Abstract
The role of Janus kinase (JAK)-3 in TLR-mediated innate immune responses is poorly understood, although the suppressive function of JAK3 inhibition in adaptive immune response has been well studied. In this study, we found that JAK3 inhibition enhanced TLR-mediated immune responses by differentially regulating pro- and anti- inflammatory cytokine production in innate immune cells. Specifically, JAK3 inhibition by pharmacological inhibitors or specific siRNA, or JAK3 gene knockout resulted in an increase in TLR-mediated production of pro-inflammatory cytokines, while concurrently decreasing the production of IL-10. Inhibition of JAK3 suppressed phosphorylation of PI3 kinase downstream effectors including Akt, mTORC1, GSK3β and CREB. Constitutive activation of Akt or inhibition of GSK3β abrogated the capability of JAK3 inhibition to enhance pro-inflammatory cytokines and suppress IL-10 production. In contrast, inhibition of PI3K enhanced this regulatory ability of JAK3 in LPS stimulated monocytes. At the transcriptional level, JAK3 knockout lead to the increased phosphorylation of STATs that could be attenuated by neutralization of de novo inflammatory cytokines. JAK3 inhibition exhibited a GSK3 activity-dependent ability to enhance phosphorylation levels and DNA binding of NF-κB p65. Moreover, JAK3 inhibition correlated with an increased CD4+ T cell response. Additionally, higher neutrophil infiltration, IL-17 expression, and intestinal epithelium erosion were observed in JAK3 knockout mice. These findings demonstrate the negative regulatory function of JAK3, and elucidate the signaling pathway by which JAK3 differentially regulates TLR-mediated inflammatory cytokine production in innate immune cells.
Keywords: JAK3, PI3K, GSK3, TLR4, Inflammatory cytokines
Introduction
Toll-like receptor (TLR)-mediated inflammatory cytokine production plays a critical role in the activation of innate and acquired immune responses. The magnitude and quality of cytokines must be fine-tuned to achieve the effective clearance of pathogens while limiting the amount of inflammation to avoid toxicity and collateral tissue damage (1–3). Thus, the mechanisms modulating TLR mediated cytokine production have attracted considerable attention. TLR engagements initiate several signaling pathways through various combinations of the adapter molecules MyD88, TIRAP, TRIF, TRAM and SARM (4). TLR4 signaling is the most complex as all 5 adapters are involved. Activation of TIRAP-MyD88 leads to the activation of IRAK4, in turn activating TRAF6 and, ultimately, leading to the activation of the NF-κB pathway that mediates the production of pro- and anti- inflammatory cytokines. In contrast, the recruitment of TRAM and TRIF mediates a signaling cascade involving the activation of two non-canonical IκB kinases, TBK-1 and IKK-ε, as well as the phospho-specific translational modification of the transcription factors NF-κB, ATF2/c-Jun, and IRF3 that subsequently induce type I IFN and an anti-viral response (5, 6). SARM acts as a negative regulator of TLR signaling by directly suppressing the function of TRIF, TRIF-dependent IRF7 and NF-κB activities (7, 8). Although a significant body of literature exists on TLR signaling, many key elements involved in the regulation of TLR signaling remain unknown, namely the role of upstream tyrosine kinases on TLR functions and how they ultimately shape cytokine profiles upon pathogen associated molecular pattern (PAMP) recognition.
Tyrosine phosphorylation is one of the earliest events noted after TLR ligation and tyrosine kinases are involved in the regulation of TLRs mediated inflammation in at least two ways. Tyrosine kinases can either act as components of TLR initiated signaling pathways and, thus, directly control cytokine production, or they can be involved in the signaling stimulated by TLR-induced cytokines (9, 10). Janus kinase is an important tyrosine kinase family that includes JAK1, JAK2, JAK3 and Tyk2 (9, 11). Past studies have showed that defects of JAK3 can result in some of the commonest inherited severe immunodeficiencies (12, 13). Although the functional role of JAK3 in adaptive immune responses has been well investigated, the impact of JAK3 on TLR-mediated innate immune responses is controversial and the underlying mechanisms remain unclear (14–17). In this regard, O’Shea et al. (14) initially reported that JAK3 knock out enhanced the production of IL-12 and IL-10 in LPS stimulated bone marrow derived dendritic cells (BMDC) which subsequently resulted in exaggerated Th1 differentiation. In contrast, Wewers et al. (17) showed that JAK3 knockout in macrophages significantly reduced TLR4-mediated IL-10 production, which resulted in enhanced ICE activity that consequently augmented the production of IL-1β. Thus, these studies showed differing effects of JAK3 on TLR4 mediated pro- and anti-inflammatory cytokine production and the function and role of JAK3 remains to be determined.
Here we report that JAK3 negatively regulates TLR4-mediated inflammatory responses through differentially regulating pro- and anti-inflammatory cytokine production. Inhibition of JAK3 attenuated TLR4-mediated activation of PI3K and then suppressed the phosphorylation of Akt and its downstream constitutive active kinase, GSK3β, which augmented pro-inflammatory cytokine level and repressed IL-10 production via enhancing NF-κB activity and decreasing CREB activity, respectively. Moreover, JAK3 inhibition resulted in the increase of CD4+ T cell responses in vitro along with enhanced PMN infiltration, IL-17 expression, as well as erosion of the intestinal epithelium. Collectively, these results identify the negative regulatory role of JAK3 in TLR4-mediated inflammatory responses and characterize the role of PI3K-Akt- GSK3β signaling pathway in JAK3-mediated control of inflammatory responses.
Material and Methods
Mice and reagents
B6129SF2 and JAK3 homozygous mutant mice (129S4-Jak3tm1Ljb/J) were purchased from The Jackson Laboratory. Mice were housed in a specific pathogen-free facility at the University of Louisville School of Medicine, and the University of Louisville Institutional Animal Care and Use Committee approved all animal protocols. Pam3CSK4, Flagelin and ultra pure LPS from E. coli 0111:B4 were purchased from Invivogen. Phosphorylated and total JAK3 antibody was purchased from Assay Biotec. Isotype-matched control antibody (IgG1) and functional grade neutralizing anti-human IL-10 (Clone JES3–9D7) mAb were purchased from eBioscience. All other antibodies were obtained from Cell Signaling Technology. The GSK3 inhibitor SB216763 (Toris) has been characterized and shown to be specific for GSK3 without discernible effects on a panel of 24 other kinases (18). JAK3 inhibitor WHIP-154 and T-1377/(CP-690550) was purchased from Tocris and LC Laboratories, respectively. PI3K inhibitor LY294002 was purchased fro LC Laboratories. Non-targeting pools of siRNA and a mixture of four pre-validated siRNA duplexes specific for GSK3β or JAK3 (ON TARGET-plus™) were purchased from Dharmacon. All ectopically expressed plasmids were purchased from Addgene. TransAM™ CREB and NF-κB p65 transcription factor ELISA kits were purchased from Active Motif. The NF-κB p65 (Ser529/536) inhibitory peptide set was purchased from IMGENEX. Cytokine ELISA kits were purchased from eBioscience.
Cell preparation
Peripheral blood mononuclear cells (PBMCs) were obtained from the venous blood of healthy donors as per protocols approved by the University of Louisville, Institutional Review Board, Human Subjects Protection Program, study number 12.0373. Monocytes were isolated by negative selection using the human monocyte isolation kit II from Miltenyi Biotec Inc. The purity of monocytes was routinely >90%, as determined by flow cytometry using a FITC-labeled anti-CD14 antibody. Memory CD4+ T cells (CD45RA-CD45RO+) were purified from PBMCs by negative selection using kits from Miltenyi Biotec. Bone marrow cells from wild type or JAK3 knock out mice were isolated by flushing femurs and tibiae with Hank’s balanced salt solution (HBSS) supplemented with 2% heat-inactivated FBS. The cells were then passed through a 70μm cell strainer. BMDM were generated as previously described (19). Cells were cultured in RPMI 1640 medium supplemented with 10% FBS (R10), 50 μM 2-mercaptoethanol, 1mM sodium pyruvate, 2 mM L-glutamine, 20 mM HEPES, 50 U/ml penicillin, and 50 μg/ml streptomycin (RPMI-complete).
Transfection, Cytokine detection and Western blot
Transfection of human monocytes was carried out by electroporation using a Nucleofector device (Amaxa, Germany) according to the manufacturer’s protocols. Briefly, purified 4 × 106 monocytes were re-suspended in 100 μl of Nucleofector solution (Human Monocytes Nucleofector kit; Amaxa) along with 2 μg of a green fluorescent protein (GFP)-coding plasmid (pCMV-GFP) and 2 μg of siRNA duplexes or ectopic plasmids for each target. Immediately after electroporation, 400 μl of pre-warmed M-199 containing 10% FCS were added to cells which were then transferred into culture plates containing pre-warmed M-199 with 10% FCS. At 48 hours post-transfection, cells were exposed to LPS (1μg/ml) with or without inhibitor.
