Abstract
OBJECTIVE
Determine if higher temperature after hypoxia-ischemia is associated with death or IQ < 70 at 6-7 yr among infants treated with intensive care without hypothermia.
DESIGN/METHODS
Control infants (non-cooled, n=106) of the NICHD Neonatal Research Network hypothermia trial had serial esophageal and skin temperatures over 72hrs. Each infant's temperature was ranked to derive an average of the upper and lower quartile, and median of each site. Temperatures were used in logistic regressions to determine adjusted associations with death or IQ < 70 at 6-7yrs. Secondary outcomes were death, IQ < 70, and moderate/severe CP. IQ and motor function were assessed with Wechsler Scales for Children and Gross Motor Function Classification System. Results are odds ratio (OR, per °C increment within the quartile or median) and 95% confidence interval (CI).
RESULTS
Primary outcome was available for 89 infants. At 6-7yrs death or IQ < 70 occurred in 54 infants (37 deaths, 17 survivors with IQ < 70) and moderate/severe CP in 15 infants. Death or IQ < 70 was associated with the upper quartile average of esophageal (OR 7.3, 95% CI 2.0-26.3) and skin temperature (OR 3.5, 95% 1.2-10.4). CP was associated with the upper quartile average of esophageal (OR 12.5, 95% CI 1.02-155) and skin temperature (OR 10.3, 95% 1.3-80.2).
CONCLUSIONS
Among non-cooled infants of a randomized trial, elevated temperatures during the first post-natal days are associated with increase odds of a worse outcome at 6-7yrs.
Keywords: encephalopathy, hypoxia-ischemia, hyperthermia, cerebral palsy
Introduction
Temperature is a critical modulator of the extent of brain injury both during and following perinatal hypoxia-ischemia.1 Relatively small reductions in core temperature are associated with prominent neuroprotection during the perinatal period. These observations triggered investigations which progressed from animal studies to clinical pilot studies to randomized clinical trials and demonstrated efficacy in reducing death or brain injury following perinatal hypoxia-ischemia.2, 3 Therapeutic hypothermia for newborn encephalopathy with an hypoxic-ischemic origin is currently widely used and the treatment continues to be disseminated throughout the neonatal community.4 Investigations of temperature as a neuroprotective treatment also revealed that elevations of core temperature after a hypoxic-ischemic event occur and are associated with an increase in the extent of brain injury.5 We previously analyzed the temperatures of non-cooled control infants enrolled in the NICHD whole body hypothermia trial and found that relatively high core temperatures occurred frequently, and were associated with an increase in the risk of death or neurodevelopmental disability at 18-22 months of age.6 Whether associations between elevated temperature and brain injury extend into childhood remains uncertain. To determine if higher temperature in the neonatal period affects early school age outcome among infants with encephalopathy cared for without hypothermia, we investigated associations between temperatures in the first days after birth and death or an Intelligence Quotient < 70 at 6-7yrs in the same cohort.
Methods
Study infants
This is a secondary observational analysis of non-cooled infants from the National Institute of Child Health and Human Development Neonatal Research Network (NRN) randomized trial of whole body cooling conducted between July 2000 and May 2003.7 The trial was performed after informed consent was obtained. Inclusion criteria for the randomized trial included a gestational age of ≥ 36 weeks, less than 6hrs post-natal age, specific physiologic and/or clinical criteria regarding acidemia, Apgar scores and need for ventilation from birth, and the presence of moderate or severe encephalopathy. The childhood outcomes of hypothermia and non cooled control infants have been published.8
Temperature control
The temperature of non-cooled infants was regulated by the specific guidelines of each NRN center's Neonatal Intensive Care Unit (NICU). Information obtained from the NRN research coordinators indicated that non-cooled infants were initially cared for on radiant warmers with servo control of abdominal skin temperature. The initial servo set point for 75% of the participating hospitals was 36.5°C and in the remaining hospitals the set point temperature was within a range of 36.0 to 36.6°C. Usual care in all NRN center NICUs included monitoring of axilla and skin temperature at frequencies that varied from every 1 to 4hrs. The range of acceptable core temperature varied among NRN center NICUs from 36.0 to 37.5°C and within individual centers the range was as large as 1°C.
All infants had an indwelling temperature probe positioned in the lower third of the esophagus with subsequent radiographic verification and temperatures were measured with a Mon-a-Therm temperature monitoring unit (Mallinckrodt, St Louis, MO). Esophageal and skin temperatures were acquired at 4hr intervals over 72hrs starting prior to 6hrs of age and corresponding to the interval of hypothermia treatment for the experimental group.7 There were a total of 19 pairs of esophageal and skin temperatures for each temperature if all temperatures were captured. Esophageal temperatures were not used in the care or management of non-cooled infants.
