Abstract
Objective
Myocardial infarction (MI) can lead to irreversible adverse left ventricular remodeling resulting in subsequent severe dysfunction. The objective of this study was to investigate the potential for biodegradable, elastomeric patch implantation to positively alter the remodeling process after MI in a porcine model.
Methods
Yorkshire pigs underwent a 60-minute catheter balloon occlusion of the left circumflex artery. Two weeks after MI animals underwent epicardial placement of a biodegradable, porous polyurethane (poly(ester urethane)urea; PEUU) patch (MI+PEUU, n = 7) or sham surgery (MI+sham, n = 8). Echocardiography before surgery and at 4 and 8 weeks after surgery measured the end-diastolic area (EDA) and fractional area change (% FAC). All animals were humanely killed 8 weeks after surgery and hearts were histologically assessed.
Results
At 8 weeks, echocardiography revealed greater EDA values in the MI+sham group (23.6 ± 6.6 cm2 , mean ± standard deviaation) than in the MI+PEUU group (15.9 ± 2.5 cm2) (P < .05) and a lower %FAC in the MI+sham group (24.8 ± 7.6) than in the MI+PEUU group (35.9 ± 7.8) (P < .05). The infarcted ventricular wall was thicker in the MI+PEUU group (1.56 ± 0.5 cm) than in the MI+sham group (0.91 ± 0.24 cm) (P < .01).
Conclusions
Biodegradable elastomeric PEUU patch implantation onto the porcine heart 2 weeks post-MI attenuated left ventricular adverse remodeling and functional deterioration and was accompanied by increased neovascularization. These findings, although limited to a 2-month follow-up, may suggest an attractive clinical option to moderate post-MI cardiac failure.
Myocardial infarction (MI) is the most frequently identified specific cause of dilated cardiomyopathy, leading to symptomatic congestive heart failure over time. Regional structural changes in left ventricular (LV) remodeling after MI can lead to global LV geometric change, which contributes to an increase in LV wall stress1 and mitral regurgitation.2 Epidemiologically, survival after MI is related to the magnitude of LV dilatation.3 Thus, therapies designed to attenuate postinfarct LV dilatation, by pharmacologic or surgical means, have been pursued to alleviate postinfarction morbidity and mortality in adverse remodeling after MI.
A spectrum of surgical procedures, cardiac resynchronization therapy (biventricular pacing),4 or pharmacologic therapy (eg, angiotensin-converting enzyme inhibitors and beta-blockers)5 have been applied in the clinical setting after MI in an effort to limit adverse LV remodeling. Surgical approachesinclude surgical ventricular restoration with an endocardial patch such as the Dor procedure6 or ventricular wrapping with an epicardial patch.7 The patches used in these procedures, however, have been made from nonbiodegradable materials with low elasticity. Such materials raise concerns about a chronic foreign-body response, potentially leading to difficulties in reoperation, or LV diastolic failure owing to nonelastic encapsulation. Microbial infection is also aconcern that arises when implanting a permanent foreign body.
In animal models for ischemic cardiomyopathy, a variety of biodegradable materials as interventional therapeutic strategies have been investigated, including epicardial patches with and without cellular constituents,8–12 intramyocardial hydrogel injectables,13,14 and intracoronary injectables.15 We have previously reported that an elastic, biodegradable cardiac patch, without cells, prevents cardiac remodeling and improves LV function after MI with a rodent model. 8 However, whether this relatively straightforward approach would serve to similarly prevent LV remodeling in a more clinically relevant large animal model has not been addressed. Namely, the efficacy of epicardial patch plasty with a degradable material in a large animal model has not been addressed to date. Our objective here was to examine the efficacy of a porous, elastic epicardial patch made from biodegradable polyurethane (poly[ester urethane]urea; PEUU), which was designed to have properties appropriate for the cardiovascular system, using a porcine ischemia–reperfusion MI model.
MATERIALS AND METHODS
Animal Preparation
Twenty-five healthy female crossbred Yorkshire swine, 4 to 5 months old and weighing 23 ± 6 kg, were used in this study. Porcine LV infarcts were created by catheter-based balloon occlusion for 60 minutes and re-perfusion of the proximal circumflex artery. Two weeks after MI, patch placement or sham surgery was performed. Before surgery, animals that survived the infarct procedure and had an infarct size meeting the selection criterion, animals with a risk area more than 25% of LV free wall, were randomly assigned to either the PEUU patch placement (MI+PEUU) or sham surgery (MI+sham) group, and were screened by echocardiography to obtain the baseline data. The animal protocol used in the study was approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh (No. 0612885). All animals received humane care in compliance with the “Guide for the Care and Use of Laboratory Animals” published by the National Institutes of Health (1996, National Academy Press, Washington, DC). Arterial blood pressure, pulse oximetry, and electrocardiograms were continuously monitored throughout the procedures.
