Abstract
The goal of resting-state functional magnetic resonance imaging (FMRI) is to investigate the brain’s functional connections by using the temporal similarity between blood oxygenation level dependent (BOLD) signals in different regions of the brain “at rest” as an indicator of synchronous neural activity. Since this measure relies on the temporal correlation of FMRI signal changes between different parts of the brain, any non-neural activity-related process that affects the signals will influence the measure of functional connectivity, yielding spurious results. To understand the sources of these resting-state FMRI confounds, this article describes the origins of the BOLD signal in terms of MR physics and cerebral physiology. Potential confounds arising from motion, cardiac and respiratory cycles, arterial CO2 concentration, blood pressure/cerebral autoregulation, and vasomotion are discussed. Two classes of techniques to remove confounds from resting-state BOLD time series are reviewed: 1) those utilising external recordings of physiology and 2) data-based cleanup methods that only use the resting-state FMRI data itself. Further methods that remove noise from functional connectivity measures at a group level are also discussed. For successful interpretation of resting-state FMRI comparisons and results, noise cleanup is an often over-looked but essential step in the analysis pipeline.
Keywords: Functional Magnetic Resonance Imaging (FMRI), resting-state, functional connectivity, noise correction, physiological noise
Introduction
Recently, resting-state FMRI has become an extremely popular area of research for neuroimagers as evidenced by the exponential growth in related publications per year (Birn, 2012). The goal of resting-state FMRI is to use the common variance of the FMRI blood oxygenation level dependent (BOLD) signals in different regions of the brain as an indicator of synchronous neural activity. The assumption is that the temporal similarity between the BOLD signals in each region demonstrates that they are in constant communication with one another and thus form a functional network. The popularity of the technique stems not only from the relative ease of data acquisition (the participants are not required to perform a task) but from the fact that resting-state networks are a phenomenon that FMRI, as a relatively young technique (~20 years), was the first to discover. Using resting-state FMRI, it is possible to simultaneously examine the relationship between multiple resting-state networks and independently measured behavioural traits, fuelling its popularity amongst neuroscientists and clinicians alike. The demonstration of resting-state networks has helped FMRI live up to its initial promise as a tool for investigating brain dynamics.
FMRI appears to be the ideal neuroimaging technique for the investigation of resting-state network characteristics. The spatial resolution is superior to other methodologies such as EEG and MEG, allowing for localization and separation of the various resting-state networks simultaneously. The relative lack of temporal resolution in the BOLD signal is not problematic since spontaneous neural fluctuations can be found in the low frequency range (Leopold et al., 2003). Furthermore, other work has demonstrated significant correlations between variations in the power of electrophysiological activity in higher frequency bands (e.g. alpha and beta) and resting-state FMRI signals (Laufs et al., 2003). However, despite the broad use of resting-state FMRI as a technique to investigate low-frequency BOLD fluctuations, the mechanisms that give rise to synchronous, spontaneous neural activity across brain regions remain largely unknown (Leopold and Maier, 2012). (These issues are addressed elsewhere in this NeuroImage special edition by Scholvinck).
Resting-state BOLD networks were first demonstrated by Biswal and colleagues in 1995 when spontaneous BOLD fluctuations in the left and right motor cortex were shown to be correlated in the absence of a task (Biswal et al., 1995). An early detailed analysis of the frequency spectrum of resting-state FMRI data demonstrated that low frequency fluctuations (defined as <0.1Hz) contributed to more than 90% of the correlation coefficient between regions of the same resting-state network (Cordes et al., 2001). Furthermore, it was demonstrated the these low-frequency fluctuations have similar properties to task-related BOLD signals (Biswal et al., 1997; Cordes et al., 2001; Lowe et al., 1998; Peltier and Noll, 2002). Using the spontaneous oscillations measured with FMRI, many resting-state networks have been discovered that correspond well to functional networks activated by a variety of tasks (Smith et al., 2009). One of the most notable and studied networks is the default mode network (DMN) which has been shown to deactivate during cognitive tasks (McKiernan et al., 2003; Raichle et al., 2001). Although it was first demonstrated using PET (Raichle et al., 2001), resting-state FMRI has become the primary tool to investigate the DMN ever since it was shown to be functionally connected at rest (Greicius et al., 2003).
One weakness of resting-state FMRI lies in an important difference between the analysis of spontaneous fluctuations and more traditional studies of task-evoked BOLD responses. In the latter, the timing and intensity of the task is known a priori and the responses of many trials are combined together to eliminate noise and to increase statistical significance (Bandettini et al., 1993; Friston et al., 1995). However, in resting-state FMRI, functional connectivity is determined by measuring the temporal similarity of the BOLD time series in voxels using some metric, commonly the correlation coefficient. For example, in the original Biswal paper (Biswal et al., 1995), the correlation coefficient between the BOLD time series of a voxel in the motor cortex and every other voxel in the brain was calculated. Voxels whose correlation coefficient passed a statistical threshold were deemed to be functionally connected, thus revealing common spontaneous fluctuations between left and right motor cortices. Since the two time series are measured simultaneously, any non-neural activity-related process that affects one or both time series will affect the measure of functional connectivity, thus yielding a spurious result. These resting-state FMRI confounds can not only increase the apparent functional connectivity by introducing spurious similarities between the time series’ but also reduce the connectivity metric if differential confounds between regions are introduced. This can be particularly problematic if the temporal similarity metric is to be used to compare connectivity between groups that display physiological or behavioural differences whilst at “rest” in the scanner (Bright and Murphy, 2013; Murphy et al., 2011; Power et al., 2012; Van Dijk et al., 2012).
To understand the source of these resting-state FMRI confounds, thus providing us with avenues for removing them, we must first understand the origins of the BOLD signal itself.
Origin of the BOLD signal
A brief description of the origin of the BOLD signal, which is reviewed more comprehensively by introductory textbooks (Buxton, 2002; Jezzard et al., 2001), follows.
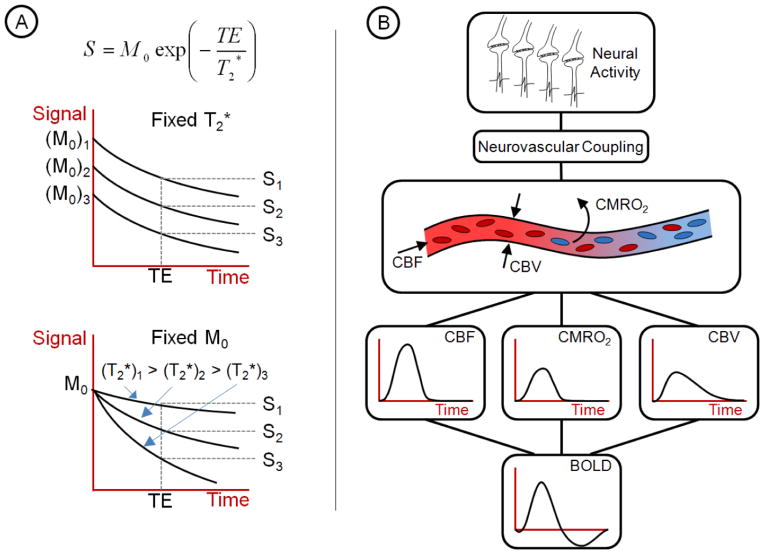
FMRI is mainly performed using gradient echo imaging techniques. The magnitude of the measured signal of a gradient echo sequence (S) depends on the initial magnetisation (M0), the T2* decay time and the time at which the image is acquired, denoted TE, the echo time (see Figure 1A).
Figure 1. Origins of the BOLD signal.
A) The magnitude of the BOLD signal depends on the initial magnetisation, M0, and the decay time, T2*. Changes in the BOLD signal strength, S, can arise from changes in either M0, T2* or a combination of both. FMRI assumes that changes in T2* are solely due to neural activity-related changes in deoxyhaemoglobin concentration. B) A schematic of the relationship between a transient increase in neural activity and the corresponding BOLD signal is shown. When neural activity causes increased oxygen consumption (CMRO2), neurovascular coupling mechanisms alter the tone of the vasculature changing CBF and CBV. The complicated interaction between these 3 parameters leads to the BOLD signal as measured with FMRI. Changes in the BOLD signal accurately reflect neural activity fluctuations, only if the intermediary vascular steps are not significantly altered. Many of the confounds in resting-state FMRI originate from physiological changes in the vasculature.
M0 depends directly on the number of excited spins in a voxel. T2* is the inverse of the relaxation rate (R2*) of the magnetisation caused by local susceptibility-induced magnetic field gradients. Changes in T2* are the basis for the blood oxygenation level dependent (BOLD) signal that is of interest in FMRI. TE, the echo time, is chosen by the experimenter to maximise the BOLD contrast which is TE-dependent: usually around 30ms for a magnetic field strength of 3T. The BOLD contrast arises from the fact that oxyhaemoglobin is diamagnetic whereas deoxyhaemoglobin is paramagnetic. An increase in deoxyhaemoglobin concentration ([dHb]) causes faster dephasing of excited spins, shortening T2*, leading to a smaller BOLD signal measured at the echo time, TE.
Neural activity is primarily an aerobic process: the production of ATP in this way means that the cerebral metabolic rate of oxygen consumption (CMRO2) closely parallels neural activity (Attwell and Laughlin, 2001). In a healthy brain, arterial blood oxygen saturation is close to 100%, that is, all haemoglobin molecules are fully loaded with oxygen. Once this blood reaches an area of neural activity in which CMRO2 has increased, the increased oxygen concentration gradient across the vessel wall causes more oxygen to unload from the passing haemoglobin. This implies that increased neural activity will lead to increased deoxyhaemoglobin concentration, [dHb], in local venous blood vessels, shortening T2* and thus reducing the BOLD signal.
