Abstract
Background
Huntington disease (HD) is associated with decline in cognition and progressive morphological changes in brain structures. Cognitive reserve may represent a mechanism by which disease-related decline may be delayed or slowed. The current study examined the relationship between cognitive reserve and longitudinal change in cognitive functioning and brain volumes among prodromal (gene expansion-positive) HD individuals.
Methods
Participants were genetically-confirmed individuals with prodromal HD enrolled in the PREDICT-HD study. Cognitive reserve was computed as the composite of performance on a lexical task estimating premorbid intellectual level, occupational status, and years of education. Linear mixed effects regression (LMER) was used to examine longitudinal changes on 4 cognitive measures and 3 brain volumes over approximately 6 years.
Results
Higher cognitive reserve was significantly associated with a slower rate of change on one cognitive measure (Trail Making Test, Part B) and slower rate of volume loss in two brain structures (caudate, putamen) for those estimated to be closest to motor disease onset. This relationship was not observed among those estimated to be further from motor disease onset.
Conclusions
Our findings demonstrate a relationship between cognitive reserve and both a measure of executive functioning and integrity of certain brain structures in prodromal HD individuals.
Keywords: Huntington disease, prodromal, cognitive reserve, cognition, caudate, putamen
1. INTRODUCTION
Huntington disease (HD) is a progressive, autosomal dominant disease caused by an expanded number of CAG repeats of the HD gene on chromosome 4 and characterized by alterations in cognition, mood, and motor functioning. Among the cognitive deficits seen in HD, decrements in executive functioning (i.e., set shifting, multi-tasking) and processing speed tend to be the most prominent and manifest early in the disease process, in some cases many years prior to clinical diagnosis based on unequivocal motor symptoms (Hahn-Barma et al., 1998; Lawrence et al., 1998; J. S. Paulsen et al., 2008; J.S. Paulsen et al., 2001; Snowden, Craufurd, Thompson, & Neary, 2002). As genetic testing procedures are able to identify those individuals who will eventually develop HD, there is great interest in identifying potential targets for therapeutic intervention that may be initiated prior to motor diagnosis.
Cognitive dysfunction is progressive in HD, and longitudinal studies have documented cognitive decline over various intervals in individuals with HD (Bachoud-Levi et al., 2001; Bamford, Caine, Kido, Cox, & Shoulson, 1995; Beglinger et al., 2010; Ho et al., 2003; Stout et al., 2012), as well as in prodromal HD (Lemiere, Decruyenaere, Evers-Kiebooms, Vandenbussche, & Dom, 2004; Tabrizi et al., 2011). In these studies, declines have been most commonly observed on measures of attention, psychomotor speed, and executive functioning, implicating frontal-striatal dysfunction.
Likewise, longitudinal morphological changes have been reported in certain brain structures in prodromal HD individuals. In particular, longitudinal changes in striatal volumes have been proposed as a potential biomarker of HD (Aylward, 2007). Specifically, significant decreases have been noted in caudate and putamen volumes over time in prodromal HD (Aylward et al., 1997; Aylward et al., 2011; Hobbs et al., 2010), with evidence of caudate atrophy at least 14 years prior to estimated motor diagnosis (Hobbs et al., 2010). Changes in frontal lobe white matter and ventricular volumes have also been observed in prodromal HD individuals (Aylward et al., 2011; Hobbs et al., 2010; Reading et al., 2005).
Additionally, significant correlations have been identified between volumes of basal ganglia structures and cognitive measures (Brandt, Bylsma, Aylward, Rothlind, & Gow, 1995; Starkstein et al., 1988). Jurgens and colleagues (2008) found that smaller putamen volumes were associated with poorer performance on measures of processing speed and task-switching (i.e., Symbol Digit Modalities Test and Trail Making Test, Part B). Thus, there is evidence of both decline in cognitive function and atrophy of brain regions among individuals with HD, with additional evidence that these two processes appear to occur in parallel. Furthermore, brain regions that typically show morphological changes in HD (e.g., basal ganglia) are those that are known to subserve cognitive functions that decline in HD (for review, see (Montoya, Price, Menear, & Lepage, 2006).
Cognitive reserve has been advanced as a mechanism by which decline related to disease may be delayed or slowed (Stern, 2009). Under the cognitive reserve hypothesis, it is theorized that individual differences exist in how effectively people are able to withstand brain pathology. Differences in both cognitive processing and underlying neural networks have both been implicated in this process (Stern, 2009). Cognitive reserve has been used as a research tool to examine the degree to which innate and environmental factors (particularly those that are modifiable) are related to progression and manifestation of disease symptoms. Among the factors most often studied in this regard are years of education, general intellectual functioning, and occupational attainment (Alexander et al., 1997; Benedict, Morrow, Weinstock Guttman, Cookfair, & Schretlen, 2010; Sumowski, Wylie, Chiaravalloti, & DeLuca, 2010).
Cognitive reserve has been examined in individuals with Alzheimer’s disease, among other populations. Some studies in this area have concluded that those with higher levels of cognitive reserve remain asymptomatic for longer periods relative to those with lower levels of cognitive reserve (e.g., (Fratiglioni & Wang, 2007). Others have reported an inverse relationship between years of education and rate of cognitive decline in patients with likely AD, such that MMSE scores in more highly educated individuals declined at slower rates (Fritsch, McClendon, Smyth, & Ogrocki, 2002). Additionally, neuropathological studies have found that individuals higher in cognitive reserve are more likely to remain cognitively intact despite accumulation of AD neuropathology (Roe, Xiong, Miller, & Morris, 2007).
Despite promising findings in the AD literature, minimal work has been conducted on this topic in prodromal HD. One study (Lopez-Sendon et al., 2011) reported a positive association between education levels and scores on clinical measures of HD (i.e., motor, cognitive, behavioral, functional capacity). However, this study used a cross-sectional design, somewhat limiting the conclusions that can be drawn. The effect of environmental enrichment has also been examined in HD transgenic mice (Nithianantharajah, Barkus, Vijiaratnam, Clement, & Hannan, 2009), with results suggesting a positive, although subtle, effect of enrichment on neuronal plasticity. Overall, however, this topic has largely gone unexamined in the HD literature.
The goal of the current study was to examine the relationship between cognitive reserve and longitudinal change in cognitive functioning and brain volumes among individuals with prodromal HD. Specifically, we used linear mixed effects regression (LMER) for longitudinal data to examine rate of change in four cognitive variables (Stroop Interference, Symbol-Digit Modalities Test, and Trail Making Test, Parts A & B) and brain volumes (caudate, putamen, frontal lobe white matter) over time in relation to a composite measure of cognitive reserve. We hypothesized that higher levels of cognitive reserve would be associated with slower rates of cognitive decline over time in our sample of prodromal HD individuals. We also predicted no significant association between cognitive reserve and longitudinal change in brain volumes, based on previous neuropathological work in Alzheimer’s disease.
2. METHODS
2.1 Participants
Participants were enrolled in the PREDICT-HD study, a multi-site, international, longitudinal study of prodromal HD (J. S. Paulsen et al., 2006; J. S. Paulsen et al., 2008). Consent was obtained according to the Declaration of Helsinki and the study protocol was approved by the Institutional Review Board at the University of Iowa as well as institutional review boards at each participating study site. All participants included in these analyses had a family history of HD and were laboratory-confirmed gene expansion-positive (at least 36 CAG repeats) individuals. Table 1 shows the descriptive statistics for the predictors at baseline. Though BDI score is a time-varying covariate, Table 1 shows results only for the baseline evaluation. Females represented sixty-four percent of the sample. Exclusion criteria included evidence of unstable medical or psychiatric illness, alcohol or drug abuse within the previous year, learning disability or mental retardation, history of special education, history of other central nervous system disease or neurological events such as seizures or head trauma, pacemaker or metallic implants, age younger than 18 years, prescription of antipsychotic medications within the past 6 months, and use of phenothiazine-derivative antiemetic medications more than 3 times per month. There was a maximum of N = 821 participants available based on these criteria. However, there was missing data and Table 2 shows descriptive statistics by visit, including the total number of data points over all visits (NV). Participants were given a baseline assessment and were then assessed annually at 6 follow-up visits, for a maximum total of 7 assessment points. Participants underwent cognitive testing at all time points. Neuroimaging data was collected at Visit 1 (baseline), Visit 3, and Visit 5. In addition, imaging data was more difficult to obtain resulting in approximately 200 fewer participants compared to the cognitive variables (see Table 2).
