Abstract
Objective
Epigallocatechin-3-gallate (EGCG), a catechin gallate ester, is the major component of green tea and has been demonstrated to inhibit tumor growth as well as inhibit smooth muscle cell migration. We evaluated the effect of the phytochemicals resveratrol, allicin, sulforaphane [SFN] and EGCG, on intimal hyperplasia in the carotid artery injury model.
Methods
Intimal hyperplasia was induced in carotid arteries of adult Sprague-Dawley rats with a wire injury. Experimental animals received intraperitoneal injections of one of the four phytochemicals daily beginning one day prior to surgery and continued for up to 4 weeks. Control animals were administered saline. Carotid specimens were harvested at 2 weeks and subjected to quantitative image analysis. In addition, EGCG specimens were analyzed for cell proliferation, immunohistochemistry, and western blot analysis.
Results
Quantitative image analysis showed significant phytochemical suppression of intimal hyperplasia at 2 and 4 weeks post-operatively with EGCG (62% decrease in intimal area). Significant decreases were also noted at 2 weeks for SFN (56%) and resveratrol (44%), whereas the decrease with allicin (24%) was not significant. Quantification of intimal hyperplasia by intima/media ratio showed similar results. Cell proliferation assay of specimens demonstrated suppression by EGCG. Immunohistochemical staining of EGCG-treated specimens showed ERK suppression but not of the jnk or p38 pathways. Western blot analysis confirmed reduced ERK activation in arteries treated with EGCG.
Conclusion
Intraperitoneal injection of the phytochemicals EGCG, SFN, resveratrol and allicin have suppressive effects on the development of intimal hyperplasia in the carotid artery injury model, with maximal effect due to EGCG. The mechanism of EGCG action may be due to inhibition of ERK activation. EGCG may affect a common pathway underlying either neoplastic cellular growth or vascular smooth muscle cellular proliferation.
Keywords: Epigallocatechin-3-gallate, intimal hyperplasia, ERK
The proliferation of studies dealing with basic mechanisms of intimal hyperplasia reflects the growing need to develop methods to prevent or suppress this biologic reaction.1–10 The passage of wires and balloons are now part of the vascular surgeon’s basic armamentarium for treatment of occlusive atherosclerosis and related pathology, but at the same time, it is ironic that these very same methods are potent inducers of intimal hyperplasia and, counter-intuitively, are re-deployed to retreat these lesions. Understanding the underlying molecular mechanisms would aid in defining appropriate modes of therapy.
Epigallocatechin-3-gallate [EGCG] the major catechin in green tea, reliably reduces intimal hyperplasia by inhibiting the proliferation of smooth muscle cells and along with other phytochemicals, has been shown to be anticarcinogenic as well as an antioxidant with antithrombotic and anti-inflammatory effects.1, 11, 12 Green tea, derived from Camellia sinensis, consists of polyphenols from the flavenoid class, generally known as catechins. By virtue of its biologic activity, it has been postulated that EGCG may provide a therapeutic option for the suppression or prevention of intimal hyperplasia. Other phytochemicals that may similarly suppress the intimal hyperplastic response include allicin, derived from garlic, resveratrol from red grapes and sulforaphane [SFN] from broccoli, cabbage, arugula and brussel sprouts. In this study, we examine the suppression of experimental intimal hyperplasia by various phytochemicals and to define potential molecular pathways of action.
Materials and Methods
The present study was performed in compliance with animal protection laws and institutional approval. Animal care complied with the Principles of Laboratory Animal Care, formulated by the National Society for Medical Research, and the Guide for the Care and Use of Laboratory Animals, (Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington: National Academy Press, 1996 [http://nap.edu/openbook.php?record_id=5140]). This study used male Sprague-Dawley rats (Harlan Laboratories, Inc.), aged seven to nine weeks and weighing between 250 and 300 grams. The rats were housed individually at 20°C±3°C with free access to food and water. Anesthesia was performed by intraperitoneal injection of a solution of saline, 100 mg/kg ketamine (Sigma-Aldrich Co., St. Louis, MO) and 10 mg/kg xylazine (Bedford Laboratories, Bedford, OH).
