Abstract
Cytochrome P450 1A1 (CYP1A1) is an extrahepatic phase I metabolizing enzyme whose expression is suppressed under physiologic conditions, but can be induced by substrates via the aryl hydrocarbon receptor (AhR). Nonetheless, recent studies show that the majority of breast tumors constitutively express CYP1A1. These findings led us to test the hypothesis that CYP1A1 promotes breast cancer progression by evaluating the effects of CYP1A1 knock down on the proliferation and survival of the MCF7 and MDA-MB-231 lines. Independently of estrogen receptor status, CYP1A1 knock down decreases cell proliferation, decreases colony formation, blocks the cell cycle at G0/G1 associated with reduction of cyclin D1, and increases apoptosis associated with reduction of survivin. CYP1A1 knock down markedly increases phosphorylation of AMP-activated protein kinase (AMPK) and decreases phosphorylation of AKT, extracellular signal-regulated kinases 1 and 2 (ERK1/2), and 70kDa ribosomal protein S6 kinase (P70S6K). AMPK inhibition by compound C partially abrogates the pro-apoptotic effects of CYP1A1siRNA, suggesting that CYP1A1siRNA effects are mediated, in part, through AMPK signaling. Consistent with CYP1A1 knock down results, pharmacologic reduction of CYP1A1 levels by the phytopolyphenol carnosol also correlates with impaired proliferation and induced AMPK phosphorylation. These results indicate that reduction of basal CYP1A1 expression is critical for inhibition of proliferation, which is not affected by alpha-naphthoflavone-mediated inhibition of CYP1A1 activity nor modulated by AhR silencing. This study supports that CYP1A1 may promote breast cancer proliferation and survival, at least in part, through AMPK signaling and that reduction of CYP1A1 levels is a potential strategy for breast cancer therapeutics.
Keywords: CYP1A1, siRNA, breast cancer, AMPK, AKT, ERK, P70S6K, AhR, carnosol, TCDD, alpha-naphthoflavone
Introduction
Cytochrome P450 1A1 (CYP1A1) is an extrahepatic phase-I enzyme that metabolizes endogenous and xenobiotic substrates. Prior studies implicating CYP1A1 in cancer have dealt primarily with the metabolic activation of pro-carcinogens including polycyclic aromatic hydrocarbons and estradiol (1–4). Other studies have focused on associations between CYP1A1 single nucleotide polymorphisms (SNPs) and increased cancer incidence. For example, the A2455G allele has been implicated with increased breast cancer risk in Caucasian populations (4, 5). Nonetheless, lack of associations between SNPs and cancer have been reported and these discrepancies have been attributed to ethnic variations between populations, variation in sample size, and lack of confirmation of protein expression (6). However, little is known about the roles of CYP1A1 in cancer biology in the absence of xenobiotic exposure.
Under physiological conditions, CYP1A1 expression is suppressed but it is induced in the presence of substrates via the transcriptional regulation of the aryl hydrocarbon receptor (AhR) (4). In recent years studies have shown that breast tumors constitutively express CYP1A1 (7, 8). Murray et al. profiled the expression levels of twenty one CYPs in 170 breast tumors from patients who had not received prior adjuvant treatment. This profiling revealed that CYP1A1 was expressed in about 90% of breast tumors. Even so, the degree of CYP1A1 expression varied among tumors and did not correlate with estrogen receptor alpha levels, tumor grade, or clinical outcome (7). Vinothini et al. reported on the expression levels of various xenobiotic-metabolizing enzymes, including CYP1A1, in 60 breast tumors from newly diagnosed patients who had not received prior adjuvant therapy. Their study showed that CYP1A1 levels were: (a) elevated in tumors compared to adjacent breast tissue, (b) higher in premenopausal compared to post-menopausal patients, and (c) positively correlated with tumor grade (8). These studies provide evidence that CYP1A1 is expressed to varying degrees in breast tumors and may be associated with cancer biology. Moreover, because of its ubiquitous expression in breast cancer and its ability to metabolize xenobiotics, interest has been shown to exploit CYP1A1 activity for breast cancer therapeutics (9–12). Nonetheless, little is known about the activities of CYP1A1 that may participate in breast cancer progression. Therefore, better understanding the function of CYP1A1 in breast cancer will assist in the development of targeted therapy and improve treatment strategies.