However, the earliest studies of neural activity using FMRI demonstrated the reverse: BOLD signal increases with increased neural activity (Bandettini et al., 1992; Ogawa et al., 1992). This indicates that [dHb] is reduced. This dichotomy is caused by neurovascular coupling (described more comprehensively elsewhere by Liu in this NeuroImage special edition). Through multiple mechanisms, neural activity causes an increase in perfusion/cerebral blood flow (CBF) through localised vasodilation (increased cerebral blood volume (CBV)) when more oxygen is in demand. The resulting increase in CBF, whilst coupled to the increased metabolism, is roughly a factor of two larger than the increase in CMRO2 (Davis et al., 1998; Hoge et al., 1999). Therefore, oxyhaemoglobin is oversupplied to the activated region leading to a reduction in deoxyhaemoglobin concentration [dHb] and, thus, an increase in the BOLD signal.
From this description of the BOLD contrast, it is clear that BOLD, rather than being a direct measure of neural activity, is a complex function of metabolism (CMRO2), CBF and CBV (see Figure 1B). Changes in the BOLD signal accurately reflect neural activity if and only if the intermediary vascular steps are not significantly altered. Phenomena that affect the complex balance between the 3 parameters CMRO2, CBF and CBV in the frequency range of resting-state fluctuations will cause changes in resting-state BOLD signals that may be spuriously correlated across regions. Similarly, phenomena that globally affect aspects of the signal other than T2* (i.e. longitudinal magnetisation – M0) will cause correlated changes in BOLD signal that may be entirely unrelated to physiology. Both magnetisation and physiological processes that change over time and that are reflected in resting BOLD signals are considered to be resting-state FMRI confounds.
Resting-state FMRI confounds
Isolating true neural activity-related BOLD signals of interest is an ongoing challenge since resting-state FMRI confounds can arise from many processes in the MRI environment. Apart from signal changes that occur due to scanner hardware instabilities (e.g. spiking), FMRI confounds arise from phenomena related to the participant that are outside the control of the experimenter. Although hardware related confounds can be fixed (at least in theory), participant-related FMRI confounds, while perhaps reduced through various strategies, will always remain and therefore must be understood to be removed. The following are descriptions of common resting-state FMRI confounds that can affect the BOLD signal by changing M0, T2* or both in a time varying way.
Motion
Motion artefacts are problematic for all types of FMRI including resting-state FMRI. When the participant moves in the magnetic field, three effects on M0 can compromise data quality. First, movement of the head causes the content of each voxel to change. Since M0 is directly proportional to the number of spins in the voxel, any alteration in voxel content will manifest itself as a change in the BOLD signal. This is particularly problematic at tissue interfaces such as gray/white matter boundaries, around large vessels and at the edges of the brain. Second, movement of the head will alter the uniformity of the magnetic field that has been shimmed for one particular head position. This changes locations of distortions and signal dropout boundaries along with directly affecting M0 itself. Finally, movement of the head within the scanner during a scan will change steady state magnetisation by changing the time between excitations in the parts of tissue that have moved from one slice to the next. This transiently influences the magnetisation M0 until steady state is reached and is often referred to as a spin history effect. The change in signal intensity due to spin history effects can often be up to twice the expected BOLD signal change (Muresan et al., 2005).
For resting-state FMRI, motion-related confounds are problematic due to their global nature. These confounds can be further compounded when comparing differences in functional connectivity between groups. Children and patient populations are usually more uncomfortable in the MR environment than young healthy controls, resulting in a greater amount of head motion. Recent studies have demonstrated that functional connectivity conclusions may be erroneous when motion artefacts have a differential effect on resting BOLD signals for between group comparisons (Power et al., In Press; Satterthwaite et al., 2012; Van Dijk et al., 2012). In these studies, subtle motion artefacts (< 0.5mm) rather than larger head movements were the cause for concern. Head motion introduced a specific bias, increasing short range and right-left connections whilst decreasing long-range and anterior-posterior connections.
Cardiac and Respiratory Physiological Noise
Although attempts to limit large movements during FMRI are commonly made using head restraints, it is not possible to limit all movement-related artefacts, even in compliant and motivated participants. Bulk motion related to the cardiac and respiration cycles will lead to similar confounds as motion of the head itself. Movement of the subject’s chest can alter the magnetic field in a time dependent manner (Brosch et al., 2002). Respiration causes bulk susceptibility variation in the lungs leading to variations in the static magnetic field within brain tissue (Raj et al., 2001). These magnetic field changes can result in a shift of the MR image in the phase-encoding direction. Small movements of the head due to breathing alter spin history in a spatially dependent manner (Friston et al., 1996). Cardiac pulsation and respiratory cycles cause the brain stem to push up into the surrounding brain tissue causing deformation and cerebrospinal fluid movement which manifest themselves in M0 changes (Dagli et al., 1999). Pulsations of the vessels caused by cardiac-related pressure changes will generate small movements in and around large blood vessels (Dagli et al., 1999). Evidence indicates that such physiological motion also affects resting-state FMRI time series through the mechanism of steady-state free precession (SSFP) disturbance (Zhao et al., 2000).
Cardiac and respiratory cycles are relatively high frequency (~1 Hz and ~0.3 Hz, respectively) compared to the low-frequency (< 0.1 Hz) BOLD fluctuations under scrutiny in resting-state FMRI. However, due to the long TR of standard resting BOLD EPI (~2–3 secs), cardiac and respiratory noise at the primary frequency are aliased into this low-frequency range (Bhattacharyya and Lowe, 2004; Lowe et al., 1998). Thus, cardiac and respiratory noise will appear as low-frequency fluctuations and may be mistaken for neural activity-related BOLD oscillations.
Furthermore, it has been demonstrated that there is a significant correlation between changes in cardiac and respiratory rates and the BOLD signal (Birn et al., 2006; Shmueli et al., 2007; Wise et al., 2004). These fluctuations in rate are within the frequency range of resting BOLD oscillations (~0.04 Hz for cardiac (Akselrod et al., 1981) and ~0.03Hz for respiration (Wise et al., 2004)) and therefore, will artificially inflate functional connectivity measures or cause spurious connectivity patterns where BOLD connectivity is not present. Rather than arising from movement-related artefacts, low-frequency fluctuations in cardiac and respiratory rate manifest themselves in resting BOLD signals through arterial CO2 concentration and blood pressure changes that are under sympathetic and parasympathetic nervous system control.
Arterial CO2 concentration
Carbon dioxide (CO2) in arterial blood is a potent vasodilator producing a global increase in CBF. CO2 is produced in tissue as a by-product of glucose metabolism and its vasodilatory properties are thought to be one of the mechanisms mediating neurovascular coupling (Raichle and Stone, 1971). However, arterial tension of CO2 can change with respiration rate, thereby changing CO2 concentration in the venous vasculature leading to BOLD signal changes that are unrelated to neural activity.
Using transcranial doppler (TCD) ultrasound, it has been demonstrated that after breathing air enriched with CO2 to cause hypercapnia (an increase in arterial CO2 concentration), blood velocity in the middle cerebral artery (MCA) changed by 2–5% per mmHg change in arterial carbon dioxide (Ide et al., 2003; Poulin et al., 1996). CO2-mediated increases in CBF caused by hypercapnia have been shown to also increase the BOLD signal (Kastrup et al., 1999; Poulin et al., 1996; Rostrup et al., 2000): the increased CBF reduces deoxyhaemoglobin concentration resulting in a longer T2*. Similarly, cued changes in participant breathing will affect the BOLD signal. Hypercapnia arising from breath-holds increases BOLD signal (Murphy et al., 2011) whilst hypocapnia arising from hyperventilation reduces it (Bright et al., 2009; Posse et al., 2001).
Breath-to-breath variations in arterial CO2 partial pressure during spontaneous breathing lead to changes in respiration rate (Modarreszadeh and Bruce, 1994). Random disturbances in CO2 concentration, although small, have significant effects on ventilation rates. Increased arterial CO2 levels activate chemoreceptors that increase subsequent breathing depth and rate (Van den Aardweg and Karemaker, 2002). An arterial baroreflex has been proposed as the cause of fluctuations in both respiratory and cardiac rates (Cohen and Taylor, 2002), suggesting that low-frequency variations in cardiac and respiratory rates are intimately linked.
The response of the vasculature to CO2, often referred to as vascular reactivity, is very sensitive, thus any changes in respiration rate and resulting CO2 variations will have consequences for resting BOLD signal fluctuations. In resting-state FMRI, we are interested in low-frequency oscillations in metabolism, however, using near-infrared spectroscopy (NIRS), is has been demonstrated that such fluctuations are also accompanied by low-frequency variations in CBF and oxygenation (Obrig et al., 2000). End-tidal CO2 partial pressure measurements are a surrogate for arterial CO2 concentration measures and have been shown to fluctuate in the 0–0.05Hz frequency range (Wise et al., 2004). Wise and colleagues demonstrated that a component of the low-frequency BOLD signal fluctuations is mediated by CO2-induced changes in CBF. Fluctuations in end-tidal CO2 of ±1.1mmHg lead to BOLD signal fluctuations of ±0.12% in grey matter. Using a measure called RVT (respiration volume per time) that attempts to capture breathing rate and depth as a surrogate for end-tidal CO2, Birn and colleagues demonstrated that related fluctuations in resting-state FMRI BOLD localised to regions with high blood volume and large blood vessels (Birn et al., 2006). It remains an ongoing mystery as to why these regions overlap well with the default mode network. A possible explanation is that brain areas that are predominantly active “at rest” have evolved to have a higher CBF and CBV.