Table 1.
Descriptive statistics for predictor variables at baseline
| Variables | N | Min | Median | Mean | Max | SD |
|---|---|---|---|---|---|---|
| Cognitive Reserve |
812 | −7.77 | 0.17 | 0.01 | 4.98 | 2.33 |
| Highest Occupation |
821 | 1.00 | 5.00 | 5.06 | 6.00 | 1.20 |
| Years of Education |
820 | 8.00 | 14.00 | 14.39 | 20.00 | 2.66 |
| ANART | 812 | 6.00 | 39.00 | 37.97 | 55.00 | 8.14 |
| CAP | 821 | 111.08 | 346.52 | 344.37 | 845.84 | 82.78 |
| Age | 821 | 18.11 | 41.10 | 41.28 | 83.73 | 10.35 |
| BDI Total | 812 | 0.00 | 4.00 | 7.25 | 48.00 | 8.24 |
ANART = American National Adult Reading Test; BDI = Beck Depression Inventory; CAP = CAG-Age Product; N = sample size
Note. Highest occupation and ANART were reverse scored, see text.
Table 2.
Descriptive statistics for response variables summarized over all time points
| Variable | N | NV | Min | Median | Mean | Max | SD |
|---|---|---|---|---|---|---|---|
| Stroop Interference | 819 | 4114 | 9.00 | 45.00 | 45.34 | 100.00 | 10.51 |
| Symbol-Digit | 819 | 4120 | 11.00 | 52.00 | 51.67 | 110.00 | 12.38 |
| Trail Making Part A | 817 | 2604 | 10.00 | 25.00 | 27.61 | 240.00 | 12.47 |
| Trail Making Part B | 817 | 2577 | 15.00 | 58.00 | 68.83 | 300.00 | 37.60 |
| Putamen | 623 | 1318 | 0.24 | 0.58 | 0.57 | 0.85 | 0.11 |
| Caudate | 623 | 1318 | 0.11 | 0.41 | 0.41 | 0.81 | 0.09 |
| Frontal White | 623 | 1318 | 7.95 | 12.93 | 12.90 | 18.67 | 1.45 |
Note. Putamen, caudate, frontal white are expressed as ratio to intracranial volume × 100; NV = total number of observations over all visits; N = sample size
2.2 Cognitive and Emotional Measures
Cognitive tests were drawn from the PREDICT-HD cognitive battery and were selected to target common cognitive deficits observed in HD (e.g., processing speed, set switching, inhibitory control). Selection of tests was also based upon the effect sizes of these measures in distinguishing individuals with prodromal HD from healthy control participants (Stout et al., 2011). Tests included in the analyses were the Stroop Color-Word Interference Task (Interference condition), Symbol Digit Modalities Test, and Trail Making Test (parts A and B). Premorbid intellectual functioning was estimated using measures of single word reading ability that varied by geographic location (see below). All tasks were scored by the examiner and rescored by two additional reviewers, and accuracy of data entry was reviewed separately by two research assistants to ensure the quality of the cognitive data. The National Adult Reading Test/American National Adult Reading Test was audio taped to allow for rescoring.
The American National Adult Reading Test (ANART; (Grober & Sliwinski, 1991) was administered to participants in the United States, Canada, and Australia, while the National Adult Reading Test (NART-2; (Nelson & Willison, 1991) was administered to participants in the United Kingdom. Both tests require participants to pronounce irregularly spelled words and are often used to estimate premorbid intelligence. The ANART has also been used previously to estimate premorbid IQ in the PREDICT-HD sample (Carlozzi et al., 2011). Performance on these measures was based on total number of words correctly pronounced. Participants in Spain were administered the Word Accentuation Test (WAT), which assesses pronunciation of low-frequency Spanish words (Del Ser, Gonzalez-Montalvo, Martinez-Espinosa, Delgado-Villapalos, & Bermejo, 1997). Participants in Germany were administered the Wortschatztest (WST), which requires individuals to discriminate written German words from non-words (Schmidt & Metzler, 1992).
The Interference subtest of the Stroop Color-Word Interference Task (Stroop, 1935) measures processing speed and inhibitory control. Participants are shown color words (e.g., “blue”) written in different colored ink (i.e., red) and are required to name the ink color aloud and inhibit the dominant response of reading the word. The score utilized was the total number of correct responses within 45 seconds.
The Symbol Digit Modalities Test (A. Smith, 1991) is a measure of visual scanning and processing speed in which participants are required to rapidly transcribe numbers to match symbols based on a reference key. Performance on this measure is based on the number of correctly transcribed items completed in 90 seconds.
The Trail Making Test (Reitan, 1958) assesses processing speed, visual scanning, response set maintenance, and set switching and is administered in two parts. In Part A, participants are required to connect numbered dots in order from 1–25 as quickly as possible. In Part B, participants are instructed to alternate between connecting numbers and letters in order. Performance on this measure is based on time to completion, with higher numbers indicating poorer (i.e., slower) performance.
Participants also completed the Beck Depression Inventory, 2nd Edition (BDI-II), a self-report measure of depression symptoms (Beck, Steer, & Brown, 1996). Participants respond to questions regarding recent symptoms of depression. The measure consists of 21 questions, each of which is rated on a 0–3 scale, based on severity. Total score on this measure ranges from 0–63, with higher scores indicating more severe depressive symptoms. This measure was included given the prominence of depressive symptoms among individuals with HD. Total BDI score was included as a covariate in statistical analyses to control for presence of depressive symptoms, which could potentially affect cognitive performance (M. M. Smith et al., in press).
2.3 Additional Measures
Cognitive reserve was computed as the composite of the task estimating premorbid intellectual level (ANART/NART/WAT/WST), highest obtained occupational status, and years of education. The ANART/NART was coded such that larger values indicated greater intellectual level. Occupational status was originally rated on a 1–6 scale (1 = Professional, 2 = Manager, 3 = Craftsman, 4 = Service, 5 = Laborer, 6 = Not in Labor Force). Occupational status was reverse coded to yield 0 = Not in Labor Force, … 6 = Professional. There were very few responses of “Not in Labor Force”. All of the variables were separately standardized prior to compositing, and thus, each variable contributed equally to the Cognitive Reserve score. Higher scores indicate greater cognitive reserve.
Individuals come into the PREDICT-HD study at different ages with different CAG expansions meaning the participants enter with different levels of progression. The variability in progression must be accounted for in the statistical analysis in order to make proper inferences. PREDICT-HD statisticians (Zhang et al., 2011) developed a proxy variable for disease progression based on an accelerated failure time (AFT) model having time to motor diagnosis as the response, and CAG, age at entry, and their interaction as predictors. The byproduct of the modeling was a “CAG-Age Product” (CAP) score, which is computed for an individual as CAP = AGE0 × (CAG – 33.7) where AGE0 is the age at study entry. CAP is a proxy for the extent of disease progression at time of study entry, and indicates the likelihood of a near-future HD diagnosis (Y. Zhang et al., 2011). CAP can also be interpreted as an index of the cumulative genetic toxicity of the mutant huntingtin gene, sometimes referred to as “genetic burden”. Zhang et al. (Ying Zhang et al., 2011) describe a optimization algorithm based on minimizing an ANOVA F-value for classifying all the individuals based on CAP score cutoffs. The resulting groups are “low”, “medium”, and “high” with the labels denoting relative levels of probability of receiving a motor diagnosis in a 5-year period initiated at study entry. Thus, low CAP group individuals are less progressed at study entry than medium CAP group individuals, who are in turn less progressed than high CAP group individuals.