The purpose of this study was to determine the biological functions and roles in signal transduction of CYP1A1 in breast cancer cells. Toward this goal we utilized small interfering RNA (siRNA) to knock down CYP1A1 in the breast cancer lines MCF7 (estrogen receptor positive; ER+) and MDA-MB-231 (triple negative; ER-/PR-/HER2-). We determined that CYP1A1 knock down decreases proliferation, decreases colony formation, blocks the cell cycle at G0/G1 associated with reduction of cyclin D1, and increases apoptosis associated with reduction of survivin. CYP1A1 silencing reduces proliferation and survival, at least in part, through increased phosphorylation of AMP-activated protein kinase (AMPK) and reduced phosphorylation of AKT, extracellular signal-regulated kinases 1 and 2 (ERK1/2), and 70kDa ribosomal protein S6 kinase (P70S6K). Consistent with CYP1A1siRNA results, pharmacologic reduction of CYP1A1 levels by carnosol also impairs proliferation and induces AMPK phosphorylation. Together, these results implicate CYP1A1 in cell proliferation and survival pathways. Additionally, modulation studies of CYP1A1 activity via 2,3,7,8-tethachlorodibenzo-p-dioxin (TCDD, inducer) and alpha-naphthoflavone (inhibitor) indicate that reduction of CYP1A1 expression levels is critical for these biological effects and cannot be substituted by modulation of its activity alone. The evidence presented here suggests that CYP1A1 silencing is a potential therapeutic strategy for breast cancer independent of estrogen receptor status.
Materials and Methods
Chemicals and reagents
MEM and cell culture supplements are from GIBCO/Invitrogen/Life Technologies (Carlsbad, CA). Charcoal/dextran-stripped serum and fetal bovine serum from HyClone (Logan, UT). ON-TARGETplus CYP1A1- and NTsiRNA pools (sequences below) are from Thermo Scientific Dharmacon (Lafayette, CO). Oligofectamine, Opti-MEM, SuperScript® III First-Strand synthesis system, and SYBR® GreenER™ qPCR SuperMixes for iCycler are from Invitrogen/Life Technology (Carlsbad, CA). All primers were purchased from Integrated DNA Technologies (Minneapolis, MN). The QiaShredder columns, RNeasy Mini Kit, QuantiTect Reverse Transcription Kit, and RNase-A are from Qiagen (Valencia, CA). The Wright-Giemsa was purchased from Thermo Fisher Scientific (Waltham, MA). Carnosol was purchased from Cayman Chemical (Ann Arbor, MI) and dissolved in DMSO to a final concentration of 40mM. Anti-actin antibody (HHF-35), MG-132, and compound C are from Calbiochem/EMD Millipore (San Diego, CA). The tetrazolium salt, propidium iodide, NP-40, cell culture grade DMSO HYBRI-MAX, ethoxyresorufin, alpha-naphthoflavone, and sodium citrate are from Sigma (St. Louis, MO). 2,3,7,8-tethachlorodibenzo-p-dioxin (TCDD) is from AccuStandard (New Haven, CT). Protease and phosphatase inhibitors are from Sigma (St. Louis, MO) and Roche (Indianapolis, IN). Human fibronectin (#356008) and the apoptosis detection kit are from BD Pharmingen (San Jose, CA). Anti-phospho-p70S6 kinase (Thr421/Ser424) is from Millipore (Billerica, MA). Antibodies raised against total and phosphorylated forms of the following proteins were obtained from Cell Signaling Technology (Beverly, MA): mTOR (p-Ser2448/clone D9C2), ERK1/2 (p-Thr202/Tyr204), Akt (total clone 40D4 and p-Ser473), LKB1 (total), AMPK (total clone F6 and p-Thr172/clone 40H9), P70S6K (total). Anti-CDK4 is from Santa Cruz Biothecnology, (Santa Cruz, CA). Anti-survivin antibody is from R&D systems (Minneapolis, MN). Anti-CYP1A1 (ab3568) was obtained from Abcam (Cambridge, MA). Anti-GAPDH antibody is from Research Diagnostics/Fitzgerald Industries (Concord, MA). The ECL Western blotting detection reagent was purchased from GE Healthcare/Biocompare (South San Francisco, CA).
Cell lines
The lines MDA-MB-231 and MCF7 were purchased from American Type Culture Collection (Manassas, VA) and grown at 37°C under 5% CO2. Unless otherwise indicated, cells were grown and maintained in complete medium (MCF7: MEM, 10% FBS, 1% L-glutamine, 1% penicillin/streptomycin, and 1% HEPES; MDA-MB-231: MEM, 10% FBS, 1% L-glutamine, 1% penicillin/streptomycin, 1% sodium pyruvate, and 2 μg/mL insulin). Because antibiotics reduce transfection efficiency, for all siRNA transfection experiments cells were passaged once in antibiotic-free medium (AFM) prior to plating for the experiments and maintained in this medium throughout the duration of the experiment. MCF7: 10% charcoal/dextran-stripped serum, phenol-red free MEM, 1% HEPES, and 1% L-glutamine; MDA-MB-231: 10% FBS, phenol-red free MEM, 1% sodium pyruvate, 1% L-glutamine, and 2 μg/mL insulin).
