5–7. While these behaviors are critically linked to improved glycemic control and the prevention of complications, integrating them into one’s daily life can be challenging8–10.
Formal diabetes self-management education helps patients integrate and improve self-care behaviors and glycemia11–14. The Diabetes Control and Complications Trial (DCCT) established the importance of diabetes educators in supporting patients’ efforts to improve self-care and glycemic control15–18. Despite these improvements, many patients from the Epidemiology of Diabetes Interventions and Complications Study (EDIC) follow-up study were not able to maintain glycemic targets19. Other diabetes interventions also have shown patients’ difficulty maintaining intervention responses over an extended period of time12,13,20. Considering the key role diabetes education and support play in self-management, a logical approach to sustaining intervention improvements may be reinforcement of diabetes education.
Reinforcement of diabetes self-care education may be a necessary component in diabetes treatment. That is, once patients have completed a basic diabetes education program, they may lack the skill sets to maintain their own self-care behaviors at different points throughout the course of their diabetes. Ongoing and repeated education (reinforcement) may be necessary to address the progressive nature of diabetes along with the multiple challenges of diabetes self-management and to maintain the self-management learning experienced during the initial education. However, few well-designed long-term randomized controlled trials have examined the value of reinforcing diabetes self-care and the best approach to accomplish reinforcement.
This study examined whether a map-based intervention was useful in reinforcing prior diabetes self-management education for patients with type 2 diabetes who had not reached glycemic targets. Factors that may be associated with an improvement/decline in glycemic control, including diabetes-related emotional distress, frustration with diabetes self-care, and diabetes quality of life were also examined. Whether diabetes self-care should be reinforced with diabetes education to help patients maintain glycemic improvements has not been clearly established. Thus, the purpose of the study was to assess the value of reinforcing diabetes self-management for improving glycemia and self-care among adults with type 2 diabetes who had prior diabetes education. The study’s hypotheses are as follows.
Hypotheses
Hypothesis 1: The map-based intervention in comparison to the control condition will lead to improved glycemic control (lowered A1C) post intervention (3 months) and at 6 and 12 months follow-up.
Hypothesis 2: The map-based intervention in comparison to the control condition will lead to improved frequency of recommended self-care behaviors post intervention (3 months) and at 6 and 12 months follow-up.
RESEARCH DESIGN AND METHODOLOGY
Design Overview
This two-arm, parallel designed trial randomized participants to either a group map-based program (Experimental Intervention) or group education on dyslipidemia and hypertension (Attention Control Intervention). Different teams of experienced diabetes nurses and dietitians, all certified diabetes educators, provided education for each arm. Written curriculum, pre-approved education materials, separate educator trainings, investigator observation of all group classes, and separate teams of trained, experienced diabetes educators to prevent carryover of education strategies ensured integrity of the two interventions. The Joslin Diabetes Center Committee on Human Subjects approved the protocol and all recruitment procedures and materials. All participants provided written informed consent prior to participation.
Setting and Participants
Participants were recruited via referrals from the clinical practice of the Joslin Clinic, advertisements in the Joslin Newsletter and website, and extensive mailings from Joslin’s mailing lists.
Adults aged 25 to 75 years diagnosed with type 2 diabetes for at least two years who were taking insulin and/or oral medication for at least one year, able to walk briskly, free of severe complications, had at least 3 hours of previous documented diabetes education, and whose Hemoglobin A1c (A1C) level >7.0% were eligible for enrollment. Exclusion criteria included inability to read and speak English, current or planned pregnancy, severe renal disease (microalbumin >300 ug/mg), severe peripheral diabetic neuropathy and/or severe peripheral vascular disease, symptomatic severe autonomic neuropathy, proliferative diabetic retinopathy based on dilated eye examination within one year of study entry, A1C levels <7.0% and A1C levels >13.0%, a history of severe unstable myocardial infarction, congestive heart failure or other severe cardiac disease, and severe hypertension (systolic ≥160 mmHg or diastolic ≥ 90 mmHg). Participants diagnosed with bipolar disorder, schizophrenia, mental retardation, organic mental disorder, and alcohol or drug abuse were excluded, as well as patients currently undergoing psychiatric treatment. These exclusions were made to avoid confounds due to concurrent changes in mental status and the effects of ongoing psychiatric treatment. Eligible participants were scheduled for a baseline and a randomization visit.
Randomization
A block randomization sequence based on a random number table was generated with randomization.com to ensure balance between the two groups at study end. Educators and study physicians had no role in randomization.
Interventions
Experimental Intervention: Conversation Maps
The US Diabetes Conversation Maps program is a set of diabetes education tools (Healthy Interactions, Inc., Chicago, IL) designed to help educators facilitate patient group discussions about diabetes self-care and goal-setting. Participants randomized to the Conversation Maps Intervention attended four one-hour sessions, each with a different map. All sessions were held in the Behavioral Research Laboratory. The four maps used for this study covered the following topics: 1) Diabetes Overview, 2) Diabetes and Healthy Eating, 3) Blood Glucose and Monitoring, and 4) The Natural Course of Diabetes (a fifth map covering gestational diabetes was not used); each map had a program manual for the group facilitator. Experienced diabetes nurses and dietitians facilitated the Map sessions. Educators helped participants focus on the diabetes information most relevant to them and their experiences. At the end of each session, educators assisted participants in setting realistic health goals and developing a plan to achieve meaningful behavior change in their lives. All Intervention educators were trained by Conversation Maps certified trainers.