Outcomes
All infants underwent follow-up evaluations in the clinics of each NRN center between 6-7yrs of age. Certified examiners were trained to reliability and were unaware of the intervention. The primary outcome was a composite of death or an Intelligence Quotient (IQ) of < 70. IQ was assessed using either the Wechsler Preschool and Primary Scale of Intelligence III for children up to age 7yrs and 3 months and the Wechsler Scale for Children IV for infants beyond 7yrs 3 months or among those who were Spanish speaking. The IQ assessment provided a verbal and performance IQ which yielded a full scale IQ where a mean (± standard deviation) score of 100±15 is normal. Secondary outcomes included components of the primary outcome (death alone and IQ < 70 alone), moderate or severe disability as previously defined by Shankaran et al8 and moderate or severe Cerebral Palsy (CP). CP was defined as a non-progressive brain disorder with abnormal muscle tone in at least one extremity and abnormal control of movement and posture that interfere with age-appropriate activities. The gross motor function classification system (GMFCS) was used to characterize functional ability.9 A severe disability was defined as either an IQ of < 55 a GMFCS of IV or V, or bilateral blindness. A moderate disability was either an IQ of 55-69, a GMFCS of III, deafness with or without amplification or epilepsy requiring anti-convulsant therapy. A mild disability was an IQ 70-84 or a GMFCS of either I or II. The absence of a disability was indicated by infants who had an IQ ≥ 85 without CP, sensory deficit or epilepsy.
Data analysis
Descriptive statistics included mean and standard deviations. Continuous and categorical variables were evaluated with t-tests and chi-squared tests, respectively. The esophageal and skin temperatures of each infant (maximum of 19 values from each site over 72hrs) were rank ordered for each site and divided into quartiles. The averages of the highest and lowest quartile of temperatures were used as an index of a high and low temperature. A midpoint temperature used the median temperature of each infant. Logistic regression was used to determine associations between temperature and outcome at 6-7yrs. Separate regressions were performed using the highest, median and lowest temperature of each infant and their respective outcome for each temperature site, esophageal and skin. Regressions that included death were adjusted for gender, level of encephalopathy and maternal race. Regressions for the outcomes of survivors were adjusted for gender, level of encephalopathy and maternal education obtained at the 6-7yr follow-up visit. Associations between temperature and outcomes were expressed as an odds ratios (OR) and 95% confidence limits (CI).
Results
There were 106 infants randomized to the non-cooled control arm of the NRN whole body cooling trial.7 Infants included in this report are illustrated in figure 1. There were 41 deaths (38·6%) and 4 infants who died did not have temperatures recorded. There were 65 survivors of which 13 were lost to follow-up. Infants included in this report include the 37 deaths with temperatures recorded, and the 52 survivors with IQ results. Thus 89 of the 106 infants were included in this report and represent 84% of the original non-cooled control group. Selected maternal and infant characteristics for the cohort in this study are compared with the 17 infants who were lost to follow-up or did not have temperatures recorded (Table 1). There were no differences except for a lower cord pH among infants of this report. Within the non-cooled control group, 8 mothers had an intra-partum temperature ≥ 38°C and 13 received antibiotics.
Figure 1.

Cohort diagram indicating the number of infants randomized to the non-cooled control group and those that were part of this investigation. Temp = temperature, FU = follow up, IQ = intelligence quotient
Table 1.
| Follow-up at 6-7 years (n = 89) | No Follow-up or Absence or Temperature (n = 17) | |
|---|---|---|
| Maternal | ||
| Racial distribution (%) | ||
| Black | 37 | 41 |
| White | 34 | 12 |
| Hispanic | 25 | 47 |
| Pregnancy complications* (%) | 39 | 29 |
| Intra-partum complications** (%) | 94 | 88 |
| Infant | ||
| Birth weight (grams) | 3343±587 | 3509±899 |
| Gestation (weeks) | 38.7±1.7 | 39.1±1.5 |
| Male (%) | 63 | 59 |
| Out-born (%) | 45 | 29 |
| Delivery room intubation (%) | 92 | 94 |
| Apgar at 10 min ≤ 5 (%) | 79 | 69 |
| Cord pH† | 6.83±0.21 | 6.98±0.24 |
| Cord Base Deficit | -20.5±8.6 | -14.7±8.2 |
| Moderate encephalopathy (%) | 58 | 76 |
| Severe encephalopathy (%) | 42 | 24 |
Pregnancy complications included chronic hypertension, pre-eclampsia, eclampsia, ante-partum hemorrhage, thyroid dysfunction and diabetes
Intra-partum complications included fetal decelerations, cord mishap, uterine rupture, shoulder dystocia, placental problems (abruption, previa, accreta), maternal problems (hemorrhage, trauma, seizures, cardio-respiratory arrest, pyrexia) and maternal antibiotics for suspected or confirmed infection
p < .05
Deaths occurred in 30 infants during the neonatal hospitalization. An additional 8 infants died following discharge from the hospital and prior to the 18-22 month follow-up visit. Three infants died between the 18-22 month and the 6-7yr follow-up visit. Follow-up was performed at an average age of 6.7yrs. Of the 52 survivors with follow-up, 35 infants had an IQ ≥ 70 and 17 Infants had an IQ < 70. The median IQ for survivors was 81 with an inter-quartile range of 48 and 91, and minimum and maximum values of 39 and 116, respectively; 11 infants were assigned a score of 39 due to severe developmental delay or cognitive impairment. There were 15 infants with CP including 9 spastic quadriplegic, 2 dystonic, 1 choreo-athetotic, 1 ataxic and 2 undesignated.