Catheterization for LV Ischemia–Reperfusion Protocol
The animals were anesthetized with ketamine (20 mg/kg) and xylazine (2 mg/kg) administered intramuscularly, followed by intubation and maintenance by mechanical ventilation with oxygen supplemented with 2.0% isoflurane. After placement of the animal in a supine position, the femoral artery was percutaneously cannulated using a 6F arterial sheath with a Sel-dinger technique under sterile conditions. A bolus of 60 mg/kg heparin and 2 mg/kg of amiodarone was intravenously administered and amiodarone was then continuously infused at 1 mg/min during the procedure to prevent lethal ventricular arrhythmias associated with this model.16 The ischemia–reperfusion model, which is commonly used in porcine studies of post-MI therapy,17 was used. The left main coronary artery was selectively engaged using an AL 1 guide catheter (6F; Cordis Corp., Miami, Fla). Using a 3.5 × 12 mm coronary dilatation balloon catheter (Guidant, Santa Clara, Calif), the proximal left circumflex artery was occluded for 60 minutes by balloon inflation (Figure 1).16 Direct-current cardioversion was performed when sustained ventricular tachycardia or ventricular fibrillation was observed. The animals were then allowed to recover from anesthesia and returned to their housing facility. Buprenorphine (0.05 mg/kg) and cefazolin (30 mg/kg) were administered intramuscularly 2 times per day for 3 days after the procedure. All animals were screened by echocardiography for infarct size as estimated by the percentage of risk area (severe hypokinetic, akinetic, or dyskinetic regions) to the LV free wall area. Animals with a risk area of less than 25% of the LV free wall were excluded from the analysis and humanely killed immediately after echography.
FIGURE 1.
Ischemia–reperfusion (I–R) injury and patch implantation protocol. Female swine underwent left circumflex artery (LCx) occlusion for 60 minutes followed by reperfusion (A to D). Black, white, and yellow arrows indicate LCx, occlusion balloon, and completely occluded LCx, respectively. *Left anterior descending (LAD) coronary artery and diagonal branches. The electrocardiogram during the LCx occlusion procedure showed elevated S-T segment and inverted Twave in lead II (D). The study protocol applied is shown in pare E. Circular poly(ester urethane)urea (PEUU) patches were implanted 2 weeks after I-R injury (F). p, Implanted PEUU patch; laa, left atrial appendage; ap, apex of heart. Macroscopic appearance for the PEUU patch implanted heart at the 8-week end point (G). The black dashed line indicates estimated edge of the remnant implanted material.
Scaffold Fabrication
PEUU was synthesized according to methods previously described from polycaprolactone (PCL, Mn = 2000, Sigma), diisocyanatobutane (BDI, Sigma), and putrescine.18 A detailed methodology for scaffold fabrication and characterization is provided in the online data supplement.
Epicardial Patch Placement Surgery
Two weeks after the MI, the animals were sedated with ketamine (20 mg/kg) and xylazine (2 mg/kg) given intramuscularly, intubated, and anesthesia was provided with oxygen with 1.5% inhaled isoflurane. Lidocaine (10 mg/kg) was given to prevent arrhythmias. The heart was exposed through an anterolateral thoracotomy at the fourth intercostal level, and the infarcted cardiac surface was lightly scraped with a surgical knife to introduce blood into the porous PEUU patch and to aid in adhering the entire patch surface onto the surface of the epicardium. The patch was then secured by continuous running suture with 6-0 polypropylene in the PEUU patch group so that the patch covered the infarct area. In the sham surgery group the heart was lightly scraped but no material was implanted. The wound was closed with 4-0 polyglactin absorbable sutures. During the procedure, bupivacaine (0.2 mL/kg) was locally injected into the thoracic wall. The animals were then allowed to recover and returned to their housing facility. Buprenorphine (0.05 mg/kg) and cefazolin (30 mg/kg) were administered intramuscularly 2 times per day for 3 days after surgery for postoperative analgesic treatment and for prophylaxis of surgical site infection, respectively. Echocardiography was performed on each animal at the 4- and 8-week time point after surgery under the anesthesia program described above, but without intubation. All animals were humanely killed at the 8-week time point with potassium chloride bolus dosing (50 mEq/kg) under anesthesia.
Echocardiography
Transthoracic echocardiograms were obtained 2 week after MI as a baseline, 4 weeks after surgery, and at the time of humane killing (8 weeks after surgery) using a Sonos 1500 platform (Hewlett-Packard Company, Palo Alto, Calif) equipped with a 2.5-MHz transducer. Short-axis views of the LV at the level of the papillary muscle were obtained from a right parasternal approach. The end-diastolic (EDA) and end-systolic (ESA) LV internal cavity areas were determined offline by tracing the endocardial border using OsiriX image processing application v.3.7.1. The LV fractional area change (%FAC) was calculated according to the following equation:
LV volumes were estimated using the following formula of Teichholz to derive LV end-systolic volume (LVESV), LV end-systolic volume (LVEDV), and LV ejection fraction (EF):
where Vand D are LV volume and diameter and were measured by M-mode echocardiography at end-systolic or end-diastolic cardiac phase. Echocardiography and offline tracing processing were done by the operators, who were blinded to the treatment assignment.
Postmortem Study
After the animals were humanely killed, adhesive noncardiac tissue was removed and the hearts were dissected. The hearts were cut along the longitudinal axis in slices 1-cm thick. The wall thickness of the risk area was measured with a digital caliper (Fisher Scientific, Pittsburgh, Pa) from 10 random locations to obtain an average for each sample. Tissue samples from the risk area were either fixed in 4% formalin and paraffin-embedded for hematoxylin and eosin staining or fixed in 4% phosphate-buffered paraformaldehyde for 4 hours, followed by immersion in 30% sucrose solution for at least 2 days for immunostaining. Samples fixed with paraformaldehyde were stained immunohistochemically with antibodies against α-smooth muscle actin (aSMA) (1:200; Sigma Chemical Co, St Louis, Mo) or CD31 (1:200; Serotec, Raleigh, NC). Nuclei were stained with 4′6-diamidino-2-phenyindole (1:10,000; Sigma). Slides were examined with an Olympus BX51 microscope and images captured digitally (Olympus America, Inc, Center Valley, Pa).