Attention Control Intervention: Heart Healthy Living
The Control arm consists of two 2-hour classes focusing on 1) dyslipidemia and 2) hypertension, but not specifically diabetes self-care. Both conditions are common in those with type 2 diabetes. These classes have been validated and are offered in The Joslin Clinic. The curriculum consists of prepared slides, a detailed curriculum manual, and specific learning activities including goal-setting. The Heart Healthy Living Program was the ideal attention control because it does not address the outcome variables of diabetes specific self-care behavior nor glycemic control yet the information provided is valuable for people with type 2 diabetes as dyslipidemia and hypertension are common comorbidities. A registered nurse (hypertension class) and a registered dietitian (lipid class) taught the classes. All Control arm educators were trained and certified by the Clinic prior to teaching.
Measures and Follow-Up
Data were collected at baseline and 3, 6, and 12 months post group interventions. The primary outcome was A1C, measured via the Turbidimetric Inhibition Immunoassay using the Roche Integra 800 Analyzer (Roche Diagnostics Operations Inc., Indianapolis, Indiana; reference range is 4.0 to 6.0%).
In addition to sociodemographic factors (age, sex, race/ethnicity, education level, marital status, occupation) and health factors (duration of diabetes, body mass index (BMI), waist circumference, blood pressure), mean 3-day pedometer readings (Omron Healthcare, Inc., Lake Forest, Illinois), a fitness assessment (6-minute walk test), and daily blood glucose meter checks were also collected.
Finally, participants completed the following:
Self-care Inventory-R (SCI-R)21 is a 15-item survey that measures the self-reported frequency of self-care behaviors on a 5-point Likert scale and has been validated for use with type 2 diabetes populations21. Four additional items inquiring about checking feet, eating heart healthy foods, looking at blood glucose patterns, and knowing about blood pressure, A1C and lipids were included.
Brief Symptom Inventory (BSI)22 is an 18-item checklist that assesses 3 primary dimensions (somatization, depression, and anxiety) and yields a total score summarizing the overall level of psychological symptoms (Cronbach’s alpha=0.89). This measure renders age and gender adjusted t-scores and is widely used with repeated assessments of its reliability and validity22. A t-score of 63 or greater suggests clinical depression.
Coping Styles23_ENREF_23_ENREF_23 is a 15-item measure assessing emotional coping and self-controlled coping. Self-controlled coping strategies include stoicism and pragmatism, indicated by statements of controlling one’s emotions and problem-solving to alleviate frustration. Emotion-based coping strategies include anger, impatience, and anxiety, indicated by angry statements, impulsive actions, anxious behaviors (nervous, worried, upset, difficulty relaxing) and avoidant behaviors (not doing something or giving up). Patients are asked to rate each item on a 4-point scale, ranging from “not at all like me” to “very much like me.” This measure is validated in diabetes populations23.
Problem Areas in Diabetes (PAID)24 is a widely used 20-item measure of diabetes-related emotional distress that assesses a broad range of feelings related to living with diabetes and its treatment, including guilt, anger, frustration, depressed mood, worry and fear24–27. The PAID has high internal reliability (alpha=.95)24,27. The PAID is sensitive to changes in glycemic control and high PAID scores are associated with lower self-reported adherence to treatment recommendations21.
Problems with Diabetes Self-Management (PDSM)26 Scale is a 5-item scale that measures frustration with diabetes self-care: glucose control, meal plan, exercise, glucose monitoring, and medications. This measure is sensitive to barriers to performance of self-care behaviors and diabetes-related emotional distress26.
Diabetes Quality of Life Scale (DQOL)28 has 46 core items rated on a five point Likert scale and yields a total score with five subscales (satisfaction, general health, impact of treatment, future effects of diabetes, and social effects). Scores are converted to a 100 point scale with 100 representing highest quality of life and zero representing lowest quality of life.28 The psychometric properties of the DQOL are well-established 28–30 and it has been tested in both type 1 and type 2 diabetes patients.29
Confidence in Diabetes Self-Care Scale (Type 2) (CIDS-2)31 is a validated 21-item measure assessing self-efficacy in diabetes, that is, the confidence individuals have in their ability to perform self-care tasks.31 Patients rate each item on a 5 point scale ranging from “No, I am sure I cannot” to “Yes, I am sure I can.” The CIDS has high internal reliability (alpha=0.90).32
Test of Functional Health Literacy in Adults (TOFHLA)33 is a complex measure of functional health literacy.33 The TOFHLA is available in English and Spanish versions and requires 20 minutes for administration. The TOFHLA assesses numeracy and reading comprehension using health-related materials. The numeracy portion consists of 17 multiple-choice questions that assess a patient's ability to interpret documents and numbers. Patients interpret with different prescription bottle labels and respond to questions regarding the dose, timing and expiration of prescriptions. The short form reading comprehension consists of two passages of text and 36 multiple-choice questions. The passages of text focus on x-ray preparation and medical insurance coverage. In each passage, selected words have been deleted and replaced with blank spaces. Patients need to choose from a response set which words make the most sense in the text.
Statistical Analysis
All analyses were conducted using SAS 9.2 (SAS Institute, Carey, NC, USA). Descriptive statistics were examined to ensure that data met statistical test assumptions. Baseline characteristics were compared using Chi-square, Wilcoxon Two-Sample or Kruskal-Wallis Test to examine between-group differences and assess the randomization procedure effectiveness.