2.4 Neuroimaging Measures
The imaging variables were derived by MRI scans obtained using a standard multi-modal protocol that included an axial 3D volumetric spoiled gradient echo series and a dual echo proton density/T2 series. Scans were processed at the University of Iowa using an automated procedure implemented in BRAINS (Magnotta et al., 2002) and artificial neural networks (Powell et al., 2008). Volume measures were determined for caudate, putamen, and frontal white matter using the automated software, AutoWorkup (Pierson et al., 2011). After completion of AutoWorkup, all scans were individually inspected for correct realignment and coregistration, tissue classification, and accuracy of brain and subcortical structures. Intra-cranial volume (ICV) was also calculated to allow for correction of structural volumes for overall head size. The variable for the analysis was the ratio of the brain structure to ICV × 100.
2.5 Statistical Analysis
Each of the variables was analyzed separately using LMER. The details of the models are presented in the appendix. Preliminary analysis not presented indicated that linear change curves were sufficient. In addition to using cognitive reserve as a predictor, the following control variables were included: age (at baseline), gender, CAP (at baseline), and BDI, which was time-varying.
The time metric for the statistical analysis was duration, defined as current age minus age at baseline. Thus, the baseline duration score was zero, and change over time was expressed in years since study entry.
The focus of the analysis was cognitive reserve by CAP effects; specifically, baseline differences (intercept differences) and differences in change over time (change curve differences). It was hypothesized that cognitive reserve differences might vary as a function of CAP. Therefore, cognitive reserve × CAP interactions were considered for intercept and linear slope.
The analytic strategy was to fit three models for each response variable and examine relative fit. Model 1 had cognitive reserve and control variable intercept and slope effects (there were no CAP effects); Model 2 had all the effects of Model 1 plus cognitive reserve × CAP intercept differences (baseline differences); Model 3 had all the effects of Model 2 plus cognitive reserve × CAP slope differences (differences in longitudinal trajectory). Each model had random intercepts, random slopes for duration, and random error. Additional details of the models are provided in the appendix.
The models were evaluated using a scaling of Akaike’s information criterion (AIC) and the likelihood ratio test (LRT). A probability scaling of the AIC, known as the AIC weight (W), was computed indicating the relative fit of each model in the group of three (see appendix). W values closer to one indicate better relative fit, whereas values closer to zero indicate worse relative fit. For this reason, W is interpreted as a global relative effect size measure (Burnham & Anderson, 2002; Long, 2011).
Since the three models of the analysis were nested, the LRT was used to test each model against the next lowest (simpler), beginning with Model 2. All pairs of nested models were tested regardless of the statistical significance of a lower order pair.
Parameter estimates and standard errors were inspected to facilitate the interpretation of the results, but the details are not presented. Comprehensive results are available from the authors. Maximum likelihood methods were used to estimate the models with the lme4 package (Bates, 2011) of the R program for statistical computing.
3. RESULTS
The descriptive statistics for the response variables are shown in Table 2. The results of the LMER model comparisons are shown in Table 3. As noted, W is the AIC weight indicating the relative fit of an individual model within the set of three (a higher value indicates better fit). The last three columns list the results of the LRT for the two-at-a-time comparison. The W column of the table shows that Model 3, containing the cognitive reserve by CAP change curve interaction, was the best fitting for Trail Making Test Part B, putamen, and caudate. The effect size for Trail Making Test Part B was very strong (W = 0.98), a bit weaker for putamen (W = 0.81), and substantially weaker for caudate (W = 0.72). The LRT for Model 2 vs. Model 3 had a relatively small p-value in each case (i.e., p < .02). Model 2, containing the cognitive reserve by CAP baseline interaction, was the best fitting for the Symbol-Digit Modalities Test, Trail Making Test Part A, and frontal white matter.
Table 3.
Model comparison for each outcome variable
| Variable | Model | K | W | Chisq | p |
|---|---|---|---|---|---|
| Stroop Int | 1 | 17 | 0.45 | ||
| Stroop Int | 2 | 18 | 0.31 | 1.31 | 0.2532 |
| Stroop Int | 3 | 19 | 0.24 | 1.47 | 0.2247 |
| Symbol Digit |
1 | 17 | 0.16 | ||
| Symbol Digit |
2 | 18 | 0.61 | 4.72 | 0.0298 |
| Symbol Digit |
3 | 19 | 0.23 | 0.05 | 0.8264 |
| Trails A | 1 | 17 | 0.30 | ||
| Trails A | 2 | 18 | 0.43 | 2.75 | 0.0971 |
| Trails A | 3 | 19 | 0.27 | 1.14 | 0.2863 |
| Trails B | 1 | 17 | 0.00 | ||
| Trails B | 2 | 18 | 0.02 | 8.76 | 0.0031 |
| Trails B | 3 | 19 | 0.98 | 10.26 | 0.0014 |
| Putamen | 1 | 17 | 0.08 | ||
| Putamen | 2 | 18 | 0.11 | 2.57 | 0.1088 |
| Putamen | 3 | 19 | 0.81 | 6.08 | 0.0137 |
| Caudate | 1 | 17 | 0.19 | ||
| Caudate | 2 | 18 | 0.09 | 0.49 | 0.4826 |
| Caudate | 3 | 19 | 0.72 | 6.34 | 0.0118 |
| Frontal White |
1 | 17 | 0.18 | ||
| Frontal White |
2 | 18 | 0.56 | 4.32 | 0.0377 |
| Frontal White |
3 | 19 | 0.25 | 0.46 | 0.4967 |
Note. K = number of model parameters; W = AIC weight; df = 1 for all chi-squared (Chisq) tests.
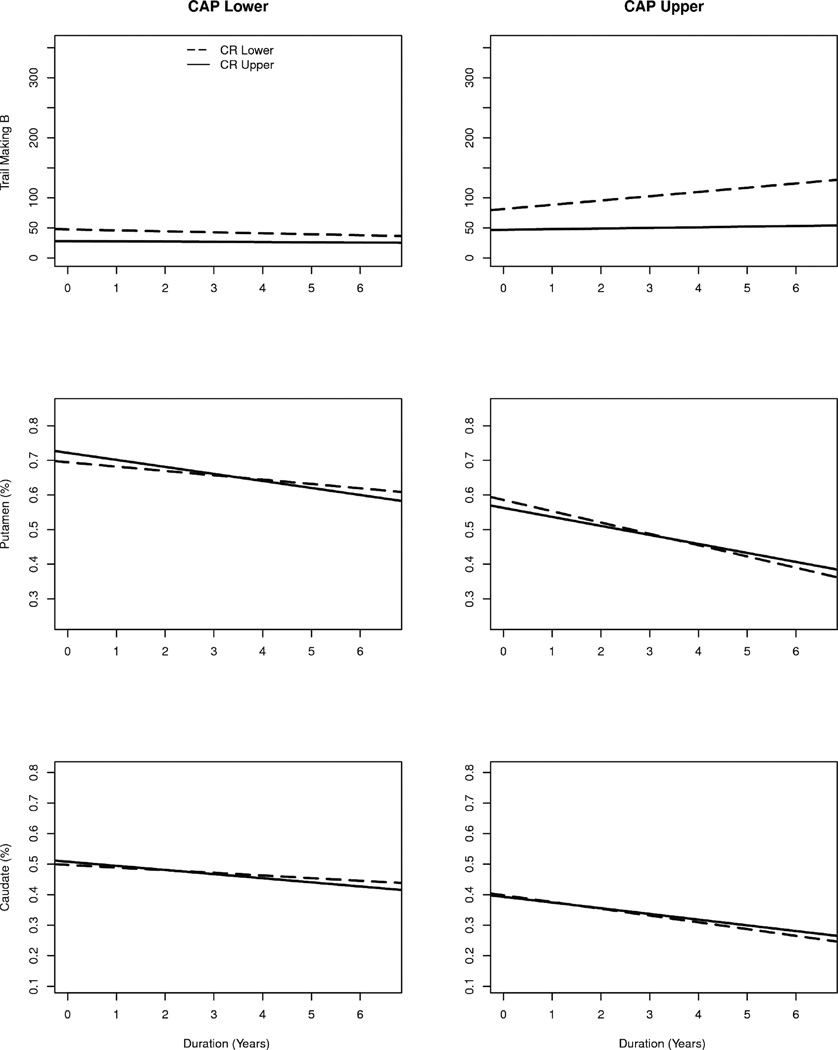
The specifics of the cognitive reserve by CAP change curve are illustrated in Figure 1. The graphs show the fitted curves as a function of two levels of CAP (lower and upper) and two levels of cognitive reserve (lower and upper). Though the entire distribution of CAP and cognitive reserve were used in the LMER analysis, specific values were substituted into the fitted models to produce predicted values for graphing. The graphing values were chosen to be the quantiles cutting off the lower and upper 10% and of the empirical distributions of the variables. The three variables (Trail Making Test Part B, putamen, caudate) mark the rows of the figure, and the two levels of CAP denote the columns. The levels of cognitive reserve are depicted by different line types (dashed = lower, solid = upper).