Cell lysates were prepared as previously described (20, 21). Images were acquired using the Kodak Image Station 4000MM system (Eastman Kodak, New Haven, CT). For siRNA studies, the levels of total JAK3 were assessed by Western blot at 3 days post-ransfection. For experiments using inhibitors for PI3K (LY294002, 25 mM), or GSK3 (SB216763, 10 mM), control cells were pre-treated for 2h with 0.01% DMSO (organic solvent control) prior to LPS (1 μg/ml) stimulation. For siRNA studies, transfected cells were stimulated with LPS (1 μg/ml) 3 days post-transfection, and cell-free supernatants were assayed for cytokine levels by ELISA (eBioscience) 20 h after the addition of LPS.
Immunohistochemistry
Intestinal tissue samples from WT and JAK3 knockout mice were fixed in 4% formaldehyde, embedded in OCT compound and stored at −80°C. Serial sections (8μm thick) were stained for neutrophils and/or IL-17 using single or double immunofluorescence. For staining, slides were rehydrated, blocked, and incubated for 1 hr at room temperature with primary FITC conjugated antibodies to mouse Ly6G, a specific neutrophil marker (FITC-conjugate; LifeSpan BioSciences) or with unconjugated human/mouse IL-17A (Santa Cruz Biotech) antibodies. AlexaFluor594-conjugated goat anti-rabbit IgG (Molecular Probes) was used as a secondary antibody for IL-17 staining. The specificity of staining was confirmed by using appropriate FITC-conjugated isotype controls or normal rabbit IgG followed by AlexaFluor594-goat anti-rabbit IgG. Images were captured using a laser-scanning confocal microscope (Olympus FV1000).
NF-κB p65 and CREB Nuclear Binding Assay
Nuclear lysates were obtained from human monocytes using a nuclear/cytosolic isolation kit purchased from Active Motif. Nuclear lysates were analyzed for DNA binding levels of NF-κB p65 and phosphorylated CREB (S133) using TransAM™ NF-κB p65 and CREB Transcription Factor ELISA Kits (Active Motif) and performed according to the manufacturer’s protocol.
Statistical analysis
Statistical significance between groups was evaluated by the analysis of variance (ANOVA) and the Tukey multiple comparison test using the InStat program (GraphPad, San Diego, CA). Differences between groups were considered significant at the level of P < 0.05.
Results
Pharmacological inhibition of JAK3 enhances TLR-mediated pro-inflammatory cytokine production while suppressing the production of IL-10
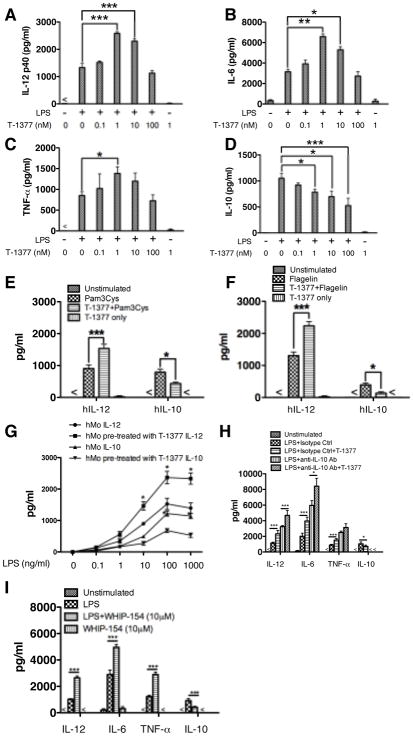
Although JAK3 has been reported to be involved in TLR mediated innate immune responses, the impact of JAK3 activity on TLR mediated inflammatory cytokine production is controversial and at times opposing results have been reported (14, 16, 17, 22). To determine the effects of JAK3 inhibition on TLR-mediated inflammatory cytokine production by innate immune cells, purified human monocytes were used to determine the production of TLR-mediated inflammatory cytokine production in the presence and absence of the JAK3 inhibitor, T-1377 (CP-690550). Since the IC50s of this inhibitor on JAK2 and JAK3 are 20 nM and 1 nM, respectively (23), a serial titration of T-1377 was utilized to minimize the influence of JAK2 on the regulatory ability of JAK3 in LPS stimulated human monocytes. As shown in Figure 1 (A–D), JAK3 inhibition with T-1377 at concentrations of 1 and 10 nM significantly enhanced the production of IL-12, TNF-α and IL-6 in LPS stimulated human monocytes, while suppressing the production of the prototypical anti-inflammatory cytokine IL-10. Moreover, JAK3 inhibition with T-1377 at concentrations of 1 nM also differentially regulated TLR2 and TLR5-mediated production of IL-12 and IL-10 in human monocytes (Fig. 1E, F). We also tested the cellular responses with different LPS doses in the context of JAK3 inhibition, and found JAK3 inhibition exhibits a significantly regulatory ability over different doses of LPS-mediated inflammatory cytokine production (Fig. 1G). To determine the influence of de novo synthesized IL-10 on JAK3 mediated inhibition, neutralizing antibody was used to block IL-10 and then the regulatory effect of JAK3 inhibition on LPS stimulation was assessed. We found JAK3 inhibition remained capable of enhancing the production of pro-inflammatory cytokine in the context of IL-10 blockade, indicating JAK3 directly affects TLR4-mediated inflammatory responses (Fig. 1H). In addition, a second JAK3 inhibitor, WHIP-154, was used to confirm the regulatory function of JAK3 in TLR4 mediated cytokine production. Similar to T-1377, WHIP-154 pretreatment enhanced TLR4 mediated pro-inflammatory cytokine production and concurrently reduced the level of IL-10 level (Fig. 1I).
Figure 1. JAK3 inhibitors differentially regulate TLR4-mediated inflammatory cytokine production in human monocytes.
Purified human monocytes were pre-treated with different JAK3 inhibitors (T-1377 or WHIP-154 (10μM)) or IL-10 neutralizing antibody (5 μg/ml) using rat IgG1 as an isotype control for 2 hours, and then stimulated with LPS (1μg/ml), Pam3CSK4 (500 ng/ml), or Flagelin (5 μg/ml). After 24 h of stimulation, the cell-free supernatants were collected and the levels of (A) IL-12p40, (B) IL-6, (C) TNF-α, and (D) IL-10 were determined by ELISA. For A–D, lower concentration of T-1377 (<10 nM) enhanced the production of IL-12p40 (A), IL-6 (B), and TNF- α (C) while suppressing IL-10 levels (D) produced by LPS-stimulated human monocytes. JAK3 inhibition by 1 nM T-1377 also differentially regulated production of IL-12 and IL-10 in TLR2- or TLR5-stimulated monocytes (E, F). With different doses of LPS, 1 nM T-1377 exhibited a similar regulatory effect on the production of IL-12 and IL-10 (G). In the context of IL-10 neutralization, JAK3 inhibition (1n M T-1377) remains capable of enhancing LPS-mediated pro-inflammatory cytokine production (H). A second JAK3 inhibitor WHIP-154 (10 μM) showed the same capability to increase or decrease the production of IL-12p40, IL-6, TNF-α and IL-10 (I), respectively. *, **, and *** indicates statistically significant at P<0.05, P<0.01, and P<0.001, respectively. Data represents the arithmetic mean ± S.D. of three biological replicates.
siRNA knockdown or genetic deletion of JAK3 results in elevated pro-inflammatory cytokine production and suppressed IL-10 production upon TLR4 stimulation
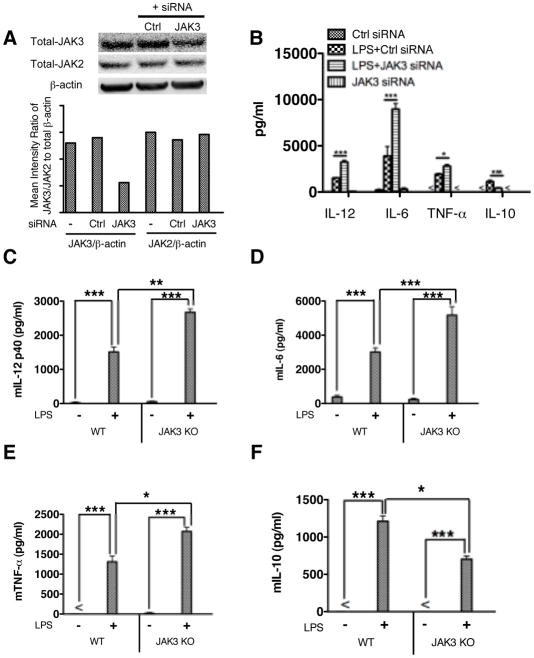
As both T-1377 (CP-690550) and WHIP-154 can have activity against JAK2 (23), to isolate the specific influences of JAK3 inhibition on TLR4 mediated inflammatory cytokine production, we utilized pre-validated siRNA to knockdown JAK3 in human monocytes. As shown in Figure 2A, JAK3 silencing by specific siRNA resulted in a substantial decrease of total JAK3 in human monocytes, as compared with control. To exclude the possible off-target effect of JAK3 siRNA, we tested the expression of total JAK2 and found no substantial change of JAK2 in JAK3 siRNA-treated monocytes. Inhibition of JAK3 significantly enhanced TLR4 mediated IL-12, TNF-α and IL-6 production and suppressed IL-10 level in LPS stimulated human monocytes (Fig. 2B). These results corroborated the cytokine data obtained with the pharmacological inhibitors. Since neither pharmacological inhibition nor siRNA can completely ablate JAK3 without effects on other isoforms of JAKs, we next utilized JAK3 knockout macrophages to determine the effect of JAK3 in TLR4 mediated inflammatory cytokine production. As compared with wild type control, JAK3 knockout resulted in significantly increased IL-12, TNF- α, and IL-6 while concurrently suppressing the production of IL-10 in LPS stimulated macrophages (Fig. 2, C–F). Taken together, these data demonstrate that JAK3 inhibition leads to elevated pro-inflammatory cytokine production and suppressed IL-10 production upon TLR4 stimulation.