As the pattern of our missing data was arbitrary, multiple imputations with the Markov Chain Monte Carlo method (SAS Proc MI) were used to input missing data34. The results presented are based on combined inferences of the 15 complete data sets. The imputation model was built using demographic, psychosocial and A1C values.
For the primary analysis of the two education interventions on A1C at baseline and the three follow-up points, a linear spline model was used to estimate the observed A1C trend over time (SAS Proc Mixed). The final model consisted of two piecewise, linear trends having different slopes within each segment but joined together at a fixed time point (3 months)35. Included in the model were time effects, group effect, and the interactions between time and group. The intercept and time effects were specified as random effects, positing that individuals vary in their baseline A1C levels and in A1C changes over time. The spline model allows the rates of change before and after 3 months to differ both within and between groups. This approach allows the testing of variables predicting the 3-month rate of change in A1C, and provides insight into whether the two education groups differ in A1C response profiles and also how they differ.
Similarly, for continuous secondary outcomes (self-care behaviors, diabetes quality of life), a linear mixed model was used for longitudinal data (SAS Proc Mixed). Included in the model were time effects, group effect, and the interactions between time and group.
RESULTS
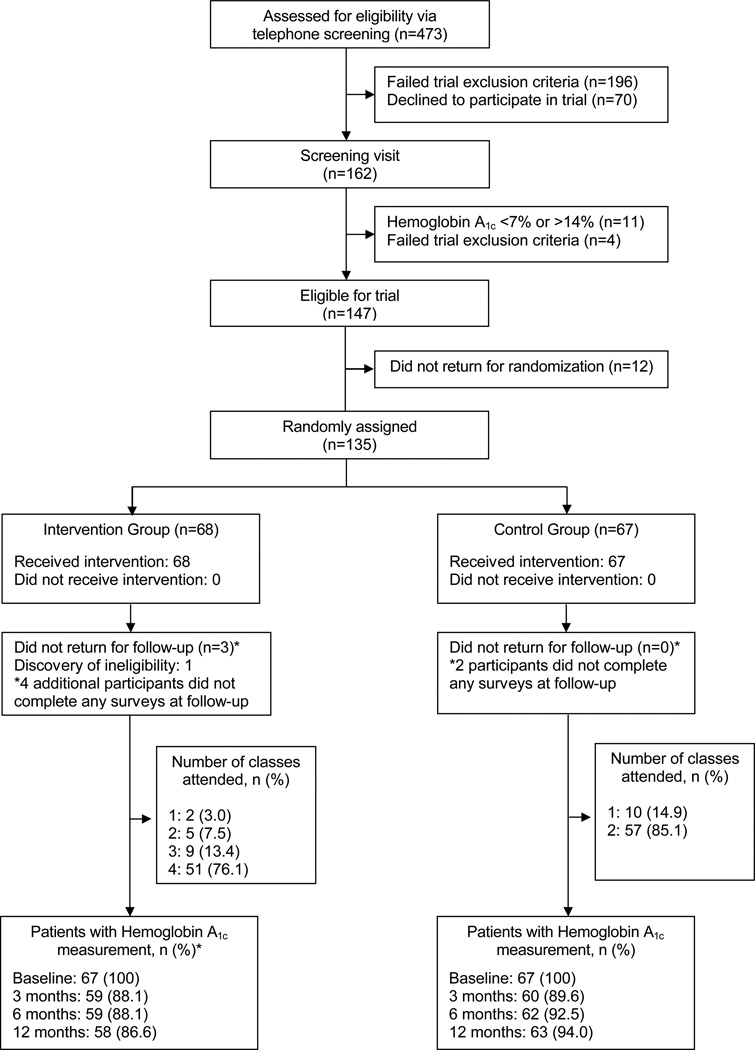
Four hundred and seventy-three adults with type 2 diabetes, of whom 162 were eligible for a screening visit, were telephone-screened; 135 were randomized (Figure 1). The most common reasons for exclusion at screening were presence of complications and/or comorbid conditions (37%), not meeting criteria for hemoglobin A1C (16%), not enough prior diabetes education (12%), inability to walk briskly (9%) or starting new insulin and/or oral medication regimen (6%). Further, twelve eligible people did not return for randomization.
Figure 1.
Study Flow Diagram
One randomized participant (Intervention group) did not meet eligibility requirements for planning pregnancy and became pregnant before her first follow-up visit. She was dropped from the study. Three other randomized participants did not return for follow-up visits. All four (including dropped participant) were randomized to the Intervention group; these participants did not differ on baseline characteristics from those who completed the study. An additional six participants (4 Intervention, 2 Control) did not complete any surveys at follow-up, but provided physiological and laboratory data. Thus, 131 of these participants have some follow-up data.
One hundred and thirty-four participants (75% Non-Hispanic White, 51% female, 59±9 years old, 15±3 years of education, 13±8 years with type 2 diabetes, A1C=8.4±1.2%; Table 1) attended the group map-based program (Intervention, n=67) or group education on dyslipidemia and hypertension (Control, n=67). Groups did not differ on baseline characteristics including A1C levels, health literacy, or self-care behaviors; however, the Intervention group had more years of academic education than Controls (15.9 vs 14.9 years, p=0.05). Both groups reported being satisfied with their diabetes education classes and educators immediately after completion of the intervention. However, two male participants stated that they were not satisfied with the Maps program.