For each retrieved sample from the infarct area, 10 different microscopic fields at 400× magnification for CD31-positive structures were photographed. To quantify the vascular density, the number of CD31-positive tubular structures was measured using digital image processing ImageJ software (National Institutes of Health, Bethesda, Md).
Statistical Analysis
Data are reported as mean ± standard deviation. For comparisons in the wall thickness and vascular density, the Student t test was performed. Two-way repeated analysis of variance followed by the Tukey test was applied to multiple comparisons in the EDA and %FAC analysis. All statistical evaluations were performed using SigmaStat (Systat Software Inc, Point Richmond, Calif).
RESULTS
Material Characteristics
The PEUU patch appeared white and spongy (Figure 2, A) with a pore size ranging from 30 to 100 µm (40 ± 22 µm, Figure 2, B) and a porosity of 86% ± 2%. The patch had a peak tensile strength of 307 ± 87 kPa with a peak tensile strain of 103% ± 13% and an initial modulus of 704 ± 100 kPa. A cyclic tensile test was performed to evaluate patch elasticity. As shown in Figure 2, C, a large hysteresis loop in the first cycle was observed, and in the next 9 cycles, smaller and overlapped hysteresis loops were recorded. No obvious unrecoverable deformation was detected.
FIGURE 2.
Macroscopic view of the porous PEUU patch (A) fabricated by thermally induced phase separation. Scanning electron micrograph (B) demonstrates the pore structure of the scaffold. Typical cyclic tensile response curves (C), with a maximum 30% strain, show a lack of obvious unrecoverable scaffold distention after 10 cycles.
Postoperative Course and Gross Observations
A total of 25 swine underwent the coronary occlusion procedure. There were 7 (28%) deaths during the 60-minute left circumflex occlusion procedure because of refractory ventricular fibrillation against direct-current shock. After the catheterization,2animals were excluded owing to their small lesions at risk. One animal was lost in the MI+sham group because of sudden death 2 weeks after the sham surgery. The final analysis thus included 7 animals in the MI+PEUU group and 8 in the MI+sham group (Table E1). The PEUU patch material tolerated suture line tension and was safely implanted with continuous suture (Figure 1, F). During systole, the elastic patch was observed to wrinkle slightly, while during diastole, qualitative stretching could be seen (Video 1). At 8 week after implantation, the PEUU patch was found to have formed no strong adhesions with the chest wall, and the region where the remnant patch was located was covered with connective tissue (Figure 1, G, Video 2).
Tissue Thickness in the Risk Zone
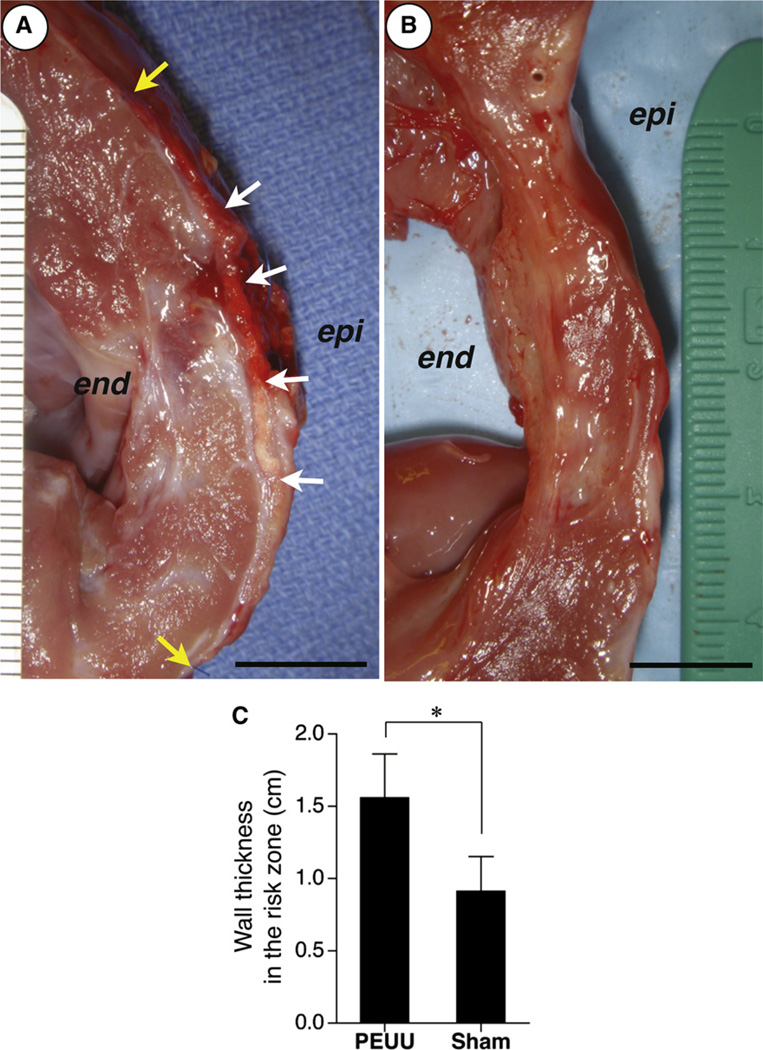
The LV wall thickness of the risk zone was measured immediately after death for both the MI+PEUU (n = 7) and MI+sham groups (n = 8). The thickness of the PEUU patched wall was significantly greater than for the sham surgery wall (1.56 ± 0.30 vs 0.91 ± 0.24 cm, PEUU vs sham, P < .01) (Figure 3).