Fig. 1.
Specifics of the cognitive reserve by “CAG-Age Product” (CAP) change curve.
The first row illustrates the interaction effect for Trail Making Test Part B. For individuals with higher (upper) scores on CAP, those who also had lower cognitive reserve (dashed lines) had a positive slope, indicating a slowing of performance over time. For individuals with higher cognitive reserve (upper; solid lines), there was a much slower rate of decline. Regarding the individuals with lower CAP scores, the slopes for both cognitive reserve levels were slightly negative and close to parallel.
The second row of Figure 1 shows the interaction for putamen, and the third row shows the interaction for caudate. In both cases, volume decreased over time, with the overall volume being lower for those with higher CAP scores (right-hand graphs). For the upper-level CAP scores depicted at right, there was a faster decrease in volume for those with lower cognitive reserve compared to those with higher cognitive reserve. The converse was true for lower CAP; those with higher cognitive reserve tended to deteriorate slightly faster than those with lower cognitive reserve. The difference in effect sizes for putamen and caudate (see Table 3) was reflected by the caudate curves that showed less separation than the putamen curves for upper CAP.
4. DISCUSSION
The goal of the current study was to examine the relationship between cognitive reserve and longitudinal change in cognitive functioning and brain volumes among prodromal HD individuals who were assessed annually over a period of up to six years. Our findings indicate that, after controlling for a number of covariates (i.e., age, gender, depression symptoms), cognitive reserve was significantly associated with linear rate of change on one cognitive measure (Trail Making Test, Part B) and in two brain structures (caudate, putamen) for those closest to estimated disease onset. Specifically, for individuals with higher CAP scores (i.e., those closer to estimated diagnosis), those with higher cognitive reserve showed a slower decline in TMT-B performance and slower rate of volume loss in caudate and putamen, relative to individuals with lower cognitive reserve. This relationship was not observed among individuals with lower CAP scores (i.e., those further from estimated diagnosis).
Although this topic has remained relatively unexplored in HD, our findings are in line with a recent study (Lopez-Sendon et al., 2011) demonstrating that higher education levels were related to better outcomes on clinical and cognitive measures. The authors attributed their findings to a possible disease-modifying effect of education on clinical manifestations and symptoms of HD. Our results add support to this hypothesis and extend it to include possible beneficial effects of other separate, but related, factors, namely occupation and general intelligence. The longitudinal design of our study also provides valuable information regarding cognitive reserve and long-term changes in cognition and brain structure that cannot be captured through studies with cross-sectional designs.
Our findings are also consistent with longitudinal studies that have reported a more rapid rate of decline in neurocognitive function over a follow-up period as prodromal HD participants approached estimated disease onset (Campodonico, Codori, & Brandt, 1996; Rupp et al., 2010; Solomon et al., 2008). For example, Campodonico and colleagues (1996) found that individuals closer to estimated HD onset had faster rate of decline on measures of sustained attention and processing speed over time than those who were estimated to be further from diagnosis. Using a cross-sectional research design, Stout and colleagues (2007) found deficits in attention, working memory, and executive function, among other domains, in individuals classified as being near clinical diagnosis, but not in those far from diagnosis. It is possible that as individuals approach diagnosis, neuropsychological measures are more likely to detect deficits in fronto-striatal circuitry. Results of our study add to a growing body of knowledge in this area and suggest that the effect of cognitive reserve is most noticeable when patients are closest to estimated disease onset.
One question raised by our findings is why the relationship between cognitive reserve and longitudinal cognitive change did not generalize to all cognitive measures. Although we initially hypothesized that higher levels of cognitive reserve would be associated with a slower rate of decline on all neuropsychological tests, Trail Making Test-B was the only cognitive measure that showed significant associations with cognitive reserve over time. Although one could argue that TMT-B is the most complex task we used (as it requires sustained attention, motor speed, visual scanning, and rapid set switching), it remains unclear how (and by what mechanism) performance on this measure alone interacts with cognitive reserve. One possibility is that TMT-B was the most intellectually demanding test administered and successful performance of the task is most highly linked with general intellectual capacity. There is some evidence to support this notion. Relative to TMT-A, for example, TMT-B is more strongly associated with fluid intelligence (Salthouse, 2011) and has stronger associations with Full Scale IQ among healthy controls (Goul & Brown, 1970). TMT-B may also have a higher sensitivity than the other measures to the types of executive functioning deficits that are particularly prevalent in HD (e.g., set switching, cognitive flexibility). Further investigation is needed on this topic to clarify some of the subtle relationships between cognitive reserve and longitudinal variations in cognition.
Results of the analyses for longitudinal brain volume change were also somewhat surprising. We initially hypothesized that cognitive reserve would show no significant association with changes in brain volumes over time. This hypothesis was based on previous findings in studies of Alzheimer’s disease, demonstrating intact cognitive functioning among patients with higher levels of cognitive reserve, despite significant accumulation of AD pathology (Roe et al., 2007). However, our results suggest that higher levels of cognitive reserve were associated with a slower rate of caudate and putamen atrophy among those who were closest to estimated disease onset. Thus, in contrast to previous AD studies, higher cognitive reserve was associated with less neuropathology (i.e., atrophy) among prodromal HD individuals who were near clinical diagnosis. These results suggest that higher levels of education, higher occupational status, and higher general intelligence may serve as protective mechanisms to both cognitive function and striatal atrophy during the period closest to disease onset, when cognition is known to decline at a more rapid rate. A caveat to this hypothesis is that the size of the effect for the slower rate of atrophy is difficult to determine. It is unknown how much of a reduction in putamen volume, for example, is associated with manifest signs of disease. Additional research is needed to address if cognitive reserve has effects specific for HD.
Our findings were also consistent with previous research that has shown morphological changes in brain structures that are known to subserve cognitive functions that decline in HD, including caudate (Brandt et al., 1995; Starkstein et al., 1988) and putamen (Jurgens et al., 2008). In one study, smaller putamen volumes correlated with poorer performance on two cognitive measures, including TMT-B (Jurgens et al., 2008). Our results suggest that cognitive reserve was associated with both rate of decline on TMT-B and rate of caudate and putamen atrophy for those closest to estimated disease onset. The mechanism by which cognitive reserve affects progression of cognitive deficits and brain structure changes in HD is not currently understood. Research in rodents suggests that environmental enrichment is associated with increased levels of brain-derived neurotrophic factor (BDNF) and improved motor and cognitive performance (Mazzocchi-Jones, Dobrossy, & Dunnett, 2011), suggesting a possible protective effect of BDNF on symptom progression in HD. However, this relationship remains unclear and warrants further investigation.