Table 1.
Baseline Characteristics of Type 2 Diabetes Participants Randomly Assigned to Intervention or Control Groups‡
| All Patients | Intervention | Control | |
|---|---|---|---|
| (N=134) | (N=67) | (N=67) | |
| Mean ± SD | Mean ± SD | Mean ± SD | |
| Demographic Variables | |||
| Hemoglobin A1c (%) | 8.4±1.2 | 8.5±1.4 | 8.3±1.0 |
| Age (years) | 59.1±8.7 | 59.9±8.5 | 58.4±9.0 |
| Females (%) | 51.5 | 47.8 | 55.2 |
| Non-Hispanic white (%) | 71.6 | 73.1 | 70.2 |
| Body mass index (kg/m2) | 34.2±7.1 | 34.6±7.0 | 33.7±7.1 |
| Diabetes duration (years) | 13.3±8.0 | 13.0±6.1 | 13.6±9.5 |
| Education (years) | 15.4±2.8 | 15.9±2.5 | 14.9±2.9* |
| Self-Care and Psychosocial Variables | |||
| Frequency of self-care (SCI-R) | 59.5±13.7 | 59.1±12.2 | 60.0±15.1 |
| Daily blood glucose checks | 1.4±1.1 | 1.2±1.0 | 1.5±1.2 |
| 6-minute walk test | 484.3±96.4 | 492.6±90.1 | 475.9±102.4 |
| Pedometer steps per day | 16,451±10,035 | 16,7943±11,895 | 16,097±7744 |
| Diabetes distress (PAID) | 34.1±21.7 | 33.3±20.3 | 34.8±23.1 |
| Frustration with diabetes self-care (PDSM) | 46.3±23.1 | 48.7±22.5 | 43.6±23.7 |
| Depressive symptoms (BSI) | 49.7±10.4 | 48.9±9.8 | 50.6±11.0 |
| Anxiety symptoms (BSI) | 49.0±10.0 | 48.1±9.1 | 49.8±10.9 |
| Diabetes quality of life (DQOL) | 67.4±11.4 | 67.9±10.6 | 66.9±12.1 |
| Self-Control Coping (Coping Styles) | 56.6±14.9 | 57.5±14.5 | 55.7±15.3 |
| Emotional Coping (Coping Styles) | 32.2±17.8 | 34.3±18.4 | 30.2±17.0 |
| Diabetes self-efficacy (CIDS-2) | 81.3±11.8 | 81.9±11.6 | 80.7±12.1 |
| Health literacy (TOFHLA) | 33.1±4.3 | 33.4±3.6 | 32.7±5.0 |
| Numeracy (TOFHLA) | 45.4±4.3 | 45.4±4.1 | 45.3±4.6 |
The randomized participant who did not meet eligibility requirements is excluded.
indicated p<0.05 based on Chi-square or Wilcoxon Two-Sample Tests
Hemoglobin A1c (A1C) levels
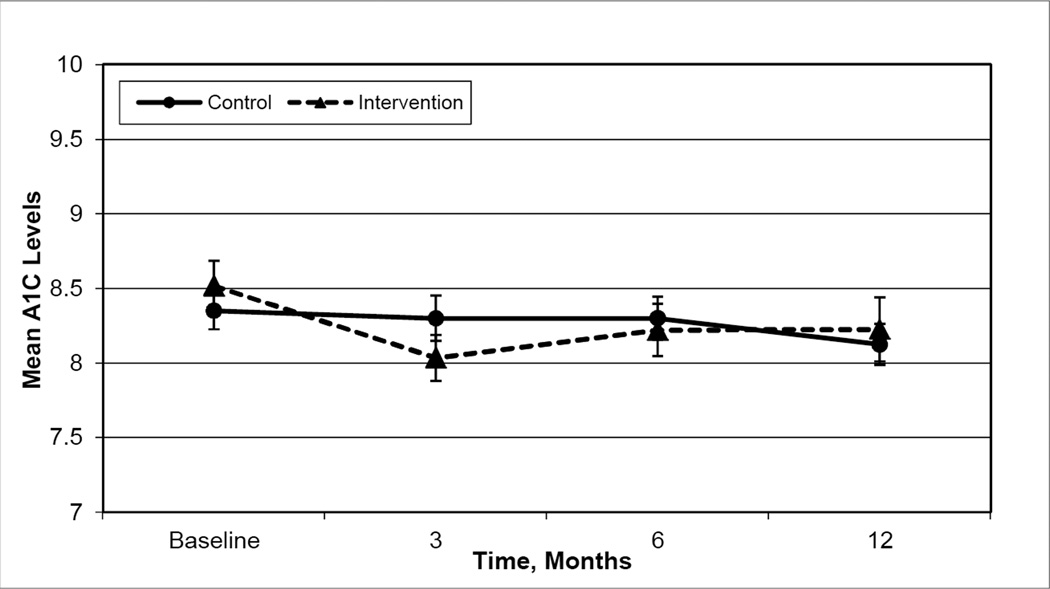
Hypothesis 1 compared glycemic improvement in the map-based intervention versus the control condition at 3, 6, and 12 months post intervention. Findings revealed participants in the Intervention arm modestly improved A1C levels at 3 months post-intervention, but not at 6 and 12 months (mean change: −0.4 points, p=0.004; −0.3 points, p=0.07; 0.04 points, p=0.8 respectively, Figure 2) while Control arm patients did not improve A1C levels at any time during follow-up (mean change: −0.02 points at 3-months, p=0.8; −0.07 points at 6-months, p=0.5; −0.2 points at 1 year, p=0.2 respectively). The linear spline model with a “knot” at 3-months, found the rate of change (beta coefficient) from baseline to 3 months for the Intervention group to be −0.114. This beta coefficient indicates a modest improvement in A1C (Table 2). However, between 3 and 12 months the parameter estimate for rate of change in A1C was 0.128 indicating the intervention’s A1C levels deteriorated. The control condition that focused on dyslipidemia and blood pressure control rather than diabetes self-management resulted in maintenance of A1C at baseline levels. Thus, the linear spline model is consistent with mean group A1C levels (Figure 2).