FIGURE 3.
Left ventricular wall thickness in the risk zone. Representative photographic images of ventricular transection for the poly(ester urethane)urea (PEUU) patched wall (A) and sham surgery wall (B) at the 8-week time point. The white and yellow arrows represent implanted PEUU patch and suture line for patch implantation, respectively. Epi, Epicardial site; end, endocardial site. Scale bars: 1 cm. The PEUU patched wall (n = 7) was significantly thicker than the sham surgery wall (n = 8) (C). *P <.01.
Histology and Immunochemistry
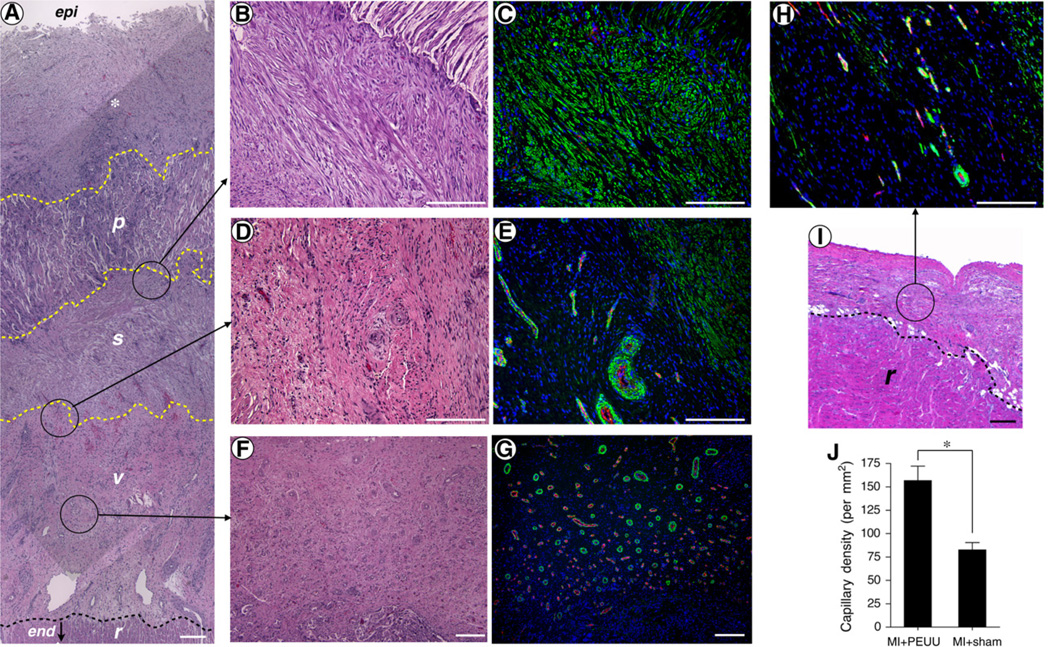
Samples for histologic assessment were obtained from all animals for both the MI+PEUU and MI+sham groups. The wall onto which the PEUU patch was placed was characterized by several distinct regions. From the endocardial side, some preserved myocardial tissue was evident; above this was a vascularized region that transitioned into a layer with diffuse cells that stained positively for αSMA. Above the layer with αSMA-stained cells, the remnant PEUU material was apparent and was infiltrated with cells also staining positively for αSMA. The remnant PEUU material appears as white voids or fragments in Figure 4, A, region p, and along the top right corner of Figure 4, B. At the epicar-dial surface, connective tissue was present above the patch (Figure 4, A-G). In contrast, the untreated, infarcted LV wall was composed of 2 regions: a layer of scar tissue that had a relatively low density of αSMA-expressing cells, and, near the endocardium, some preserved myocardial tissue comparable with that seen in the hearts receiving the patch (Figure 4, H and I). Comparing the region above the preserved myocardial tissue in the MI+sham hearts with the similar region above this tissue in the MI+PEUU group, the latter group had a significantly greater density of vascular structures with a wide variety of diameters compared with the MI+sham group (157±16vs83±8 per mm2, PEUU vs sham; P < .01) (Figure 4, J).
FIGURE 4.
Hematoxylin and eosin staining and immunostaining for α-smooth muscle actin (αSMA) and CD31. The poly(ester urethane)urea (PEUU) patched wall exhibited an αSMA rich layer (s) beneath the implanted PEUU patch (p). Below the aSMA rich layer was a vascular rich layer (v) and then a myocardial remnant (r) region at the endocardial side (A). epi, Epicardial site; end, endocardial site. *Connective tissue formed above the implanted PEUU. The dashed lines approximate each boundary. Looking at a higher magnification of the boundary area between the PEUU patch and αSMA rich layer showed that the PEUU had partially degraded and cellular infiltration had occurred with αSMA-positive cells (B and C). The junction between αSMA and vascular rich layers is highlighted in (D and E) and the center of the vascular rich layer is showed in parts F and G. The hematoxylin and eosin staining in the MI+sham group is shown in part I. The αSMA-positive structure and vessels in the MIsham group are shown in part H. Capillary density was greater in the MI-PEUU group than in the MI+sham group (*P < .01) (J). Scale bars: 200 µm.