There were 1598 esophageal temperatures from 89 infants during the intervention with a distribution of 7.8% < 36.5°C, 20.6% between 36.5 and < 37°C, 42.2% between 37 and < 37.5°C, 20.0% between 37.5 and < 38°C and 9.4% ≥ 38°C. The number of infants with a temperature ≥ 38°C (Figure 2) was 25, 4 and 0 for the average of the highest quartile, median and average of the lowest quartile, respectively. The distribution of skin temperature (data not shown) is similar to that of the esophageal temperature but with a smaller number of infants with temperatures ≥ 38°C in the highest quartile and median temperature. Infants with at least one esophageal temperature ≥ 38°C (n = 44) differed from infants with all esophageal temperatures < 38°C (n = 45) in birth weight (3558±556 vs 3133±545 grams for infants with temperatures ≥ 38°C versus < 38°C, respectively, p=.0005). However, there were no differences in gestational age, gender, delivery room intubation, Apgar at 10 minutes, cord pH and base deficit, and level of encephalopathy. Infants born to mothers with pyrexia had a baseline temperature of 37.4±.7°C (n=8) compared to 37.0±1.1°C for infants whose mothers did not have pyrexia (n=77, 4 infants with missing data). Esophageal temperatures > 38°C occurred in 3 of 8 compared to 34 of 80 during the 72 intervention interval among infants whose mothers did and did not have pyrexia. Isolated esophageal temperatures ≥ 38°C was recorded in 16 infants and occurred in the first 24hrs in 10 infants. Infants with multiple continuous esophageal temperatures ≥ 38°C (n=23) had a median onset of 8hrs from baseline (interquartile range 4, 28 hours) while infants whose multiple elevated temperatures were not continuous (n=5) occurred throughout the 72hr intervention.
Figure 2.
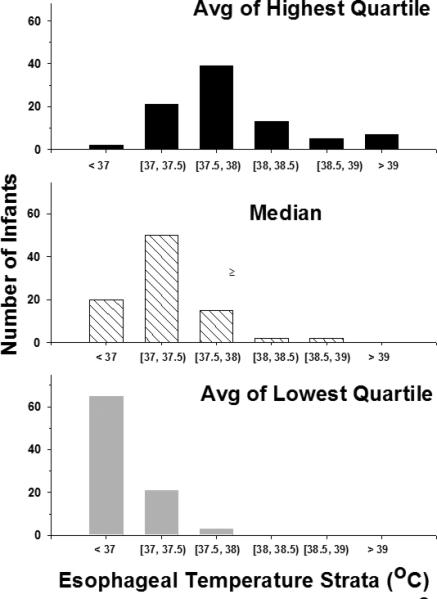
This plot displays the distribution of the non-cooled control infants among 0·5°C strata of esophageal temperature along the horizontal axis and the number of infants along the vertical axis. Interval notation is used along the horizontal axis where brackets are used to show inclusion and parentheses are used to indicate exclusion. The top, middle and lower panels display the results for the average of the highest quartile, the median and the average of the lowest quartile of esophageal temperature, respectively.
Associations between temperature and outcomes are listed in Table 2. The average of the highest quartile for esophageal and skin temperatures were associated with a 7.3 and 3.5 fold increase, respectively in the odds of death or an IQ < 70 per degree centigrade increase in temperature. The average of the highest quartile for esophageal temperature, the median esophageal temperature and the highest quartile of skin temperature were all associated with increased odds of death alone. There were no associations between temperature and an IQ < 70 alone. The average of the highest quartile for esophageal and skin temperatures were associated with a 12.5 and 10.3 fold increase, respectively in the odds of moderate or severe CP relative to the absence or mild CP. Similarly, the averages of the highest quartile for esophageal and skin temperature were associated with death or CP (OR, 95% CI, 6.76, 1.95-23.4 and 3.81, 1.3-11.4, respectively for esophageal and skin sites). In unadjusted analyses the highest quartiles for esophageal and skin temperatures were associated with an increase in the odds of moderate or severe disability relative to the absence or mild disability. These associations did not persist after adjustment for covariates. There were no associations between the lowest quartile of esophageal or skin temperature and 6-7yr outcome.
Table 2.