Figure 2. JAK3 deficiency enhances the production of IL-12, TNF-α, and IL-6 while decreasing IL-10 levels in TLR4 stimulated cells.
Purified human monocytes were pre-treated with non-target or JAK3 specific siRNA for 72 hours and then stimulated with LPS for 24 h. Whole cell lysates and cell-free supernatants were collected to determine the transfection efficiency and cytokine levels, respectively. (A) siRNA mediated knockdown of JAK3 protein levels was assessed by Western Blot. Total JAK2 levels were also tested to exclude an off-target effect of JAK2. The ratio of total-JAK3 or JAK2 to total β-actin was determined by densitometry. (B) siRNA silencing-mediated JAK3 inhibition enhances the production of IL-12, TNF-α, and IL-6 while decreasing IL-10 levels in LPS stimulated cells. For C to F, wild type and JAK3 knockout BMDM were generated and stimulated with LPS for 24 h. Cell-free supernatants were collected and assayed for cytokine levels by ELISA. JAK3 knockout enhanced TLR4 induced production of IL-12(C), IL-6 (D), TNF-α (E) and reduced IL-10 levels (F) in LPS stimulated BMDM. *, **, and *** indicates statistically significant difference at P<0.05, P<0.01 and P<0.001, respectively. Data represent the arithmetic mean ± S.D. of three biological replicates.
The effects of JAK3 inhibition on the activation of the PI3K-Akt pathway and its downstream constitutive active kinase GSK3β in LPS stimulated innate immune cells
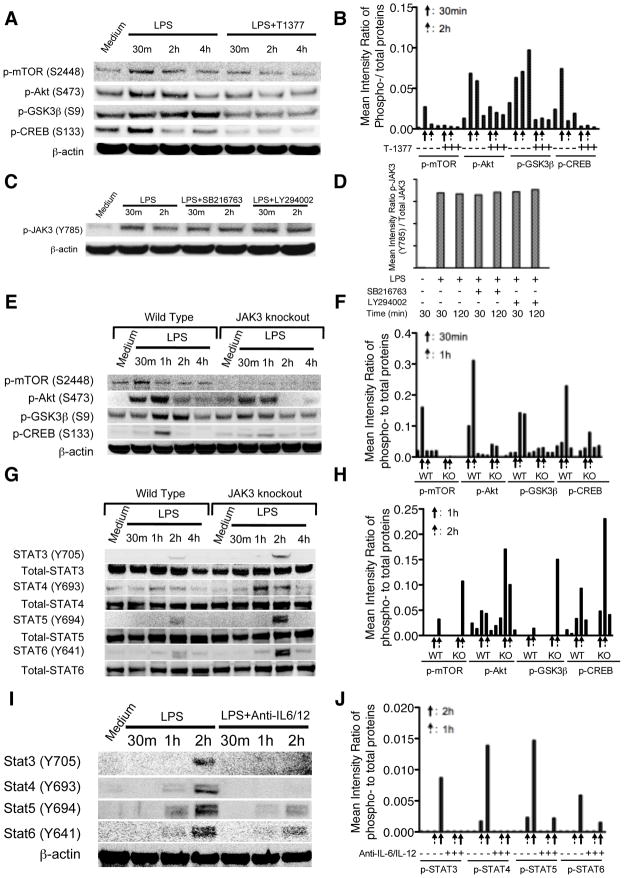
The PI3-Kinase pathway has been demonstrated to modulate TLR4 mediated inflammatory immune responses via differentially regulating the levels of pro- and anti-inflammatory cytokines (24–27). Our recent studies found that a downstream constitutive active kinase of the PI3K-Akt pathway, GSK3β, is responsible for the ability of the PI3K-Akt pathway to suppress the levels of pro-inflammatory cytokines while augmenting anti-inflammatory cytokine production upon TLR stimulation (28). Since we have observed the differential regulatory ability of JAK3 inhibition on TLR4 mediated inflammatory cytokines and previous reports shown the possible links between the JAK3 and PI3K pathways (22, 29–31), we next sought to determine whether JAK3 inhibition regulates the TLR4 mediated inflammatory response through PI3K and its downstream signaling components such as Akt, mTORC1, GSK3β, and CREB. As shown in Figure 3A and B, compared with the control group, T-1377 treated monocytes exhibited reduced phosphorylation of mTORC1, Akt, GSK3β, and CREB over time upon LPS stimulation. In contrast, inhibition of Akt or GSK3β has no discernible effect on the phosphorylated levels of JAK3, compared with cells stimulated with LPS alone (Fig. 3C, D). We next used JAK3 knockout BMDM to confirm the functional role of JAK3. As shown in Figure 3E and F, compared with wild type control, JAK3 knockout BMDM exhibited substantially decreased phosphorylation of mTORC1, Akt, GSK3β, and CREB over time upon LPS stimulation. Because numerous studies have shown JAKs-STATs play a critical role in the cytokine mediated signaling pathway (32–35), we next examined whether JAK3 knockout modified autocrine inflammatory cytokine production would subsequently affect STATs phosphorylation. JAK3 knockout cells and wild type control were stimulated with LPS at different time points, and then tyrosine phosphorylation of STAT3, STAT4, STAT5, and STAT6 were examined. As shown in Figure 3G and H, JAK3 knockout potently increased the levels of tyrosine phosphorylation of STAT3, STAT4, STAT5, and STAT6 at late time points upon LPS stimulation, as compared with wild type control. This result is consistent with and expand upon an earlier study showing JAK3 inhibition resulted in enhanced STAT3 phosphorylation (35). Our previous study has shown that TLR induced phosphorylation of STATs was mainly caused by de novo synthesized cytokines (36). Hence, we next investigated the effect of the neutralizing antibodies against inflammatory cytokines (including IL-6, IL-12) on STAT phosphorylation in the context of JAK3 inhibition. Compared with control cells, neutralization of inflammatory cytokines clearly ameliorated the ability of JAK3 inhibition to enhance tyrosine phosphorylation of STATs (Fig. 3I, J). These data verified our findings that JAK3 inhibition was capable of differentially regulating inflammatory cytokines and these de novo synthesized cytokines contribute to the subsequent tyrosine phosphorylation of STATs in TLR4 stimulated cells.
Figure 3. JAK3 inhibition suppresses TLR4-mediated activity of the PI3K pathway while enhancing the phosphorylation levels of STATs.
Purified human monocytes were pre-treated with JAK3 inhibitor (1nM T-1377) for 2 h and then stimulated with LPS over a 4 h time course. (A) Total cell lysates were probed for the levels of phosphorylated mTORC1, Akt, GSK3β, and CREB by Western Blot and the ratio of phospho- to total proteins was determined by densitometry (B). (C) Purified human monocytes were stimulated with LPS in the presence or absence of PI3K or GSK3 inhibitor. The levels of phosphorylated JAK3 were analyzed by Western Blot and the ratio to total JAK3 was determined by densitometry (D). From E to H, wild type and JAK3 knockout BMDM were treated with LPS for up to 4 h, total cell lysates were collected at the given time points, and probed for the levels of phosphorylated mTORC1, Akt, GSK3β, CREB (E), STAT3, STAT4, STAT5, and STAT6 (G) by Western Blot and the ratio of phospho- to total protein determined by densitometry (F, H). Anti-IL-12 and anti-IL-6 antibody cocktails were used to determine the effect of JAK3 inhibition on the tyrosine phosphorylation of STAT3, STAT4, STAT5, and STAT6 upon the neutralization of IL-12 and IL-6 signaling. Wild type and JAK3 knockout BMDM were pre-treated with neutralizing anti-IL6 and anti-IL-12 cocktail for 2 h and then stimulated with LPS. Whole cell lysates were probed for the levels of phosphorylated STATs and the ratio of phospho- to total STATs determined by densitometry (I, J). Data are representative of three to five biological replicates.