Despite previous reports describing significant changes in frontal lobe white matter among individuals with prodromal HD (Aylward et al., 2011; Reading et al., 2005), we found no evidence that rate of frontal white matter atrophy was related to cognitive reserve in our study sample. Again, there is very little empirical data from other studies of HD against which to compare our findings. Others have described an association between measures of cognitive reserve and white matter integrity in studies of MCI and AD (Arenaza-Urquijo et al., 2011; Teipel et al., 2009). These studies have generally concluded that white matter integrity is an important aspect of cognitive reserve and may be related to risk of developing dementia. However, these studies used diffusion tensor imaging (DTI) techniques to calculate a measure of white matter integrity (i.e., fractional anisotropy; FA), whereas our study examined gross volumes of the frontal lobe white matter. It is possible that methodological differences account for the differential findings between the current study and previous work in this area.
The current study had some limitations. First, the cohort of participants included in this study may not be an entirely representative sample, as it was restricted to individuals with prodromal HD who had undergone predictive genetic testing and who were willing to participate in an intensive, longitudinal study. However, it should be noted that this by far represents the largest sample of prodromal HD individuals ever studied. As mentioned previously, the neuroimaging methodology used may have altered our findings with regard to white matter. Our study used measures of white matter volume, which may have lacked the sensitivity to detect more subtle changes in white matter in this population. Additionally, while we found significant associations between cognitive reserve and rate of change in caudate and putamen volumes, the effect sizes of these findings were relatively small, and it is unclear whether this represents a clinically meaningful outcome. Our cognitive reserve measure also consisted of a combination of various premorbid intellectual measures that may provide different estimates of general cognitive skills, potentially introducing additionally variability into the cognitive reserve variable and making it more difficult to achieve significant correlations. In particular, the use of different measures to estimate premorbid verbal intelligence in different countries (i.e., NART, ANART, WAT, WST) may have produced subtle variation in the data and affected study outcome to some degree. Lastly, neuropsychological tests were administered to participants annually over the course of six years. It is possible that familiarity with the tests (i.e., practice effects) attenuated potential findings of cognitive decline over time and influenced our results to some degree.
Overall, the current study demonstrated a relationship between cognitive reserve and longitudinal changes in cognitive functioning and brain structures. Our results lend support to the hypothesis that higher levels of cognitive reserve confer some protection against the cognitive decline that precedes motor onset of HD. Furthermore, increased cognitive reserve was associated with a slower rate of atrophy in brain structures implicated in the development of HD, though the meaning of the slower rate is difficult to interpret.
Lastly, our findings carry implications regarding disease development in HD. As described previously, the construct of cognitive reserve is intended to capture the influence of both innate ability and cognitively enriching experiences on disease onset and development. This suggests that cognitive reserve represents a potentially modifiable factor and a potential target for future studies looking to delay conversion to HD. Since the genetic risk of developing HD is known, interventions to improve reserve may be initiated years before predicted onset of the disease and may provide more years of intact functioning and independence. It is also possible that earlier intervention via enriching experiences may be beneficial in limiting some of the precipitous decline that is characteristic of the years closely preceding symptom onset in HD. Engagement in cognitively enriching activities and regular physical exercise delay onset of HD and cognitive decline in rodents (Pang, Stam, Nithianantharajah, Howard, & Hannan, 2006) and may prove to be protective factors in humans. An additional possibility is that individuals who are higher in cognitive reserve initially may take better care of themselves (e.g., better diet, less alcohol use, more exercise, etc.), which in turn may lead to less brain atrophy and reduced cognitive decline. However, additional work is clearly needed to address these questions. Future investigations should attempt to further clarify the complex role that cognitive reserve plays in the development of HD, as well as its specificity to HD, including possible mechanisms through which cognitive reserve may affect changes in both cognitive function and brain structure among those with prodromal HD.
Supplementary Material
Acknowledgment
We thank the PREDICT-HD sites, the study participants, and the National Research Roster for Huntington Disease Patients and Families.
Funding
This research is supported by the National Institutes for Health, National Institute of Neurological Disorders and Stroke (NS040068) and CHDI Foundation, Inc (A3917).
PREDICT-HD Investigators, Coordinators, Motor Raters, Cognitive Raters
Thomas Wassink, MD, Stephen Cross, BA, Mycah Kimble, BA, Patricia Ryan, MSW, LISW, MA, and Eric A. Epping, MD, PhD (University of Iowa, Iowa City, Iowa, USA);
Edmond Chiu, MD, Olga Yastrubetskaya, PhD, Joy Preston, Anita Goh, D.Psych, Stephanie Antonopoulos and Samantha Loi (St. Vincent’s Hospital, The University of Melbourne, Kew, Victoria, Australia);
Phyllis Chua, MD, and Angela Komiti, BS, MA (The University of Melbourne, Royal Melbourne Hospital, Melbourne, Australia);
Lynn Raymond, MD, PhD, and Joji Decolongon, MSC, CCRP (University of British Columbia, Vancouver, British Columbia, Canada);
Christopher A. Ross, MD, PhD, Mark Varvaris, BA, and William M. Mallonee, MD, and Greg Suter, BA (Hereditary Neurological Disease Centre, Wichita, Kansas, USA);
Ali Samii, MD, and Alma Macaraeg, BS (University of Washington and VA Puget Sound Health Care System, Seattle, Washington, USA);
Randi Jones, PhD, Cathy Wood-Siverio, MS, Stewart A. Factor, DO, and Claudia Testa, MD, PhD (Emory University School of Medicine, Atlanta, Georgia, USA);
Roger A. Barker, BA, MBBS, MRCP, and Sarah Mason, BSC (Cambridge Centre for Brain Repair, Cambridge, UK);
Elizabeth McCusker, MD, Jane Griffith, RN, Clement Loy, MD, and David Gunn, BS (Westmead Hospital, Sydney, Australia);
Michael Orth MD, PhD, Sigurd Süβmuth, MD, RN, Katrin Barth, RN, and Sonja Trautmann, RN (University of Ulm, Ulm, Germany);
Kimberly Quaid, PhD, Melissa Wesson, MS, and Joanne Wojcieszek, MD (Indiana University School of Medicine, Indianapolis, IN);
Mark Guttman, MD, Alanna Sheinberg, BA, and Irita Karmalkar, BSc (Centre for Addiction and Mental Health, University of Toronto, Markham, Ontario, Canada);
Susan Perlman, MD, and Brian Clemente, (Los Angeles Medical Center, Los Angeles, California, USA);
Michael D. Geschwind, MD, PhD, and Gail Kang, MD (University of California San Francisco, California, USA);
Gabriela Satris, BA, Tom Warner, MD, PhD, Maggie Burrows, RN, BA (National Hospital for Neurology and Neurosurgery, London, UK);
Anne Rosser, MD, PhD, MRCP, Kathy Price, RN, and Sarah Hunt, BSc (Cardiff University, Cardiff, Wales, UK);
Frederick Marshall, MD, Amy Chesire, LCSW-R, MSG, Mary Wodarski, BA, and Charlyne Hickey, RN, MS (University of Rochester, Rochester, New York, USA);
Peter Panegyres, MB, BS, PhD, Joseph Lee, and Steve Andrew (Neurosciences Unit, Graylands, Selby-Lemnos & Special Care Health Services, Perth, Australia);
Joel Perlmutter, MD, Stacey Barton, MSW, LCSW, and Amy Schmidt (Washington University, St. Louis, Missouri, USA);
Zosia Miedzybrodzka, MD, PhD, Daniela Rae, RN, and Mariella D’Alessandro, PhD (Clinical Genetics Centre, Aberdeen, Scotland, UK);
David Craufurd, MD, Judith Bek, PhD, and Elizabeth Howard, MD (University of Manchester, Manchester, UK);
Pietro Mazzoni, MD, PhD, Karen Marder, MD, MPH, and Paula Wasserman, MA (Columbia University Medical Center, New York, New York, USA);
Rajeev Kumar, MD and Diane Erickson, RN (Colorado Neurological Institute, Englewood, Colorado, USA);
Vicki Wheelock, MD, Terry Tempkin, RNC, MSN, and Lisa Kjer, MSW (University of California Davis, Sacramento, California, USA);
Wayne Martin, MD, Pamela King, BScN, RN, Marguerite Wieler and Satwinder Sran, BSC (University of Alberta, Edmonton, Alberta, Canada); Oksana Suchowersky, MD, FRCPC,
Anwar Ahmed, PhD, Stephen Rao, PhD, Christine Reece, BS, Alex Bura, BA, Lyla Mourany, and Juliet Schulz (Cleveland Clinic Foundation, Cleveland, Ohio, USA).