Figure 2.
Mean Hemoglobin A1C Levels over Time for the Intervention and Control Groups
Table 2.
Spline Model Estimating Rate of Change in A1C Over Time‡
| Estimate | Standard Error |
t-value | p-value | |
|---|---|---|---|---|
| Intercept | 8.431 | 0.106 | 79.93 | <.0001 |
| Month (Baseline to 3 month) | −0.114 | 0.037 | −3.06 | 0.003 |
| Month (3 month to 12 month) | 0.128 | 0.048 | 2.68 | 0.008 |
| Month (Baseline to 3 month) * Education Class | 0.106 | 0.050 | 2.11 | 0.038 |
| Month (3 month to 12 month) * Education Class | −0.144 | 0.065 | −2.21 | 0.029 |
The map-based education served as the reference group.
Hypothesis 2 compared frequency of recommended self-care behaviors in the map-based intervention versus the control condition at 3, 6, and 12 months post intervention. Both the Intervention and Control arms improved self-reported frequency of self-care following the intervention; however, these improvements did not differ by type of intervention (Table 3). Objective measures of self-care (6-minute walk test, mean 3-day pedometer readings, number of glucose check per day) did not change over time (Table 3). Importantly, diabetes quality of life, diabetes-related distress, and frustration with diabetes self-care improved in both groups. None of the improvements in secondary outcomes differed by type of intervention.
Table 3.
Linear Mixed Model with Interactions Predicting Psychosocial Outcomes over Time
| Intervention Group (N=67) |
Control Group (N=67) |
Mixed Model Analysis with Interactions |
||
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Effect | P-value | |
| Self-Care Inventory-R | ||||
| Baseline | 59.1±12.2 | 60.0±15.1 | Time | <0.001 |
| 3 Month | 61.9±12.6 | 65.6±14.4 | Group | 0.51 |
| 6 Month | 64.5±10.4 | 65.1±15.3 | Group*Time | 0.52 |
| 12 Month | 64.7±13.3 | 68.2±14.1 | ||
| Glucose checks per day | ||||
| Baseline | 1.2±1.0 | 1.5±1.2 | Time | 0.50 |
| 3 Month | 1.3±1.0 | 1.5±1.3 | Group | 0.12 |
| 6 Month | 1.2±0.9 | 1.7±1.3 | Group*Time | 0.26 |
| 12 Month | 1.2±1.0 | 1.8±1.6 | ||
| 6-minute walk test | ||||
| Baseline | 492.6±90.1 | 475.9±102.4 | Time | 0.55 |
| 3 Month | 488.0±84.8 | 472.6±104.3 | Group | 0.21 |
| 6 Month | 475.8±86.4 | 472.4±107.5 | Group*Time | 0.66 |
| 12 Month | 485.3±79.5 | 476.1±94.1 | ||
| Pedometer readings | ||||
| Baseline | 16,794±11,895 | 16,097±7744 | Time | 0.89 |
| 3 Month | 17,567±15,711 | 14,633±8157 | Group | 0.25 |
| 6 Month | 16,548±10,042 | 14.679±8707 | Group*Time | 0.83 |
| 12 Month | 16,619±10,452 | 15,350±8745 | ||
| Body Mass Index | ||||
| Baseline | 34.6±7.0 | 33.7±7.1 | Time | 0.63 |
| 3 Month | 34.1±6.2 | 33.7±7.1 | Group | 0.34 |
| 6 Month | 34.4±7.2 | 33.0±7.2 | Group*Time | 0.86 |
| 12 Month | 34.4±7.5 | 33.4±6.4 | ||
| Diabetes Quality of Life | ||||
| Baseline | 67.9±10.6 | 66.9±12.1 | Time | 0.03 |
| 3 Month | 69.7±10.8 | 69.9±12.4 | Group | 0.63 |
| 6 Month | 71.1±10.0 | 70.9±12.1 | Group*Time | 0.86 |
| 12 Month | 71.0±10.6 | 71.1±12.3 | ||
| Problem Areas in Diabetes | ||||
| Baseline | 33.3±20.3 | 34.8±23.1 | Time | 0.003 |
| 3 Month | 27.7±16.9 | 28.9±21.1 | Group | 0.45 |
| 6 Month | 26.1±15.3 | 28.3±22.5 | Group*Time | 0.98 |
| 12 Month | 25.0±16.0 | 25.7±22.7 | ||
| Frustration with Self-Care | ||||
| Baseline | 48.7±22.5 | 43.6±23.7 | Time | <0.001 |
| 3 Month | 39.7±23.3 | 36.2±23.5 | Group | 0.36 |
| 6 Month | 36.3±21.0 | 35.2±27.0 | Group*Time | 0.72 |
| 12 Month | 34.8±23.4 | 32.2±25.1 | ||
Linear Mixed Model showed no between group differences for any variable over time.