Echocardiography
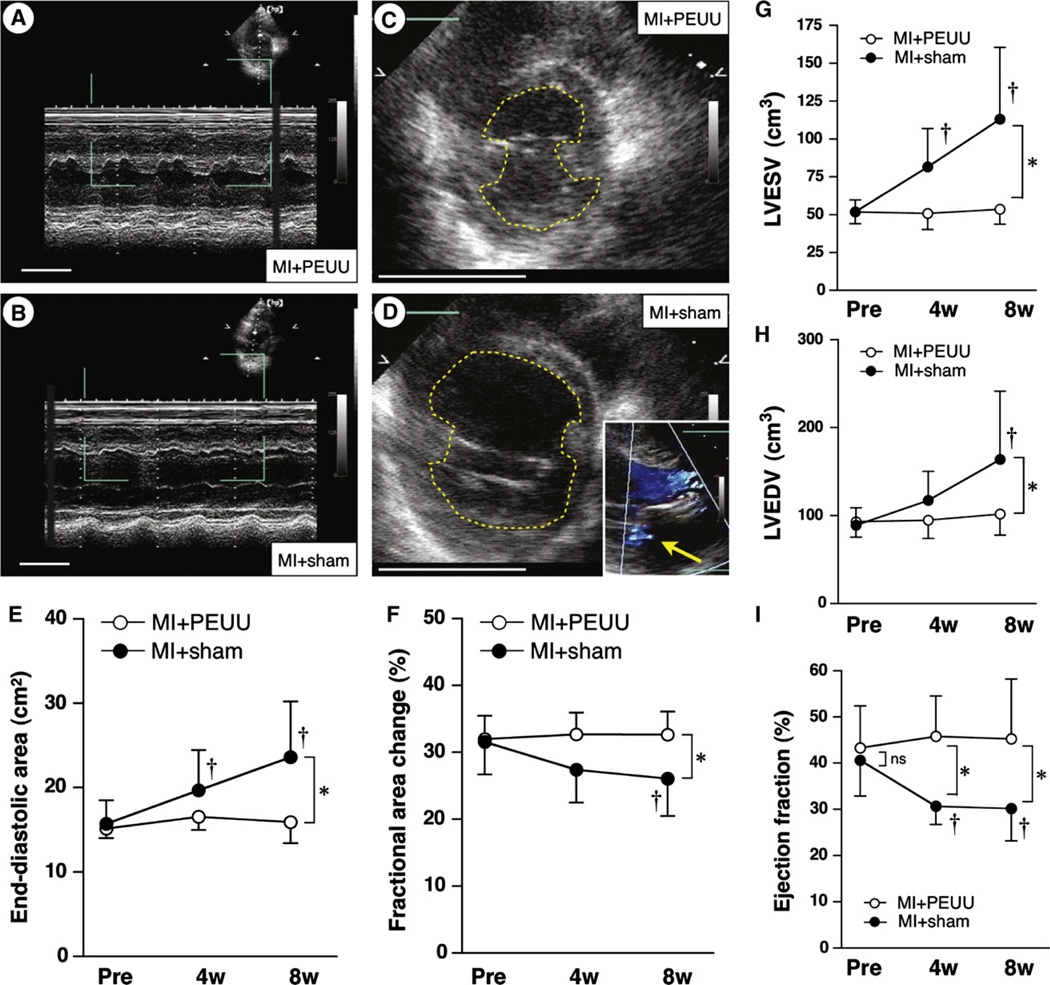
Transthoracic echocardiograms were obtained in all animals in the MI+PEUU group (n = 7) and MI+sham group (n = 8) at all 3 time points. One case of trivial mitral regurgitation was observed in the MI+sham group at the 8-week time point. A significant interaction was found to exist between the groups and observed time points (P <.05). The MI+PEUU group did not experience a significant change in either EDA or %FAC after patch implantation at any of the time points; the EDA of the MI+sham group at 4 and 8 weeks was significantly increased and the % FAC at 8 weeks was decreased versus the 0-week point when compared for time factor within group. There were no statistically significant differences in the EDA and % FAC between the 2 groups at the time of surgery and 4 weeks after surgery. In contrast, 8 weeks after surgery, the EDA in the MI+sham group was greater than that for the MI+PEUU group (15.9 ± 2.5 vs 23.6 ± 6.6 cm2, PEUU vs sham; P < .05) and the %FAC in the MI+PEUU group was greater than that of the MI+sham group (35.9 ± 7.8 vs 24.8 ± 7.6%; P < .05) (Figure 5, A to F). Consistently, LV volume analysis using parameters estimated by LV inner diameters revealed greater LVESV (113 ±47 cm3) and LVEDV (164 ± 77) in the MI+sham group than those (54 ±10 and 102± 24, respectively) in the MI+PEUU group (P < .05). The MI+sham group had decreased EF at 4 and 8 weeks (30% ± 4% and 30% ± 7%, respectively) than the MI+PEUU group (46 ± 9 and 45 ± 13, respectively) (P < .05) (Figure 5, G to I).
FIGURE 5.
