Abstract
Patients with inherited bone marrow failure syndromes (IBMFS) have “stress erythropoiesis”, with anaemia, macrocytosis, increased fetal haemoglobin (Hb F) and high erythropoietin levels. In haemoglobinopathies, Hb F levels are regulated by 3 quantitative trait loci, HBS1L-MYB, BCL11A and Xmn1-HBG2. In our study of 97 patients with an IBMFS, increased Hb F was associated with young age, male gender, anaemia, high erythropoietin levels, and alternative alleles in Xmn1-HBG2 [adjusted p = 0.04 for the total group, driven by Fanconi anaemia (p=0.02) and dyskeratosis congenita (p=0.09)]. Thus Hb F is regulated in IBMFS by Xmn1-HBG2 as it is in the haemoglobinopathies.
Keywords: Fetal haemoglobin, inherited bone marrow failure syndromes, quantitative trait loci, Fanconi anaemia, dyskeratosis congenita
Introduction
Patients with inherited bone marrow failure syndromes (IBMFS) (Shimamura & Alter, 2010) frequently have manifestations of what has been called “stress haematopoiesis” (Alter, 1979) whose features include macrocytosis (increased mean cell volume [MCV]), increased fetal haemoglobin (Hb F) and erythropoietin (Epo) levels higher than predicted by the degree of anaemia (red blood cell count [RBC]) (Young & Alter, 1994). Common variations in HbF levels in healthy adults and in patients with sickle cell anaemia and thalassaemia are regulated by 3 quantitative trait loci (QTL), located at HBS1L-MYB on chromosome 6q, BCL11A on chromosome 2p and Xmn1-HBG2 representing the HBB cluster on chromosome 11p (Thein et al, 2009).
Whereas these 3 QTLs have been shown to be very important determinants of Hb F levels in patients with haemoglobinopathies and in healthy adults (Thein et al, 2009), the potential role of these QTLs in modulating elevated Hb F levels in patients with an IBMFS has not been previously reported. Here, we investigated parameters that might be associated with elevated Hb F in patients with the 4 major IBMFS: Diamond-Blackfan anaemia (DBA), dyskeratosis congenita (DC), Fanconi anaemia (FA), and Shwachman-Diamond syndrome (SDS). These parameters included gender, age, Hb F QTLs and potential components of stress erythropoiesis, such as anaemia, macrocytosis and erythropoietin level.
Methods
The analyses reported here were performed on material from 97 untransplanted patients enrolled in the National Cancer Institute (NCI) Inherited Bone Marrow Failure Syndromes study NCI 02-C-0052 [NCT00027274] (http://www.marrowfailure.cancer.gov). The study was approved by the NCI institutional review board, and written informed consent forms were signed. All patients were seen at the National Institutes of Health Clinical Center. Routine complete blood counts were obtained, and the percent Hb F was measured by high performance liquid chromatography; elevated Hb F% was defined as above 1%. Absolute Hb F (g/l) was calculated by multiplication of Hb F% and total Hb in g/l in order to include data from transfused patients, and log-transformed to approximate a normal distribution. Anaemia and macrocytosis were defined according to standards for age (Dallman & Siimes, 1979). A patient was scored as “anaemic” if the Hb was below the normal range for age, or if he/she was receiving transfusions or medical treatment for anaemia (corticosteroids for DBA, androgens for DC and FA). Epo levels were log-transformed due to the wide range (8 – 1800 mu/ml); above 25 mu/ml was considered elevated.