Experimental design
Rats were randomly divided into a saline control group (n=5) and experimental groups, EGCG (n=5), SFN (n=6), resveratrol (n=5), and allicin (n=6). Treatment began one day prior to surgery and continued daily until animals were sacrificed; the treatment regimen consisted of 1ml/kg intraperitoneal injections of either saline, 1 mg/kg EGCG, 0.9 mg/kg allicin, 3 mg/kg resveratrol, or 0.48 mg/kg SFN.
Injury to the common carotid artery was performed on all anesthetized animals as described by Clowes1 and Tulis13 but modified to use a guidewire. A slightly right of midline incision of approximately 2 cm in length was made from immediately below the mandible to just above the sternum. Carotid artery exposure was obtained and isolated with 5-0 Prolene sutures placed around the common and internal carotid arteries; 6-0 Prolene sutures were placed around the external carotid artery. Through an arteriotomy in the external carotid artery, a 0.034 in. uncoated guidewire was inserted and passed 8 times. Following removal of the wire, the external carotid was tied off and the internal carotid circulation restored.
Rats were sacrificed after excision of the carotid artery specimen with a lethal dose of anesthesia followed by placement into a CO2 chamber. Specimens for histology were ligated and excised at 2 weeks post injury, rinsed with saline and fixed in 10% formalin. Specimens for western blot analysis were perfused with saline at 2 weeks post injury and immediately frozen in liquid nitrogen.
Histology and morphometry
Specimens were embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Four sections of each specimen were selected at random and photographed at 40x magnification. Cross-sectional areas of the intima and media were digitally measured in pixels using Image J (NIH, Bethesda, MD). Intimal area was defined as the area encompassed by the internal elastic lamina minus the lumen area. The outer margin of the media was defined by the interface between the circular smooth muscle cells of the media and the connective tissue of the adventitia. Each defined cross-sectional area was manually traced with the software package.
Immunohistochemistry analysis
Immunohistochemistry staining was performed specific for the proteins extracellular signal-regulated kinase (ERK), c-jun N-terminal kinase (JNK) and phosphorylated-p38 (Santa Cruz Biotechnology, Inc). These antibodies are known to have an interspecies cross-reactivity with rat antigens. Immunohistochemistry was performed as follows: formalin-fixed paraffin sections (5 μm thick) were cut and air-dried on polyL-lysine-coated slides (Histology Control Systems Inc, Glen Head, NY). After deparaffinization and rehydration, tissue sections were digested with a Proteinase K solution (DAKO) to unmask some fixated antigenic sites. The specimens were then incubated with 3% hydrogen peroxide to block endogenous peroxidase and reduce nonspecific binding. Primary antibodies were incubated with the specimens for 30 minutes at room temperature. Subsequently, the slides were covered with biotinylated antimouse secondary antibody and incubated with streptavidin peroxidase to form avidin-biotin complexes. Prepared AEC and DAB substrate-chromogen solutions were applied to cover specimens. Sections were counterstained with hematoxylin. Specimens were mounted and coverslipped with a glycerol-mounting medium. In the control slides, incubation of the primary antibody was omitted; all other steps were identical.
Ki-67 Proliferation Index analysis was performed at 2 weeks after injury using similar immunohistochemistry techniques on formalin-fixed, paraffin-embedded tissue sections. Following antigen retrieval with Target Retrieval Solution (DAKO), specimens were labeled with mouse anti-rat Ki-67 antigen (DAKO), stained using biotinylated rabbit anti-mouse immunoglobulins (DAKO) and streptavidin/HRP, and then visualized using DAB+ (DAKO). The Ki-67 proliferation index was assessed by point counting and reported as percent positive. At least 4 sections were examined for each group; n=6 for each group.
Western blot analysis was performed on eight specimens (five controls and three experimental EGCG specimens), collected at 2 weeks after injury; specimens were different than those examined for histology. Densitometry was performed on the phosphorylated or total ERK1 (p44), ERK2 (p42), or the phosphorylated p38 band. The value for the treated artery (left) was normalized by subtracting the value obtained from the control artery (right).