siRNA transfection
To knock down CYP1A1 or AhR levels, cells were transfected with a pool of siRNA designed by Dharmacon (sequences below). Cells were seeded at 30% confluence on 6-well plate and incubated overnight. Prior to transfection cells were washed twice with phenol-red free MEM and 0.8mL of OptiMEM was added to each well. A transfection solution of 60nM siRNA suspended in 0.3% oligofectamine/OptiMEM was gently added to the cells (200uL/well). After overnight incubation, AFM medium was added to obtain 10% serum medium. For transfection quality purposes a separate well was transfected with siGLO green transfection indicator # D-001630-01. Sequences: human CYP1A1siRNA ON-TARGETplus SMARTpool #L-004790-00-0005: UCGACAAGGUGUUAAGUGA, AAAUGCAGCUGCGCUCUUA, CUACAGGUAUGUGGUGGUA, GAACUGCUUAGCCUAGUCA. ON-TARGETplus Human AhRsiRNA SMART pool # L-004990-00-0005: GCAAGUUAAUGGCAUGUUU, GAACUCAAGCUGUAUGGUA, GCACGAGAGGCUCAGGUUA,GCAACAAGAUGAGUCUAUU. Non-targeting siRNA ON-TARGETplus #1 D-001810-10: UGGUUUACAUGUCGACUAA, UGGUUUACAUGUUGUGUGA, UGGUUUACAUGUUUUCUGA, UGGUUUACAUGUUUUCCUA.
Quantitative RT-PCR
To evaluate CYP1A1 mRNA knock down efficiency, total RNA was collected in RLT buffer and beta-mercaptoethanol, passed through a QiaShredder column, and purified using the RNeasy Mini Kit according to the manufacturer’s protocol. First strand cDNA was made using the QuantiTect Reverse Transcription kit according to the manufacturer’s protocol. The quantitative RT-PCR reactions were prepared using SYBR® GreenER™ qPCR SuperMixes for iCycler and performed at as follow: 50°C for 2min, 95°C for 8.5min, 40cycles of 95°C for 15sec and 60°C for 1min. The comparative Ct value method was used for data analysis (13). GAPDH was utilized for normalization. Primer sequences: CYP1A1 forward: 5′-GCT GAC TTC ATC CCT ATT CTT CG-3′, reverse: 5′:TTT TGT AGT GCT CCT TGA CCA TCT-3′; GAPDH forward: 5′-GGG AAG GTG AAG GTC GGA GT-3′, reverse: 5′-GAG TTA AAA GCA GCC CTG GTG A-3′.
Semi-quantitative RT-PCR
To evaluate siRNA specificity for CYP1A1 compared to other CYPs, total mRNA was collected and purified as described above. The cDNA was prepared using the First Strand Synthesis Kit according to the manufacturer’s protocol (Invitrogen). Semi-quantitative RT-PCR analysis of CYP1A1, CYP1A2, and CYP1B1 was performed using SuperScript® III Reverse Transcriptase (Invitrogen) and Taq Master Mix kit according to manufacturer’s protocol (Qiagen) in a PX2 Thermal Cycler (Thermo) (94°C for 2min, 50 cycles: 94°C/30sec, 55°C/30sec, 72°C/45sec, 72°C for 10min and 4°C for 5min). The PCR products were resolved in a 1.5% agarose gel and GAPDH was used to normalize samples. Primer sequences [Gene (amplicon size), F = Forward primer (5′–3′), R = Reverse primer (5′–3′)]: GAPDH (609bp), F: CAC AGT CCA TGC CAT CAC TGC, R: GGT CTA CAT GGC AAC TGT GAG; CYP1A1 (294bp), F: CTT CAT CCT GGA GAC CTT CC, R: AAG ACC TCC CAG CGG GCA A; CYP1A2 (371bp), F: CAA TCA GGT GGT GGT GTC AG, R: GCT CCT GGA CTG TTT TCT GC; CYP1B1 (350bp), F; TCA ACC GCA ACT TCA GCA ACT T, R: ATA GGG CAG GTT GGG CTG GTC A.
Western blot assay
To evaluate protein levels, cell extracts were prepared using RIPA extraction buffer (50mM Tris pH 7.5, 150mM NaCl, 1% NP-40, 0.5% DOC, 0.1% SDS, 2mM EDTA pH 8, 2mM EGTA pH 8, 1mM DTT, 1mM PMSF, 5uM PAO, 25mM NaF, 2mM Na3VO4, 100uM leupeptin, 2uM pepstatin, 2.8uM TPCK, 2.7uM TLCK, 4.17uM AEBSF, 4.17uM chymostatin, 2uM aprotinin, 2uM antipain, 5mM N-ethyl-maliemide, 40uM MG-132) and processed according to standard Western blot analysis. GAPDH was used as loading control and relative protein amounts were quantified by densitometry of x-ray film exposures using an Alpha-Innotec densitometer.