JAK3 differentially regulates LPS induced cytokines production in a GSK3β-dependent manner
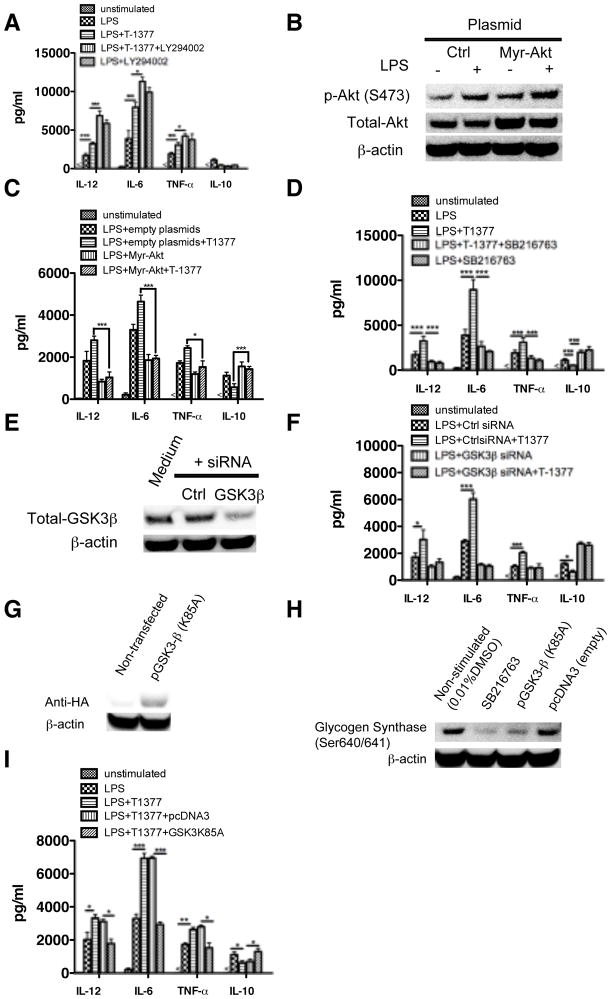
The PI3K pathway has been shown to play a critical regulatory role in TLR4 mediated inflammatory cytokine production via phosphorylating and then inhibiting the activity of a downstream kinase, GSK3β (28). Phosphorylation and inactivation of GSK3 is effectuated by Akt after activation of PI3K. Because our data have shown that inhibition of JAK3 differentially regulated LPS induced cytokine production and decreased the LPS induced phosphorylation of Akt and GSK3β, we next investigated the functional role of this pathway in JAK3-mediated regulation of inflammatory cytokine production. As shown in Figure 4A, inhibition of PI3K with the pharmacological inhibitor LY294002 significantly enhanced the ability of JAK3 inhibition to augment pro-inflammatory cytokine production and suppress IL-10 levels in LPS stimulated monocytes. We next asked whether over-expression of ectopic constitutively active Akt would attenuate the hyper inflammatory response induced by JAK3 inhibition. As compared with cells expressing empty vector, expression of constitutively active Akt resulted in significantly lower production of IL-12, TNF-α and IL-6 in LPS stimulated monocytes (Fig. 4B, C). Moreover, over-expression of constitutively active Akt also abrogated the effect of JAK3 inhibition upon LPS stimulation (Fig. 4C). It suggested that the regulatory ability of JAK3 is dependent on the activity of PI3K. Since GSK3β has been shown to play a critical role in TLR4 mediated inflammatory cytokine production and we have observed that JAK3 inhibition attenuated the phosphorylation of GSK3β upon LPS stimulation (Fig. 3A), we next determined whether GSK3β activity impacts the JAK3 inhibition-mediated cytokine production. After pre-treating JAK3 inactivated monocytes with GSK3 inhibitor (SB216763) and then stimulating with LPS, we found GSK3 inactivation significantly abrogated JAK3 inhibition mediated regulation of inflammatory cytokines production (Fig. 4D). Similarly, siRNA mediated GSK3β silencing (Fig. 4E) also significantly attenuated the capability of JAK3 to differentially regulate LPS induced cytokine production, as compared with non-specific siRNA treated human monocytes (Fig. 4F). As confirmatory evidence, we found TLR4 stimulation of monocytes expressing a kinase dead mutant encoding GSK3β (K85A) significantly (P<0.05) attenuated the production of IL-12, IL-6, TNF-α and enhanced IL-10 level upon JAK3 inhibitor pre-treatment (Fig. 4, G–I). Taken together, these results demonstrated that the ability of JAK3 inhibition to differentially regulate LPS induced inflammatory cytokine production is regulated by PI3K and its downstream kinases, Akt and GSK3β.
Figure 4. The ability of JAK3 inhibition to regulate TLR4 mediated inflammatory cytokine production is dependent on the activity of PI3K and GSK3β.
The levels of IL-12, TNF-α, IL-6, and IL-10 were assayed by ELISA in LPS stimulated human monocytes. The regulatory effect of JAK3 inhibition was (A) significantly enhanced by PI3K inhibition (LY294002) and (B, C) abrogated by constitutive expression of Akt. Inhibition of GSK3 mediated by pharmacological inhibitor (D) or specific siRNA (E, F) attenuated the regulatory ability of JAK3 on LPS-mediated inflammatory cytokine production in human monocytes. (G) HA expression levels were detected by Western Blot 48 h post-transfection in nontransfected monocytes and monocytes transfected to confirm the transfection efficiency of kinase dead (K85A) plasmid encoding GSK3β. (H) monocytes transfected with kinase dead (K85A) plasmid exhibited a loss in the phosphorylation levels of the GSK3 specific substrate glycogen synthase (GS) (S640/641). (I) As compared with monocytes transfected with empty vector control, the kinase dead (K85A) GSK3β mutant abrogated the ability of JAK3 inhibition to increase the production of TNF-α, IL-12, IL-6, and decrease the IL-10 level in LPS stimulated cells. *, **, and *** indicates statistically significant at P<0.05, P<0.01 and P<0.001, respectively. Data represent the arithmetic mean ± S.D. of three biological replicates.
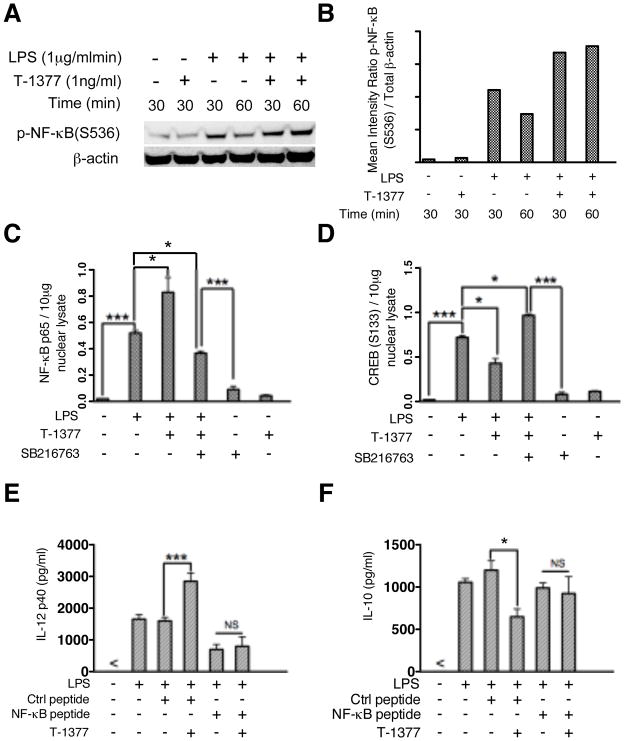
JAK3 inhibition modifies TLR4-mediated DNA binding activity of NF-κB and CREB and consequently regulates inflammatory cytokine induction
We next examined whether JAK3 inhibition of Akt and GSK3β could affect downstream signaling molecules/transcription factors involved in the control of LPS mediated inflammatory cytokine production. Our previous study (28) demonstrated that GSK3 inactivation differentially regulates TLR mediated inflammatory cytokine production. GSK3 increases the phosphorylation of CREB that displaces NF-κB from CBP, leading to diminished pro-inflammatory cytokine production (28). We therefore tested whether JAK3 inhibition could affect LPS mediated DNA binding activity of NF-κB and CREB, and whether this effect was dependent on the activity of GSK3. As expected, compared with LPS simulation alone, JAK3 inhibition enhanced phosphorylation of NF-κB p65 (S536) (Fig. 5A and B) and its DNA binding activity (Fig. 5C) but reduced that of CREB in LPS stimulated monocytes (Fig. 5D). We also observed that inhibition of GSK3 was able to reverse the effect of JAK3 inhibition of NF-κB and CREB DNA binding (Fig. 5, C and D). Since our previous studies have shown that GSK3 inhibition suppresses pro-inflammatory cytokine production by LPS-stimulated cells via its capacity to reduce the association of CBP with NF-κB (28), we predicted TLR4 mediated activity of NF-κB would affect the capability of JAK3 inhibition to enhance the production of inflammatory cytokine production in LPS stimulated monocytes. To test this hypothesis, we utilized NF-κB P65 inhibitory peptide and observed that inhibition of NF-κB p65 prevented the ability of T-1377 to increase IL-12 and decrease IL-10 production in LPS-stimulated monocytes, as compared with control (Fig. 5, E and F).
Figure 5. JAK3 inhibition affects the phosphorylation levels and DNA binding activity of NF-κB and CREB in monocytes.
Purified human monocytes were pretreated with JAK3 inhibitor (T-1377, 10 nM), control peptide, or NF-κB inhibitory peptide and then stimulated with LPS. Whole cell lysates, cytosoplasmic and nuclear cell fractions were collected after stimulation with LPS at the time points indicated. (A) JAK3 inhibition increased the phosphorylation of NF-κB (S536) upon LPS stimulation. (B) Densitometer scans of phospho-NF-κB (S536) and total β-actin were performed and recorded as the ratio of phospho-NF-κB (S536): total β-actin. NF-κB and CREB DNA binding assays were performed to measure the levels of total NF-κBp65 (C) (10 μg nuclear lysate) and CREB (S133) (D) (20 μg nuclear lysate) in monocytes stimulated with LPS for 4 h in the presence and absence of the JAK3 inhibitor (T-1377), GSK3 inhibitor (SB216763) or both. For E and F, the functional effect of JAK3 inhibition on NF-κB P65 activity in LPS stimulated monocytes was assessed by pre-treatment of cells with a control peptide or NF-κB inhibitor peptide (100 μM) followed by stimulation with LPS for 20 h; The levels of IL-12P40 (E) and IL-10 (F) were determined by ELISA. For C, D, E, and F, data represents the arithmetic mean ± S.D. of three biological replicates. * and *** indicates statistically significant at P<0.05 and P<0.001, respectively.