CONCLUSIONS AND IMPLICATIONS
This single center randomized controlled trial studying 134 adults with type 2 diabetes in poor glycemic control represented a comparison of a map-based group intervention with an attention control group focused on dyslipidemia and blood pressure. The map-based intervention helped poorly controlled diabetes patients who had received prior diabetes self-management education realize a modest improvement in glycemic control at three months post-education; however, these participants were not able to maintain that improvement over time (that is, at 6- and 12-months follow-up). The Control arm did not show improvement or deterioration in A1C levels at any time during the follow-up period. Interestingly, self-care and diabetes quality of life did not differ by type of intervention at follow-up. However, neither the Intervention nor Control arms negatively impacted participants’ self-reported frequency of self-care, diabetes quality of life, diabetes-related distress, or frustration with diabetes self-care over time.
To our knowledge, this study is the first of its kind to formally examine reinforcement of diabetes education. Contrary to the findings of Sperl-Hillen and colleagues36, this study found modest improvements in A1C levels at 3-months post-intervention in the map-based group. These data suggest reinforcing diabetes education for patients who have not met glycemic targets is helpful and important; however, a map-based group education program may not be the most effective program for reinforcing and importantly maintaining improvements over time. One possibility is that patients who are struggling with self-management issues and glycemic control need more highly structured programs to help them develop and maintain new lifestyle behaviors that will last over a longer term. Structured education that incorporates scaffolding, modeling, and problem-solving skills helps patients who are struggling to integrate self-care into their daily lives14. Further, structured education has been shown to support maintenance of diabetes self-care and glycemic control over time14. More work is required to determine if highly structured diabetes self-care education embedded with behavioral strategies is more suitable than standard group education alone for reinforcement of diabetes education.
One dose of education, whether it is a full course over several weeks or one session, appears not to be enough to support diabetes self-care throughout a patients’ lifetime. Patients with diabetes face important stressors/events at different points throughout the course of their illness that can impact how they manage and cope with diabetes37–39. Four periods necessitate special attention: 1) onset of diabetes, 2) health maintenance and prevention of complications, 3) early onset of complications, and 4) the stage of illness where complications dominate38,39. Each period has educational and psychological implications for the patient, family, and clinician regarding the prevention and treatment of diabetes and its complications. For example, diagnosis of a complication may evoke heightened anxiety, feelings of helplessness, and changes in self-care routines. Patients facing stressors/events typically employ coping strategies they have used in the past that have varying levels of effectiveness37; however, these strategies (e.g., denial, anger) may interfere with their diabetes self-management23. Thus, ongoing and repeated education and support that focuses on potential stressors, diagnosis/treatment of complications, and varying self-care regimens is needed to help patients maintain glycemic control and employ effective coping strategies and prevention strategies as their diabetes progresses. More research is needed to determine the amount or “dose” of education required for long-term maintenance.
Study limitations include homogeneity of the study sample with regards to race/ethnicity (72% Non-Hispanic white), age and education, participant self-selection, and self-reported data. This trial examined the impact of only one program of group education on reinforcing diabetes education. Other group education has been shown to improve glycemic control in both the short-term and long-term40; however, an individual approach may be more effective for some type 2 diabetes patients36. Also, the Intervention and Control arms did not have follow-up support built into the programs, which may partially explain the modest glycemic improvements or lack of maintenance observed in the intervention group. Finally, the design of this randomized controlled trial did not allow for examination of the dose of the intervention or learning thresholds in patients with prior diabetes education who had not reached A1C targets. Future research aimed at reinforcing education should focus on rigorous, randomized controlled trials that compare the effectiveness of education methods and address the important issue of sustainability in behavioral change.
Diabetes self-care education is an important part of diabetes care for all people with diabetes, and our findings support the notion that people who are struggling with their diabetes self-care need ongoing and repeated education to help them improve and maintain their diabetes control. Thus, reinforcing diabetes self-management education for patients who have not met glycemic targets is a necessary component of ongoing diabetes care. The results of our study show the map-based program helped patients improve their A1C at 3 months but not over the long-term. The most effective self-care education program for maintaining improvements over the long-term needs to be determined.
Acknowledgments
Funding: This work was supported by the American Diabetes Association (ADA) grant 7-08-CR-62, the Diabetes and Endocrinology Research Core NIH P30 DK36836, and the NIH Training Grant No. T32 DK007260. Bayer HealthCare LLC (Tarrytown, New York) contributed glucose meters and test strips. ADA and these companies had no role in the conduct of the study or preparation of this manuscript.
Footnotes
Contributors: The researchers thank the patients who participated in the study and the nurses and staff at the Joslin Clinical Research Center. We also thank donors who have contributed to Behavioral Research and to the Clinical Research Center.
Conflict of Interest: Om P. Ganda, MD has provided consulting services to Abbott; received payment for lectures from Merck, Abbott, GlaxoSmithKline, Bristol-Myers Squibb, Astra-Zeneca, and Daiichi-Sankyo; and has stock options with BMS. No other author has anything to declare.