Statistical analysis
Data are expressed as the arithmetic mean ± the standard error of the mean (SEM). Densitometry of western blots was performed with the Mann-Whitney U test. A statistic corresponding to P less than 0.05 was considered statistically significant.
RESULTS
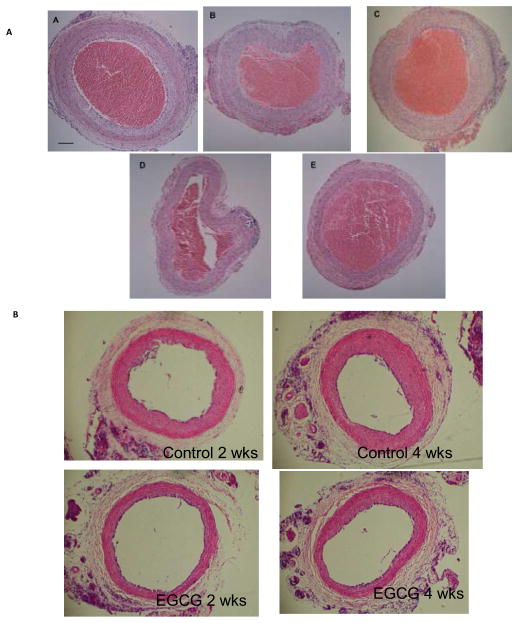
Representative cross sections of wire-injured rat carotid arteries harvested at 2 weeks shows suppression of intimal hyperplasia compared to controls (Figure 1A). Morphometric analysis showed suppression of the intimal area by 62%, 56%, 44% and 24% by EGCG, SFN, resveratrol and allicin, respectively vs. control, P<.05 for all except allicin. Intima:media ratios were similarly decreased, with maximal suppression of the intima:media ratio with EGCG (P<0.01)(Figure 2). The suppression of EGCG on intimal hyperplasia was sustained for up to 4 weeks (Figure 1B); the reduction of intimal area was significant (2 weeks: control 0.174 ± 0.09 vs. EGCG 0.103 ± 0.03; p<0.05; 4 weeks: control 0.287 ± 0.06 vs. EGCG 0.143 ± 0.03; p<0.01).
Figure 1.
(A) Representative cross sections of rat carotid arteries harvested at two weeks post injury and stained with hematoxylin and eosin. Images show extent of intimal hyperplasia in the Control (A) and suppression by phytochemical inhibitors: Allicin (B), Resveratrol (C), SFN (D), and EGCG (E). Unflushed blood occupies the lumen of the specimens. Size bar represents 50 microns. n=5–6 for each group. (B) Representative cross sections of rat carotid arteries harvested at 2 or 4 weeks after injury, stained with H&E. n=6 for each group.
Figure 2.
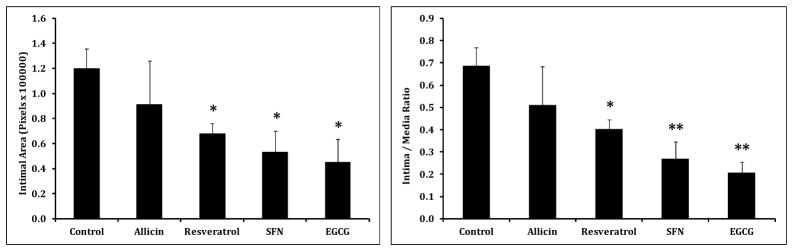
Histogram depicting intimal area and intima: media ratios at 2 weeks after wire injury for control, allicin, resveratrol, SFN and EGCG groups. (A) Intimal area (measured in pixels) was significantly lower in the resveratrol, SFN, and EGCG groups than in the control group. (B) Intima:media ratios were similar. Results are expressed as mean ± SEM, *P<.05 vs the control group, **P<.01 vs the control group. n=5–6 for each group.
Since EGCG is a profound suppressor of intimal hyperplasia, we determined whether EGCG suppresses cell proliferation too. In EGCG treated arteries, the Ki-67 proliferation index was almost half that of the corresponding control specimens at 2 weeks (control 6.023 ± 1.28 vs. EGCG 3.33 ± 1.02, P<.05), although no difference was present at 4 weeks (control 2.26 ± 0.87 vs. EGCG 1.89 ± 1.01, p>0.05). This suppression is consistent with reduction of cell proliferation as a mechanism of EGCG action.