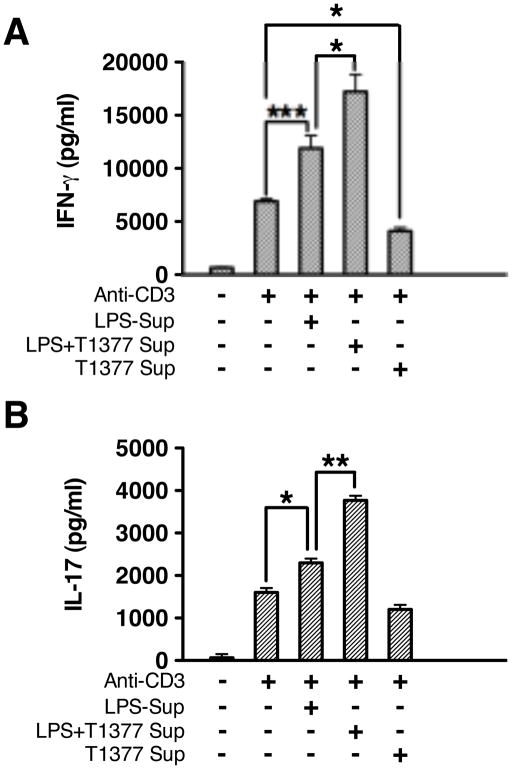
JAK3 inhibition positively regulates T cell responses by controlling inflammatory cytokine production
Several previous studies have shown that low doses of JAK3 inhibitor enhanced T cell responses, whereas many others reported that JAK3 inhibition suppresses adaptive immune responses by disrupting autocrine cytokine signaling of T and B cells (16, 37–39). Since we observed pharmacological inhibition, siRNA, or genetic deletion of JAK3 enhanced pro-inflammatory cytokine production in innate immune cells, we next examined whether the regulatory function of JAK3 inhibition in innate immune cells also impacted subsequent T cell responses. To this end, we cultured autologous memory CD4+ T cells with supernatants isolated from human monocytes under different stimulation scenarios. We observed that supernatant from monocytes stimulated with LPS alone had the ability to enhance CD4+ memory T cell responses by augmenting IFN-γ and IL-17 production (Fig. 6, A and B). Moreover, using supernatants from monocyte pre-treatment with JAK3 inhibitor and LPS reinforced the enhancement of IFN-γ and IL-17, while supernatant from monocyte pre-treated with JAK3 inhibitor alone reduced the production of IFN-γ and IL-17 (Fig. 6, A and B). These results indicate that JAK3 inhibition in innate immune cells can positively regulate subsequent T cells responses by enhancing the production of IFN-γ and IL-17.
Figure 6. Modulation of inflammatory cytokines by JAK3 in human monocytes enhanced Th1 and Th17 cytokine production by memory CD4+ T cells.
Human monocytes and autologous memory CD4+ T cells were isolated from PBMC. Monocytes were stimulated with LPS in the presence and absence of JAK3 inhibitor (T-1377) for 24 hours. Cell-free supernatant isolated from non-stimulated monocytes, LPS stimulated monocytes, or monocytes stimulated with LPS in the presence of JAK3 inhibitor were transferred to culture with autologous memory CD4+ T cells plated in 96 well plates (2×105/well) pre-coated with or without anti-CD3 (1 μg/ml). After 96 hours of co-culture, cell-free supernatants were collected and assayed for IFN-γ or IL-17 by ELISA. Data represent the arithmetic mean ± S.D. of three biological replicates * and *** indicates statistically significant at P<0.05 and P<0.001, respectively.
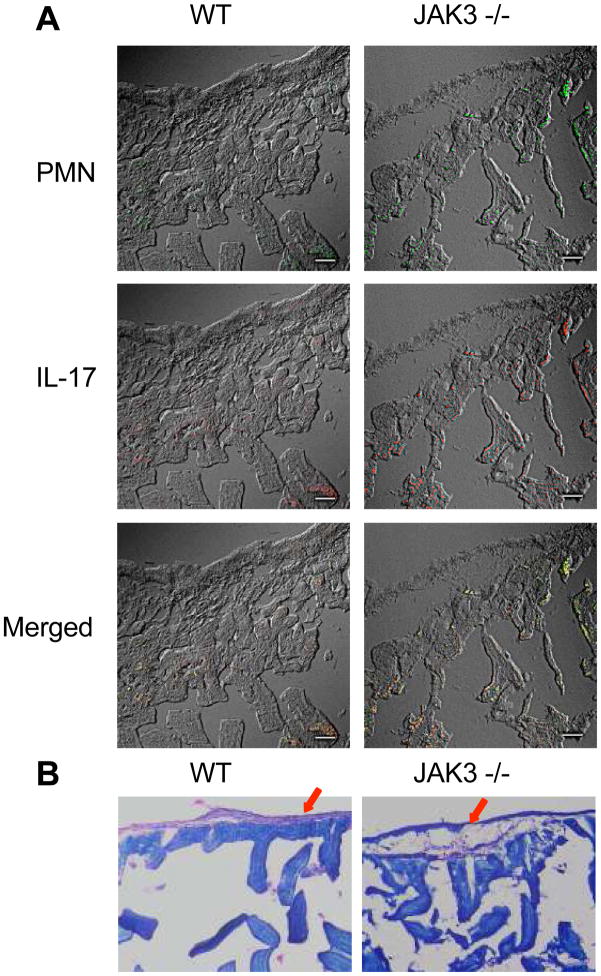
JAK3 knockout mice display high levels of intestinal inflammation and PMN infiltration
It has been reported that the intestinal lamina propria is the largest reservoir of CD4+ lymphocytes, and that either IL-6 and/or IL-17 can play a crucial role in the initiation and progression of intestine bowel diseases (40–44). In the present study, we observed in vitro that JAK3 inhibition enhanced pro-inflammatory cytokine production including IL-6 and IL-17. Moreover, compared with wild type mice, more than 90% knockout mice exhibited some symptoms of intestine bowel diseases (IBD) including lower body weight and severe rectal prolapses after 20 weeks (data not shown). These observations suggest that JAK3 inhibition may enhance intestinal microbiota-induced inflammatory responses. In an effort to investigate this possibility, we assessed the presence of IL-17 and polymorphonuclear neutrophil (PMN) infiltration in the lamina propria of the intestine of JAK3 deficiency mice. We discovered a high degree of PMN infiltration and a mild increase in IL-17 expression in the lamina propria of JAK3 knockout mice, as compared with wild type mice (Fig 7. A). Moreover, obvious damage in the intestinal epithelial layer was also found in JAK3 knockout mice (Fig 7. B). Considering previous studies have demonstrated the functional role of PMN infiltration and IL-17 expression in the initiation and development of intestine bowel diseases (40, 45), these data indicated that JAK3 may participate in regulation of the initiation and development of intestine inflammation.
Figure 7. JAK3 knockout aggravated the erosion of intestinal epithelium and enhanced the infiltration of PMN and expression of IL-17.
(A) PMN infiltration and IL-17 expression were assessed by staining sections of intestinal tissue with FITC conjugated anti-Ly6G (a specific neutrophil marker) and AlexaFluor 594-conjugated IL-17 (magnification, 40×, scale bar = 50μm). (B) Hematoxylin and Eosin (H & E) staining of serial sections of small intestine from WT mice and JAK3 knockout mice showing the integrity of intestinal epithelium (magnification, 20×)
Discussion
In the present study, we demonstrated the negative regulatory role of JAK3 in TLR4 mediated inflammatory responses, and characterized the cell-signaling pathway by which JAK3 inhibition enhanced the production of pro-inflammatory cytokines while concurrently suppressing the anti-inflammatory cytokine IL-10 in innate immune cells. We found inhibition of JAK3 attenuated TLR4 mediated activity of PI3K and then suppressed the phosphorylation of Akt and its downstream kinase GSK3β. Inhibition of JAK3 diminished the phosphorylation level of GSK3β resulting in enhanced GSK3β activity and subsequent elevated TLR4 mediated inflammatory responses. This finding identified GSK3β as a downstream signaling target of JAK3 in TLR4 stimulated innate immune cells. By analyzing downstream transcription factors of GSK3β, we observed that JAK3 inhibition enhanced the DNA binding activity of NF-κB and attenuated that of CREB upon LPS stimulation and this effect was dependent on the activity of GSK3β. Moreover, the functional role of NF-κB was verified by the finding that inhibitory peptide-mediated blocking of NF-κB attenuated the ability of JAK3 inhibition to enhance TLR4 mediated pro-inflammatory cytokine production. Additionally, we found JAK3 inhibition was capable of increasing CD4+ T cell responses in vitro and enhancing PMN infiltration, as well as IL-17 expression in intestine tissues. Collectively, these findings establish a negative regulatory role for JAK3 in TLR4 mediated pro-inflammatory responses and characterize the involvement of the PI3K-Akt- GSK3β signaling pathway in JAK3-mediated control of inflammatory responses.