MTT Cell Proliferation Assay
To assess viability, cells were incubated with 0.1mg/mL of tetrazolium (3, [4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) for 2hrs at 37°C under 5% CO2. The supernatant was replaced with DMSO. Proliferating cells reduce the yellow tetrazollium into DMSO-soluble purple formazan which was read in a BioTek plate reader at an absorbance of 540nm.
Anchorage-dependent clonogenic assay
To assess cell survival, two hundred transfected cells were seeded onto fibronectin-coated 6-well plates and incubated for 14 days until visible colonies were observed. Colonies were fixed and stained with Wright-Giemsa and total colony number was counted. Plate preparation: 1mL fibronectin (10 μg/mL in PBS) incubated overnight at 4°C, then blocked with 3% BSA in PBS for 1hr at RT, and rinsed with PBS prior to use.
Cell cycle assay
To evaluate the cell cycle distribution, cellular DNA content was measured by propidium iodide staining. Because MCF7 cells form clumps in the presence of ethanol, two different methods were utilized for fixing and staining. MDA-MB-231 method: cells were collected by trypsinization, washed with ice cold PBS, resuspended in 200 μL of ice cold 70% ethanol, incubated overnight at 4°C, centrifuged, washed with PBS, and finally resuspended in 0.5mL of Nicoletti buffer (50 μg/ml propidium iodide, 0.1% sodium citrate, 0.1% Triton X-100 and 1 mg/ml RNase-A in PBS). MCF7 method: cells were washed twice with PBS over ice, scraped off the plate with 1mL of PBS, collected and centrifuged for 5mins, resuspended in 0.5mL of staining solution (0.1% sodium citrate, 50 μg/mL propidium iodide, 0.01% NP-40, and 1 μg/mL RNase A in water), and incubated overnight at 4°C. Following the respective staining methods propidium iodide content was profiled using a FACScalibur flow cytometer (BD Bioscience). Cell cycle distribution was determined using the Watson Pragmatic algorithm in the FlowJo software.
Apoptosis assay
To evaluate cell death cells were collected by trypsinization, washed with PBS, and stained with propidium iodide and Annexin-V/FITC for 15mins at RT. Stained cells were evaluated using a FACScalibur flow cytometer (BD Bioscience) and the results were analyzed with FlowJo software (Tree Star).
Ethoxyresorufin-O-deethylase (EROD) assay
To assess CYP1A1 activity, an EROD assay adapted from (14, 15) was utilized. Cells were plated onto 6-well plates, treated as desired, washed 2× with 1xPBS, then incubated at 37°C for 60mins with 2mM ethoxyresorufin in buffer (50mM Tris, 0.1M NaCl, 6.25mM MgCl2, pH 7.8, warmed to 37°C). A 100uL aliquot of the assay supernatant was transferred to a black-wall clear bottom 96-well plate and fluorescence was read in a BioTek fluorescence plate reader at excitation λ (530nm)/emission λ (590nm). When necessary fluorescent counts were normalized to total cell number.
Statistics
All experiments were reproducible and performed at least in triplicate. Statistical significance (p values) was calculated using two-tailed Student’s t-test. Equality of variance was determined by F test.
Results
CYP1A1 knock down impairs cell proliferation and survival
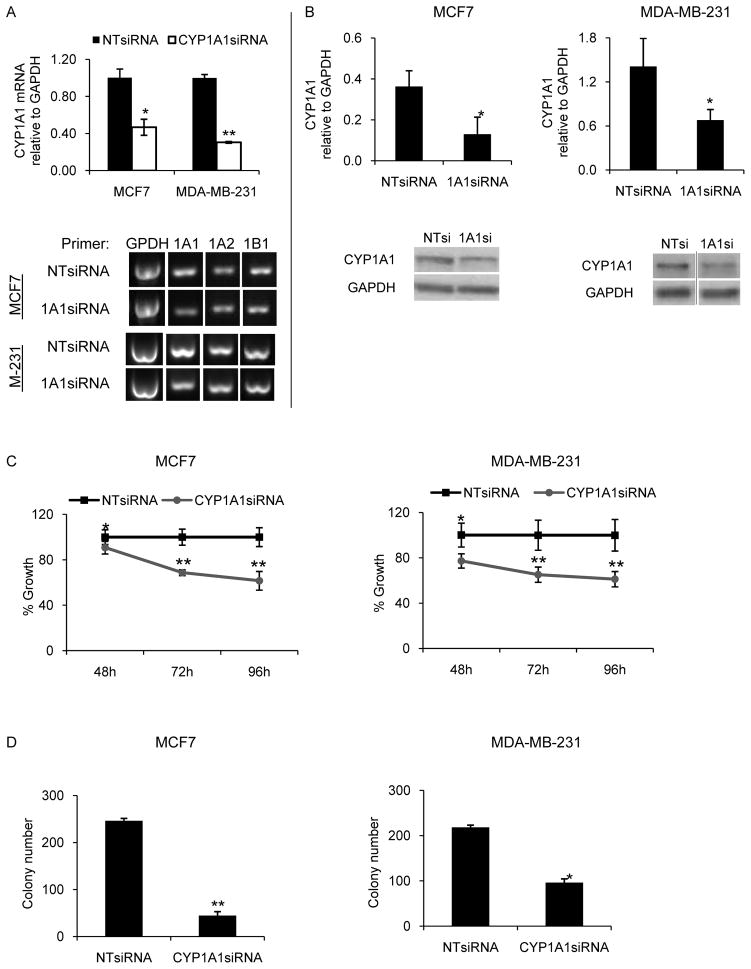