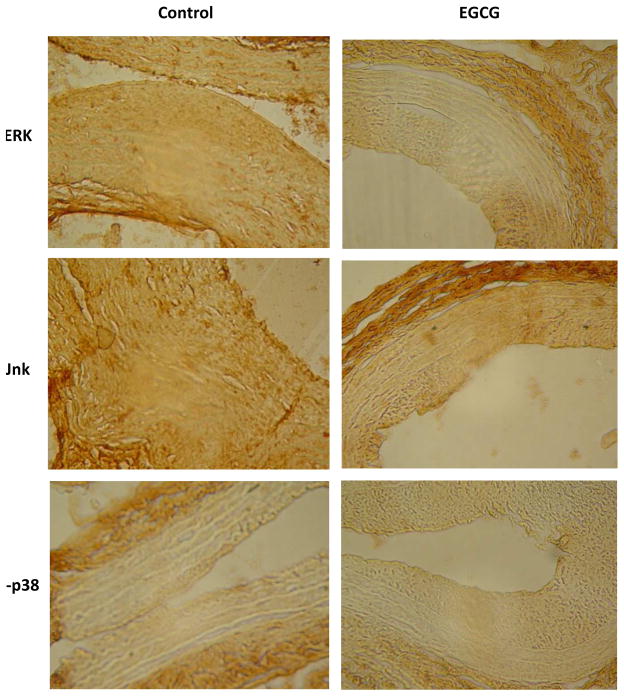
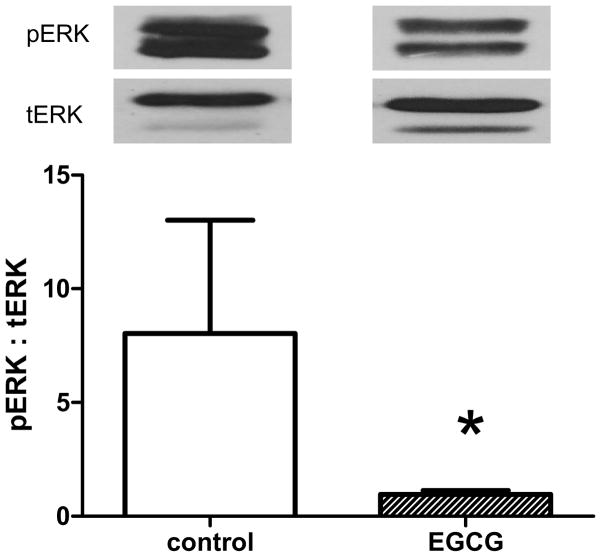
Since EGCG may reduce intimal hyperplasia by reduction of cell proliferation, we determined whether EGCG has an effect on the MAPK signal transduction pathways, as a basis for its reduction of cell proliferation. Immunohistochemistry showed reduced ERK immunoreactivity only in the EGCG-treated specimens, with no suppression in jnk or p-p38 immunoreactivity (Figure 3); densitometry confirmed reduced ERK immunoreactivity at both 2 and 4 weeks (Table 1). Western blot analysis confirmed diminished ERK activity in the EGCG-treated arteries, with a diminished phospho:total ERK ratio in the EGCG-treated arteries (P=.036, Mann-Whitney U test; Figure 4). The diminished ERK activity was specific, as there was no change in phosphorylated p38 activity between control and EGCG-treated arteries (6817 ± 2274 vs. 5111 ± 468 units, P=.57, Mann-Whitney U test).
Figure 3.
Photomicrographs depicting representative images of immunohistochemistry, 100X magnification. N=6 for each group.
Table 1.
Immunohistochemistry Densitometry
| 2 weeks | 4 weeks | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Control | EGCG | p-value | Control | EGCG | p-value | |
|
| ||||||
| ERK | 19372135 | 14761568 | P<.05 | 20348544 | 12436546 | P<0.02 |
| Jnk | 17166680 | 18159448 | NS | 14961339 | 12493368 | NS |
| p38 | 15822508 | 17294588 | NS | 12527016 | 12658844 | NS |
Values measured in arbitrary units.