Associations between temperature and outcome at 6-7 years*
| Death or IQ < 70 N=89‡ | Death N=89‡ | IQ < 70 N=45¶ | Cerebral Palsy (moderate or severe)† N=45¶ | Disability (moderate or severe)† N=43ε | |
|---|---|---|---|---|---|
| Esophageal Temperature | |||||
| Highest quartile | 7.3 (2.03-26.3) | 4.8 (1.65-13.9) | 9.11 (.74-112) | 12.5 (1.02-155) | 10.3 (.78-136) |
| Median | 3.26 (.87-12.2) | 4.35 (1.15-16.5) | .74 (.07-7.84) | .65 (.06-7.20) | .58 (.06-5.87) |
| Lowest quartile | 1.06 (.44-2.58) | 1.12 (.48-2.63) | .67 (.16-2.81) | .62 (.15-2.59) | .67 (.17-2.74) |
| Skin Temperature | |||||
| Highest quartile | 3.47 (1.16-10.4) | 2.71 (1.02-7.16) | 5.36 (.82-34.9) | 10.3 (1.33-80.2) | 7.29 (.97-54.9) |
| Median | 1.28 (.48-3.41) | 1.25 (.48-3.27) | 1.72 (.26-11.2) | 2.43 (.31-18.9) | 1.28 (.23-7.08) |
| Lowest quartile | 1.08 (.64-1.83) | 1.0 (.60-1.64) | 1.31 (.43-3.98) | 1.73 (.44-6.89) | 1.09 (.42-2.79) |
All results are expressed as an odds ratio per degree centigrade change in temperature and the 95% confidence interval after adjustment for gender, level of encephalopathy, and maternal race (regressions including death) or maternal education (regressions among survivors).
Associations of CP and Disability were for infants with moderate or severe extent relative to none or mild in extent
Sample size was 89 for all except the highest quartile of esophageal and skin temperature where the n was 87
Sample size was 45 for all except the highest quartile of esophageal temperature where the n was 44
Sample size was 43 for all except the highest quartile of esophageal temperature where the n was 42
Discussion
The non-cooled control infants of the NRN Hypothermia trial represent a unique cohort of patients to examine the effects of temperature in the early neonatal period on childhood outcome. The results of this investigation extend prior associations between elevated temperatures and adverse outcome reported at 18-22 months of age.6, 10 Elevated esophageal and skin temperatures occurring over the first three days following newborn encephalopathy secondary to hypoxia-ischemia was associated with increased odds of death or an IQ < 70 at 6-7yrs. The results for this composite outcome were largely driven by associations between increasing temperature and death, but the odds ratio for an IQ < 70 alone were large and showed similar trends as death. These associations can not distinguish whether elevated temperature is a marker of brain injury, is in the causal pathway leading to brain injury, or represents a combination of both possibilities.
The frequency of elevated temperatures at delivery and over the first days following birth among infants with encephalopathy is unclear but was unexpectedly high among infants enrolled in three early trials of hypothermia. A core temperature greater than 38°C occurred at least once in 39%, 31% and 9% of non-cooled control infants in the NICHD, the CoolCap and the TOBY trials, respectively.7, 10, 11 The etiology for the elevated temperature remains unclear. Infants in the current report (NICHD trial) were cared for on radiant warmers with servo control of skin temperature and adjustment in set point temperature based on axilla temperatures. Inappropriate use or monitoring of radiant warmers may result in elevated temperatures given the heat gain associated with these devices and the limited ability of newborns to dissipate heat.12 Data is not available to assess proper use of radiant warmers. Proven bloodstream infection is a cause of elevated temperatures secondary to endogenous and exogenous pyrogens but occurred in only 1 infant of this cohort during the 72hrs following study initiation.7 A greater birth weight among infants with at least one esophageal temperature ≥ 38°C parallels observations from the CoolCap trial. As suggested by Wyatt et al,10 a greater birth weight may impede heat dissipation and predispose to elevated temperatures. Seizures and associated muscle activity can elevate core temperature and clinical seizures were frequent among infants in this trial.7 However, clinical seizures were not associated with death or disability at 18-22 months.13 Maternal fever has been associated with neonatal seizures14 and neonatal encephalopathy 15, 16 but the frequency of elevated intra-partum maternal temperature was 9% in the current report, far lower than the primary outcome. Among adult patients brain temperature can be increased after injury in neurosurgical patients,17 and there are consistent associations between the occurrence of elevated core temperature and either ischemic stroke,18 traumatic brain injury19 or intracranial hemorrhage.20 The occurrence of elevated temperatures among newborns with encephalopathy parallels the presence of fever in adults with neurological injury.