The purpose of this study was to understand the biological roles of CYP1A1 in MCF7 and MDA-MB-231 breast cancer lines. We chose ER+ and triple-negative breast cancer because there is a significant unmet need for new treatments for metastatic hormone therapy refractory and triple negative breast cancer. To achieve the goal of defining the role of CYP1A1 in breast cancer growth, pooled siRNA was utilized to knock down CYP1A1 mRNA and protein. To validate the knock down efficiency, CYP1A1 mRNA and protein levels were measured by quantitative RT-PCR and by Western blot, respectively. Significant reductions of CYP1A1 mRNA and protein levels were observed between 48h and 120h. CYP1A1 mRNA levels were reduced by 53% (p=0.002) in the MCF7 line and by 70% (p=6×10−6) in MDA-MB-231 line (Fig. 1A, top panel). CYP1A1 protein levels were reduced by 64% (p=0.02) in the MCF7 line and by 52% (p=0.04) in the MDA-MB-231 line (Fig. 1B). Because CYP1A1 shares 73% identity with CYP1A2 and 41% identity with CYP1B1, we also evaluated by semi-quantitative RT-PCR whether CYP1A1siRNA alters the mRNA levels of these CYP1 family members. No change was observed in the levels of these CYPs indicating that the biological effects observed are due specifically to reduction of CYP1A1 (Fig. 1A, bottom panel).
Figure 1. CYP1A1 knock down impairs breast cell proliferation and survival.
The efficiency of CYP1A1siRNA knock down in MCF7 and MDA-MB-231 cells was evaluated by quantitative RT-PCR (A, top panel) and by western blot (B). To evaluate siRNA specificity semi-quantitative RT-PCR was performed with the indicated primers and the products were run in 1.5% agarose gel (representative samples shown in A, bottom panel). C, Proliferation experiments for lines MCF7 and MDA-MB-231 after transfection with CYP1A1siRNA for the indicated times. D, Clonogenicity was evaluated by transfection for 48hrs followed by seeding of two-hundred transfected cells onto fibronectin-coated 6-well plates. Visible colonies were stained and counted after 14 days of culture. (n=3, *p<0.05, **p<0.001)
To investigate the functional roles of CYP1A1 in breast cancer, we determined the effects of CYP1A1 knock down on two “hallmarks of cancer”: uncontrolled cell proliferation and loss of inhibition of cell death. First, we examined the effects of CYP1A1 knock down on the ability of MCF7 and MDA-MB-231 cells to proliferate. After transfection for 48h, 72h, and 96h, viable cells were measured by MTT assay. CYP1A1siRNA reduces the proliferation of both lines. At 96h of transfection with CYP1A1siRNA, proliferation was reduced by ~40% for the MCF7 and MDA-MB-231 lines (Fig. 1C, p< 0.001).
To determine the effects of CYP1A1 knock down on cell survival we performed a clonogenic assay to count cells able to survive and proliferate to form visible colonies. MCF7 and MDA-MB-231 cells were transfected for 48hrs, seeded at low density onto fibronectin-coated plates, and colonies were counted after two weeks of culture. Compared to NTsiRNA control, CYP1A1siRNA inhibited colony formation of MCF7 line by 82% (p=4×10−6) and of MDA-MB-231 line by 56% (p=0.03) (Fig. 1D). Together these results indicate that CYP1A1 silencing impairs proliferation and survival of breast cancer cells.