Temporal echocardiographic assessment for end-diastolic area (EDA) and fractional area change (%FAC) after the patch implantation or sham surgery. Representative M-mode (A and B) and short-axis view of the left ventricle (C and D) at the 8-week time point. Scale bars: 5 cm. The broken lines in parts C and D indicate the inner surface of the left ventricle. The inset in part D shows trivial mitral regurgitation (yellow arrow), a case observed in the MI+sham group. The EDA was significantly greater in the MI+sham than in the MI+PEUU group (E) and %FAC was significantly preserved in the PEUU versus the MI+sham group (n = 7 or 8 for each group) (F). *P < .05 between groups at 8-week time point; †P < .05 versus just before implantation (Pre) within group. The volumetric analysis using estimates derived from left ventricular diameters showed greater increases in left ventricular volume in the MI+sham group at the 8-week point (G and H) and preserved ejection fraction in the MI+PEUU group at 4- and 8-week points (I). LVESV, Left ventricular end-systolic volume; LVEDV, left ventricular end-diastolic volume; ns, not significant.
DISCUSSION
The results demonstrated that PEUU patch implantation prevented LV adverse remodeling and cardiac functional loss for an 8-week period after placement over an ischemia–reperfusion injury in a porcine model. The implanted PEUU patch partially degraded in this period and an αSMA-positive cellular infiltrate in the remnant patch material was observed. Below the patch, similarly labeled cells were observed together with significantly increased neovascularization and LV wall thickening, the latter of which would theoretically contribute to decreased wall stress explained by the LaPlace law. These findings are consistent with our previous study using a smaller patch of the same material in a rodent ischemic cardiomyopathy model.8
Whereas nonbiodegradable, biventricular wrapping devices have already been evaluated in the clinic,7 a localized, elastic biodegradable patch has theoretical advantages over these earlier approaches. Whole epicardial wrapping raises concerns about reoperation complexity owing to extensive pericardial adhesions and the potential for coronary blood flow impairment.19 Furthermore, the placement of a permanent foreign body is associated with a chronic infection risk and potential calcification nidus. A regional, degradable patch plasty approach would limit such concerns and, through the selection of an appropriate degradable elastic polymer, could offer the potential to control the degradation rate20 and to incorporate pharmaceuticals for localized controlled release.21 Although several studies have reported a variety of biodegradable, epicardial patch approaches to limit LV remodeling after ischemic injury,8–12 these approaches have used cellular components and few have been translated to the more clinically relevant large animal models.
There are 2 notable earlier reports in which regional epicardial patch plasty was evaluated in a large animal model, although both reports used nonbiodegradable and nonelastic synthetic materials.19,22 Kelley and colleagues22 placed a commercial knitted polypropylene mesh over the anticipated LV infarct region before MI and showed limited LV dilation and functional deterioration, such as LV end-diastolic pressure, in an ovine model at 8 weeks. Although the results from this early study were encouraging, the clinical relevance of patch placement before MI raised some questions regarding the likely effects of a patch placed at a more clinically relevant time after MI. More recently, Liao and colleagues19 placed a composite synthetic mesh made of a polypropylene mesh inner layer and expanded polytetrafluoroethylene outer layer, with the expanded polytetrafluoroethylene side toward the pericardium to minimize the risk of pericardial adhesions. The patch was used in a porcine model 8 weeks after MI to evaluate the impact on the chronic phase of LV remodeling. Using an elegant array of magnetic resonance imaging and catheter-based hemodynamic analysis, these investigators showed that regional, nonbiodegradable patch placement attenuated LV dilation, increased LV wall thickness, and improved LV ejection fraction and +dP/dt. At 12 weeks after patch placement, significant LV dilation was observed, in contrast to our findings. The timing of patch placement (2 weeks after MI in our study vs 8 weeks in their study) and whether the peri-infarct (border) zone was covered with material (the PEUU patch covered both the risk and border zone) might account for the discrepancy in the LV dilation after patching. Both of these earlier reports provide encouraging results regarding the potential for regional patch plasty and raised the question whether such benefits could be achieved without placement of a permanent foreign body on the epicardium and whether an elastic patch material might not be better suited mechanically for the task of altering the mechanical environment of the remodeling ischemic LV wall.