REFERENCES
- 1.Conn VS, Hafdahl AR, Mehr DR, Lemaster JW, Brown SA, Nielsen PJ. Metabolic effects of interventions to increase exercise in adults with type 2 diabetes. Diabetologia. 2007 May;50(5):913–921. doi: 10.1007/s00125-007-0625-0. [DOI] [PubMed] [Google Scholar]
- 2.Maiorana A, O'Driscoll G, Goodman C, Taylor R, Green D. Combined aerobic and resistance exercise improves glycemic control and fitness in type 2 diabetes. Diabetes Res Clin Pract. 2002 May;56(2):115–123. doi: 10.1016/s0168-8227(01)00368-0. [DOI] [PubMed] [Google Scholar]
- 3.Karter AJ, Ackerson LM, Darbinian JA, et al. Self-monitoring of blood glucose levels and glycemic control: the Northern California Kaiser Permanente Diabetes registry*. The American Journal of Medicine. 2001;111(1):1–9. doi: 10.1016/s0002-9343(01)00742-2. [DOI] [PubMed] [Google Scholar]
- 4.Pi-Sunyer FX, Maggio CA, McCarron DA, et al. Multicenter randomized trial of a comprehensive prepared meal program in type 2 diabetes. Diabetes Care. 1999 Feb;22(2):191–197. doi: 10.2337/diacare.22.2.191. [DOI] [PubMed] [Google Scholar]
- 5.Jacobson AM, Adler AG, Derby L, Anderson BJ, Wolfsdorf JI. Clinic attendance and glycemic control Study of contrasting groups of patients with IDDM. Diabetes Care. 1991 Jul;14(7):599–601. doi: 10.2337/diacare.14.7.599. [DOI] [PubMed] [Google Scholar]
- 6.Dyer PH, Lloyd CE, Lancashire RJ, Bain SC, Barnett AH. Factors associated with clinic non-attendance in adults with type 1 diabetes mellitus. Diabet Med. 1998 Apr;15(4):339–343. doi: 10.1002/(SICI)1096-9136(199804)15:4<339::AID-DIA577>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 7.Khan H, Lasker SS, Chowdhury TA. Exploring reasons for very poor glycaemic control in patients with Type 2 diabetes. Prim Care Diabetes. 2011;5(5):251–255. doi: 10.1016/j.pcd.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Gafarian CT, Heiby EM, Blair P, Singer F. The Diabetes Time Management Questionnaire. Diabetes Educ. 1999 Jul-Aug;25(4):585–592. doi: 10.1177/014572179902500411. [DOI] [PubMed] [Google Scholar]
- 9.Wdowik MJ, Kendall PA, Harris MA. College students with diabetes: using focus groups and interviews to determine psychosocial issues and barriers to control. Diabetes Educ. 1997 Sep-Oct;23(5):558–562. doi: 10.1177/014572179702300507. [DOI] [PubMed] [Google Scholar]
- 10.Rubin RR. M.P. Psychological issues and treatment for people with diabetes. Journal of Clinical Psychology. 2001;57(4):457–478. doi: 10.1002/jclp.1041. [DOI] [PubMed] [Google Scholar]
- 11.Ismail K, Winkley K, Rabe-Hesketh S. Systematic review and meta-analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. Lancet. 2004 May 15;363(9421):1589–1597. doi: 10.1016/S0140-6736(04)16202-8. [DOI] [PubMed] [Google Scholar]
- 12.Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-management education for adults with type 2 diabetes: a meta-analysis of the effect on glycemic control. Diabetes Care. 2002 Jul;25(7):1159–1171. doi: 10.2337/diacare.25.7.1159. [DOI] [PubMed] [Google Scholar]
- 13.Norris SL, Engelgau MM, Narayan KM. Effectiveness of self-management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes Care. 2001 Mar;24(3):561–587. doi: 10.2337/diacare.24.3.561. [DOI] [PubMed] [Google Scholar]
- 14.Weinger K, Beverly EA, Lee Y, Sitnokov L, Ganda OP, Caballero AE. The effect of a structured behavioral intervention on poorly controlled diabetes: a randomized controlled trial. Arch Intern Med. 2011 Dec 12;171(22):1990–1999. doi: 10.1001/archinternmed.2011.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diabetes Control and Complications Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993 Sep 30;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 16.Ahern J, Grove N, Strand T, et al. The impact of the Trial Coordinator in the Diabetes Control and Complications Trial (DCCT) The DCCT Research Group. Diabetes Educ. 1993 Nov-Dec;19(6):509–512. doi: 10.1177/014572179301900606. [DOI] [PubMed] [Google Scholar]
- 17.Diabetes Control and Complications Research Group. Implementation of treatment protocols in the Diabetes Control and Complications Trial. Diabetes Care. 1995 Mar;18(3):361–376. doi: 10.2337/diacare.18.3.361. [DOI] [PubMed] [Google Scholar]
- 18.Lorenz RA, Bubb J, Davis D, et al. Changing behavior Practical lessons from the diabetes control and complications trial. Diabetes Care. 1996 Jun;19(6):648–652. doi: 10.2337/diacare.19.6.648. [DOI] [PubMed] [Google Scholar]