Figure 4.
Bar graph showing mean of control and EGCG-treated arteries, examined by Western blot for phospho-ERK1: total-ERK1 ratio. Above each bar are representative images of the immunoreactive bands detected by Western blot. Control, n=5; EGCG, n=3.
DISCUSSION
The intimal hyperplastic response to vascular injury is a complex process, intensely investigated over several decades.1–12 Yet, no pharmacologic medications exist that can reliably and predicatively suppress or prevent this pathologic process. In this study, we examined the suppressive effects of four phytochemicals on intimal hyperplasia induced by wire injury to the rat carotid artery. Our data shows that EGCG had a moderate suppressive effect on the development of intimal hyperplasia and supports the observations of other investigators.14–17 However, compared, to allicin, resveratrol or SFN, EGCG is the most powerful repressor of intimal hyperplasia in this experimental model.
A number of mechanisms have been proposed to explain the suppressive effect of EGCG on intimal hyperplasia. These include increases in tissue inhibitor of matrix metalloproteinase (TIMP-2),18 or through the inhibition of protein kinase C,19, 20 platelet derived growth factor induced vascular endothelial growth factor (PDGF-induced VEGF),21 or Rho-signaling with reduction of mitogen-activated protein kinases (MAPK) including ERK, JNK, and p38.22 Intraperitoneal delivery of EGCG appeared to decrease ERK expression (Figures 3 and 4). Interestingly, EGCG-mediated ERK inhibition was detected at 2 weeks after injury, consistent with our previous data that ERK activation is sustained after injury.23 Further studies are required to assess the relationship of EGCG to other important signal transduction pathways, such as those that control matrix metalloproteinase expression, as well as to clarify potential synergistic or antagonistic activity among the different phytochemicals.24
Finally it is important to consider the limitations of carotid injury as a model of intima hyperplasia. The animals used in this study had healthy blood vessel which lacked atherogenic or vasoproliferative pathology. Although the response to intervention among healthy and diseased vessels shares many common cellular and molecular signals, the two are different and independent processes. In addition, this in vivo model carries certain inherent anatomical constraints which include lower percentage of medial wall elastin, condensed subintimal layer, lack of vasa vasorum and decreased ERK expression.13, 25 In addition, intra-peritoneal administration of the studied drugs, necessary to ensure equivalent bioavailability in the animal groups, is not an exact mimic of the oral consumption route, including post-absorption metabolism, of human consumers of green tea. Nevertheless, the finding of EGCG as a powerful suppressor of intimal hyperplasia in vivo suggests that the model is powerful enough to detect these effects, and warrants additional testing in other animal models of disease.
CONCLUSION
Intraperitoneal injection of the phytochemical EGCG has suppressive effects on the development of intimal hyperplasia in the experimental murine carotid artery model. Other phytochemicals showed lesser effects. Further studies are required to analyze the relationship of phytochemicals and the biologic activity induced by vascular wall trauma. The relationship of these phytochemicals with other cytoactive compounds as well as the role of synergistic or antagonistic activity are also subjects for further study.
Clinical Relevance.
Intimal hyperplasia remains an unsolved problem in the treatment of occlusive vascular diseases. Migration of smooth muscle cells and proliferation in the intima are currently thought to be the primary mechanisms leading to intimal hyperplasia. Among the numerous agents investigated to suppress this pathologic entity, green tea has been considered as a potential remedy. We investigated this role for epigallocatechin-3-gallate (EGCG), the major component of green tea, and compared it to other phytochemicals derived from garlic, grapes and broccoli. EGCG proved to be the most potent of these phytochemicals by inhibition of ERK activation. These results suggest that EGCG may be useful to suppress or prevent intimal hyperplasia.