Executive Committee
Jane Paulsen, PhD, Principal Investigator, Eric A. Epping, MD, PhD, Hans Johnson, PhD, Megan Smith, PhD, Jeffrey D. Long, PhD, (University of Iowa, Iowa City, IA); Elizabeth Aylward, PhD (Seattle Children's Research Institute, WA); Kelsey Montross, BA, Jeremy Bockholt, BS, MS, Steve Blanchard, MSHA.
Scientific Consultants
Bio Markers: Ken Evans, PhD (Ontario Cancer Biomarker Network); Wayne Matson, PhD (VA Medical Center, Bedford, MA); Asa Peterson, MD, PhD (Lund University, Sweden); James A. Mills, MS (University of Iowa)
Brain: Jean Paul Vonsattell, PhD (Chair), and Carol Moskowitz, ANP, MS (Columbia University Medical Center); Stacie Vik, BA (University of Iowa).
Cognitive: Deborah Harrington, PhD (Chair), Gabriel Castillo, BS, Jessica Morison, BS, and Jason Reed, BS (University of California, San Diego), Michael Diaz, PhD, Ian Dobbins, PhD, Tamara Hershey, PhD, Erin Foster, OTD, and Deborah Moore, BA (Washington University Cognitive Science Battery Development); Holly Westervelt, PhD (Chair, Quality Control and Training, Alpert Medical School of Brown University), Jennifer Davis, PhD, and Geoff Tremont, PhD, MS (Scientific Consultants, Alpert Medical School of Brown University); Megan Smith, PhD (Chair, Administration), David J. Moser, PhD (University of Iowa); Carissa Gehl, PhD (VA Medical Center, Iowa City, IA); Kirsty Matheson (University of Aberdeen); Karen Siedlecki, PhD (Fordham University); Marleen Van Walsem (EHDN); Greg Elias, BA, Mary Gover, and Rachel Bernier, (Rhode Island Hospital); Noelle Carlozzi (University of Michigan); Kevin Duff, PhD (University of Utah); Nellie Georgiou-Karistianis (St. Vincent’s Hospital, The University of Melbourne, Australia); and Kate Papp (University of Connecticut).
Functional: Janet Williams, PhD Eunyoe Ro, MA, Lee Anna Clark, Nancy Downing, RN, PhD, Michelle Harreld, BS, and Stacie Vik, BA (University of Iowa); Rebecca Ready, PhD (University of Massachusetts); Anthony Vaccarino, PhD (Ontario Cancer Biomarker Network); Sarah Farias, PhD (University of California, Davis); Noelle Carlozzi, PhD (University of Michigan); and Carissa Gehl, PhD (VA Medical Center, Iowa City, IA).
Genetics: Marcy MacDonald, PhD Jim Gusella, PhD, and Rick Myers, PhD (Massachusetts General Hospital); Tom Wassink, MD (Co-Chair) Eric A. Epping, MD, PhD, and James Mills, MS, (University of Iowa); Zosia Miedzybrodzka, MD, PhD (University of Aberdeen); and Christopher Ross, MD, PhD (Johns Hopkins University).
Imaging: Administrative: Kathy Jones, BS, Jacquie Marietta, BS, Greg Harris, BS, Eun Young Kim, MS, Hans Johnson, PhD, Greg Ennis, MA, and Thomas Wassink, MD (University of Iowa); John Ashburner, PhD (Functional Imaging Lab, London); Steve Potkin, MD (University of California, Irvine); and Arthur Toga, PhD (University of California, Los Angeles). Striatal: Elizabeth Aylward, PhD (Chair, Seattle Children's Research Institute). Surface Analysis: Eric Axelson, BSE (University of Iowa). Shape Analysis: Christopher A. Ross (Chair), MD, PhD, Michael Miller, PhD, and Sarah Reading, MD (Johns Hopkins University); Mirza Faisal Beg, PhD (Simon Fraser University). DTI: Vincent A. Magnotta, PhD (Chair, University of Iowa); Karl Helmer, PhD (Massachusetts General Hospital); Kelvin Lim, MD (University of Ulm, Germany); Mark Lowe, PhD (Cleveland Clinic); Sasumu Mori, PhD (Johns Hopkins University); Allen Song, PhD (Duke University); and Jessica Turner, PhD (University of California, Irvine). fMRI: Steve Rao, PhD (Chair), Erik Beall, PhD, Katherine Koenig, PhD, Michael Phillips, MD, Christine Reece, BS, and Jan Zimbelman, PhD, PT (Cleveland Clinic); Kathy Jones, (University of Iowa).
Psychiatric: Eric A. Epping, MD, PhD (Chair), Nancy Downing, RN, PhD, Jess Fiedorowicz, MD, Robert Robinson, MD, Megan Smith, PhD, Leigh Beglinger, PhD, James Mills, MS, Michelle Harreld, BS, Stacie Vik, BA, Janet Williams, PhD, Dawei Liu, PhD, David Moser, PhD, and Kelly Rowe (University of Iowa); Karen Anderson, MD (University of Maryland); David Craufurd, MD (University of Manchester); Mark Groves, MD (Columbia University); Anthony Vaccarino, PhD and Ken Evans, PhD (Ontario Cancer Biomarker Network); Hugh Rickards, MD (Queen Elizabeth Psychiatric Hospital); Eric van Duijn, MD (Leiden University Medical Center, Netherlands).
Core Sections
Biostatistics: Jeffrey D. Long, PhD, Ji-In Kim, PhD, James A. Mills, MS, Ying Zhang, PhD, Dawei Liu, PhD, Wenjing Lu, Craig Stout, and Spencer Lourens (University of Iowa).
Recruitment/Retention: Martha Nance, MD (Chair, University of Minnesota); Mycah Kimble, BA, Patricia Ryan, MSW, LISW, MA, Sean Thompson, BA (University of Iowa).
Ethics: Cheryl Erwin, JD, PhD, (Chair, McGovern Center for Health, Humanities and the Human Spirit); Eric A. Epping, MD, PhD Janet Williams, PhD, James Mills, MS, and Stacie Vik, BA (University of Iowa); Martha Nance, MD (University of Minnesota); and Lisa Hughes, MEd (University of Texas Medical School at Houston).
IT/Management: Jeremy Bockholt, Jim Smith, AS, Roland Zschiegner, Ryan Wyse, Owen Wade, Jason Evans, and Derek Weston (University of Iowa).
Program Management
Administrative: Chris Werling-Witkoske (Chair), Greg Ennis, MA, Stacie Vik, BA, Karla Anderson, BS, Brittany Lichty, BA, and Leann Davis (University of Iowa).
Financial: Steve Blanchard, MSHA, Kelsey Montross, BA, and Phil Danzer (University of Iowa).