Blood Pressure and Cerebral Autoregulation
Cerebral autoregulation is the intrinsic dynamic ability of cerebral vessels to maintain steady-state CBF despite fluctuations in arterial blood pressure (Lagopoulos et al., 2006). Cerebral autoregulation protects the vasculature from excessive blood flow, which may cause haemorrhage leading to ischemia and cell death. The arterial and arteriolar systems are responsible for maintaining stable CBF during times of increased blood pressure and do so by changing vascular tone through vasoconstriction and vasodilation processes (Ekstrom-Jodal et al., 1971; Failla et al., 1999). In the healthy brain, CBF is held constant at 40–50 ml/100g/min over the range of arterial blood pressure from 50 to 150 mmHg (Kontos et al., 1978).
Like arterial CO2 concentration, arterial blood pressure also fluctuates over time. Blood pressure variations are a key source of CBF fluctuations due to the delay in dynamic autoregulation (Diehl et al., 1995; Lang et al., 1999). Blood pressure is under the control of the sympathetic and parasympathic nervous systems that modulate arterial vascular tone (Failla et al., 1999) and heart rate in the low-frequency range (Akselrod et al., 1981; Katura et al., 2006). Low-frequency oscillations in oxyhaemoglobin stem from both heart rate and blood pressure fluctuations, with the former explaining 20% of variance, the latter 5% and a common contribution of approximately 10% on the variance using Optical Topography data (Katura et al., 2006). However, non-linear modeling demonstrates that blood pressure accounts for 60% of the total predictive power of CBF fluctuations whereas CO2 accounts for only 17% (Mitsis et al., 2004). Therefore, fluctuations in arterial blood pressure are likely to have a greater influence on vasculature than fluctuations in CO2 concentration and, thus, on resting-state BOLD signal variations.
Evidence of the influence of blood pressure oscillations on resting-state FMRI fluctuations in humans is sparse. Blood pressure levels in rats have been shown to affect evoked fMRI responses, with transient hypertension increasing BOLD (Wang et al., 2006) and CBF (Qiao et al., 2007) signals. Under hypotension, neural activity-evoked CBV increases in visual cortex are negligible compared to ~10% at normal blood pressure levels (Nagaoka et al., 2006). Increases in the amplitude of low-frequency BOLD fluctuations have been demonstrated with a drop in mean arterial pressure (Biswal and Kannurpatti, 2009). As supporting evidence in humans, BOLD signal correlates of heart rate and pulse height in the low frequency range have been discovered with fluctuations in cardiac rate explaining up to 11% of the variance in the resting-state BOLD signal (Chang et al., 2009; de Munck et al., 2008; Shmueli et al., 2007).
Vasomotion
In addition to the autoregulatory response to blood pressure changes, vascular tone throughout the body exhibits low-frequency oscillations (< 0.1 Hz) in the absence of any stimulus (Aalkjaer et al., 2011). This phenomenon, known as vasomotion, is still poorly understood, particularly in the brain. Controversy exists in the literature over whether vasomotion is an independent process from CO2 and blood pressure fluctuations. Vasomotion is directly affected by baseline vascular tone (Morita-Tsuzuki et al., 1992), is independent of cardiac and respiratory cycles and is increased when cerebral perfusion is challenged (Biswal and Kannurpatti, 2009; Hudetz et al., 1998), the most potent stimuli being hypotension, hyperventilation and vasoconstriction. Vasomotion fluctuations are absent in ischemic brain territories and dependent on intravascular pressure suggesting that these oscillations stem from a myogenic mechanism, that is, the smooth muscle of the arterioles contracting due to the mechanical stress of increased blood volume (Hudetz et al., 1998). If vasomotion is independent of cardiac, respiratory, arterial CO2 concentration and blood pressure fluctuations, its low-frequency characteristics will present another confound for resting-state FMRI BOLD oscillations.
Resting-state FMRI cleanup
The BOLD signal is an indirect measure of neural activity. The goal of resting-state FMRI is to use synchronous low-frequency resting-state BOLD fluctuations from disparate regions of the brain as an indicator of synchronous neural activity. As outlined in the previous section, the BOLD signal can be confounded by many processes also low in frequency but not related to neural activity. Thus, interpretation of functional connectivity measures is difficult unless these confounds are removed. Fortunately, due in no small part to the popularity of the field, much effort has been focussed on addressing these issues. Although it is not currently possible to entirely remove all confounds, application of one or more of the noise removal techniques allows for more subtle measures to be made and can serve to increase confidence in resting-state FMRI results and interpretations. One important caveat is that some variations in physiology and other confounding processes may be temporally coupled with variations in the neural activity of interest. Removing such variance will also remove signals of interest. All attempts to cleanup resting-state FMRI data should bear this caveat in mind.
For individual resting-state dataset cleanup, noise removal methods can be roughly placed into two categories: 1) those utilising external recordings of physiology and 2) data-based cleanup methods that only use the resting-state FMRI data itself. Techniques from both categories can be used in conjunction when appropriate. Once functional connectivity measures are calculated on an individual basis, further cleanup can be performed at the group level.
Time-series Cleanup with Physiological Recordings
As demonstrated in the “Resting-state FMRI confounds” section, many of the noise sources that can affect functional connectivity measures originate from physiological processes. For well over 100 years, scientists have used external recordings of these processes to understand the workings of the human body. Many physiological recordings methods can be translated into the MR environment. By concurrently recording cardiac, respiratory, end-tidal CO2 and blood pressure traces, estimates of the related BOLD fluctuations can be modelled. The majority of the cleanup techniques that utilise physiological recordings remove these estimates from each voxels’ time series using linear regression, thus “cleaning up” the resting-state FMRI data. The influence of each physiological process on the BOLD signal is modelled as a time series and is fit to the data using a linear regression procedure. This fit is then subtracted from the data to remove the associated physiological variance.
Most MRI scanners come with a finger/toe pulse oximeter and a respiratory bellows, making it relatively easy and painless to record cardiac and respiratory traces during resting-state FMRI data acquisitions. Methods to remove the primary effects of cardiac and respiratory cycles have been developed for both k-space (Hu et al., 1995) and image space data (Glover et al., 2000). The former suffers from the problem that correction of single k-space points affects all voxels, potentially introducing spatially correlated noise whilst allowing high spatial frequency confounds to remain. The latter, dubbed RETROICOR (standing for RETROspective Image CORrection) estimates the phase of the cardiac and respiratory cycles at which an imaging slice is acquired. Low-order Fourier series’ of the phase data are fit to each voxels’ time series using a general linear model and removed. Since the phases of the cardiac and respiration cycles are matched to the timing of each imaging slice, the technique can remove the primary cardiac and respiratory frequencies that are aliased into the low-frequencies of interest (Lowe et al., 1998). Recently, it has been shown that averaged over gray matter, the cardiac and respiratory regressors modelled in RETROICOR account for only a small amount of time course variance, ~5% (Bianciardi et al., 2009; Jo et al., 2010). (It is important to note that the proportion of overall variance attributable to the underlying physiological noise increases with the signal-to-noise ratio (SNR) and so may be smaller or larger depending on voxel size and field strength (Bodurka et al., 2007; Kruger and Glover, 2001; Murphy et al., 2007; Triantafyllou et al., 2005)). The original implementation of RETROICOR did not fit Fourier series’ of orders greater than 2. More recently, it has been demonstrated that model fitting with higher orders along with interaction terms improves the fit but may suffer from a loss of some signal of interest (Harvey et al., 2008). One drawback of the RETROICOR technique is that changes in the time interval between cardiac beats are primarily associated with time variation in the diastolic rather than the systolic part of the cardiac cycle. This makes it difficult to determine an exact cardiac phase for each slice accurately (Dagli et al., 1999).
Low-frequency BOLD fluctuations related to variations in cardiac and respiratory rates can also be removed from the data with the same physiological traces. Birn and colleagues developed a measure called RVT (respiration volume per time) that attempts to capture changes in respiration depth and rate which cause periodic fluctuations in arterial CO2 concentration (Birn et al., 2006). RVT is calculated by generating envelopes of the maximum and minimum respiratory belt positions at the peaks of inspiration and expiration, respectively. The difference of these envelopes is divided by the breath-to-breath period of respiration. Using this as a regressor in a linear model, Birn and colleagues could remove the variance of respiration induced changes from resting-state BOLD signals. Convolution of the RVT regressor with a respiration response function further improved the fit to breathing-induced changes in BOLD data (Birn et al., 2008). Low-frequency changes in heart rate (HR) can also be removed from resting-state BOLD data using regression (Shmueli et al., 2007), a process further improved by convolution of the HR regressor with a cardiac response function (Chang et al., 2009). Combining both the RVT and HR methods into a single regression step can explain ~16% of resting-state BOLD variance with each explaining ~8% individually (Chang et al., 2009).