Acknowledgments
This study was supported in part by the Perella Foundation, the Douglas Francis Memorial Fund, the Englewood Hospital Medical Staff Research Fund, and the National Institutes of Health (R01-HL095498 to A.D.) as well as the resources and the use of facilities at the VA Connecticut Healthcare System, West Haven, CT (A.D.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clowes AW, Reidy MA, Clowes MM. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983;49(3):327–33. [PubMed] [Google Scholar]
- 2.Eefting D, Bot I, de Vries MR, Schepers A, van Bockel JH, Van Berkel TJ, et al. Local lentiviral short hairpin RNA silencing of CCR2 inhibits vein graft thickening in hypercholesterolemic apolipoprotein E3-Leiden mice. J Vasc Surg. 2009;50(1):152–60. doi: 10.1016/j.jvs.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 3.Furuyama T, Komori K, Shimokawa H, Matsumoto Y, Uwatoku T, Hirano K, et al. Long-term inhibition of Rho kinase suppresses intimal thickening in autologous vein grafts in rabbits. J Vasc Surg. 2006;43(6):1249–56. doi: 10.1016/j.jvs.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 4.Hamdan AD, Misare B, Contreras M, LoGerfo FW, Quist WC. Evaluation of anastomotic hyperplasia progression using the cyclin specific antibody MIB-1. Am J Surg. 1996;172(2):168–70. doi: 10.1016/S0002-9610(96)00143-2. discussion 70–1. [DOI] [PubMed] [Google Scholar]
- 5.Kallenbach K, Salcher R, Heim A, Karck M, Mignatti P, Haverich A. Inhibition of smooth muscle cell migration and neointima formation in vein grafts by overexpression of matrix metalloproteinase-3. J Vasc Surg. 2009;49(3):750–8. doi: 10.1016/j.jvs.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kodama A, Komori K, Hattori K, Yamanouchi D, Kajikuri J, Itoh T. Sarpogrelate hydrochloride reduced intimal hyperplasia in experimental rabbit vein graft. J Vasc Surg. 2009;49(5):1272–81. doi: 10.1016/j.jvs.2008.11.071. [DOI] [PubMed] [Google Scholar]
- 7.Lyon CA, Koutsouki E, Aguilera CM, Blaschuk OW, George SJ. Inhibition of N-cadherin retards smooth muscle cell migration and intimal thickening via induction of apoptosis. J Vasc Surg. 2010;52(5):1301–9. doi: 10.1016/j.jvs.2010.05.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfeiffer T, Wallich M, Sandmann W, Schrader J, Godecke A. Lipoplex gene transfer of inducible nitric oxide synthase inhibits the reactive intimal hyperplasia after expanded polytetrafluoroethylene bypass grafting. J Vasc Surg. 2006;43(5):1021–7. doi: 10.1016/j.jvs.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Qu Y, Shi X, Zhang H, Sun W, Han S, Yu C, et al. VCAM-1 siRNA reduces neointimal formation after surgical mechanical injury of the rat carotid artery. J Vasc Surg. 2009;50(6):1452–8. doi: 10.1016/j.jvs.2009.08.050. [DOI] [PubMed] [Google Scholar]
- 10.Wu YJ, Sala-Newby GB, Shu KT, Yeh HI, Nakayama KI, Nakayama K, et al. S-phase kinase-associated protein-2 (Skp2) promotes vascular smooth muscle cell proliferation and neointima formation in vivo. J Vasc Surg. 2009;50(5):1135–42. doi: 10.1016/j.jvs.2009.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tipoe GL, Leung TM, Hung MW, Fung ML. Green tea polyphenols as an anti-oxidant and anti-inflammatory agent for cardiovascular protection. Cardiovasc Hematol Disord Drug Targets. 2007;7(2):135–44. doi: 10.2174/187152907780830905. [DOI] [PubMed] [Google Scholar]