- 19.Epidemiology of Diabetes Interventions and Complications (EDIC) Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999 Jan 1;22(1):99–111. doi: 10.2337/diacare.22.1.99. 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davidson MB. Editor's comment: "It ain't necessarily so". Diabetes Care. 2002 Nov;25(11):2116. doi: 10.2337/diacare.25.11.2116. [DOI] [PubMed] [Google Scholar]
- 21.Weinger K, Butler HA, Welch GW, La Greca AM. Measuring diabetes self-care: a psychometric analysis of the Self-Care Inventory-Revised with adults. Diabetes Care. 2005 Jun;28(6):1346–1352. doi: 10.2337/diacare.28.6.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Derogatis LR. BSI 18: Brief Symptom Inventory. Administration, Scoring and Procedures Manual. Minneapolis, MN: National Computer Systems, Inc; 2000. [Google Scholar]
- 23.Peyrot M, McMurry JF, Jr, Kruger DF. A biopsychosocial model of glycemic control in diabetes: stress, coping and regimen adherence. J Health Soc Behav. 1999 Jun;40(2):141–158. [PubMed] [Google Scholar]
- 24.Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care. 1995 Jun;18(6):754–760. doi: 10.2337/diacare.18.6.754. [DOI] [PubMed] [Google Scholar]
- 25.Welch G, Weinger K, Anderson B, Polonsky WH. Responsiveness of the Problem Areas In Diabetes (PAID) questionnaire. Diabet Med. 2003 Jan;20(1):69–72. doi: 10.1046/j.1464-5491.2003.00832.x. [DOI] [PubMed] [Google Scholar]
- 26.Weinger K, Jacobson AM. Psychosocial and quality of life correlates of glycemic control during intensive treatment of type 1 diabetes. Patient Educ Couns. 2001 Feb;42(2):123–131. doi: 10.1016/s0738-3991(00)00098-7. [DOI] [PubMed] [Google Scholar]
- 27.Welch GW, Jacobson AM, Polonsky WH. The Problem Areas in Diabetes Scale An evaluation of its clinical utility. Diabetes Care. 1997 May;20(5):760–766. doi: 10.2337/diacare.20.5.760. [DOI] [PubMed] [Google Scholar]
- 28.Jacobson AM. the DCCT Research Group. The Diabetes Quality of Life Measure. In: Bradley C, editor. Handbook of Psychology and Diabetes. London: J. Wiley; 1994. [Google Scholar]
- 29.Jacobson AM, de Groot M, Samson JA. The evaluation of two measures of quality of life in patients with type I and type II diabetes. Diabetes Care. 1994 Apr;17(4):267–274. doi: 10.2337/diacare.17.4.267. [DOI] [PubMed] [Google Scholar]
- 30.DCCT. Reliability and validity of a diabetes quality-of-life measure for the diabetes control and complications trial (DCCT). The DCCT Research Group. Diabetes Care. 1988 Oct;11(9):725–732. doi: 10.2337/diacare.11.9.725. [DOI] [PubMed] [Google Scholar]
- 31.van der Ven NC, Weinger K, Yi J, et al. The confidence in diabetes self-care scale: psychometric properties of a new measure of diabetes-specific self-efficacy in Dutch and US patients with type 1 diabetes. Diabetes Care. 2003 Mar;26(3):713–718. doi: 10.2337/diacare.26.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weinger K, Yi J. Psychological influences on glycemic control and self-management in type 1 diabetes [Abstract] Diabetes. 2000;48(Supplement):A318. [Google Scholar]
- 33.Nurss JR, Parker RM, Baker DW. Test of Functional Health Literacy in Adults. 2 ed. Snow Camp, NC: Peppercorn Books & Press; 2001. [Google Scholar]
- 34.Little RJA, Rubin DB. Statistical Analysis with Missing Data. Hoboken, NY: John Wiley & Sons, Inc; 2002. [Google Scholar]
- 35.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Second ed. Hoboken, New Jersey: John Wiley & Sons, Inc; 2011. [Google Scholar]
- 36.Sperl-Hillen J, Beaton S, Fernandes O, et al. Comparative effectiveness of patient education methods for type 2 diabetes: a randomized controlled trial. Arch Intern Med. 2011 Dec 12;171(22):2001–2010. doi: 10.1001/archinternmed.2011.507. [DOI] [PubMed] [Google Scholar]
- 37.Hamburg BA, Inoff GE. Coping with predictable crises of diabetes. Diabetes Care. 1983 Jul-Aug;6(4):409–416. doi: 10.2337/diacare.6.4.409. [DOI] [PubMed] [Google Scholar]
- 38.Weinger K, Welch G, Jacobson A. Psychological and psychiatric issues in diabetes mellitus. In: Poretsky L, editor. Principles of Diabetes Mellitus. Norwell, Massachusetts: Kluwer Academic Publishers; 2002. pp. 639–654. [Google Scholar]
- 39.Weinger K, McMurrich Greenlaw S. Behavioral strategies for improving self-management. In: Childs B, Cypress M, Spollett G, editors. Complete Nurse's Guide to Diabetes Care. 2nd Edition. Alexandria, VA: American Diabetes Association; 2009. [Google Scholar]
- 40.Deakin T, McShane CE, Cade JE, Williams RD. Group based training for self-management strategies in people with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005;(2) doi: 10.1002/14651858.CD003417.pub2. CD003417. [DOI] [PubMed] [Google Scholar]