The association between elevated temperature during the immediate neonatal period and an increase in the odds of death or an IQ < 70 at 6-7yrs supports the importance of temperature as either a marker of brain injury, an effector of the extent of injury, or both of these considerations. Although this association is largely driven by the occurrence of death, the impact of temperature among survivors is indicated by the association with the secondary outcome of CP. Disabling CP among infants with a birth weight > 2500 grams has been associated with maternal fever (temperature > 38°C) in a population based case controlled study examining maternal infection during admission for delivery.21 One or more indicators of maternal infection were present in 37% of infants with spastic quadriplegia in contrast to only 2.9% in control infants. The odds of an association between spastic CP and either fever > 38°C in labor or a clinical diagnosis of chorioamnionitis was identical (9.3, 95% confidence intervals of 2.7-31). In the setting of chorioamnionitis, it is difficult to separate the effects of fever from an underlying maternal infection. As noted in a comprehensive meta-analysis of chorioamnionitis as a risk factor for CP, studies that evaluated fever alone were heterogeneous reflecting the diverse etiologies of fever during labor.22 An additional case control study nested within a large birth cohort from a health care organization (231,582 singleton births of ≥ 36 weeks gestation) also support clinical chorioamnionitis as an independent risk factor for CP.23 The current investigation adds new information on the association of temperature and outcome of encephalopathic infants who had a low frequency of exposure to clinical chorioamnionitis. Although the numbers are small and the confidence intervals are wide, elevations in either esophageal or skin temperature are associated with increasing risk of CP at 6-7yrs. As in other reports of associations between potentially asphyxiating conditions and CP, the majority of the CP type was spastic quadriplegia.24
The data from this investigation suggest that temperature may act independently of an underlying systemic inflammatory process such as chorioamnionitis. There is consistent data in both newborn and adult rodents to demonstrate that hyperthermia occurring either during or following hypoxia-ischemia exacerbates the extent of brain injury compared to similar insults under normothermic conditions.25-27 Furthermore, induced elevated temperature even at 24hrs post-forebrain ischemia in adult rats resulted in a dramatic increase in ischemic neurons of the hippocampus.28 In contrast, preventing hyperthermia decreased histological brain injury following hypoxic-ischemic seizures in 10 day rat pups.29 Similarly brain ischemia in adult rats was followed by temperature elevations 21-63hrs following recirculation.30 Anti-pyretic medications or active cooling minimized temperature elevations and was associated with less brain injury. We could not demonstrate an effect of time after birth on associations between temperature and outcome in our study. The results of the current investigation however do not establish a causal link between elevated temperature and CP. It is possible that injury to specific regions of the brain (temperature regulatory centers of the hypothalamus) mediate elevated systemic temperatures as noted for ischemia and trauma31, 32 and result in a different hypothalamic set point.33 Thus, elevated temperatures may only represent a marker of injury. Furthermore, the association between temperature and 6-7yr outcome may in part reflect the beneficial effects of lower temperature rather than detrimental effects of higher temperature.
There are limitations to this study. The non-cooled control arm of the hypothermia trial is a small group which limits power to evaluate exploratory hypotheses. Logistic regressions that included death could not be adjusted for measures of maternal socio-economic status (education, income) due to missing data. Mortality was high and 16% of the group did not have available outcome or temperature data. However, all follow-up evaluations were performed at a consistent age, by personnel who were trained to reliability and used standardized tests.
This investigation confirms associations between elevated temperature and brain injury including CP. The results also aid the design of clinical trials of neuroprotective strategies. Active surveillance for elevated temperatures in non-cooled infants and an algorithm to minimize elevated temperatures has been incorporated in a trial of hypothermia initiated after 6hrs of age (ClinicalTrials.gov-NCT 00614744). Similarly surveillance and avoidance of elevated temperatures in the days following therapeutic hypothermia is part of an ongoing trial to test the optimal duration and temperature of a hypothermia regimen (ClinicalTrials.gov -NCT 01192776). Randomized trials of pharmacologic therapies for newborn encephalopathy are likely to be combined with therapeutic hypothermia. Comparable temperature profiles of study groups during and following hypothermia will be critical for proper evaluation of drug treatments.
Acknowledgements
The National Institutes of Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) provided grant support for the Neonatal Research Network's Whole-Body Hypothermia Trial and its 6-7 Year School-age Follow-up.
Data collected at participating sites of the NICHD Neonatal Research Network (NRN) were transmitted to RTI International, the data coordinating center (DCC) for the network, which stored, managed and analyzed the data for this study. On behalf of the NRN, Dr. Abhik Das (DCC Principal Investigator) and Mr. Scott A. McDonald (DCC Statistician) had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. The following investigators, in addition to those listed as authors, participated in this study:
NRN Steering Committee Chairs: Alan H. Jobe, MD PhD, University of Cincinnati (2003-2006); Michael S. Caplan, MD, University of Chicago, Pritzker School of Medicine (2006-2011).
Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island (U10 HD27904) – William Oh, MD; Theresa M. Leach, MEd CAES; Angelita M. Hensman, RN BSN; Lucy Noel; Victoria E. Watson, MS CAS.
Case Western Reserve University, Rainbow Babies & Children's Hospital (U10 HD21364, M01 RR80) – Michele C. Walsh, MD MS; Avroy A. Fanaroff, MD; Deanne E. Wilson-Costello, MD; Nancy Bass, MD; Harriet G. Friedman MA; Nancy S. Newman, BA RN; Bonnie S. Siner, RN.
Cincinnati Children's Hospital Medical Center and University of Cincinnati Hospital (U10 HD27853, M01 RR8084) – Kurt Schibler, MD; Edward F. Donovan, MD; Kimberly Yolton, PhD; Kate Bridges, MD; Jean J. Steichen, MD; Barbara Alexander, RN; Cathy Grisby, BSN CCRC; Holly L. Mincey, RN BSN; Jody Hessling, RN; Teresa L. Gratton, PA.
Duke University School of Medicine, University Hospital, Alamance Regional Medical Center, and Durham Regional Hospital (U10 HD40492, M01 RR30) – Ronald N. Goldberg, MD; C. Michael Cotten, MD MHS; Ricki F. Goldstein, MD; Kathryn E. Gustafson, PhD; Kathy J. Auten, MSHS; Katherine A. Foy, RN; Kimberley A. Fisher, PhD FNP-BC IBCLC; Sandy Grimes, RN BSN; Melody B. Lohmeyer, RN MSN.