- 12.Wallitt EJ, Jevon M, Hornick PI. Therapeutics of vein graft intimal hyperplasia: 100 years on. Ann Thorac Surg. 2007;84(1):317–23. doi: 10.1016/j.athoracsur.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 13.Tulis DA. Rat carotid artery balloon injury model. Methods Mol Med. 2007;139:1–30. doi: 10.1007/978-1-59745-571-8_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han DW, Jung DY, Park JC, Cho HH, Hyon SH, Han DK. Underlying mechanism for suppression of vascular smooth muscle cells by green tea polyphenol EGCG released from biodegradable polymers for stent application. J Biomed Mater Res A. 2010;95(2):424–33. doi: 10.1002/jbm.a.32870. [DOI] [PubMed] [Google Scholar]
- 15.Kim DW, Park YS, Kim YG, Piao H, Kwon JS, Hwang KK, et al. Local delivery of green tea catechins inhibits neointimal formation in the rat carotid artery injury model. Heart Vessels. 2004;19(5):242–7. doi: 10.1007/s00380-004-0768-6. [DOI] [PubMed] [Google Scholar]
- 16.Won SM, Park YH, Kim HJ, Park KM, Lee WJ. Catechins inhibit angiotensin II-induced vascular smooth muscle cell proliferation via mitogen-activated protein kinase pathway. Exp Mol Med. 2006;38(5):525–34. doi: 10.1038/emm.2006.62. [DOI] [PubMed] [Google Scholar]
- 17.Zheng Y, Song HJ, Kim CH, Kim HS, Kim EG, Sachinidis A, et al. Inhibitory effect of epigallocatechin 3-O-gallate on vascular smooth muscle cell hypertrophy induced by angiotensin II. J Cardiovasc Pharmacol. 2004;43(2):200–8. doi: 10.1097/00005344-200402000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Cheng XW, Kuzuya M, Sasaki T, Kanda S, Tamaya-Mori N, Koike T, et al. Green tea catechins inhibit neointimal hyperplasia in a rat carotid arterial injury model by TIMP-2 overexpression. Cardiovasc Res. 2004;62(3):594–602. doi: 10.1016/j.cardiores.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 19.Yang J, Han Y, Sun H, Chen C, He D, Guo J, et al. (−)-Epigallocatechin gallate suppresses proliferation of vascular smooth muscle cells induced by high glucose by inhibition of PKC and ERK1/2 signalings. J Agric Food Chem. 2011;59(21):11483–90. doi: 10.1021/jf2024819. [DOI] [PubMed] [Google Scholar]
- 20.Weber AA, Neuhaus T, Skach RA, Hescheler J, Ahn HY, Schror K, et al. Mechanisms of the inhibitory effects of epigallocatechin-3 gallate on platelet-derived growth factor-BB-induced cell signaling and mitogenesis. Faseb J. 2004;18(1):128–30. doi: 10.1096/fj.03-0007fje. [DOI] [PubMed] [Google Scholar]
- 21.Park JS, Kim MH, Chang HJ, Kim KM, Kim SM, Shin BA, et al. Epigallocatechin-3-gallate inhibits the PDGF-induced VEGF expression in human vascular smooth muscle cells via blocking PDGF receptor and Erk-1/2. Int J Oncol. 2006;29(5):1247–52. [PubMed] [Google Scholar]
- 22.Higashi N, Kohjima M, Fukushima M, Ohta S, Kotoh K, Enjoji M, et al. Epigallocatechin-3-gallate, a green-tea polyphenol, suppresses Rho signaling in TWNT-4 human hepatic stellate cells. J Lab Clin Med. 2005;145(6):316–22. doi: 10.1016/j.lab.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 23.Yamashita A, Hanna AK, Hirata S, Dardik A, Sumpio BE. Antisense basic fibroblast growth factor alters the time course of mitogen-activated protein kinase in arterialized vein graft remodeling. J Vasc Surg. 2003;37:866–873. doi: 10.1067/mva.2003.130. [DOI] [PubMed] [Google Scholar]
- 24.Kim CH, Moon SK. Epigallocatechin-3-gallate causes the p21/WAF1-mediated G(1)-phase arrest of cell cycle and inhibits matrix metalloproteinase-9 expression in TNF-alpha-induced vascular smooth muscle cells. Arch Biochem Biophys. 2005;435(2):264–72. doi: 10.1016/j.abb.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 25.Sims FH. A comparison of structural features of the walls of coronary arteries from 10 different species. Pathology. 1989;21(2):115–24. doi: 10.3109/00313028909059547. [DOI] [PubMed] [Google Scholar]