Genomic DNA was extracted from leucocytes by standard methods. The candidate QTLs were then evaluated by TaqMan genotyping of representative single nucleotide polymorphisms (SNPs): five for HBS1L-MYB, two for BCL11A, and one for Xmn1-HBG2 (Creary et al, 2009) (Table I). Standard linear models and R-Square values were used to summarize scatter plots of Hb F in g/l versus age by gender and syndrome, as well as scatter plots of Hb F versus other features by syndrome. Because Hb F values were non-negative with a very wide range, we used a generalized linear model (GLM) approach appropriate for non-negative data with a constant coefficient of variation (Mccullagh & Nelder, 1989). Specifically, in the GLM, the relationships between mean Hb F levels and various explanatory factors including SNP genotypes were assumed to be multiplicative (i.e. a log link function in the GLM), and also the relationship between the variance of the observations and the mean was equal to a constant times the square of the mean. Consequently, the estimated standard errors around the mean values increased in direct proportion to the mean values, i.e. there was more scatter expected for groups with a high predicted mean Hb F level and less for groups with a low predicted mean Hb F level. Because this was a descriptive study, no p-value adjustments were made for multiple testing. Analyses were performed with Excel 2010 (Microsoft, Redmond, WA, USA), Stata 12.1 (StataCorp, College Station, TX, USA), and Matlab 2008b software (The MathWorks, Natick, MA, USA).
Table I.
Study Population and Hb F Regulation
| Total | DBA | DC | FA | SDS | Global p | |||
|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||
| Number, N | 97 | 31 | 35 | 25 | 6 | |||
| Age at Study, years | 17 (2–58) | 22 (3–58) | 16 (2–48) | 21 (5–56) | 13 (8–42) | 0.44 | ||
| Male:female | 56:41 | 19:12 | 28:7 | 6:19 | 3:3 | <0.001 | ||
| Anaemic, n (%) | 55 (57%) | 19 (62%) | 23 (66%) | 12 (48%) | 1 (17%) | 0.12 | ||
| Macrocytic, n (%) | 59 (61%) | 15 (48%) | 25 (71%) | 18 (72%) | 1 (17%) | 0.02 | ||
| Epo >25 mu/ml, n (%) | 70 | 18 (69%) | 28 (80%) | 19 (79%) | 5 (83%) | 0.77 | ||
| On transfusions, n (%) | 13 (13%) | 7 (23%) | 4 (11%) | 2 (8%) | 0 | 1.0 | ||
| On drug treatment, n (%) | 18 (19%) | 7 (23%) | 6 (17%) | 5 (20%)1 | 0 | 1.0 | ||
| Hb F >1%, n (%) | 68 (70%) | 15 (48%) | 29 (83%) | 19 (76%) | 5 (83%) | 0.02 | ||
| QTL Alternative Alleles: Frequency of Increased Hb F and Alternative Alleles (Heterozygous plus Homozygous)2 | ||||||||
| Gene | SNP | Allele Change (+>-frequency)2 | Total | DBA | DC | FA | SDS | p for Hb F2 |
| HBS1L-MYB | rs9376090 | T>C (0.39) | 43/87 | 15/26 | 20/32 | 6/24 | 2/5 | 0.11 |
| HBS1L-MYB | rs9399137 | T>C (0.36) | 43/81 | 15/25 | 21/31 | 5/20 | 2/5 | 0.31 |
| HBS1L-MYB | rs9389269 | T>C (0.39) | 43/84 | 15/25 | 21/31 | 5/23 | 2/5 | 0.37 |
| HBS1L-MYB | rs9402686 | G>A (0.39) | 44/84 | 15/25 | 21/31 | 6/23 | 2/5 | 0.41 |
| HBS1L-MYB | rs9494142 | T>C (0.35) | 41/83 | 13/25 | 21/31 | 5/22 | 2/5 | 0.78 |