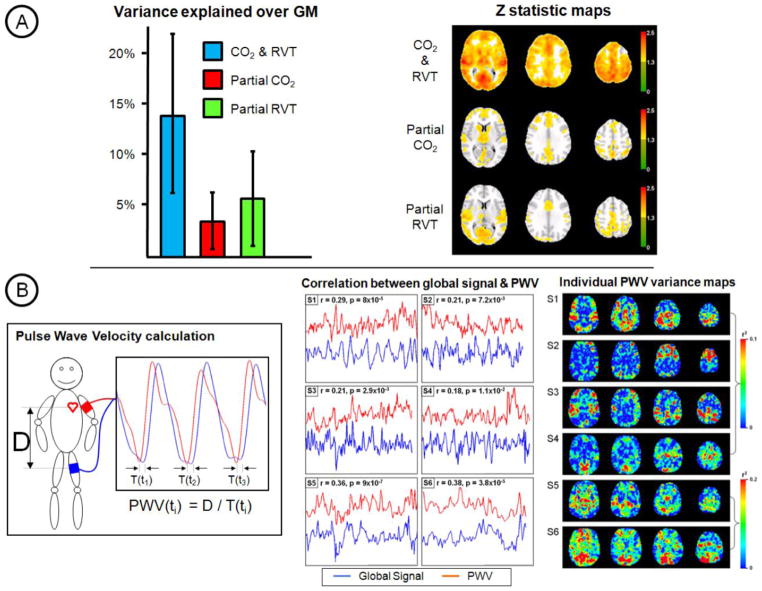
End-tidal CO2 partial pressure is a surrogate measurement for arterial CO2 concentration. A significant correlation between resting-state BOLD signals and end-tidal CO2 measured with a capnograph can be found. Wise and colleagues first demonstrated that ~16% of resting-state BOLD variance can be explained in grey matter (Wise et al., 2004) and thus removed. They also found that doppler ultrasound of the middle cerebral artery demonstrated that blood velocity lagged the low-frequency CO2 fluctuations by 6.3s and that the BOLD signal fluctuations were mediated by CO2-induced changes in CBF. The RVT correction method was developed to emulate end-tidal CO2 noise removal in situations where a capnograph is unavailable. As such, both regressors are highly correlated and explain similar spatial and temporal BOLD signal variance (Chang and Glover, 2009b). Figure 2A demonstrates in a cohort of 12 subjects that although end-tidal CO2 and RVT regressors remove common variance, each also captures separate components of the noise. RVT is not a perfect substitute for end-tidal CO2 correction. However, if both options are available then both noise correction strategies should be performed.
Figure 2. CO2 and PWV resting-state FMRI confounds.
A) A comparison of CO2 and RVT correction is shown for a cohort of 12 subjects. Together, RVT and end-tidal CO2 traces can explain ~15% of the variance in resting BOLD fluctuations. These confounds are widespread throughout gray matter. Although, common variance is removed, each type of correction captures a separate component of the noise. Complementary variance is explained in spatially similar and distinct regions. (Data first presented as a poster at the ISMRM 2009 meeting – Murphy, K., Harris, A.D. Niazy, R.K., Evans, C.J. & Wise, R.G. Low-Frequency Respiration Related Signals in Resting State fMRI: a comparison of end-tidal CO2 and respiration volume per time.) B) Pulse wave velocity (PWV) can be measured using partially inflated blood pressure cuffs. This measure of arterial stiffness reflects sympathetic tone that is related to fluctuations in arterial blood pressure. Although PWV measures are inherently noisy, large correlations with the global BOLD signal can be observed (over 6 subjects). Voxel-wise correlations of BOLD signal with PWV show localisation of explained variance in highly vascular regions for some subjects. This demonstrates that blood pressure fluctuations may be a large source of confound for resting-state FMRI that, to date, has been largely ignored by researchers. (Data first presented as an e-poster at the ISMRM 2011 meeting – Murphy, K., Coulson, J., Harris, A.D. Fjordorova, M. & Wise, R.G. The association between pulse wave velocity, as a marker of sympathetic tone, and resting state BOLD signals.)
Near-infrared spectroscopy (NIRS) acquired simultaneously with BOLD FMRI can provide information about blood flow and oxygenation. By using a concurrent NIRS recording at multiple delays to build six regressors in a method called Regressor Interpolation at Progressive Time Delays (RIPTiDe), Frederick and colleagues demonstrated that 10.5% of resting-state BOLD variance could be explained throughout the brain (Frederick et al., 2012). Comparisons with a combination of RETROICOR and RVT corrections that could only explain 6.8% of the variance show that this method may provide a superior correction technique. However, one major drawback of this technique is that care must be taken to ensure that the NIRS regressors do not contain neural activity-related fluctuations of interest. It is unclear how this could be achieved in practice.
A more interventionalist approach to reducing CO2-mediated resting-state BOLD fluctuations can be taken by controlling arterial gases. By utilising dynamic end-tidal forcing or prospective targeting to limit fluctuations in arterial CO2 and O2, related BOLD variance can be reduced (Madjar et al., 2012; Slessarev et al., 2007; Wise et al., 2007). However, it is unclear what effects these interventions will have on resting-state neural activity. Also dynamic, and even slice-specific, shimming of the B0 magnetic field can reduce the effect of chest motion and fluctuations in CO2 concentration on the BOLD signal which is especially problematic at higher field strengths (van Gelderen et al., 2007).
It is likely that blood pressure fluctuations will have an equal or greater influence on resting-state BOLD signals than respiration-related variations (see above). If MR-compatible continuous blood pressure devices were available such variance could be removed using similar regression techniques. Fluctuations in arterial blood pressure are controlled by the sympathetic nervous system through changes in arterial vascular tone (Failla et al., 1999). Sympathetic tone may be monitored by estimating arterial stiffness through pulse wave velocity (PWV) measurements. Partially inflated non-invasive blood pressure cuffs placed around the bicep and thigh are used to monitor the heart beat-induced pressure wave. Timing delays between the pressure wave traces divided by the arterial distance between the cuffs provide the measure of arterial stiffness or PWV. Figure 2B demonstrates that significant variance in the global BOLD signal can be explained by variance in PWV measurements. The almost instantaneous influence of PWV on resting-state BOLD signals is expected if this variance can be attributed to short-term fluctuations in CBF driven by variations in blood pressure. The relationship between PWV and BOLD signals is widespread throughout the brain and can be seen to be localised to more vascular regions in some subjects in Figure 2B. However, the inherent noisy nature of PWV measurements make voxel-wise comparisons difficult. These results demonstrate that blood pressure fluctuations are a confound in resting-state BOLD signals. Once an MR-compatible continuous blood pressure device has been developed, removal of such noise will be possible by following standard regression techniques.
Data-based time series cleanup techniques
There are many reasons why physiological recordings may not be available when analysing resting-state FMRI data; lack of equipment, data corruption and non-compliant/uncomfortable participants, to name but a few. Large databases of freely downloadable resting-state FMRI data exist such as the 1000 Functional Connectomes Project (http://fcon_1000.projects.nitrc.org/), the Autism Brain Imaging Data Exchange (ABIDE - http://fcon_1000.projects.nitrc.org/indi/abide/) and Alzheimer’s Disease Neuroimaging Initiative (ADNI - http://www.adni-info.org/). Unfortunately, varying degrees of corresponding physiological recordings accompany this data with the vast majority having none at all. Lack of physiological recordings renders each of the previously described cleanup methods impossible to perform. If, however, researchers find themselves in this situation, many methods exist that attempt to estimate and remove resting-state FMRI confounds using only the resting-state data itself.
The simplest strategy is to bandpass filter the data so that fluctuations outside the frequency range of interest do not affect functional connectivity measures. Most studies of resting-state BOLD fluctuations perform this step as standard (even when physiological data has been recorded), usually filtering out frequencies lower than ~0.01Hz and greater than ~0.1Hz. Removal of primary cardiac (~1Hz) and respiration (~0.3Hz) frequencies was first demonstrated with such a technique dubbed IMPACT – Image-based Physiological Artifacts estimation and Correction Technique (Chuang and Chen, 2001). However, this is only possible when the TR is short enough (<0.5s) since these confounding frequencies are aliased into the lower frequency range at longer, more standard TRs (2s – 3s) (Lowe et al., 1998). Accounting for non-neural noise by using high sampling rates was popular in the early days of resting-state FMRI (Biswal et al., 1995; Cordes et al., 2001; De Luca et al., 2006; Lowe et al., 1998). Kiviniemi and colleagues, taking advantage of this aliasing of physiological noise, demonstrated that low-frequency BOLD signal fluctuations exist that are unrelated to the aliased cardiac and respiratory frequencies (Kiviniemi et al., 2005). One drawback of low-TR sampling is limited spatial coverage. Recent advances in image acquisition techniques have allowed high-frequency temporal sampling at the same time as whole brain coverage (Domsch et al., 2012; Moeller et al., 2010). Sampling faster than the haemodynamic response may be advantageous for resting-state cleanup strategies. However, any frequency filtering strategy cannot remove low-frequency variations related to physiology, such as arterial CO2 concentration and blood pressure variations, which are in the same frequency range as neural activity-related resting-state BOLD oscillations.
Motion of the participant in the scanner is problematic for all types of FMRI, introducing noise into task-related designs and biasing correlations between regions in resting-state FMRI. Motion correction is a standard preprocessing technique in all FMRI data analyses. Usually six motion regressors are estimated from the data (3 translations, 3 rotations) by determining the amount of movement required to optimally register each brain volume to the previous volume (Friston et al., 1996; Jenkinson et al., 2002). Variance related to the 6 motion regressors can be removed from the BOLD time series’ using a multiple linear regression. Lund and colleagues investigated the effects of residual movement artefacts on intra-subject and inter-subject variability and demonstrated that inclusion of motion parameters in the analyses significantly reduce both types of variance (Lund et al., 2005).