Emory University, Grady Memorial Hospital and Emory University Hospital Midtown (U10 HD27851, M01 RR39) – Barbara J. Stoll, MD; David P. Carlton, MD; Ira Adams-Chapman, MD; Lucky Jain, MD; Ann M. Blackwelder, RNC BS MS; Ellen C. Hale, RN BS CCRC; Sobha Fritz, PhD.
Eunice Kennedy Shriver National Institute of Child Health and Human Development – Linda L. Wright, MD; Elizabeth M. McClure, MEd; Stephanie Wilson Archer, MA.
Indiana University, University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services (U10 HD27856, M01 RR750) – Brenda B. Poindexter, MD MS; James A. Lemons, MD; Anna M. Dusick, MD FAAP; Diana D. Appel, RN BSN; Jessica Bissey, PsyD HSPP; Dianne E. Herron, RN; Lucy C. Miller, RN BSN CCRC; Leslie Richard, RN; Leslie Dawn Wilson, BSN CCRC.
RTI International (U10 HD36790) – W. Kenneth Poole, PhD; Jeanette O'Donnell Auman, BS; Margaret Cunningham, BS; Jane Hammond, PhD; Betty K. Hastings; Carolyn M. Petrie Huitema, MS; Jamie E. Newman, PhD MPH; Carolyn M. Petrie Huitema, MS; Scott E. Schaefer, MS; Kristin M. Zaterka-Baxter, RN BSN.
Stanford University and Lucile Packard Children's Hospital (U10 HD27880, M01 RR70) – Krisa P. Van Meurs, MD; Susan R. Hintz, MD MS Epi; David K. Stevenson, MD; M. Bethany Ball, BS CCRC; Maria Elena DeAnda, PhD.
University of Alabama at Birmingham Health System and Children's Hospital of Alabama (U10 HD34216, M01 RR32) – Waldemar A. Carlo, MD; Namasivayam Ambalavanan, MD; Myriam Peralta-Carcelen, MD MPH; Kathleen G. Nelson, MD; Monica V. Collins, RN BSN MaEd; Shirley S. Cosby, RN BSN; Vivien A. Phillips, RN BSN; Laurie Lou Smith, EdS NCSP.
University of California-San Diego Medical Center and Sharp Mary Birch Hospital for Women (U10 HD40461) - Yvonne E. Vaucher, MD MPH; Martha G. Fuller, RN MSN; Radmila West PhD; Neil N. Finer, MD; David Kaegi, MD; Maynard R. Rasmussen, MD; Kathy Arnell, RNC; Chris Henderson, RCP CRTT; Wade Rich, BSHS RRT.
University of Miami Holtz Children's Hospital (U10 HD21397, M01 RR16587) – Shahnaz Duara, MD; Charles R. Bauer, MD; Sylvia Hiriart-Fajardo, MD; Mary Allison, RN; Maria Calejo, MS;Ruth Everett-Thomas, RN MSN; Silvia M. Frade Eguaras, MA; Susan Gauthier, BA.
University of New Mexico Health Sciences Center (U10 HD27881)- Lu-Ann Papile, MD; Conra Backstrom Lacy, RN.
University of Rochester Medical Center, Golisano Children's Hospital (U10 HD40521, M01 RR44) – Dale L. Phelps, MD; Gary J. Myers, MD; Ronnie Guillet, MD PhD; Diane Hust, MS RN CS; Linda J. Reubens, RN CCRC.
University of Texas Southwestern Medical Center at Dallas, Parkland Health & Hospital System, and Children's Medical Center Dallas (U10 HD40689, M01 RR633) – Pablo J. Sánchez, MD; R. Sue Broyles, MD; Abbot R. Laptook, MD; Charles R. Rosenfeld, MD; Walid A. Salhab, MD; Roy J. Heyne, MD; Cathy Boatman, MS CIMI; Cristin Dooley, PhD LSSP; Gaynelle Hensley, RN; Jackie F. Hickman, RN; Melissa H. Leps, RN; Susie Madison, RN; Nancy A. Miller, RN; Janet S. Morgan, RN; Lizette E. Torres, RN; Alicia Guzman.
University of Texas Health Science Center at Houston Medical School, Children's Memorial Hermann Hospital, and Lyndon Baines Johnson General Hospital/Harris County Hospital District (U10 HD21373, M01 RR2588) – Jon E. Tyson, MD MPH; Kathleen A. Kennedy, MD MPH; Esther G. Akpa, RN BSN; Patty A. Cluff, RN; Claudia I. Franco, RN BSN; Anna E. Lis, RN, BSN; Georgia E. McDavid, RN; Patti L. Pierce Tate, RCP; Nora I. Alaniz, BS; Pamela J. Bradt, MD MPH; Magda Cedillo; Susan Dieterich, PhD; Patricia W. Evans, MD; Charles Green, PhD; Margarita Jiminez, MD; Terri Major-Kincade, MD MPH; Brenda H. Morris, MD; M. Layne Poundstone, RN BSN; Stacey Reddoch, BA; Saba Siddiki, MD; Maegan C. Simmons, RN; Laura L. Whitely, MD; Sharon L. Wright, MT; Lourdes M. Valdés PhD.