The aryl hydrocarbon receptor (AhR) localizes to the cytosol of MCF7 cells and translocates to the nucleus upon ligand activation. In contrast, MDA-MB-231 cells display primarily nuclear AhR expression. In both lines, nuclear AhR is responsible for the inducible transcriptional regulation of CYP1A1. Given the important role of AhR in the regulation of CYP1A1, it is possible that the biological functions of CYP1A1 may be affected by or be dependent on the AhR status. To determine whether AhR is required for the roles of CYP1A1 on cell proliferation, we investigated the impact of siRNA-mediated knock down of AhR on basal CYP1A1 expression and cell proliferation. While AhRsiRNA reduces AhR levels by at least 70% (p < 0.05) in both lines, the levels of CYP1A1 remain unaffected (Supplementary Fig. S1A). Therefore, this system allows us to distinguish AhR-specific effects. As assessed by MTT assay, the proliferation of these lines is not impaired by AhR knock down (Supplementary Fig. S1B), suggesting that the effects of CYP1A1 knock down on cell proliferation and survival (Fig. 1) may be independent of the AhR expression status of these lines.
CYP1A1 knock down blocks the cell cycle
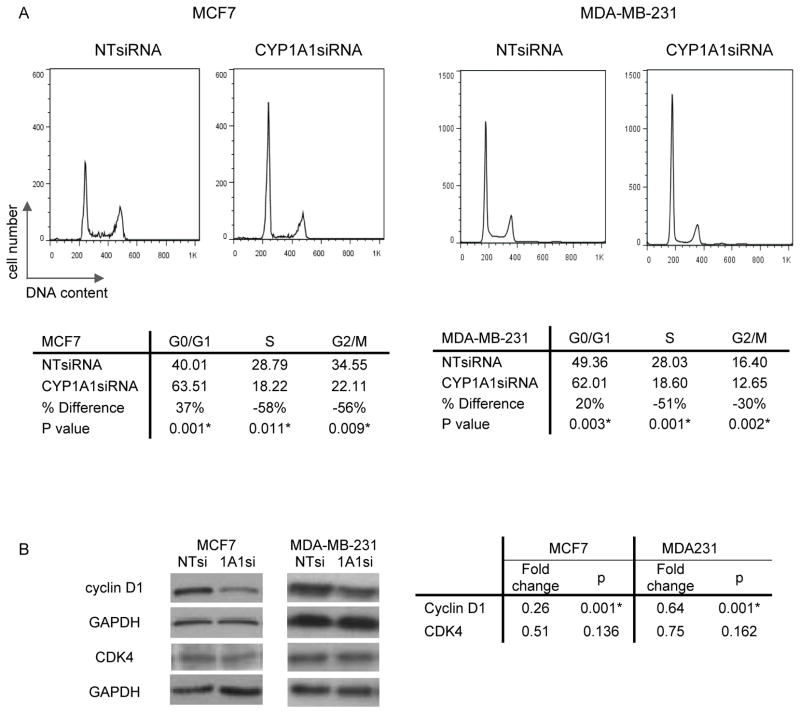
The ability of cells to proliferate depends on the balance between cell growth, division and death. For this reason we investigated the effect of CYP1A1 knock down on the ability of MCF7 and MDA-MB-231 lines to progress through the cell cycle. To achieve this goal, cells were transfected for 48hrs, permeabilized, stained with propidium iodide, and cell cycle distribution was analyzed by flow cytometry. The G0/G1 populations of MCF7 and MDA-MB-231 lines increased by 37% (p=0.001) and 20% (p=0.003) respectively, consistent with a block in the cell cycle at G0/G1 (Fig. 2A).
Figure 2. CYP1A1 knock down blocks the cell cycle in breast cancer cells.
A, Cell cycle distribution was assessed by flow cytometry of MDA-MB-231 and MCF7 cells transfected for 48hr and stained with propidium iodine. B, Cyclin D1 and CDK4 proteins were visualized by western blot. Fold changes are expressed relative to CYP1A1siRNA knock down (n=3, p values shown, * indicates statistical significance).
Because the cyclin D1/CDK4 complex regulates the G1/S transition of the cell cycle (16), we investigated the effects of CYP1A1 knock down on these regulatory proteins. Cyclin D1 levels were reduced by 74% (p=0.001) in MCF7 line and by 36% (p= 0.001) in MDA-MB-231 line (Fig. 2B). CDK4 levels were not significantly reduced in both lines, although a trend toward reduction is observed. These results suggest that CYP1A1 knock down may block the cell cycle, at least in part, through downregulation of cyclin D1.