Although inclusion of motion parameters can reduce motion-related FMRI confounds, recent studies have shown that subtle movement artefacts, rather than large head motion artefacts, remain that bias functional connectivity comparisons between groups (Power et al., 2012; Van Dijk et al., 2012). This phenomenon is particularly pronounced when comparing healthy controls to children or patient populations as these groups are more likely to move in the scanner. Power and colleagues proposed a volume censoring technique to deal with this problem dubbed “scrubbing” whereby volumes that were deemed to be affected by excessive motion (using metrics called framewise displacement and DVARS; the first being a measure of instantaneous motion at each time point, the second being a measure of the rate of change of BOLD signal at each time point) are simply ignored in the functional connectivity analyses (Power et al., 2012). There is some debate in the literature about when this scrubbing technique should be applied (Carp, In Press; Power et al., In Press). If scrubbing is performed after temporal filtering of the time series then artefactual motion-related noise from the censored volumes will be smoothed into neighbouring time points (Carp, In Press). It was suggested that instead, “bad” time points should be replaced by interpolating adjacent time points before temporal filtering. However, Power and colleagues argue that the large chunks of data that are replaced may have characteristics of interest that will be lost (Power et al., In Press). Also, variation in degrees-of-freedom lost across subjects using the scrubbing technique must be accounted for. Regression of up to 36 motion-derived parameters along with motion spike regressors to account for movement in individual time points has also been proposed (Satterthwaite et al., 2013). However, the authors note that the procedure does not result in the elimination of motion artefact and so recommend that subject motion be explicitly reported as an outcome measure in resting-state FMRI studies.
Other methods for removing these subtle motion-related confounds require an adjustment to the acquisition of resting-state FMRI data. Bright and Murphy introduced a dual-echo approach in which the time series of the shorter echo is regressed from the longer BOLD echo on a voxelwise basis (Bright and Murphy, 2013). If the TE of the first echo is short enough to minimise BOLD weighting, motion-related and physiological confounds can be successfully removed from resting-state FMRI data. Multi-echo approaches in which M0 and T2* fluctuations can be separated effectively could further enhance this method (Barth et al., 1999; Kundu et al., 2012; Posse et al., 1999; Speck and Hennig, 1998).
The majority of methods from the “Time-series Cleanup with Physiological Recordings” section derive time series from physiological traces and remove the associated variance from BOLD time series using regression methods. Confound regressors can also be derived from the resting-state dataset itself. Global signal regression (GSReg) removes the global mean BOLD signal computed across all voxels in the brain (Desjardins et al., 2001; Greicius et al., 2003; Macey et al., 2004). The assumption is that any process that affects BOLD signals globally must be unrelated to neural activity and, therefore, must be a confound. GSRreg was widely used in early resting-state FMRI studies because it revealed a more consistent and focal pattern of functional connectivity across the brain (Fox et al., 2005; Fox et al., 2009; Greicius et al., 2003). The utility of GSReg as a confound correction technique has been called into question over recent years. Functional connectivity analyses, using the posterior cingulate (PCC) as a seed region, maps the default mode network (DMN). It has been demonstrated that fluctuations in the DMN are anti-correlated with a network that routinely exhibits task-related activations, named the task-positive network (TPN) (Fox et al., 2005). The implication is that these two networks are diametrically opposed at rest with one showing decreasing activation when the other increases neural activity. However, Murphy and colleagues demonstrated mathematically that the process of GSReg forces the sum of correlation values across the brain to be less than or equal to zero and that this anti-correlated network disappears from the results when GSReg is not performed (Murphy et al., 2009).
Many studies have tried to determine whether these anticorrelations are a true reflection of functional connectivity or an artefact of the GSReg technique (Anderson et al., 2011; Chai et al., 2012; Chang and Glover, 2009a; Fox et al., 2009; Fransson, 2005). The first paper to claim that global signal regression uncovers truly anti-correlated networks argued that their spatial distribution, consistency across subjects, presence when the regressed signal was from a subset of all voxels and their existence before global signal regression demonstrated a biological basis (Fox et al., 2009). Since then, studies have attempted to detect the anti-correlated networks with other imaging methods.
Electrophysiological studies in the cat homologues of the DMN and TPN show that the two networks are anti-correlated at most 20% of the time (Popa et al., 2009). In humans, no negative correlations between the dorsal attention network and the DMN were observed in MEG signal (de Pasquale et al., 2010). Furthermore, it has been demonstrated that local field potentials from a single cortical site in monkeys at rest exhibit widespread positive correlations with BOLD signals across the entire brain (Scholvinck et al., 2010). This indicates that the global BOLD signal is tightly coupled with underlying neural activity and so the assumption that it purely represents a confound is questionable. All the evidence suggests that, at best, GSReg will reduce positive correlations that may or may not be spurious but will introduce negative correlations that can not be trusted. Indeed, by modelling group comparisons, it has been shown GSReg can alter local and long-range correlations, potentially spreading group differences that exist only in one region to regions that show no true functional connectivity differences (Saad et al., 2012). Interpretation of functional connectivity results after GSReg is difficult and, therefore, this resting-state FMRI cleanup method should be avoided.
The problems with global signal regression stem from the fact that the fluctuations of interest, that is, the BOLD fluctuations related to neural activity on which we would like to base our functional connectivity measure, contribute to the global signal confound regressor. To this end, alternative methods have been devised that attempt to circumvent that problem. BOLD signals related to neural activity fluctuations should be predominantly present in gray matter. Nuisance regressors derived from other areas such as CSF, white matter, the sagittal sinus or the edges of the brain that are unlikely to show neural activity-induced BOLD signal fluctuations have been employed with some success (Birn et al., 2009; Bright and Murphy, 2013; Weissenbacher et al., 2009). White matter and CSF signal regression improves the specificity of functional connectivity maps (Weissenbacher et al., 2009). Partial volume with gray matter can cause problems, therefore erosion of the white matter and ventricular CSF masks is advised (Jo et al., 2010). Similarly, a method called CompCor derives confound regressors from the principal components of the BOLD signal in white matter and CSF to avoid generating a time series that is modulated by neural activity (Behzadi et al., 2007). Nuisance regressors can also be constructed from soft tissue far from the brain such as the face and the calvarium which can represent significant noise variance in the BOLD data (Anderson et al., 2011).
Independent component analyses (ICA) is a successful technique for detecting consistent resting-state networks (Bartels and Zeki, 2004; Beckmann et al., 2005; Damoiseaux et al., 2006; Kiviniemi et al., 2003). The ICA method separates resting-state FMRI data into spatial components that are mathematically independent and determines the associated time series. If noise sources are independent from neural activity-related BOLD fluctuations, ICA is able to isolate one type of signal from the other. However, the results of ICA yield one time course per resting-state network and don’t allow the detailed analysis of connectivity between nodes of the same network that seed correlation approaches provide. Various noise correction methods use ICA to isolate physiological noise components to remove them from resting-state data allowing further seed region analyses. CORSICA uses spatial ICA to identify and remove signal fluctuations that match known spatial patterns of physiological noise (Perlbarg et al., 2007). PESTICA, using a similar approach, estimates cardiac and respiratory fluctuations from resting-state data with temporal ICA, generating spatial weight matrices that can be applied to other resting-state data (Beall and Lowe, 2007). Using multi-echo BOLD acquisition, Kundu and colleagues have demonstrated an automated ICA sorting algorithm that can separate BOLD from non-BOLD signals (Kundu et al., 2012).
Group level cleanup
The resting-state FMRI cleanup techniques described in the last two sections are concerned with resting-state confound correction in BOLD signal time series. After removal of these confounds, one would expect functional connectivity measures to be less biased. However, physiological differences between groups may still confound group comparisons. Changes in vascular structure and integrity with age and disease cause many changes in neurovascular coupling: atherosclerosis, increased tortuosity, changes in collateral circulation after recanalization of occluded cerebral vessels, reduction in resting CBF, changes in vascular reactivity, lowered resting CMRO2 (D’Esposito et al., 2003). Each of these age-related changes can affect how a given amount of fluctuation in neural activity is translated into a BOLD signal fluctuation.
Focussing on vascular reactivity, decreased vascular responsiveness to hypercapnia has been observed in aged rats (Tamaki et al., 1995). This suggests that for a given amount of neural fluctuation, the corresponding CBF fluctuations will be reduced in amplitude in older people. Results presented by Biswal and colleagues showing changes in connectivity and fluctuation amplitude with age may be evidence of this (Biswal et al., 2010). Murphy and colleagues have demonstrated that by measuring an individual’s vascular reactivity using a breath-hold task, variance in across group measures of functional connectivity can be reduced as evidenced by increased spatial extent and statistical significance of resting-state networks (Murphy et al., 2011).
An example of altered vasculature affecting resting-state FMRI measures can be found in pharmacological studies where the drugs may alter vascular properties such as resting CBF levels and vascular reactivity (Iannetti and Wise, 2007). Caffeine has been shown to reduce resting-state BOLD functional connectivity measures in the visual cortex (Rack-Gomer et al., 2009). However, corresponding reductions in baseline CBF demonstrate that this phenomenon may have a vascular along with a neural component. A recent study into psilocybin examining functional connectivity differences caused by the drug presented more convincing results by accounting for CBF alterations and vascular reactivity differences between drug and placebo sessions (Carhart-Harris et al., 2012).
Other group-level cleanup methods could also be performed. Any physiological quantity that varies across a group and that is thought to affect the BOLD signal change to neural activity could be used. For example, resting heart rate, resting systolic and diastolic blood pressure, resting end-tidal CO2 levels and even age may be used as covariates at a group level. It is important to bear in mind that there may be a linear relationship between these confounds and the underlying true neural activity levels across the group, for example, the underlying neural activity levels may decrease with age (D’Esposito et al., 2003). Therefore, caution should be used when interpreting results after such group level corrections.