Wayne State University, Hutzel Women's Hospital, and Children's Hospital of Michigan (U10 HD21385) – Athina Pappas, MD; Yvette R. Johnson, MD MPH; Laura A. Goldston, MA; Rebecca Bara, RN BSN; Geraldine Muran, RN BSN; Deborah Kennedy, RN BSN; Patrick J. Pruitt, BS.
Yale University, Yale-New Haven Children's Hospital (U10 HD27871, M01 RR125, UL1 RR24139) – Richard A. Ehrenkranz, MD; Patricia Gettner, RN; Monica Konstantino, RN BSN; JoAnn Poulsen, RN; Elaine Romano, MSN; Joanne Williams, RN BSN; Susan DeLancy, MA CAS.
Footnotes
Disclosures
Dr. Cotten reports having served on the data and safety monitoring board for the Inhibitex phase 3 study of Vernonate for the prevention of infections in preterm infants. Dr. Donovan reports having received support from the Environmental Protection Agency (Lanphear) and the Gerber Foundation. Dr. Carlo reports having served on the advisory board of Pediatrix Medical Group and ParadigmHealth and holding stock options at the Pediatrix Medical Group. Dr. Stevenson reports having received research support from Pfizer.
References
- 1.Drury PP, Bennet L, Gunn AJ. Mechanisms of hypothermic neuroprotection. Semin Fetal Neonatal Med. 2010;15:287–292. doi: 10.1016/j.siny.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Higgins RD, Raju T, Edwards AD, et al. Hypothermia and other treatment options for neonatal encephalopathy: an executive summary of the Eunice Kennedy Shriver NICHD workshop. J Pediatr. 2011;159:851–858. e851. doi: 10.1016/j.jpeds.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah PS. Hypothermia: a systematic review and meta-analysis of clinical trials. Semin Fetal Neonatal Med. 2010;15:238–246. doi: 10.1016/j.siny.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Azzopardi D, Strohm B, Edwards AD, et al. Treatment of asphyxiated newborns with moderate hypothermia in routine clinical practice: how cooling is managed in the UK outside a clinical trial. Arch Dis Child Fetal Neonatal Ed. 2009;94:F260–264. doi: 10.1136/adc.2008.146977. [DOI] [PubMed] [Google Scholar]
- 5.Dietrich WD, Bramlett HM. Hyperthermia and central nervous system injury. Prog Brain Res. 2007;162:201–217. doi: 10.1016/S0079-6123(06)62011-6. [DOI] [PubMed] [Google Scholar]
- 6.Laptook A, Tyson J, Shankaran S, et al. Elevated temperature after hypoxic-ischemic encephalopathy: risk factor for adverse outcomes. Pediatrics. 2008;122:491–499. doi: 10.1542/peds.2007-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 8.Shankaran S, Pappas A, McDonald SA, et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med. 2012;366:2085–2092. doi: 10.1056/NEJMoa1112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palisano RJ, Cameron D, Rosenbaum PL, et al. Stability of the gross motor function classification system. Dev Med Child Neurol. 2006;48:424–428. doi: 10.1017/S0012162206000934. [DOI] [PubMed] [Google Scholar]
- 10.Wyatt JS, Gluckman PD, Liu PY, et al. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics. 2007;119:912–921. doi: 10.1542/peds.2006-2839. [DOI] [PubMed] [Google Scholar]
- 11.Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349–1358. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 12.Sinclair JC. Management of the Thermal Environment. In: Sinclair JC, Bracken MB, editors. Effective Care of the Newborn Infant. Oxford University Press; New York: 1992. pp. 40–58. [Google Scholar]
- 13.Kwon JM, Guillet R, Shankaran S, et al. Clinical seizures in neonatal hypoxic-ischemic encephalopathy have no independent impact on neurodevelopmental outcome: secondary analyses of data from the neonatal research network hypothermia trial. Journal of child neurology. 2011;26:322–328. doi: 10.1177/0883073810380915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lieberman E, Eichenwald E, Mathur G, et al. Intrapartum fever and unexplained seizures in term infants. Pediatrics. 2000;106:983–988. doi: 10.1542/peds.106.5.983. [DOI] [PubMed] [Google Scholar]
- 15.Impey L, Greenwood C, MacQuillan K, et al. Fever in labour and neonatal encephalopathy: a prospective cohort study. BJOG. 2001;108:594–597. doi: 10.1111/j.1471-0528.2001.00145.x. [DOI] [PubMed] [Google Scholar]