The advantage of using an elastic patch material such as PEUU as opposed to a stiff polymer has not been definitely shown, although there is reason to believe that the mechanical properties of the patch material matter. Using a rodent model, we8 have shown that an unpatched infarcted LV wall had the least compliance, whereas the PEUU-patched LV was significantly more compliant, better approximating the passive properties of healthy cardiac tissue. Also,in com-paring an expanded polytetrafluoroethylene versus a PEUU patch applied to an infarcted rat LV, the PEUU patched wall was thicker and those animals had significantly improved functional reserve under dobutamine stress.23 It is speculated that the elastic PEUU material allows myofibers in both the risk zone and border zone to stretch during diastole while also providing a reduction in wall stress. The advantage of elasticity and distensibilty in the infarcted LV has been implicated previously, wherein improvement in LV function was attributed to augmented elastin network formation by mononuclear cell injection24 or elastin gene therapy.25 Even though the original mechanical properties of the applied PEUU patch will necessarily be reduced as material degradation proceeds, the induced tissue formed beneath the patch increases wall thickness, leading to reduction in wall stress, and in the rat model, is mechanically softer under tensile loading than the unpatched infarcted wall.8
Several limitations of the current report should be mentioned. Although patch placement was associated with an alteration in the extent of adverse LV remodeling, this intervention becomes the starting point of an altered remodeling process. Hence, a longer follow-up period after patch implantation would provide additional insights into the influence of epicardial patch plasty on extended functional preservation and LV morphology. Although patch mechanical support properties will be lost earlier than complete material degradation, an end point after traces of the material are completely removed would elucidate whether the temporal LV support effects on ventricular geometry endure for a prolonged period. Second, detailed assessments could be performed using several techniques to provide better insight into the nature of functional benefits associated with the procedure: echocardiography to track regional LV wall motion and provide more detailed geometric analysis (eg, sphericity index); magnetic resonance imaging to investigate LV geometry and provide accurate LV volumes with the cardiac cycle; positron emission tomography/magnetic resonance imaging to assess cardiac metabolism; and invasive hemodynamic monitoring to obtain functional parameters such as dP/dt max or preload/afterload-corrected echocardiographic evaluation. Third, postmortem infarct size and fibrosis measurements are lacking in the study. Finally, this study does not address the optimal timing for patch implantation or the optimal rate for material degradation. Performing the patch plasty procedure sooner after MI (eg, within days as opposed to 2 weeks) may produce better functional preservation. Whether a faster or slower degrading patch would provide improved benefit is also undetermined, as is the optimal elastic stiffness of the material to be applied.
In conclusion, PEUU patch implantation prevented LV dilatation, preserved contractile function, and improved the retention of wall thickness in the infarcted LV wall. Improved vascularity was observed in the patched LV wall. These data in a large animal model are consistent with our previous results in small animals and suggest that PEUU patch placement may be efficacious for cardiomyoplasty of the chronically infarcted LV wall by altering the adverse remodeling process and, at least temporarily, preserving myocardial function.
We thank Jennifer Debarr, Keisuke Takanari, Hong-bin Jiang, and Deanna L. Rhoads for their help with the tissue histological assessment, David Fischer, Judith L. Thoma, and Takeyoshi Ota for their excellent assistance with the animal procedures, Tomomi Yamada for her statistical evaluation, and Joseph E. Pichamuthu for his help on the cyclic tensile testing.
Supplementary Material
Acknowledgments
Funded by the National Heart, Lung and Blood Institute, National Institutes of Health grant HL069368.
Abbreviations and Acronyms
- αSMA
α-smooth muscle actin
- EDA
end-diastolic area
- EF
ejection fraction
- ESA
end-systolic area
- %FAC
fractional area change
- LV
left ventricular (ventricle)
- LVEDV
left ventricular end-diastolic volume
- LVESV
left ventricular end-systolic volume
- MI
myocardial infarction
- PEUU
poly(ester urethane)urea;
Footnotes
Disclosures: Authors have nothing to disclose with regard to commercial support.
References
- 1.Adhyapak SM, Parachuri VR. Architecture of the left ventricle: insights for optimal surgical ventricular restoration. Heart Fail Rev. 2010;15:73–83. doi: 10.1007/s10741-009-9151-0. [DOI] [PubMed] [Google Scholar]
- 2.Mesana T. Ischemic mitral regurgitation: the challenge goes on. Curr Opin Cardiol. 2012;27:108–110. doi: 10.1097/HCO.0b013e32834fdc22. [DOI] [PubMed] [Google Scholar]
- 3.White HD, Norris RM, Brown MA, Brandt PW, Whitlock RM, Wild CJ. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987;76:44–51. doi: 10.1161/01.cir.76.1.44. [DOI] [PubMed] [Google Scholar]
- 4.Aiba T, Tomaselli G. Electrical remodeling in dyssynchrony and resynchronization. J Cardiovasc Transl Res. 2012;5:170–179. doi: 10.1007/s12265-012-9348-9. [DOI] [PubMed] [Google Scholar]
- 5.Gajarsa JJ, Kloner RA. Left ventricular remodeling in the post-infarction heart: a review of cellular, molecular mechanisms, and therapeutic modalities. Heart Fail Rev. 2011;16:13–21. doi: 10.1007/s10741-010-9181-7. [DOI] [PubMed] [Google Scholar]