CYP1A1 knock down increases cell death
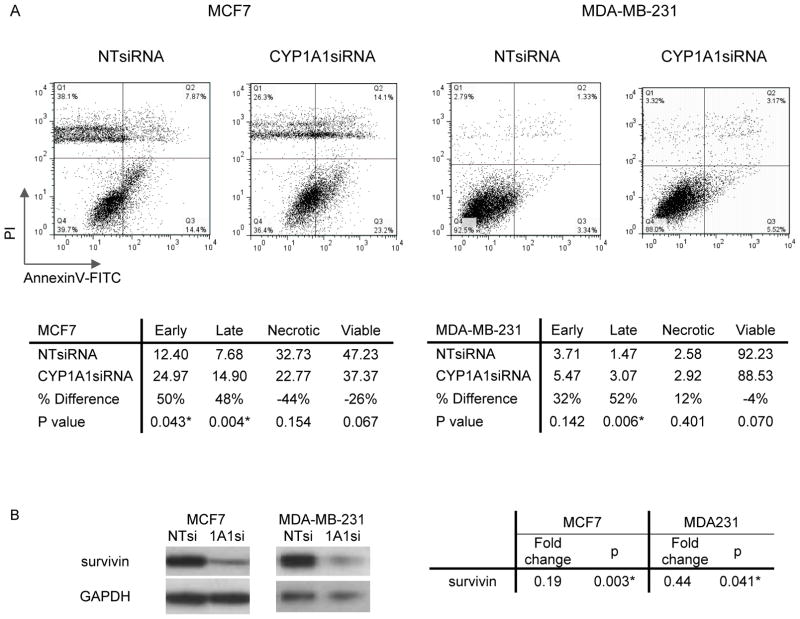
To further understand the anti-proliferative effects of CYP1A1 silencing, apoptosis was measured by flow cytometry. In this assay, cells were transfected for 48hrs, stained with propidium iodide and Annexin-V/FITC, and analyzed by flow cytometry. CYP1A1 silencing of the MCF7 line correlated with a 50% increase in the early (lower right) and late (upper right quadrant) apoptotic populations (Fig. 3A; p < 0.05). CYP1A1 silencing of the MDA-MB-231 line correlated with a 50% increase in the late apoptotic population (Fig. 3A; p = 0.006).
Figure 3. CYP1A1 knock down increases cell death.
A, Apoptosis was assessed by flow cytometry of MDA-MB-231 or MCF7 cells transfected for 48hr and stained with propidium iodine and Annexin V-FITC. B, Survivin protein was visualized by western blot. Fold changes are expressed relative to CYP1A1siRNA knock down (n=3, p values shown, * indicates statistical significance). Legend: Early, early apoptotic population in the lower right quadrant; Late, late apoptotic population in the upper right quadrant; Necrotic, necrotic population in the upper left quadrant; Viable, alive population in the lower left quadrant.
Survivin is an anti-apoptotic protein of the inhibitor of apoptosis (IAP) family whose importance in breast cancer, including MCF7 and MDA-MB-231 lines, has been established (17, 18). Therefore, to further understand the role of CYP1A1 in apoptosis, we measured the effects of CYP1A1 knock down on the levels of survivin. Consistent with increased apoptosis, we observed that CYP1A1 knock down was associated with an 80% reduction of survivin (p = 0.003) in MCF7 line and a 56% reduction (p= 0.04) in MDA-MB-231 line (Fig. 3B).
CYP1A1 knock down inhibits the ERK1/2 and AKT pathways
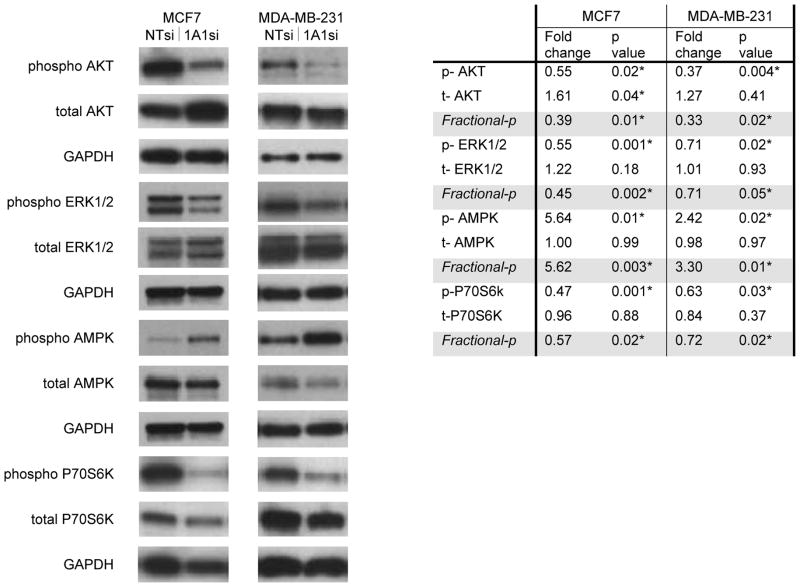
Because the MEK/ERK and PI3K/AKT signaling pathways promote the growth of breast cancer (19–22), we determined whether CYP1A1 knock down inhibits ERK1/2 and/or AKT phosphorylation in these lines (Fig. 4). CYP1A1 knock down reduced phosphorylation of ERK in both lines, most notably by 45% (p=0.001) in the MCF7 line (Fig. 4). CYP1A1 knock down resulted in reduction of AKT phosphorylation by 45% (p=0.02) in MCF7 line and by 65% in MDA-MB-231 line (p=0.004) (Fig. 4). These reductions in phosphorylation correlate with downstream inhibition of the protein synthesis regulator P70S6K (23, 24). Phosphorylation of P70S6K was reduced by 53% (p=0.001) in the MCF7 line and by 37% (p=0.03) in the MDA-MB-231 line (Fig. 4). These results implicate CYP1A1 upstream of the ERK and PI3K/AKT pathways.