- 16.Blume HK, Li CI, Loch CM, Koepsell TD. Intrapartum fever and chorioamnionitis as risks for encephalopathy in term newborns: a case-control study. Dev Med Child Neurol. 2008;50:19–24. doi: 10.1111/j.1469-8749.2007.02007.x. [DOI] [PubMed] [Google Scholar]
- 17.Schwab S, Spranger M, Aschoff A, et al. Brain temperature monitoring and modulation in patients with severe MCA infarction. Neurology. 1997;48:762–767. doi: 10.1212/wnl.48.3.762. [DOI] [PubMed] [Google Scholar]
- 18.Reith J, Jorgensen HS, Pedersen PM, et al. Body temperature in acute stroke: relation to stroke severity, infarct size, mortality, and outcome. Lancet. 1996;347:422–425. doi: 10.1016/s0140-6736(96)90008-2. [DOI] [PubMed] [Google Scholar]
- 19.Cairns CJ, Andrews PJ. Management of hyperthermia in traumatic brain injury. Curr Opin Crit Care. 2002;8:106–110. doi: 10.1097/00075198-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Oliveira-Filho J, Ezzeddine MA, Segal AZ, et al. Fever in subarachnoid hemorrhage: relationship to vasospasm and outcome. Neurology. 2001;56:1299–1304. doi: 10.1212/wnl.56.10.1299. [DOI] [PubMed] [Google Scholar]
- 21.Grether JK, Nelson KB. Maternal infection and cerebral palsy in infants of normal birth weight. JAMA. 1997;278:207–211. [PubMed] [Google Scholar]
- 22.Wu YW, Colford JM., Jr. Chorioamnionitis as a risk factor for cerebral palsy: A meta-analysis. JAMA. 2000;284:1417–1424. doi: 10.1001/jama.284.11.1417. [DOI] [PubMed] [Google Scholar]
- 23.Wu YW, Escobar GJ, Grether JK, et al. Chorioamnionitis and cerebral palsy in term and near-term infants. JAMA. 2003;290:2677–2684. doi: 10.1001/jama.290.20.2677. [DOI] [PubMed] [Google Scholar]
- 24.Nelson KB, Grether JK. Potentially asphyxiating conditions and spastic cerebral palsy in infants of normal birth weight. Am J Obstet Gynecol. 1998;179:507–513. doi: 10.1016/s0002-9378(98)70387-4. [DOI] [PubMed] [Google Scholar]
- 25.Tomimatsu T, Fukuda H, Kanagawa T, et al. Effects of hyperthermia on hypoxic-ischemic brain damage in the immature rat: its influence on caspase-3-like protease. Am J Obstet Gynecol. 2003;188:768–773. doi: 10.1067/mob.2003.163. [DOI] [PubMed] [Google Scholar]
- 26.Fukuda H, Tomimatsu T, Kanagawa T, et al. Postischemic hyperthermia induced caspase-3 activation in the newborn rat brain after hypoxia-ischemia and exacerbated the brain damage. Biol Neonate. 2003;84:164–171. doi: 10.1159/000071952. [DOI] [PubMed] [Google Scholar]
- 27.Minamisawa H, Smith ML, Siesjo BK. The effect of mild hyperthermia and hypothermia on brain damage following 5, 10, and 15 minutes of forebrain ischemia. Ann Neurol. 1990;28:26–33. doi: 10.1002/ana.410280107. [DOI] [PubMed] [Google Scholar]
- 28.Baena RC, Busto R, Dietrich WD, et al. Hyperthermia delayed by 24 hours aggravates neuronal damage in rat hippocampus following global ischemia. Neurology. 1997;48:768–773. doi: 10.1212/wnl.48.3.768. [DOI] [PubMed] [Google Scholar]
- 29.Yager JY, Armstrong EA, Jaharus C, et al. Preventing hyperthermia decreases brain damage following neonatal hypoxic-ischemic seizures. Brain Res. 2004;1011:48–57. doi: 10.1016/j.brainres.2004.02.070. [DOI] [PubMed] [Google Scholar]
- 30.Coimbra C, Boris-Moller F, Drake M, Wieloch T. Diminished neuronal damage in the rat brain by late treatment with the antipyretic drug dipyrone or cooling following cerebral ischemia. Acta Neuropathol. 1996;92:447–453. doi: 10.1007/s004010050545. [DOI] [PubMed] [Google Scholar]
- 31.Abraham H, Somogyvari-Vigh A, Maderdrut JL, et al. Rapidly activated microglial cells in the preoptic area may play a role in the generation of hyperthermia following occlusion of the middle cerebral artery in the rat. Exp Brain Res. 2003;153:84–91. doi: 10.1007/s00221-003-1572-8. [DOI] [PubMed] [Google Scholar]
- 32.Thompson HJ, Hoover RC, Tkacs NC, et al. Development of posttraumatic hypothermia after traumatic brain injury in rats is associated with increased periventricular inflammation. J Cereb Blood Flow Metab. 2005;25:163–176. doi: 10.1038/sj.jcbfm.9600008. [DOI] [PubMed] [Google Scholar]
- 33.Childs C. Human brain temperature: regulation, measurement and relationship with cerebral trauma: part 1. Br J Neurosurg. 2008;22:486–496. doi: 10.1080/02688690802245541. [DOI] [PubMed] [Google Scholar]