| BCL11A | rs11886868 | C>T (0.91) | 74/85 | 21/25 | 29/31 | 20/24 | 4/5 | 0.62 |
| BCL11A | rs4671393 | A>G (0.99) | 81/82 | 25/25 | 29/30 | 23/23 | 4/4 | 0.30 |
| Xmn1-HBG2 | rs7482144 | G>A (0.55) | 44/84 | 11/25 | 16/31 | 13/23 | 4/5 | 0.08 |
| Multiplicative Effect on Hb F, Univariate3 | ||||||||
| Xmn1-HBG2 | 1.38 (0.08) | 1.41 (0.31) | 1.12 (NS) | 2.09 (0.07) | ||||
| Age, per year | 0.97 (<0.001) | 0.98 (0.02) | 0.97 (<0.001) | 0.97 (<0.001) | ||||
| Gender, female vs male | 0.70 (0.04) | 0.8 (NS) | 0.59 (0.08) | 0.72 (NS) | ||||
| RBC, per 1012/l | 0.61 (<0.001) | 0.72 (NS) | 0.62 (0.001) | 0.53 (0.01) | ||||
| MCV, per fl | 1.01 (NS) | 1.01 (NS) | 1.0 (NS) | 1.02 (NS) | ||||
| Epo, per natural log | 1.36 (<0.001) | 1.27 (0.02) | 1.32 (<0.001) | 1.44 (<0.001) | ||||
| DBA vs FA | 0.79 (NS) | |||||||
| DC vs FA | 1.25 (NS) | |||||||
| SDS vs FA | 0.54 (0.08) | |||||||
| Multiplicative Effect on Hb F, Multivariate3 | ||||||||
| Xmn1-HBG2 | 1.32 (0.04) | 0.96 (NS) | 1.54 (0.09) | 1.58 (0.06) | ||||
| Age, per year | 0.97 (<0.001) | 0.98 (0.1) | 0.97 (<0.001) | 0.97 (<0.001) | ||||
| Gender, female vs male | 0.76 (0.09) | 1.34 (NS) | 0.76 (NS) | 0.60 (0.04) | ||||
| RBC, per 1012/l | 1.14 (NS) | 2.54 (NS) | 1.17 (NS) | 0.84 (NS) | ||||
| MCV, per fl | 1.01 (NS) | 1.01 (NS) | 1.02 (NS) | 1.02 (NS) | ||||
| Epo, per natural log | 1.34 (<0.001) | 1.65 (0.04) | 1.34 (0.06) | 1.27 (0.08) | ||||
| DBA vs FA | 0.94 (NS) | |||||||
| DC vs FA | 0.94 (NS) | |||||||
| SDS vs FA | 0.70 (NS) | |||||||
| Multiplicative Effect on Hb F, Final Models4 | ||||||||
| Xmn1-HBG2 |
1.32 (0.04) 1.02–1.71 |
0.84 (0.61) 0.43–1.65 |
1.48 (0.09) 0.94–2.33 |
1.68 (0.02) 1.08–2.61 |
||||
| Age, per year |
0.97 (<0.001) 0.97–0.98 |
0.98 (0.04) 0.96–1.0 |
0.97 (0.001) 0.95–0.99 |
0.97 (<0.001) 0.95–0.98 |
||||
| Gender, female vs male |
0.76 (0.04) 0.58–0.99 |
0.89 (NS) 0.47–1.67 |
0.72 (NS) 0.40–1.30 |
0.61 (0.03) 0.39–0.96 |
||||
| Epo, per natural log |
1.30 (<0.001) 1.21–1.41 |
1.26 (0.02) 1.04–1.53 |
1.32 (<0.001) 1.16–1.51 |
1.38 (<0.001) 1.21–1.58 |
||||
DBA, Diamond-Blackfan anaemia; DC, Dyskeratosis congenita; FA; Fanconi anaemia; SDS, Shwachman-Diamond syndrome; Epo, erythropoietin; RBC, red blood cell count; MCV, mean cell volume. Ages are median and range. In some syndromes the denominator for a particular variable was less than the total number for that syndrome. The male:female ratio was significantly less in FA, the proportion of patients with macrocytic red cells was low in SDS, and the proportion of patients with increased Hb F % was low in DBA relative to the other syndromes.
One patient with FA was on transfusions and androgens.
+>-, usual to alternative alleles. Frequencies for European individuals with one or two of the alternative alleles are in brackets. P is for significant association of the alternative SNPs with increased Hb F in the total group, unadjusted for any other factors.
Data are contribution and (p value). SDS not analysed separately due to small numbers (n = 6). Significant data are in bold.