Caveats
Resting-state FMRI confound removal should increase confidence in functional connectivity results, however, we must be aware of two caveats. The first is that cleanup methods may result in a variable loss of degrees-of-freedom that can be offset by adjusting the length of the resting-state scan. This may not be troublesome if all data in a study are processed so that each subject/group suffers an identical reduction in the degrees-of-freedom. The second is more serious. Variations in cardiac rate, respiration, arterial CO2 concentration or blood pressure may be correlated with the variations in neural activity that we would like the BOLD signal to capture. For example, heart rate variability is an indicator of emotional arousal and autonomic nervous system activity (Macefield, 2009) and breathing variations are often tied to emotional state (Shea, 1996). If neural activity in the resting-state network under investigation is linked to, or synchronous with, any of these physiological processes, removing the physiological confound will also remove these neural activity-related BOLD fluctuations. Since resting-state FMRI is based on spontaneous fluctuations in BOLD and the relationship between the resting-state network and physiological processes may not be known, resting-state FMRI confound removal should always be performed with this caveat in mind.
Conclusions
The BOLD signal arises from a complicated interaction between neural activity and vascular processes. Resting-state FMRI uses the temporal similarity of intrinsic BOLD signal fluctuations across brain regions as an indicator of synchronous neural activity. However, the influence of noise confounds on measures of functional connectivity is too often ignored. The utility of resting-state FMRI lies in examining differences between sessions or groups to determine if there is a relationship between altered functional connectivity and behavioural/clinical symptoms. Unfortunately for the field, many of the sources of resting-state FMRI confounds, as outlined in this paper, also vary between sessions or groups, rendering the results of such studies difficult to interpret. The resting-state FMRI cleanup methods described in this paper go some way to providing useful confound removal. However, many have focussed on cardiac and respiratory processes that are easy to record and have largely ignored other sources such as arterial CO2 concentration and blood pressure that may provide complementary information. Further refinement of resting-state FMRI noise removal is of paramount importance, always keeping in mind the caveat that the noise may be coupled to the signals of interest. The field of resting-state FMRI would certainly benefit from an increased effort in noise correction techniques, paving the way for more advanced applications to the healthy and diseased brain.
Highlights.
Resting-state FMRI measures temporal similarity between BOLD signals
Confounds can arise that affect the BOLD signals leading to spurious results
Motion, cardiac/respiration, arterial CO2 concentration & blood pressure are sources
Techniques to remove resting-state FMRI confounds are reviewed
Noise correction is an essential step for resting-state FMRI analyses
Acknowledgments
Kevin Murphy is supported by a Wellcome Trust Research Career Development Fellowship. Rasmus Birn is funded in part by NIH grant RC1MH090912 and by the Health Emotions Research Institute. Peter Bandettini is funded by the National Institute of Mental Health Intramural Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aalkjaer C, Boedtkjer D, Matchkov V. Vasomotion - what is currently thought? Acta Physiol (Oxf) 2011;202:253–269. doi: 10.1111/j.1748-1716.2011.02320.x. [DOI] [PubMed] [Google Scholar]
- Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213:220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- Anderson JS, Druzgal TJ, Lopez-Larson M, Jeong EK, Desai K, Yurgelun-Todd D. Network anticorrelations, global regression, and phase-shifted soft tissue correction. Hum Brain Mapp. 2011;32:919–934. doi: 10.1002/hbm.21079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. Processing strategies for time-course data sets in functional MRI of the human brain. Magn Reson Med. 1993;30:161–173. doi: 10.1002/mrm.1910300204. [DOI] [PubMed] [Google Scholar]
- Bandettini PA, Wong EC, Hinks RS, Tikofsky RS, Hyde JS. Time course EPI of human brain function during task activation. Magn Reson Med. 1992;25:390–397. doi: 10.1002/mrm.1910250220. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The chronoarchitecture of the human brain--natural viewing conditions reveal a time-based anatomy of the brain. Neuroimage. 2004;22:419–433. doi: 10.1016/j.neuroimage.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Barth M, Metzler A, Klarhofer M, Roll S, Moser E, Leibfritz D. Functional MRI of the human motor cortex using single-shot, multiple gradient-echo spiral imaging. Magn Reson Imaging. 1999;17:1239–1243. doi: 10.1016/s0730-725x(99)00087-9. [DOI] [PubMed] [Google Scholar]
- Beall EB, Lowe MJ. Isolating physiologic noise sources with independently determined spatial measures. Neuroimage. 2007;37:1286–1300. doi: 10.1016/j.neuroimage.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya PK, Lowe MJ. Cardiac-induced physiologic noise in tissue is a direct observation of cardiac-induced fluctuations. Magn Reson Imaging. 2004;22:9–13. doi: 10.1016/j.mri.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Bianciardi M, Fukunaga M, van Gelderen P, Horovitz SG, de Zwart JA, Shmueli K, Duyn JH. Sources of functional magnetic resonance imaging signal fluctuations in the human brain at rest: a 7 T study. Magn Reson Imaging. 2009;27:1019–1029. doi: 10.1016/j.mri.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM. The role of physiological noise in resting-state functional connectivity. Neuroimage. 2012;62:864–870. doi: 10.1016/j.neuroimage.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Birn RM, Diamond JB, Smith MA, Bandettini PA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage. 2006;31:1536–1548. doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- Birn RM, Murphy K, Handwerker DA, Bandettini PA. fMRI in the presence of task-correlated breathing variations. Neuroimage. 2009;47:1092–1104. doi: 10.1016/j.neuroimage.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Smith MA, Jones TB, Bandettini PA. The respiration response function: the temporal dynamics of fMRI signal fluctuations related to changes in respiration. Neuroimage. 2008;40:644–654. doi: 10.1016/j.neuroimage.2007.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Kannurpatti SS. Resting-state functional connectivity in animal models: modulations by exsanguination. Methods Mol Biol. 2009;489:255–274. doi: 10.1007/978-1-59745-543-5_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kotter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SA, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, Whitfield-Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang HY, Castellanos FX, Milham MP. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodurka J, Ye F, Petridou N, Murphy K, Bandettini PA. Mapping the MRI voxel volume in which thermal noise matches physiological noise--implications for fMRI. Neuroimage. 2007;34:542–549. doi: 10.1016/j.neuroimage.2006.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright MG, Bulte DP, Jezzard P, Duyn JH. Characterization of regional heterogeneity in cerebrovascular reactivity dynamics using novel hypocapnia task and BOLD fMRI. Neuroimage. 2009;48:166–175. doi: 10.1016/j.neuroimage.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright MG, Murphy K. Removing motion and physiological artifacts from intrinsic BOLD fluctuations using short echo data. Neuroimage. 2013;64:526–537. doi: 10.1016/j.neuroimage.2012.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosch JR, Talavage TM, Ulmer JL, Nyenhuis JA. Simulation of human respiration in fMRI with a mechanical model. IEEE Trans Biomed Eng. 2002;49:700–707. doi: 10.1109/TBME.2002.1010854. [DOI] [PubMed] [Google Scholar]
- Buxton RB. Introduction to funtional magnetic resonance imaging: principles and techniques. Cambridge University Press; Cambridge, UK: 2002. [Google Scholar]
- Carhart-Harris RL, Erritzoe D, Williams T, Stone JM, Reed LJ, Colasanti A, Tyacke RJ, Leech R, Malizia AL, Murphy K, Hobden P, Evans J, Feilding A, Wise RG, Nutt DJ. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci U S A. 2012;109:2138–2143. doi: 10.1073/pnas.1119598109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp J. Optimizing the order of operations for movement scrubbing: Comment on Power et al. Neuroimage. doi: 10.1016/j.neuroimage.2011.12.061. In Press. [DOI] [PubMed] [Google Scholar]