- 6.Dor V, Montiglio F, Sabatier M, Coste P, Barletta G, Di Donato M, et al. Left ventricular shape changes induced by aneurysmectomy with endoventricular circular patch plasty reconstruction. Eur Heart J. 1994;15:1063–1069. doi: 10.1093/oxfordjournals.eurheartj.a060629. [DOI] [PubMed] [Google Scholar]
- 7.Mann DL, Kubo SH, Sabbah HN, Starling RC, Jessup M, Oh JK, et al. Beneficial effects of the CorCap cardiac support device: five-year results from the Acorn Trial. J Thorac Cardiovasc Surg. 2011;143:1036–1042. doi: 10.1016/j.jtcvs.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujimoto KL, Tobita K, Merryman WD, Guan J, Momoi N, Stolz DB, et al. An elastic, biodegradable cardiac patch induces contractile smooth muscle and improves cardiac remodeling and function in subacute myocardial infarction. J Am Coll Cardiol. 2007;49:2292–2300. doi: 10.1016/j.jacc.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ke Q, Yang Y, Rana JS, Chen Y, Morgan JP, Xiao YF. Embryonic stem cells cultured in biodegradable scaffold repair infarcted myocardium in mice. Sheng Li Xue Bao. 2005;57:673–681. [PubMed] [Google Scholar]
- 10.Lancaster J, Juneman E, Hagerty T, Do R, Hicks M, Meltzer K, et al. Viable fibroblast matrix patch induces angiogenesis and increases myocardial blood flow in heart failure after myocardial infarction. Tissue Eng Part A. 2010;16:3065–3073. doi: 10.1089/ten.TEA.2009.0589. [DOI] [PubMed] [Google Scholar]
- 11.Miyagi Y, Zeng F, Huang XP, Foltz WD, Wu J, Mihic A, et al. Surgical ventricular restoration with a cell- and cytokine-seeded biodegradable scaffold. Biomaterials. 2010;31:7684–7694. doi: 10.1016/j.biomaterials.2010.06.048. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Q, Zhou JY, Zheng Z, Zhang H, Hu SS. A novel vascularized patch enhances cell survival and modifies ventricular remodeling in a rat myocardial infarction model. J Thorac Cardiovasc Surg. 2010;140:1388–1396. doi: 10.1016/j.jtcvs.2010.02.036. e3. [DOI] [PubMed] [Google Scholar]
- 13.Nelson DM, Ma Z, Fujimoto KL, Hashizume R, Wagner WR. Intra-myocardial biomaterial injection therapy in the treatment of heart failure: materials, outcomes and challenges. Acta Biomater. 2011;7:1–15. doi: 10.1016/j.actbio.2010.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujimoto KL, Ma Z, Nelson DM, Hashizume R, Guan J, Tobita K, et al. Synthesis, characterization and therapeutic efficacy of a biodegradable, thermorespon-sive hydrogel designed for application in chronic infarcted myocardium. Biomaterials. 2009;30:4357–4368. doi: 10.1016/j.biomaterials.2009.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leor J, Tuvia S, Guetta V, Manczur F, Castel D, Willenz U, et al. Intracoronary injection of in situ forming alginate hydrogel reverses left ventricular remodeling after myocardial infarction in swine. J Am Coll Cardiol. 2009;54:1014–1023. doi: 10.1016/j.jacc.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Toma C, Letts DP, Tanabe M, Gorcsan J3rd, Counihan PJ. Positive effect of darbepoetin on peri-infarction remodeling in a porcine model of myocardial ischemia-reperfusion. J Mol Cell Cardiol. 2007;43:130–136. doi: 10.1016/j.yjmcc.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 17.van der Spoel TI, Jansen of Lorkeers SJ, Agostoni P, van Belle E, Gyongyosi M, Sluijter JP, et al. Human relevance of pre-clinical studies in stem cell therapy: systematic review and meta-analysis of large animal models of ischaemic heart disease. Cardiovasc Res. 2011;91:649–658. doi: 10.1093/cvr/cvr113. [DOI] [PubMed] [Google Scholar]
- 18.Guan J, Sacks MS, Beckman EJ, Wagner WR. Synthesis, characterization, and cytocompatibility of elastomeric, biodegradable poly(ester-urethane)ureas based on poly(caprolactone) and putrescine. J Biomed Mater Res. 2002;61:493–503. doi: 10.1002/jbm.10204. [DOI] [PubMed] [Google Scholar]
- 19.Liao SY, Siu CW, Liu Y, Zhang Y, Chan WS, Wu EX, et al. Attenuation of left ventricular adverse remodeling with epicardial patching after myocardial infarction. J Card Fail. 2010;16:590–598. doi: 10.1016/j.cardfail.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Hong Y, Guan J, Fujimoto KL, Hashizume R, Pelinescu AL, Wagner WR. Tailoring the degradation kinetics of poly(ester carbonate urethane)urea thermoplastic elastomers for tissue engineering scaffolds. Biomaterials. 2010;31:4249–4258. doi: 10.1016/j.biomaterials.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelson DM, Baraniak PR, Ma Z, Guan J, Mason NS, Wagner WR. Controlled release of IGF-1 and HGF from a biodegradable polyurethane scaffold. Pharm Res. 2011;28:1282–1293. doi: 10.1007/s11095-011-0391-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelley ST, Malekan R, Gorman JH3rd, Jackson BM, Gorman RC, Suzuki Y, et al. Restraining infarct expansion preserves left ventricular geometry and function after acute anteroapical infarction. Circulation. 1999;99:135–142. doi: 10.1161/01.cir.99.1.135. [DOI] [PubMed] [Google Scholar]
- 23.Fujimoto K, Tobita K, Guan J, Hashizume R, Takanari K, Alfieri CM, et al. Placement of an elastic, biodegradable cardiac patch on a sub-acute infarcted heart leads to cellularization with early developmental cardiomyocyte characteristics. J Card Fail. 2012;18:585–595. doi: 10.1016/j.cardfail.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lichtenauer M, Mildner M, Baumgartner A, Hasun M, Werba G, Beer L, et al. Intravenous and intramyocardial injection of apoptotic white blood cell suspensions prevents ventricular remodelling by increasing elastin expression in cardiac scar tissue after myocardial infarction. Basic Res Cardiol. 2011;106:645–655. doi: 10.1007/s00395-011-0173-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mizuno T, Yau TM, Weisel RD, Kiani CG, Li RK. Elastin stabilizes an infarct and preserves ventricular function. Circulation. 2005;112:I81–I88. doi: 10.1161/01.CIRCULATIONAHA.105.523795. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.