JAK3 has been demonstrated to be involved in cytokine signaling through coupling with the γc receptor and subsequently to play a critical role in the development, proliferation, and differentiation of B cells and T cells (38, 46). Despite numerous studies noting the immunosuppressive effect of JAK3 inhibition in the treatment of organ transplantation and several other inflammatory diseases(47–49), there is no consistent evidence for a role of JAK3 inhibition in TLRs mediated inflammatory responses. Yamaoka et al. (14) reported that a genetic deficiency of JAK3 or its γc chain receptor resulted in the elevation of inflammatory cytokines including IL-12 and IL-10 in LPS stimulated mice bone marrow derived dendritic cells, while other groups have shown JAK3 inhibition or knockout reduced the production of IL-10 in TLR4 mediated innate immune cells(17). In our current study, using primary human monocytes and gene knockout JAK3 macrophages, we found JAK3 inhibition or genetic deficiency resulted in enhanced production of TNF-α, IL-6, IL-12 but reduced IL-10 production. The discrepancy in IL-10 production among the studies is likely due to several factors such as different cell types, different maturation stage of dendritic cells, and the influence of JAK3 deficiency on other signaling pathways. Our current findings highlight the functional role of JAK3 in innate immune cells and elucidate the signaling pathway by which JAK3 negatively regulates TLR mediated inflammatory cytokine production.
The PI3K-Akt pathway has been demonstrated to negatively regulate TLR mediated-inflammatory responses (28). However, how TLRs initiate the activation of PI3K pathway and the possible role of tyrosine kinases like JAKs in this process are less known. Previous studies have demonstrated that activation of PI3K occurs via its regulatory subunit p85 binding to a phosphotyrosine residue (YXXM) present within activated cellular receptors (50). Upon TLR stimulation, PI3K was activated through binding with the YXXM motif of the adaptor molecules such as MyD88 or the intracellular component of TLR receptors such as TLR2/3 (51, 52). Several lines of evidence showed JAK family members are involved in the regulation of TLR signaling (53, 54). However, no study to date has identified how JAKs are involved in the signaling events of TLRs, if JAKs are involved in the tyrosine phosphorylation of TLRs, or how they may act in a synergistic, independent or an opposing manner in innate immune cells. In this study, we demonstrated JAK3 inhibition suppressed activity of the PI3K-Akt-GSK3β pathway, which enhances the production of pro-inflammatory cytokines while decreasing IL-10 levels in innate immune cells. Our unpublished data showed that JAK3 inhibition attenuated tyrosine phosphorylation of TLR4 and JAK3 at early time points, which indicated JAK3-mediated tyrosine phosphorylation is involved in TLR-initiated signaling events. Although the connectivity between JAK3 and TLR4 is still not known, TLR4 receptor stimulation induces a conformational change, which may bring JAK3 into close proximity, resulting in activation via trans phosphorylation. Upon TLRs-mediated phosphorylation, activated JAK3 could phosphorylate tyrosine residues within the cytoplasmic domains of associated receptor or adaptor molecules that allow for the recruitment of downstream signaling molecules.
Mapping of the PI3K pathway by several groups revealed that the ability of the PI3K pathway to dictate regulation of pro- and anti-inflammatory cytokines largely depended upon the inactivation of the serine/threonine kinase GSK3β (19, 20, 28). Several groups have shown that JAK3 activation is involved in the activation of the PI3K pathway following various stimuli (22, 29–31). In particular, inhibition of JAK3 was shown to down-regulate PI3K activation and Akt phosphorylation in mast cells in the present of antigen stimulation (30). However, it was unclear if JAK3 was required for the TLR-mediated action of the PI3K pathway. Our present study has provided evidence for the first time demonstrating the PI3K-Akt-GSK3β pathway is responsible for JAK3 mediated differential regulation of inflammatory cytokine production by LPS stimulated innate immune cells. Moreover, we observed several signaling molecules, such as mTORC1 and P70S6Kinase, involved in JAK3 mediated regulation of cytokine production (data not shown). This finding is consistent with our previous published results on the regulatory effect of the PI3K-mTORC1 pathway and its downstream serine/threonine kinases P70S6K and GSK3β in TLR4 mediated human monocytes(36, 55). Since our previous publication has demonstrated that mTORC1 inhibition led to differential cytokine production (36), and since inhibition of JAK3 resulted in de-phosphorylation of mTORC1 and its downstream signaling molecules P70S6K and GSK3β, it is reasonable to predict that JAK3 exhibits its regulatory role through this pathway. Our current data support this concept, since either pharmacological inhibition or ectopic expression of kinase dead GSK3β abrogated the differential regulatory ability of JAK3 inhibition upon LPS stimulation. Considering the similarity of JAK3 and mTORC1 inhibitors on suppressing adaptive immune responses while enhancing TLR4 mediated pro-inflammatory cytokine levels, our study suggested this could be a common feature for molecules that possess the capability to suppress adaptive immune responses while concurrently enhancing the immune responses of innate immune cells. At the transcriptional level, we found that JAK3 inhibition differentially altered the TLR4 mediated DNA binding activity of CREB and NF-κB. Furthermore, peptide-mediated inhibition of NF-κB suppressed pro-inflammatory cytokine production in JAK3 inhibited cells stimulated with LPS. These observations suggest that JAK3 differentially regulates TLR4 mediated inflammatory responses through modifying the activity of NF-κB and CREB. Taken together, our current study characterized the signaling pathway, PI3K-Akt-GSK3β, that JAK3 depends upon to affect TLR4 mediated inflammatory cytokine production.
Although numerous reports have shown JAK3 inhibition suppresses inflammation responses in several autoimmune diseases (47, 49), whether this is truly attributable to inhibition of JAK3 has not yet been established. It has been reported that the IC50 value of the most widely investigated JAK3 inhibitor, T-1377 (CP-690550), is 1 nM for JAK3 while 20 nM for JAK2 in lymphocytes(23). Additionally, as accurate concentrations of inhibitors are difficult to achieve in vivo, it is unlikely that all the inflammatory suppressive effects of T-1377 can be attributed to JAK3 inhibition. Since several recent studies (56–58) have established a suppressive function for JAK2 inhibition in TLR mediated immune responses, the suppressive effect of T-1377 on inflammatory responses might be mainly due to JAK2, not JAK3, inhibition. A recent study by Yoshida substantiated this possibility, showing that a low dose of T-1377 enhanced the inflammatory responses and accelerated the onset of experimental autoimmune encephalomyelitis (16). A very recent published abstract also reported that specific inhibition of JAK1 and JAK2 is more effective than inhibition of JAKs in protecting mice from acute Graft-versus-Host Disease (aGvHD) by significantly decreasing alloreactive CD4+ T-cells (59). These observations indicated JAK2 and JAK3 might have distinct regulatory function in inflammatory responses. Our current study demonstrated JAK3 inhibition, either by titrated concentrations of pharmacological inhibitor or genetic knockdown, had an ability to enhance the pro-inflammatory cytokine production and related T cell responses. As a negative regulator of inflammation, JAK3 knockout also resulted in the high expression of IL-17 and infiltration of PMN in the lamina propria of the distal intestine. These results identified the negative regulatory role of JAK3 in TLR induced inflammatory cytokine production.
Whereas numerous studies have reported that pharmacological inhibition of JAK3 was capable of suppressing the inflammatory immune response by disrupting the development of lymphocytes, the effect of JAK3 on innate immune cells and the cellular mechanism responsible for this effect have remained obscure. As it is well known that the innate immune responses prime the adaptive immune cells to enhance the host defense ability, elucidating the indirect influence of JAK3 inhibition on the responses of autologous CD4+ T cells will be helpful to comprehensively assess the regulatory effect of JAK3 inhibition on TLR-mediated immune responses. The work presented in this study demonstrated that JAK3 inhibition enhanced TLR4 mediated pro-inflammatory cytokine production while concurrently reducing the IL-10 levels in innate immune cells. We also discovered that the PI3K-Akt-GSK3β signaling pathway is utilized by JAK3 to differentially regulate TLR4 mediated cytokine production. JAK3 inhibition induced enhancement of pro-inflammatory cytokine production and this correlated with elevated CD4+ T cell responses and the inflammation status of the intestine. Our further studies elucidated that JAK3 inhibition enhanced GSK3β activity and was capable of altering the DNA binding activity of NF-κB and CREB, which resulted in elevated production of TLR4 mediated pro-inflammatory cytokines. Thus, the function of JAK3 in immune cells related to the dampening of the pro-inflammatory responses and elevating production of anti-inflammatory cytokine IL-10. Understanding the mechanism regulating inflammatory cytokines by JAK3 inhibition provides a logical strategy for designing novel treatment strategies to combat immune-related inflammatory diseases.