- Chai XJ, Castanon AN, Ongur D, Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. Neuroimage. 2012;59:1420–1428. doi: 10.1016/j.neuroimage.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Cunningham JP, Glover GH. Influence of heart rate on the BOLD signal: the cardiac response function. Neuroimage. 2009;44:857–869. doi: 10.1016/j.neuroimage.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Glover GH. Effects of model-based physiological noise correction on default mode network anti-correlations and correlations. Neuroimage. 2009a;47:1448–1459. doi: 10.1016/j.neuroimage.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Glover GH. Relationship between respiration, end-tidal CO2, and BOLD signals in resting-state fMRI. Neuroimage. 2009b;47:1381–1393. doi: 10.1016/j.neuroimage.2009.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang KH, Chen JH. IMPACT: image-based physiological artifacts estimation and correction technique for functional MRI. Magn Reson Med. 2001;46:344–353. doi: 10.1002/mrm.1197. [DOI] [PubMed] [Google Scholar]
- Cohen MA, Taylor JA. Short-term cardiovascular oscillations in man: measuring and modelling the physiologies. J Physiol. 2002;542:669–683. doi: 10.1113/jphysiol.2002.017483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat Rev Neurosci. 2003;4:863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- Dagli MS, Ingeholm JE, Haxby JV. Localization of cardiac-induced signal change in fMRI. Neuroimage. 1999;9:407–415. doi: 10.1006/nimg.1998.0424. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TL, Kwong KK, Weisskoff RM, Rosen BR. Calibrated functional MRI: mapping the dynamics of oxidative metabolism. Proc Natl Acad Sci U S A. 1998;95:1834–1839. doi: 10.1073/pnas.95.4.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage. 2006;29:1359–1367. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- de Munck JC, Goncalves SI, Faes TJ, Kuijer JP, Pouwels PJ, Heethaar RM, Lopes da Silva FH. A study of the brain’s resting state based on alpha band power, heart rate and fMRI. Neuroimage. 2008;42:112–121. doi: 10.1016/j.neuroimage.2008.04.244. [DOI] [PubMed] [Google Scholar]
- de Pasquale F, Della Penna S, Snyder AZ, Lewis C, Mantini D, Marzetti L, Belardinelli P, Ciancetta L, Pizzella V, Romani GL, Corbetta M. Temporal dynamics of spontaneous MEG activity in brain networks. Proc Natl Acad Sci U S A. 2010;107:6040–6045. doi: 10.1073/pnas.0913863107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins AE, Kiehl KA, Liddle PF. Removal of confounding effects of global signal in functional MRI analyses. Neuroimage. 2001;13:751–758. doi: 10.1006/nimg.2000.0719. [DOI] [PubMed] [Google Scholar]
- Diehl RR, Linden D, Lucke D, Berlit P. Phase relationship between cerebral blood flow velocity and blood pressure. A clinical test of autoregulation. Stroke. 1995;26:1801–1804. doi: 10.1161/01.str.26.10.1801. [DOI] [PubMed] [Google Scholar]
- Domsch S, Lemke A, Weingartner S, Schad LR. A novel temporal filtering strategy for functional MRI using UNFOLD. Neuroimage. 2012;62:59–66. doi: 10.1016/j.neuroimage.2012.03.064. [DOI] [PubMed] [Google Scholar]
- Ekstrom-Jodal B, Haggendal E, Linder LE, Nilsson NJ. Cerebral blood flow autoregulation at high arterial pressures and different levels of carbon dioxide tension in dogs. Eur Neurol. 1971;6:6–10. doi: 10.1159/000114457. [DOI] [PubMed] [Google Scholar]
- Failla M, Grappiolo A, Emanuelli G, Vitale G, Fraschini N, Bigoni M, Grieco N, Denti M, Giannattasio C, Mancia G. Sympathetic tone restrains arterial distensibility of healthy and atherosclerotic subjects. J Hypertens. 1999;17:1117–1123. doi: 10.1097/00004872-199917080-00011. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick B, Nickerson LD, Tong Y. Physiological denoising of BOLD fMRI data using Regressor Interpolation at Progressive Time Delays (RIPTiDe) processing of concurrent fMRI and near-infrared spectroscopy (NIRS) Neuroimage. 2012;60:1913–1923. doi: 10.1016/j.neuroimage.2012.01.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R. Analysis of fMRI time-series revisited. Neuroimage. 1995;2:45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magn Reson Med. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44:162–167. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AK, Pattinson KT, Brooks JC, Mayhew SD, Jenkinson M, Wise RG. Brainstem functional magnetic resonance imaging: disentangling signal from physiological noise. J Magn Reson Imaging. 2008;28:1337–1344. doi: 10.1002/jmri.21623. [DOI] [PubMed] [Google Scholar]
- Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex. Proc Natl Acad Sci U S A. 1999;96:9403–9408. doi: 10.1073/pnas.96.16.9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Le TH, Parrish T, Erhard P. Retrospective estimation and correction of physiological fluctuation in functional MRI. Magn Reson Med. 1995;34:201–212. doi: 10.1002/mrm.1910340211. [DOI] [PubMed] [Google Scholar]
- Hudetz AG, Biswal BB, Shen H, Lauer KK, Kampine JP. Spontaneous fluctuations in cerebral oxygen supply. An introduction. Adv Exp Med Biol. 1998;454:551–559. doi: 10.1007/978-1-4615-4863-8_66. [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Wise RG. BOLD functional MRI in disease and pharmacological studies: room for improvement? Magn Reson Imaging. 2007;25:978–988. doi: 10.1016/j.mri.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Ide K, Eliasziw M, Poulin MJ. Relationship between middle cerebral artery blood velocity and end-tidal PCO2 in the hypocapnic-hypercapnic range in humans. J Appl Physiol. 2003;95:129–137. doi: 10.1152/japplphysiol.01186.2002. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jezzard P, Matthews PM, Smith SM. Functional MRI: an introduction to methods. Oxford University Press; Oxford, UK: 2001. [Google Scholar]
- Jo HJ, Saad ZS, Simmons WK, Milbury LA, Cox RW. Mapping sources of correlation in resting state FMRI, with artifact detection and removal. Neuroimage. 2010;52:571–582. doi: 10.1016/j.neuroimage.2010.04.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastrup A, Kruger G, Glover GH, Neumann-Haefelin T, Moseley ME. Regional variability of cerebral blood oxygenation response to hypercapnia. Neuroimage. 1999;10:675–681. doi: 10.1006/nimg.1999.0505. [DOI] [PubMed] [Google Scholar]
- Katura T, Tanaka N, Obata A, Sato H, Maki A. Quantitative evaluation of interrelations between spontaneous low-frequency oscillations in cerebral hemodynamics and systemic cardiovascular dynamics. Neuroimage. 2006;31:1592–1600. doi: 10.1016/j.neuroimage.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Kiviniemi V, Kantola JH, Jauhiainen J, Hyvarinen A, Tervonen O. Independent component analysis of nondeterministic fMRI signal sources. Neuroimage. 2003;19:253–260. doi: 10.1016/s1053-8119(03)00097-1. [DOI] [PubMed] [Google Scholar]
- Kiviniemi V, Ruohonen J, Tervonen O. Separation of physiological very low frequency fluctuation from aliasing by switched sampling interval fMRI scans. Magn Reson Imaging. 2005;23:41–46. doi: 10.1016/j.mri.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Kontos HA, Wei EP, Navari RM, Levasseur JE, Rosenblum WI, Patterson JL., Jr Responses of cerebral arteries and arterioles to acute hypotension and hypertension. Am J Physiol. 1978;234:H371–383. doi: 10.1152/ajpheart.1978.234.4.H371. [DOI] [PubMed] [Google Scholar]
- Kruger G, Glover GH. Physiological noise in oxygenation-sensitive magnetic resonance imaging. Magn Reson Med. 2001;46:631–637. doi: 10.1002/mrm.1240. [DOI] [PubMed] [Google Scholar]
- Kundu P, Inati SJ, Evans JW, Luh WM, Bandettini PA. Differentiating BOLD and non-BOLD signals in fMRI time series using multi-echo EPI. Neuroimage. 2012;60:1759–1770. doi: 10.1016/j.neuroimage.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagopoulos J, Malhi GS, Cahill AM, Lang EW, Mudalier Y, Dorsch N, Yam A, Griffith J, Mulvey J. Cerebrovascular autoregulation as a neuroimaging tool. Acta Neuropsychiatrica. 2006;18:100–104. doi: 10.1111/j.1601-5215.2006.00133.x. [DOI] [PubMed] [Google Scholar]
- Lang EW, Diehl RR, Timmermann L, Baron R, Deuschl G, Mehdorn HM, Zunker P. Spontaneous oscillations of arterial blood pressure, cerebral and peripheral blood flow in healthy and comatose subjects. Neurol Res. 1999;21:665–669. doi: 10.1080/01616412.1999.11740995. [DOI] [PubMed] [Google Scholar]
- Laufs H, Krakow KSP, Eger E, Beyerle A, Salek-Haddadi A, Kleinschmidt A. Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. Proc Natl Acad Sci U S A. 2003;100:11053–11058. doi: 10.1073/pnas.1831638100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold DA, Maier A. Ongoing physiological processes in the cerebral cortex. Neuroimage. 2012;62:2190–2200. doi: 10.1016/j.neuroimage.2011.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold DA, Murayama Y, Logothetis NK. Very slow activity fluctuations in monkey visual cortex: implications for functional brain imaging. Cereb Cortex. 2003;13:422–433. doi: 10.1093/cercor/13.4.422. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- Lund TE, Norgaard MD, Rostrup E, Rowe JB, Paulson OB. Motion or activity: their role in intra- and inter-subject variation in fMRI. Neuroimage. 2005;26:960–964. doi: 10.1016/j.neuroimage.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Macefield VG. Developments in autonomic research: a review of the latest literature. Clin Auton Res. 2009;19:193–196. doi: 10.1007/s10286-009-0024-3. [DOI] [PubMed] [Google Scholar]
- Macey PM, Macey KE, Kumar R, Harper RM. A method for removal of global effects from fMRI time series. Neuroimage. 2004;22:360–366. doi: 10.1016/j.neuroimage.2003.12.042. [DOI] [PubMed] [Google Scholar]