References
- Alexander GE, Furey ML, Grady CL, Pietrini P, Brady DR, Mentis MJ, Schapiro MB. Association of premorbid intellectual function with cerebral metabolism in Alzheimer's disease: implications for the cognitive reserve hypothesis. Am J Psychiatry. 1997;154(2):165–172. doi: 10.1176/ajp.154.2.165. [DOI] [PubMed] [Google Scholar]
- Arenaza-Urquijo EM, Bosch B, Sala-Llonch R, Sole-Padulles C, Junque C, Fernandez-Espejo D, Bartres-Faz D. Specific anatomic associations between white matter integrity and cognitive reserve in normal and cognitively impaired elders. Am J Geriatr Psychiatry. 2011;19(1):33–42. doi: 10.1097/JGP.0b013e3181e448e1. [DOI] [PubMed] [Google Scholar]
- Aylward EH. Change in MRI striatal volumes as a biomarker in preclinical Huntington's disease. Brain Res Bull. 2007;72(2–3):152–158. doi: 10.1016/j.brainresbull.2006.10.028. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Li Q, Stine OC, Ranen N, Sherr M, Barta PE, Ross CA. Longitudinal change in basal ganglia volume in patients with Huntington's disease. Neurology. 1997;48(2):394–399. doi: 10.1212/wnl.48.2.394. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Nopoulos PC, Ross CA, Langbehn DR, Pierson RK, Mills JA, Paulsen JS. Longitudinal change in regional brain volumes in prodromal Huntington disease. J Neurol Neurosurg Psychiatry. 2011;82(4):405–410. doi: 10.1136/jnnp.2010.208264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachoud-Levi AC, Maison P, Bartolomeo P, Boisse MF, Dalla Barba G, Ergis AM, Peschanski M. Retest effects and cognitive decline in longitudinal follow-up of patients with early HD. Neurology. 2001;56(8):1052–1058. doi: 10.1212/wnl.56.8.1052. [DOI] [PubMed] [Google Scholar]
- Bamford KA, Caine ED, Kido DK, Cox C, Shoulson I. A prospective evaluation of cognitive decline in early Huntington's disease: functional and radiographic correlates. Neurology. 1995;45(10):1867–1873. doi: 10.1212/wnl.45.10.1867. [DOI] [PubMed] [Google Scholar]
- Bates DM. lme4: Mixed-Effects Modeling with R. New York: Springer; 2011. [Google Scholar]
- Beck AT, Steer RA, Brown GK. BDI-II, Beck depression inventory: manual. 2nd ed. Boston: Harcourt Brace; 1996. [Google Scholar]
- Beglinger LJ, Duff K, Allison J, Theriault D, O'Rourke JJF, Leserman A, Paulsen JS. Cognitive change in patients with Huntington disease on the Repeatable Battery for the Assessment of Neuropsychological Status. Journal of Clinical and Experimental Neuropsychology. 2010;32:573–578. doi: 10.1080/13803390903313564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict RH, Morrow SA, Weinstock Guttman B, Cookfair D, Schretlen DJ. Cognitive reserve moderates decline in information processing speed in multiple sclerosis patients. J Int Neuropsychol Soc. 2010;16(5):829–835. doi: 10.1017/S1355617710000688. [DOI] [PubMed] [Google Scholar]
- Brandt J, Bylsma FW, Aylward EH, Rothlind J, Gow CA. Impaired source memory in Huntington's disease and its relation to basal ganglia atrophy. J Clin Exp Neuropsychol. 1995;17(6):868–877. doi: 10.1080/01688639508402436. [DOI] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. Model Selection and Multimodal Inference. New York: Springer; 2002. [Google Scholar]
- Campodonico JR, Codori AM, Brandt J. Neuropsychological stability over two years in asymptomatic carriers of the Huntington's disease mutation. J Neurol Neurosurg Psychiatry. 1996;61(6):621–624. doi: 10.1136/jnnp.61.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlozzi NE, Stout JC, Mills JA, Duff K, Beglinger LJ, Aylward EH Group, t. P.-H. I. o. t. H. S. Estimating premorbid IQ in the prodromal phase of a neurodegenerative disease. The Clinical Neuropsychologist. 2011;25:757–777. doi: 10.1080/13854046.2011.577811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Ser T, Gonzalez-Montalvo JI, Martinez-Espinosa S, Delgado-Villapalos C, Bermejo F. Estimation of premorbid intelligence in Spanish people with the Word Accentuation Test and its application to the diagnosis of dementia. Brain Cogn. 1997;33(3):343–356. doi: 10.1006/brcg.1997.0877. [DOI] [PubMed] [Google Scholar]
- Fratiglioni L, Wang HX. Brain reserve hypothesis in dementia. J Alzheimers Dis. 2007;12(1):11–22. doi: 10.3233/jad-2007-12103. [DOI] [PubMed] [Google Scholar]
- Fritsch T, McClendon MJ, Smyth KA, Ogrocki PK. Effects of educational attainment and occupational status on cognitive and functional decline in persons with Alzheimer-type dementia. Int Psychogeriatr. 2002;14(4):347–363. doi: 10.1017/s1041610202008554. [DOI] [PubMed] [Google Scholar]
- Goul WR, Brown M. Effects of age and intelligence on trail making test performance and validity. Perceptual and Motor Skills. 1970;30:319–326. doi: 10.2466/pms.1970.30.1.319. [DOI] [PubMed] [Google Scholar]
- Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. J Clin Exp Neuropsychol. 1991;13(6):933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- Hahn-Barma V, Deweer B, Durr A, Dode C, Feingold J, Pillon B, Dubois B. Are cognitive changes the first symptoms of Huntington's disease? A study of gene carriers. Journal of Neurology, Neurosurgery, and Psychiatry. 1998;64:172–177. doi: 10.1136/jnnp.64.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho AK, Sahakian BJ, Brown RG, Barker RA, Hodges JR, Ane MN, Bodner T. Profile of cognitive progression in early Huntington's disease. Neurology. 2003;61(12):1702–1706. doi: 10.1212/01.wnl.0000098878.47789.bd. [DOI] [PubMed] [Google Scholar]
- Hobbs NZ, Barnes J, Frost C, Henley SM, Wild EJ, Macdonald K, Tabrizi SJ. Onset and progression of pathologic atrophy in Huntington disease: a longitudinal MR imaging study. AJNR Am J Neuroradiol. 2010;31(6):1036–1041. doi: 10.3174/ajnr.A2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgens CK, van de Wiel L, van Es AC, Grimbergen YM, Witjes-Ane MN, van der Grond J, Roos RA. Basal ganglia volume and clinical correlates in 'preclinical' Huntington's disease. J Neurol. 2008;255(11):1785–1791. doi: 10.1007/s00415-008-0050-4. [DOI] [PubMed] [Google Scholar]
- Lawrence AD, Hodges JR, Rosser AE, Kershaw A, ffrench-Constant C, Rubinsztein DC, Sahakian BJ. Evidence for specific cognitive deficits in preclinical Huntington's disease. Brain. 1998;121:1329–1341. doi: 10.1093/brain/121.7.1329. [DOI] [PubMed] [Google Scholar]
- Lemiere J, Decruyenaere M, Evers-Kiebooms G, Vandenbussche E, Dom R. Cognitive changes in patients with Huntington's disease (HD) and asymptomatic carriers of the HD mutation--a longitudinal follow-up study. J Neurol. 2004;251(8):935–942. doi: 10.1007/s00415-004-0461-9. [DOI] [PubMed] [Google Scholar]
- Long JD. Longitudinal Data Analysis for the Behavioral Sciences Using R. Thousand Oaks, CA: Sage Publications Inc; 2011. [Google Scholar]
- Lopez-Sendon JL, Royuela A, Trigo P, Orth M, Lange H, Reilmann R, de Yebenes JG. What is the impact of education on Huntington's disease? Mov Disord. 2011;26(8):1489–1495. doi: 10.1002/mds.23385. [DOI] [PubMed] [Google Scholar]
- Magnotta VA, Harris G, Andreasen NC, O'Leary DS, Yuh WT, Heckel D. Structural MR image processing using the BRAINS2 toolbox. Comput Med Imaging Graph. 2002;26(4):251–264. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- Mazzocchi-Jones D, Dobrossy M, Dunnett SB. Environmental enrichment facilitates long-term potentiation in embryonic striatal grafts. Neurorehabilitation and Neural Repair. 2011;25:548–557. doi: 10.1177/1545968311402090. [DOI] [PubMed] [Google Scholar]