Acknowledgments
1. This research was supported by Grant R01 DE017921 (R.J.L.) from the National Institute of Dental Research.
3. Abbreviations used in this paper
- JAK3
Janus Kinase-3
- GSK3β
glycogen synthase kinase 3-beta
- mTORC1
mammalian target of rapamycin complex 1
- CREB
cAMP Response Element-Binding Protein
- PMNs
Polymorphonuclear leukocytes
- NF-κB
Nuclear Factor-kappa B
- TLR
Toll-like receptor
- siRNA
small interfering RNA
- MyD88
Myeloid differentiation primary response gene 88
- TIRAP
toll-interleukin 1 receptor (TIR) domain containing adaptor protein
- TRIF
toll-interleukin 1 receptor (TIR)-domain-containing adapter-inducing interferon-β
- TRAM
TRIF-related adaptor molecule
- SARM
sterile a motif (SAM) and armadillo repeat motif (ARM)-Containing Protein
- TRAF
TNF receptor associated factors
- IRAK
IL-1R-associated kinase
- TBK
TANK-binding kinase
- IKK
I kappa B kinase
- ATF-2
Activating Transcription Factor-2
- IRF
interferon regulatory factor (IRF)
Footnotes
Conflict-of-Interest Disclosure
The authors declare no competing financial interests
Wang H., Lamont R., and Suttles J. designed research, analyzed and interpreted data. Wang H., Lamont R., Scott D. and Liang S. performed research, analyze and interpret data, and wrote the manuscript. Wang H., Zhou, H., Brown, J, Gao S., and Jotwani R. performed research.
References
- 1.O’Neill LA. ‘Fine tuning’ TLR signaling. Nat Immunol. 2008;9:459–461. doi: 10.1038/ni0508-459. [DOI] [PubMed] [Google Scholar]
- 2.Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447:972–978. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- 3.Garlanda C, Di Liberto D, Vecchi A, La Manna MP, Buracchi C, Caccamo N, Salerno A, Dieli F, Mantovani A. Damping excessive inflammation and tissue damage in Mycobacterium tuberculosis infection by Toll IL-1 receptor 8/single Ig IL-1-related receptor, a negative regulator of IL-1/TLR signaling. J Immunol. 2007;179:3119–3125. doi: 10.4049/jimmunol.179.5.3119. [DOI] [PubMed] [Google Scholar]
- 4.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Lee MS, Kim YJ. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu Rev Biochem. 2007;76:447–480. doi: 10.1146/annurev.biochem.76.060605.122847. [DOI] [PubMed] [Google Scholar]
- 6.O’Neill LA. How Toll-like receptors signal: what we know and what we don’t know. Curr Opin Immunol. 2006;18:3–9. doi: 10.1016/j.coi.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Szretter KJ, Samuel MA, Gilfillan S, Fuchs A, Colonna M, Diamond MS. The immune adaptor molecule SARM modulates tumor necrosis factor alpha production and microglia activation in the brainstem and restricts West Nile Virus pathogenesis. J Virol. 2009;83:9329–9338. doi: 10.1128/JVI.00836-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belinda LW, Wei WX, Hanh BT, Lei LX, Bow H, Ling DJ. SARM: a novel Toll-like receptor adaptor, is functionally conserved from arthropod to human. Mol Immunol. 2008;45:1732–1742. doi: 10.1016/j.molimm.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 9.Page TH, Smolinska M, Gillespie J, Urbaniak AM, Foxwell BM. Tyrosine kinases and inflammatory signalling. Curr Mol Med. 2009;9:69–85. doi: 10.2174/156652409787314507. [DOI] [PubMed] [Google Scholar]
- 10.Novogrodsky A, Vanichkin A, Patya M, Gazit A, Osherov N, Levitzki A. Prevention of lipopolysaccharide-induced lethal toxicity by tyrosine kinase inhibitors. Science. 1994;264:1319–1322. doi: 10.1126/science.8191285. [DOI] [PubMed] [Google Scholar]
- 11.Gray P, Dunne A, Brikos C, Jefferies CA, Doyle SL, O’Neill LA. MyD88 adapter-like (Mal) is phosphorylated by Bruton’s tyrosine kinase during TLR2 and TLR4 signal transduction. J Biol Chem. 2006;281:10489–10495. doi: 10.1074/jbc.M508892200. [DOI] [PubMed] [Google Scholar]
- 12.Macchi P, Villa A, Giliani S, Sacco MG, Frattini A, Porta F, Ugazio AG, Johnston JA, Candotti F, O’Shea JJ, et al. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID) Nature. 1995;377:65–68. doi: 10.1038/377065a0. [DOI] [PubMed] [Google Scholar]
- 13.Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273–287. doi: 10.1111/j.1600-065X.2008.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamaoka K, Min B, Zhou YJ, Paul WE, O’Shea J. Jak3 negatively regulates dendritic-cell cytokine production and survival. Blood. 2005;106:3227–3233. doi: 10.1182/blood-2005-02-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sareila O, Korhonen R, Karpanniemi O, Nieminen R, Kankaanranta H, Moilanen E. Janus kinase 3 inhibitor WHI-P154 in macrophages activated by bacterial endotoxin: differential effects on the expression of iNOS, COX-2 and TNF-alpha. Int Immunopharmacol. 2008;8:100–108. doi: 10.1016/j.intimp.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida H, Kimura A, Fukaya T, Sekiya T, Morita R, Shichita T, Inoue H, Yoshimura A. Low dose CP-690,550 (tofacitinib), a pan-JAK inhibitor, accelerates the onset of experimental autoimmune encephalomyelitis by potentiating Th17 differentiation. Biochem Biophys Res Commun. 418:234–240. doi: 10.1016/j.bbrc.2011.12.156. [DOI] [PubMed] [Google Scholar]
- 17.Kim HJ, Hart J, Knatz N, Hall MW, Wewers MD. Janus kinase 3 down-regulates lipopolysaccharide-induced IL-1 beta-converting enzyme activation by autocrine IL-10. J Immunol. 2004;172:4948–4955. doi: 10.4049/jimmunol.172.8.4948. [DOI] [PubMed] [Google Scholar]
- 18.Cross DA, Culbert AA, Chalmers KA, Facci L, Skaper SD, Reith AD. Selective small-molecule inhibitors of glycogen synthase kinase-3 activity protect primary neurones from death. J Neurochem. 2001;77:94–102. doi: 10.1046/j.1471-4159.2001.t01-1-00251.x. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Garcia CA, Rehani K, Cekic C, Alard P, Kinane DF, Mitchell T, Martin M. IFN-beta production by TLR4-stimulated innate immune cells is negatively regulated by GSK3-beta. J Immunol. 2008;181:6797–6802. doi: 10.4049/jimmunol.181.10.6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rehani K, Wang H, Garcia CA, Kinane DF, Martin M. Toll-like receptor-mediated production of IL-1Ra is negatively regulated by GSK3 via the MAPK ERK1/2. J Immunol. 2009;182:547–553. doi: 10.4049/jimmunol.182.1.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia CA, Wang H, Benakanakere MR, Barrett E, Kinane DF, Martin M. c-jun controls the ability of IL-12 to induce IL-10 production from human memory CD4+ T cells. J Immunol. 2009;183:4475–4482. doi: 10.4049/jimmunol.0901283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirata N, Yanagawa Y, Iwabuchi K, Onoe K. Selective regulation of interleukin-10 production via Janus kinase pathway in murine conventional dendritic cells. Cell Immunol. 2009;258:9–17. doi: 10.1016/j.cellimm.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Changelian PS, Flanagan ME, Ball DJ, Kent CR, Magnuson KS, Martin WH, Rizzuti BJ, Sawyer PS, Perry BD, Brissette WH, McCurdy SP, Kudlacz EM, Conklyn MJ, Elliott EA, Koslov ER, Fisher MB, Strelevitz TJ, Yoon K, Whipple DA, Sun J, Munchhof MJ, Doty JL, Casavant JM, Blumenkopf TA, Hines M, Brown MF, Lillie BM, Subramanyam C, Shang-Poa C, Milici AJ, Beckius GE, Moyer JD, Su C, Woodworth TG, Gaweco AS, Beals CR, Littman BH, Fisher DA, Smith JF, Zagouras P, Magna HA, Saltarelli MJ, Johnson KS, Nelms LF, Des Etages SG, Hayes LS, Kawabata TT, Finco-Kent D, Baker DL, Larson M, Si MS, Paniagua R, Higgins J, Holm B, Reitz B, Zhou YJ, Morris RE, O’Shea JJ, Borie DC. Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor. Science. 2003;302:875–878. doi: 10.1126/science.1087061. [DOI] [PubMed] [Google Scholar]
- 24.Fukao T, Tanabe M, Terauchi Y, Ota T, Matsuda S, Asano T, Kadowaki T, Takeuchi T, Koyasu S. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat Immunol. 2002;3:875–881. doi: 10.1038/ni825. [DOI] [PubMed] [Google Scholar]
- 25.Fukao T, Yamada T, Tanabe M, Terauchi Y, Ota T, Takayama T, Asano T, Takeuchi T, Kadowaki T, Hata Ji J, Koyasu S. Selective loss of gastrointestinal mast cells and impaired immunity in PI3K-deficient mice. Nat Immunol. 2002;3:295–304. doi: 10.1038/ni768. [DOI] [PubMed] [Google Scholar]
- 26.Guha M, Mackman N. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J Biol Chem. 2002;277:32124–32132. doi: 10.1074/jbc.M203298200. [DOI] [PubMed] [Google Scholar]