Second line in final model shows 95% confidence intervals.
Results
There were 31 patients with DBA, 35 with DC, 25 with FA, and 6 with SDS. Five of the 97 were not Caucasian (one was African American, one was half African American, one was Asian, and two were half Asian). The ages ranged from 2 to 58 years, with a median age of 17 (Table I). The male:female ratio depended on the syndrome, with an excess of males in DC and DBA, and females in FA. Anaemia was more common in DBA and DC, and macrocytosis in DC and FA. Seven DBA and two DC patients were receiving routine transfusions. Hb F was elevated (Hb F >1%) in 70% of the total group of patients: 48% of DBA, 83% of DC, 76% of FA and 83% of SDS. In the pooled group of 97 IBMFS patients, 59 (61%) were macrocytic for age, 55 (57%) were anaemic for age, and 70 (77%) had elevated Epo.
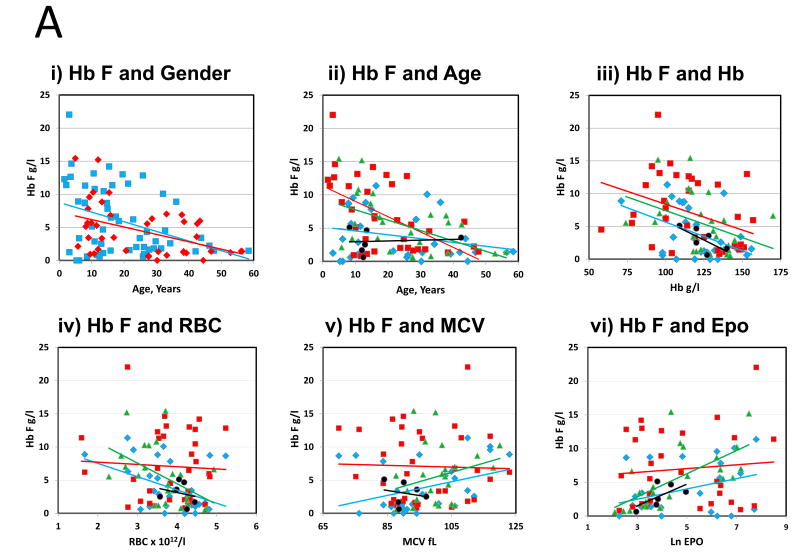
The associations of Hb F and gender, age, Hb, RBC, MCV, and Epo are shown in Figure 1A. Hb F was higher in males than females; decreased with age at a similar rate in both genders, particularly in DC and FA; was higher in anaemic patients and in those with elevated MCV and those with elevated Epo. The association of higher Hb F with manifestations of anaemia, macrocytosis, and increased Epo is consistent with the concept of “stress erythropoiesis” (Alter, 1979).
Figure 1.
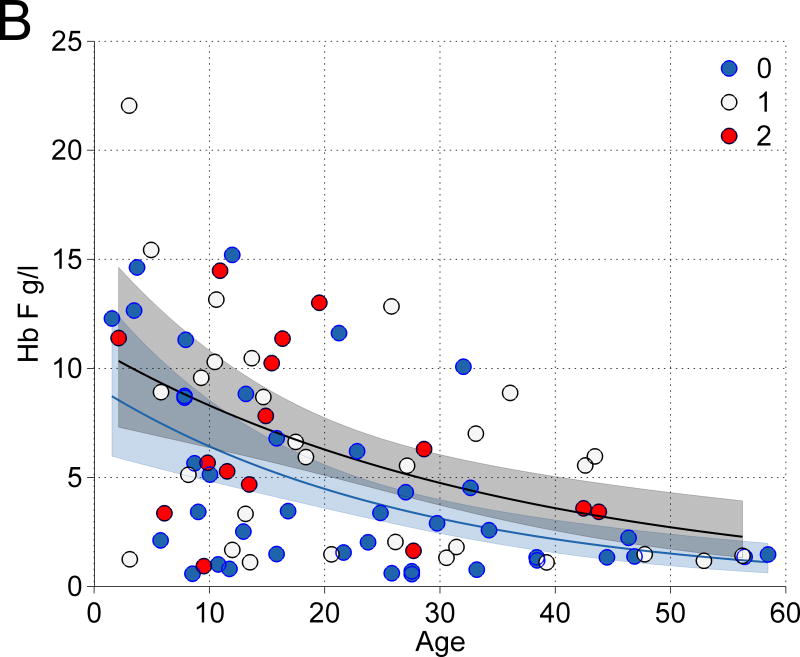
(A) The association of Hb F with other features: (i) Hb F and gender vs age: Blue square, male, R2 = 0·1424, P = 0·004. Red diamond, female, R2 = 0·1991, P = 0·003, (ii) Hb F and age by syndrome: Blue diamond, Diamond-Blackfan anaemia (DBA), Red square, Dyskeratosis congenita (DC). Green triangle, Fanconi anaemia (FA). Black circle, Shwachman-Diamond syndrome (SDS). Respective R2 = 0·0664; 0·2973; 0·2699; 0·0074; P = 0·16; 0·001; 0·008; 0·087. Overall R2 = 0·1734; P = <0·001, (iii) Hb F and Hb by syndrome: Respective R2 = 0·2099; 0·1462; 0·1514; 0·4772; P = 0·01; 0·02; 0·06; 0·13. Overall R2 = 0·1620; P = <0·001, (iv) Hb F and red blood cell count (RBC) by syndrome: Respective R2 = 0·1727; 0·0028; 0·2464; 0·0521; P = 0·02; 0·02; 0·012; 0·7. Overall R2 = 0·2152; P ≤ 0·001, (v) Hb F and mean cell volume (MCV) by syndrome: Respective R2 = 0·1309; 0·0103; 0·0748; 0·0409; P = 0·045; 0·6; 0·2; 0·7. Overall R2 = 0·0722; P = 0·008, and (vi) Hb F and erythropoietin (Epo) by syndrome: Respective R2 = 0·136; 0·2023, 0·4127, 0·3832. P = 0·06; 0·007; 0·001; 0·2. Overall R2 = 0·2375; P ≤ 0·001. (B) The association of Xmn1-HBG2 and elevated Hb F. In univariate analyses, the combination of 1 and 2 alternative alleles in Xmn1-HBG2 showed the strongest trend toward an association with increased Hb F. Blue, +/+, wild type; white, +/−, one alternative allele; red, −/−, two alternative alleles.
The frequencies of carriers of the alternative alleles (in heterozygous or homozygous form) for the QTLs were 50% for HBS1L-MYB, >90% for BCL11A, and 52% for Xmn1-HBG2 in the entire group (Table I). The relationship between the alternative alleles for Xmn1-HBG2 and the age-related decline in Hb F in the total cohort is shown in Figure 1B. At all ages, the expected level of Hb F (black curve) was higher in those with 1 or 2 alternative alleles (red and white circles) than with 2 wild-type alleles (blue curve and blue circles, respectively). Also, at younger ages there was noticeably more scatter around the age-related mean values than at older ages (both groups); this feature is accounted for in the error bands (grey and blue shaded regions) via the GLM approach.
In univariate GLM analyses, significant multiplicative modifiers of Hb F levels included age, gender, RBC and Epo; the Xmn1-HBG2 SNP was of borderline significance (p=0.08) (Table I). Inclusion of all parameters in a multivariate GLM model maintained significance of age and Epo, and increased the significance of the p value for the Xmn1-HBG2 SNP to 0.04. The final multivariate model for Hb F in the total group of IBMFS included the alternative allele for the Xmn1-HBG2SNP (p = 0.04), younger age (p<0.001), male sex (p=0.04), and increased Epo (p<0.001). In this model, the alternative allele for the Xmn1-HBG2QTL was associated with a 32% increase in the level of Hb F in the entire group (Table I). Subset analyses indicated that the strongest association of the Xmn1-HBG2QTL was in FA and DC (increased Hb F by 68% and 48% respectively, p-values 0.02 and 0.09). There was no effect in DBA (decreased Hb F by 18%, p = 0.6). Data including the other Hb F QTLs were not significant.