- Montoya A, Price BH, Menear M, Lepage M. Brain imaging and cognitive dysfunctions in Huntington's disease. J Psychiatry Neurosci. 2006;31(1):21–29. [PMC free article] [PubMed] [Google Scholar]
- Nelson HE, Willison J. The National Adult Reading Test (NART): Test manual. 2nd ed. Windsor, England: NFER Nelson; 1991. [Google Scholar]
- Nithianantharajah J, Barkus C, Vijiaratnam N, Clement O, Hannan AJ. Modeling brain reserve: experience-dependent neuronal plasticity in healthy and Huntington's disease transgenic mice. Am J Geriatr Psychiatry. 2009;17(3):196–209. doi: 10.1097/JGP.0b013e318196a632. [DOI] [PubMed] [Google Scholar]
- Pang TY, Stam NC, Nithianantharajah J, Howard ML, Hannan AJ. Differential effects of voluntary physical exercise on behavioral and brain-dervied neurotrophic factor expression deficits in Huntington's disease transgenic mice. Neuroscience. 2006;141:569–584. doi: 10.1016/j.neuroscience.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Paulsen JS, Hayden M, Stout JC, Langbehn DR, Aylward E, Ross CA Group, P.-H. I. o. t. H. S. Preparing for preventative clinical trials: the Predict-HD study. Arch Neurol. 2006;63:883–890. doi: 10.1001/archneur.63.6.883. [DOI] [PubMed] [Google Scholar]
- Paulsen JS, Langbehn DR, Stout JC, Aylward E, Ross CA, Nance M, Hayden M. Detection of Huntington's disease decades before diagnosis: the Predict-HD study. J Neurol Neurosurg Psychiatry. 2008;79(8):874–880. doi: 10.1136/jnnp.2007.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen JS, Zhao H, Stout JC, Brinkman RR, Guttman M, Ross CA Group, T.H.S. Clinical markers of early disease in persons near onset of Huntington’s disease. Neurology. 2001;57:658–662. doi: 10.1212/wnl.57.4.658. [DOI] [PubMed] [Google Scholar]
- Pierson RK, Johnson HJ, Harris G, Keefe H, Paulsen J, Andreasen NC, Magnotta VA. Fully automated analysis using BRAINS: AutoWorkup. Neuroimage. 2011;54:328–336. doi: 10.1016/j.neuroimage.2010.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell S, Magnotta VA, Johnson H, Jammalamadaka VK, Pierson R, Andreasen NC. Registration and machine learning-based automated segmentation of subcortical and cerebellar brain structures. Neuroimage. 2008;39(1):238–247. doi: 10.1016/j.neuroimage.2007.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reading SA, Yassa MA, Bakker A, Dziorny AC, Gourley LM, Yallapragada V, Ross CA. Regional white matter change in pre-symptomatic Huntington’s disease: a diffusion tensor imaging study. Psychiatry Res. 2005;140(1):55–62. doi: 10.1016/j.pscychresns.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8:271–276. [Google Scholar]
- Roe CM, Xiong C, Miller JP, Morris JC. Education and Alzheimer disease without dementia: support for the cognitive reserve hypothesis. Neurology. 2007;68(3):223–228. doi: 10.1212/01.wnl.0000251303.50459.8a. [DOI] [PubMed] [Google Scholar]
- Rupp J, Blekher T, Jackson J, Beristain X, Marshall J, Hui S, Foroud T. Progression in prediagnostic Huntington disease. J Neurol Neurosurg Psychiatry. 2010;81(4):379–384. doi: 10.1136/jnnp.2009.176982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. What cognitive abilities are involved in trail-making performance? Intelligence. 2011;39:222–232. doi: 10.1016/j.intell.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K, Metzler P. Wortschatztest (WST) Weinheim: Beltz Verlag; 1992. [Google Scholar]
- Smith A. Symbol Digit Modalities Test. Los Angeles: Western Psychological Services; 1991. [Google Scholar]
- Smith MM, Mills JA, Epping EA, Westervelt HJ, Paulsen JS Group, T.P-H. I. a. C. o. t. H. S. Depressive symptom severity is related to poorer cognitive performance in prodromal Huntington disease. Neuropsychology. doi: 10.1037/a0029218. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden JS, Craufurd D, Thompson J, Neary D. Psychomotor, executive, and memory function in preclinical Huntington's disease. Journal of Clinical and Experimental Neuropsychology. 2002;24(2):133–145. doi: 10.1076/jcen.24.2.133.998. [DOI] [PubMed] [Google Scholar]
- Solomon AC, Stout JC, Weaver M, Queller S, Tomusk A, Whitlock KB, Foroud T. Ten-year rate of longitudinal change in neurocognitive and motor function in prediagnosis Huntington disease. Mov Disord. 2008;23(13):1830–1836. doi: 10.1002/mds.22097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkstein SE, Brandt J, Folstein S, Strauss M, Berthier ML, Pearlson GD, Folstein M. Neuropsychological and neuroradiological correlates in Huntington's disease. J Neurol Neurosurg Psychiatry. 1988;51(10):1259–1263. doi: 10.1136/jnnp.51.10.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47(10):2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout JC, Jones R, Labuschagne I, O’Regan AM, Say MJ, Dumas EM, Frost C. Evaluation of longitudinal 12 and 24 month cognitive outcomes in premanifest and early Huntington's disease. Journal of Neurology, Neurosurgery, and Psychiatry. 2012;83:687–694. doi: 10.1136/jnnp-2011-301940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout JC, Paulsen JS, Queller S, Solomon AC, Whitlock KB, Campbell JC, Aylward EH. Neurocognitive signs in prodromal Huntington disease. Neuropsychology. 2011;25(1):1–14. doi: 10.1037/a0020937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout JC, Weaver M, Solomon AC, Queller S, Hui S, Johnson SA, Foroud T. Are cognitive changes progressive in prediagnostic HD? Cogn Behav Neurol. 2007;20(4):212–218. doi: 10.1097/WNN.0b013e31815cfef8. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference and serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- Sumowski JF, Wylie GR, Chiaravalloti N, DeLuca J. Intellectual enrichment lessens the effect of brain atrophy on learning and memory in multiple sclerosis. Neurology. 2010;74(24):1942–1945. doi: 10.1212/WNL.0b013e3181e396be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabrizi SJ, Scahill RI, Durr A, Roos RA, Leavitt BR, Jones R, Investigators T-H. Biological and clinical changes in premanifest and early stage Huntington's disease in the TRACK-HD study: the 12-month longitudinal analysis. Lancet Neurology. 2011;10:31–42. doi: 10.1016/S1474-4422(10)70276-3. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Meindl T, Wagner M, Kohl T, Burger K, Reiser MF, Hampel H. White matter microstructure in relation to education in aging and Alzheimer's disease. J Alzheimers Dis. 2009;17(3):571–583. doi: 10.3233/JAD-2009-1077. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Long JD, Mills JA, Warner JH, Lu W, Paulsen JS. Indexing disease progression at study entry with individuals at-risk for Huntington disease. Am J Med Genet B Neuropsychiatr Genet. 2011;156B(7):751–763. doi: 10.1002/ajmg.b.31232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Long JD, Mills JA, Warner JH, Lu W, Paulsen JS Group, t. P.-H. I. a. C. o. t. H. S. Indexing disease progression at study entry with individuals at risk for Huntington disease. American journal of medical genetics. 2011;156(7):751–763. doi: 10.1002/ajmg.b.31232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.