- Madjar C, Gauthier CJ, Bellec P, Birn RM, Brooks JC, Hoge RD. Task-related BOLD responses and resting-state functional connectivity during physiological clamping of end-tidal CO(2) Neuroimage. 2012;61:41–49. doi: 10.1016/j.neuroimage.2012.02.080. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Mitsis GD, Poulin MJ, Robbins PA, Marmarelis VZ. Nonlinear modeling of the dynamic effects of arterial pressure and CO2 variations on cerebral blood flow in healthy humans. IEEE Trans Biomed Eng. 2004;51:1932–1943. doi: 10.1109/TBME.2004.834272. [DOI] [PubMed] [Google Scholar]
- Modarreszadeh M, Bruce EN. Ventilatory variability induced by spontaneous variations of PaCO2 in humans. J Appl Physiol. 1994;76:2765–2775. doi: 10.1152/jappl.1994.76.6.2765. [DOI] [PubMed] [Google Scholar]
- Moeller S, Yacoub E, Olman CA, Auerbach E, Strupp J, Harel N, Ugurbil K. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn Reson Med. 2010;63:1144–1153. doi: 10.1002/mrm.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita-Tsuzuki Y, Bouskela E, Hardebo JE. Vasomotion in the rat cerebral microcirculation recorded by laser-Doppler flowmetry. Acta Physiol Scand. 1992;146:431–439. doi: 10.1111/j.1748-1716.1992.tb09444.x. [DOI] [PubMed] [Google Scholar]
- Muresan L, Renken R, Roerdink JB, Duifhuis H. Automated correction of spin-history related motion artefacts in fMRI: simulated and phantom data. IEEE Trans Biomed Eng. 2005;52:1450–1460. doi: 10.1109/TBME.2005.851484. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Bodurka J, Bandettini PA. How long to scan? The relationship between fMRI temporal signal to noise ratio and necessary scan duration. Neuroimage. 2007;34:565–574. doi: 10.1016/j.neuroimage.2006.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Harris AD, Wise RG. Robustly measuring vascular reactivity differences with breath-hold: normalising stimulus-evoked and resting state BOLD fMRI data. Neuroimage. 2011;54:369–379. doi: 10.1016/j.neuroimage.2010.07.059. [DOI] [PubMed] [Google Scholar]
- Nagaoka T, Zhao F, Wang P, Harel N, Kennan RP, Ogawa S, Kim SG. Increases in oxygen consumption without cerebral blood volume change during visual stimulation under hypotension condition. J Cereb Blood Flow Metab. 2006;26:1043–1051. doi: 10.1038/sj.jcbfm.9600251. [DOI] [PubMed] [Google Scholar]
- Obrig H, Neufang M, Wenzel R, Kohl M, Steinbrink J, Einhaupl K, Villringer A. Spontaneous low frequency oscillations of cerebral hemodynamics and metabolism in human adults. Neuroimage. 2000;12:623–639. doi: 10.1006/nimg.2000.0657. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Tank DW, Menon R, Ellermann JM, Kim SG, Merkle H, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci U S A. 1992;89:5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlbarg V, Bellec P, Anton JL, Pelegrini-Issac M, Doyon J, Benali H. CORSICA: correction of structured noise in fMRI by automatic identification of ICA components. Magn Reson Imaging. 2007;25:35–46. doi: 10.1016/j.mri.2006.09.042. [DOI] [PubMed] [Google Scholar]
- Popa D, Popescu AT, Pare D. Contrasting activity profile of two distributed cortical networks as a function of attentional demands. J Neurosci. 2009;29:1191–1201. doi: 10.1523/JNEUROSCI.4867-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posse S, Kemna LJ, Elghahwagi B, Wiese S, Kiselev VG. Effect of graded hypo- and hypercapnia on fMRI contrast in visual cortex: quantification of T(*)(2) changes by multiecho EPI. Magn Reson Med. 2001;46:264–271. doi: 10.1002/mrm.1187. [DOI] [PubMed] [Google Scholar]
- Posse S, Wiese S, Gembris D, Mathiak K, Kessler C, Grosse-Ruyken ML, Elghahwagi B, Richards T, Dager SR, Kiselev VG. Enhancement of BOLD-contrast sensitivity by single-shot multi-echo functional MR imaging. Magn Reson Med. 1999;42:87–97. doi: 10.1002/(sici)1522-2594(199907)42:1<87::aid-mrm13>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Poulin MJ, Liang PJ, Robbins PA. Dynamics of the cerebral blood flow response to step changes in end-tidal PCO2 and PO2 in humans. J Appl Physiol. 1996;81:1084–1095. doi: 10.1152/jappl.1996.81.3.1084. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Steps toward optimizing motion artifact removal in functional connectivity MRI; a reply to Carp. Neuroimage. doi: 10.1016/j.neuroimage.2012.03.017. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao M, Rushforth D, Wang R, Shaw RA, Tomanek B, Dunn JF, Tuor UI. Blood-oxygen-level-dependent magnetic resonance signal and cerebral oxygenation responses to brain activation are enhanced by concurrent transient hypertension in rats. J Cereb Blood Flow Metab. 2007;27:1280–1289. doi: 10.1038/sj.jcbfm.9600436. [DOI] [PubMed] [Google Scholar]
- Rack-Gomer AL, Liau J, Liu TT. Caffeine reduces resting-state BOLD functional connectivity in the motor cortex. Neuroimage. 2009;46:56–63. doi: 10.1016/j.neuroimage.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Stone HL. Cerebral blood flow autoregulation and graded hypercapnia. Eur Neurol. 1971;6:1–5. doi: 10.1159/000114443. [DOI] [PubMed] [Google Scholar]
- Raj D, Anderson AW, Gore JC. Respiratory effects in human functional magnetic resonance imaging due to bulk susceptibility changes. Phys Med Biol. 2001;46:3331–3340. doi: 10.1088/0031-9155/46/12/318. [DOI] [PubMed] [Google Scholar]
- Rostrup E, Law I, Blinkenberg M, Larsson HB, Born AP, Holm S, Paulson OB. Regional differences in the CBF and BOLD responses to hypercapnia: a combined PET and fMRI study. Neuroimage. 2000;11:87–97. doi: 10.1006/nimg.1999.0526. [DOI] [PubMed] [Google Scholar]
- Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, Cox RW. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2012;2:25–32. doi: 10.1089/brain.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Eickhoff SB, Hakonarson H, Gur RC, Gur RE, Wolf DH. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, Gur RC, Gur RE. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60:623–632. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholvinck ML, Maier A, Ye FQ, Duyn JH, Leopold DA. Neural basis of global resting-state fMRI activity. Proc Natl Acad Sci U S A. 2010;107:10238–10243. doi: 10.1073/pnas.0913110107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea SA. Behavioural and arousal-related influences on breathing in humans. Exp Physiol. 1996;81:1–26. doi: 10.1113/expphysiol.1996.sp003911. [DOI] [PubMed] [Google Scholar]
- Shmueli K, van Gelderen P, de Zwart JA, Horovitz SG, Fukunaga M, Jansma JM, Duyn JH. Low-frequency fluctuations in the cardiac rate as a source of variance in the resting-state fMRI BOLD signal. Neuroimage. 2007;38:306–320. doi: 10.1016/j.neuroimage.2007.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slessarev M, Han J, Mardimae A, Prisman E, Preiss D, Volgyesi G, Ansel C, Duffin J, Fisher JA. Prospective targeting and control of end-tidal CO2 and O2 concentrations. J Physiol. 2007;581:1207–1219. doi: 10.1113/jphysiol.2007.129395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck O, Hennig J. Functional imaging by I0- and T2*-parameter mapping using multi-image EPI. Magn Reson Med. 1998;40:243–248. doi: 10.1002/mrm.1910400210. [DOI] [PubMed] [Google Scholar]
- Tamaki K, Nakai M, Yokota T, Ogata J. Effects of aging and chronic hypertension on cerebral blood flow and cerebrovascular CO2 reactivity in the rat. Gerontology. 1995;41:11–17. doi: 10.1159/000213657. [DOI] [PubMed] [Google Scholar]
- Triantafyllou C, Hoge RD, Krueger G, Wiggins CJ, Potthast A, Wiggins GC, Wald LL. Comparison of physiological noise at 1.5 T, 3 T and 7 T and optimization of fMRI acquisition parameters. Neuroimage. 2005;26:243–250. doi: 10.1016/j.neuroimage.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Van den Aardweg JG, Karemaker JM. Influence of chemoreflexes on respiratory variability in healthy subjects. Am J Respir Crit Care Med. 2002;165:1041–1047. doi: 10.1164/ajrccm.165.8.2104100. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gelderen P, de Zwart JA, Starewicz P, Hinks RS, Duyn JH. Real-time shimming to compensate for respiration-induced B0 fluctuations. Magn Reson Med. 2007;57:362–368. doi: 10.1002/mrm.21136. [DOI] [PubMed] [Google Scholar]
- Wang R, Foniok T, Wamsteeker JI, Qiao M, Tomanek B, Vivanco RA, Tuor UI. Transient blood pressure changes affect the functional magnetic resonance imaging detection of cerebral activation. Neuroimage. 2006;31:1–11. doi: 10.1016/j.neuroimage.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, Windischberger C. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. Neuroimage. 2009;47:1408–1416. doi: 10.1016/j.neuroimage.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Wise RG, Ide K, Poulin MJ, Tracey I. Resting fluctuations in arterial carbon dioxide induce significant low frequency variations in BOLD signal. Neuroimage. 2004;21:1652–1664. doi: 10.1016/j.neuroimage.2003.11.025. [DOI] [PubMed] [Google Scholar]
- Wise RG, Pattinson KT, Bulte DP, Chiarelli PA, Mayhew SD, Balanos GM, O’Connor DF, Pragnell TR, Robbins PA, Tracey I, Jezzard P. Dynamic forcing of end-tidal carbon dioxide and oxygen applied to functional magnetic resonance imaging. J Cereb Blood Flow Metab. 2007;27:1521–1532. doi: 10.1038/sj.jcbfm.9600465. [DOI] [PubMed] [Google Scholar]
- Zhao X, Bodurka J, Jesmanowicz A, Li SJ. B(0)-fluctuation-induced temporal variation in EPI image series due to the disturbance of steady-state free precession. Magn Reson Med. 2000;44:758–765. doi: 10.1002/1522-2594(200011)44:5<758::aid-mrm14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]