- 27.Martin M, Schifferle RE, Cuesta N, Vogel SN, Katz J, Michalek SM. Role of the phosphatidylinositol 3 kinase-Akt pathway in the regulation of IL-10 and IL-12 by Porphyromonas gingivalis lipopolysaccharide. J Immunol. 2003;171:717–725. doi: 10.4049/jimmunol.171.2.717. [DOI] [PubMed] [Google Scholar]
- 28.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linwong W, Hirasawa N, Aoyama S, Hamada H, Saito T, Ohuchi K. Inhibition of the antigen-induced activation of rodent mast cells by putative Janus kinase 3 inhibitors WHI-P131 and WHI-P154 in a Janus kinase 3-independent manner. Br J Pharmacol. 2005;145:818–828. doi: 10.1038/sj.bjp.0706240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El Mabrouk M, Sylvester J, Zafarullah M. Signaling pathways implicated in oncostatin M-induced aggrecanase-1 and matrix metalloproteinase-13 expression in human articular chondrocytes. Biochim Biophys Acta. 2007;1773:309–320. doi: 10.1016/j.bbamcr.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Zhi W, Wareski P, Weng NP. IL-15 activates telomerase and minimizes telomere loss and may preserve the replicative life span of memory CD8+ T cells in vitro. J Immunol. 2005;174:4019–4024. doi: 10.4049/jimmunol.174.7.4019. [DOI] [PubMed] [Google Scholar]
- 32.Wang KS, Frank DA, Ritz J. Interleukin-2 enhances the response of natural killer cells to interleukin-12 through up-regulation of the interleukin-12 receptor and STAT4. Blood. 2000;95:3183–3190. [PubMed] [Google Scholar]
- 33.Wang KS, Zorn E, Ritz J. Specific down-regulation of interleukin-12 signaling through induction of phospho-STAT4 protein degradation. Blood. 2001;97:3860–3866. doi: 10.1182/blood.v97.12.3860. [DOI] [PubMed] [Google Scholar]
- 34.Liu Z, Qiu YH, Li B, Ma SH, Peng YP. Neuroprotection of interleukin-6 against NMDA-induced apoptosis and its signal-transduction mechanisms. Neurotox Res. 19:484–495. doi: 10.1007/s12640-010-9215-x. [DOI] [PubMed] [Google Scholar]
- 35.Jang YN, Lee IJ, Park MC, Baik EJ. Role of JAK3 in myogenic differentiation. Cell Signal. 24:742–749. doi: 10.1016/j.cellsig.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 36.Wang H, Brown J, Gu Z, Garcia CA, Liang R, Alard P, Beurel E, Jope RS, Greenway T, Martin M. Convergence of the mammalian target of rapamycin complex 1- and glycogen synthase kinase 3-beta-signaling pathways regulates the innate inflammatory response. J Immunol. 186:5217–5226. doi: 10.4049/jimmunol.1002513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamaoka K, Tanaka Y. Jak inhibitor; possibility and mechanism as a new disease modifying anti-rheumatic drug. Nihon Rinsho Meneki Gakkai Kaishi. 2009;32:85–91. doi: 10.2177/jsci.32.85. [DOI] [PubMed] [Google Scholar]
- 38.O’Shea JJ. Targeting the Jak/STAT pathway for immunosuppression. Ann Rheum Dis. 2004;63(Suppl 2):ii67–ii71. doi: 10.1136/ard.2004.028290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Shea JJ, Husa M, Li D, Hofmann SR, Watford W, Roberts JL, Buckley RH, Changelian P, Candotti F. Jak3 and the pathogenesis of severe combined immunodeficiency. Mol Immunol. 2004;41:727–737. doi: 10.1016/j.molimm.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 40.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, Murphy E, Sathe M, Cua DJ, Kastelein RA, Rennick D. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 43.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carey R, Jurickova I, Ballard E, Bonkowski E, Han X, Xu H, Denson LA. Activation of an IL-6:STAT3-dependent transcriptome in pediatric-onset inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:446–457. doi: 10.1002/ibd.20342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ina K, Kusugami K, Hosokawa T, Imada A, Shimizu T, Yamaguchi T, Ohsuga M, Kyokane K, Sakai T, Nishio Y, Yokoyama Y, Ando T. Increased mucosal production of granulocyte colony-stimulating factor is related to a delay in neutrophil apoptosis in Inflammatory Bowel disease. J Gastroenterol Hepatol. 1999;14:46–53. doi: 10.1046/j.1440-1746.1999.01807.x. [DOI] [PubMed] [Google Scholar]
- 46.Yao Z, Cui Y, Watford WT, Bream JH, Yamaoka K, Hissong BD, Li D, Durum SK, Jiang Q, Bhandoola A, Hennighausen L, O’Shea JJ. Stat5a/b are essential for normal lymphoid development and differentiation. Proc Natl Acad Sci U S A. 2006;103:1000–1005. doi: 10.1073/pnas.0507350103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paniagua R, Si MS, Flores MG, Rousvoal G, Zhang S, Aalami O, Campbell A, Changelian PS, Reitz BA, Borie DC. Effects of JAK3 inhibition with CP-690,550 on immune cell populations and their functions in nonhuman primate recipients of kidney allografts. Transplantation. 2005;80:1283–1292. doi: 10.1097/01.tp.0000177643.05739.cd. [DOI] [PubMed] [Google Scholar]
- 48.Vijayakrishnan L, Venkataramanan R, Gulati P. Treating inflammation with the Janus kinase inhibitor CP-690,550. Trends Pharmacol Sci. 32:25–34. doi: 10.1016/j.tips.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 49.Chang BY, Zhao F, He X, Ren H, Braselmann S, Taylor V, Wicks J, Payan DG, Grossbard EB, Pine PR, Bullard DC. JAK3 inhibition significantly attenuates psoriasiform skin inflammation in CD18 mutant PL/J mice. J Immunol. 2009;183:2183–2192. doi: 10.4049/jimmunol.0804063. [DOI] [PubMed] [Google Scholar]
- 50.Toker A, Cantley LC. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature. 1997;387:673–676. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- 51.Gelman AE, LaRosa DF, Zhang J, Walsh PT, Choi Y, Sunyer JO, Turka LA. The adaptor molecule MyD88 activates PI-3 kinase signaling in CD4+ T cells and enables CpG oligodeoxynucleotide-mediated costimulation. Immunity. 2006;25:783–793. doi: 10.1016/j.immuni.2006.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sarkar SN, Peters KL, Elco CP, Sakamoto S, Pal S, Sen GC. Novel roles of TLR3 tyrosine phosphorylation and PI3 kinase in double-stranded RNA signaling. Nat Struct Mol Biol. 2004;11:1060–1067. doi: 10.1038/nsmb847. [DOI] [PubMed] [Google Scholar]
- 53.Kimura A, Naka T, Muta T, Takeuchi O, Akira S, Kawase I, Kishimoto T. Suppressor of cytokine signaling-1 selectively inhibits LPS-induced IL-6 production by regulating JAK-STAT. Proc Natl Acad Sci U S A. 2005;102:17089–17094. doi: 10.1073/pnas.0508517102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Okugawa S, Ota Y, Kitazawa T, Nakayama K, Yanagimoto S, Tsukada K, Kawada M, Kimura S. Janus kinase 2 is involved in lipopolysaccharide-induced activation of macrophages. Am J Physiol Cell Physiol. 2003;285:C399–408. doi: 10.1152/ajpcell.00026.2003. [DOI] [PubMed] [Google Scholar]
- 55.Weichhart T, Costantino G, Poglitsch M, Rosner M, Zeyda M, Stuhlmeier KM, Kolbe T, Stulnig TM, Horl WH, Hengstschlager M, Muller M, Saemann MD. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–577. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 56.Zhong J, Yang P, Muta K, Dong R, Marrero M, Gong F, Wang CY. Loss of Jak2 selectively suppresses DC-mediated innate immune response and protects mice from lethal dose of LPS-induced septic shock. PLoS One. 5:e9593. doi: 10.1371/journal.pone.0009593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stump KL, Lu LD, Dobrzanski P, Serdikoff C, Gingrich DE, Dugan BJ, Angeles TS, Albom MS, Ator MA, Dorsey BD, Ruggeri BA, Seavey MM. A highly selective, orally active inhibitor of Janus kinase 2, CEP-33779, ablates disease in two mouse models of rheumatoid arthritis. Arthritis Res Ther. 13:R68. doi: 10.1186/ar3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fridman JS, Scherle PA, Collins R, Burn TC, Li Y, Li J, Covington MB, Thomas B, Collier P, Favata MF, Wen X, Shi J, McGee R, Haley PJ, Shepard S, Rodgers JD, Yeleswaram S, Hollis G, Newton RC, Metcalf B, Friedman SM, Vaddi K. Selective inhibition of JAK1 and JAK2 is efficacious in rodent models of arthritis: preclinical characterization of INCB028050. J Immunol. 184:5298–5307. doi: 10.4049/jimmunol.0902819. [DOI] [PubMed] [Google Scholar]
- 59.Gimondi S, Vaccaroli R, Vendramin A, Bermema A, Radaelli E, Mariotti J, Corradini P. Inhibition of JAK1/JAK2 Is More Effective Than Inhibition of JAK3 in Protecting Mice From Acute Graft-Versus-Host Disease (aGvHD) by Significantly Decreasing Alloreactive CD4+ T-Cells. 54th American Society of Hematology Annual Meeting and Exposition; Atlanta, GA. 2012. [Google Scholar]