Discussion
The association of high Hb F with age, degree of anaemia, MCV and Epo in patients with an IBMFS was not unexpected. The higher mean Hb F in males was not predicted and might be a false positive finding. The novel observation was that the alternative allele at Xmn1-HBG2 was associated with an increased level of Hb F in the total IBMFS cohort; this was seen in FA and DC, but not DBA, after adjustment for age, sex and Epo level. This allele was the same as found to be associated with increased Hb F in haemoglobinopathies (Thein et al, 2009), and it is interesting to note that the regulation of Hb F is conserved despite varying degrees of marrow failure and stress.
The availability of almost 100 patients with a well-characterized IBMFS is a strength of the study, although a limitation is that the total number and number within each syndrome is still small. A role of the other QTLs related to Hb F regulation might be identified in future larger studies. It is important to point out that a low level of Hb F should not exclude the diagnosis of an IBMFS in a patient who has other signs of stress erythropoiesis (anaemia with increased MCV and Epo), because that patient may not have the variant allele associated with increased Hb F. Thus, while elevated Hb F might suggest strong consideration of an inherited marrow failure syndrome, the converse of a low level of Hb F should not be construed to eliminate an inherited syndrome from the differential diagnosis. Future longitudinal studies are required to determine whether the level of Hb F changes over time, and whether it is predictive of evolution of bone marrow failure. In summary, this is the first study to show regulation of Hb F by the same QTL in FA and DC as in sickle cell anaemia and thalassaemia, thus linking Hb F regulation across disparate haematological disorders.
Acknowledgments
We are extremely grateful to all subjects for their enthusiastic participation in the IBMFS study. This research was supported in part by the Intramural Research Program of the National Institutes of Health and the National Cancer Institute (B.P.A., N.G., S.A.S., P.S.R.), and by contracts N02-CP-91026, N02-CP-11019 and HHSN261200655001C with Westat, Incorporated; and the Medical Research Council (MRC, UK) G0001249 ID 56477 (S.L.T.).
Footnotes
BPA and SLT designed the study, analysed data, and wrote the paper. TD and SM performed the research and analysed data. PSR analysed data and wrote the paper. NG and SAS provided clinical material and samples. All authors reviewed and edited the final manuscript.
References
- Alter BP. Fetal erythropoiesis in stress hematopoiesis. Exp Hematol. 1979;7(Suppl 5):200–209. [PubMed] [Google Scholar]
- Creary LE, Ulug P, Menzel S, McKenzie CA, Hanchard NA, Taylor V, Farrall M, Forrester TE, Thein SL. Genetic variation on chromosome 6 influences F cell levels in healthy individuals of African descent and HbF levels in sickle cell patients. PLoS One. 2009;4:e4218. doi: 10.1371/journal.pone.0004218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman PR, Siimes MA. Percentile curves for hemoglobin and red cell volume in infancy and childhood. J Pediatr. 1979;94:26–31. doi: 10.1016/s0022-3476(79)80344-3. [DOI] [PubMed] [Google Scholar]
- Mccullagh P, Nelder JA. Generalized Linear Models. 2. Chapman and Hall; Boca Raton: 1989. p. 532. [Google Scholar]
- Shimamura A, Alter BP. Pathophysiology and management of inherited bone marrow failure syndromes. Blood Rev. 2010;24:101–122. doi: 10.1016/j.blre.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thein SL, Menzel S, Lathrop M, Garner C. Control of fetal hemoglobin: new insights emerging from genomics and clinical implications. Hum Mol Genet. 2009;18:R216–R223. doi: 10.1093/hmg/ddp401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young NS, Alter BP. Aplastic Anemia: Acquired and Inherited. W B Saunders; Philadelphia PA: 1994. p. 410. [Google Scholar]