Figure 4. CYP1A1 knock down inhibits ERK1/2 and AKT and activates AMPK.
The effects of CYP1A1siRNA on downstream signaling were evaluated by western blot of cells transfected for 72–120hrs. Fold changes are expressed relative to CYP1A1siRNA knock down (n=3, p values shown, * indicates statistical significance). Fractional phosphorylation results are included (Fractional-p= phosphorylated/total protein).
CYP1A1 knock down induces the AMPK pathway
While reduction of P70S6K phosphorylation caused by CYP1A1 silencing could be related to loss of ERK1/2 and PI3K/AKT signaling, this inhibition could also be due to activation of AMPK, a major regulator of cellular bioenergetics and metabolic tumor suppressor (25). CYP1A1 knock down increases phosphorylation of AMPK 5.6-fold (p=0.01) in the MCF7 line and 2.4-fold (p=0.02) in MDA-MB-231 line (Fig. 4). The activation of AMPK phosphorylation by CYP1A1 silencing in conjunction with concomitant down-regulation of ERK1/2 and AKT phosphorylation is consistent with known crosstalk with AMPK (26–28) and potentially places CYP1A1 as a candidate upstream regulator of these kinases. The levels of LKB1, a known regulator of AMPK (29), were determined by Western blot in the MCF7 line. Although an initial marginal reduction of LKB1 by CYP1A1siRNA was observed (48h transfection fold change= 0.70, p= 0.047), this reduction was not sustained at longer time points (72–96h; 96h transfection fold change= 1.01, p= 0.98). This suggests that LKB1-dependent and LKB1-independent mechanisms of AMPK activation may be involved. A possible model summarizing these mechanisms is presented in Figure 7.
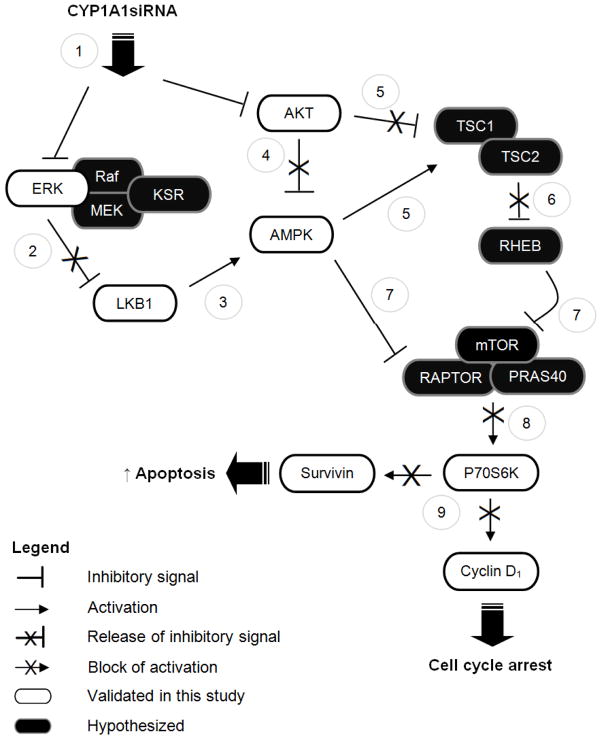
Figure 7. Possible mechanisms of action of CYP1A1 knock down.
CYP1A1 knock down by siRNA results in the dual inactivation ERK and AKT (1). Inactivation of ERK could presumably remove the suppressive pressure that this exerts on LKB1 (2), thereby allowing activation of AMPK (3). LKB1-independent mechanisms may also be possible. Additionally, inhibition of AKT could result in the release of its suppressive effect on AMPK (4). Alternatively, inhibition of AKT by CYP1A1siRNA could result in the release of the TSC1/2 complex from suppression (5), followed by release of RHEB (6), and subsequent inhibition of mTOR signaling (7). Alternatively, AMPK could directly activate TSC2 (5) and inhibit RAPTOR (7), thereby inhibiting mTOR signaling(8). In this manner CYP1A1siRNA treatment results in cell cycle arrest and increased apoptosis (9). In summary, we propose that CYP1A1siRNA-mediated inhibition of ERK1/2 and/or AKT results in AMPK activation, thereby inhibiting the mTOR/P70S6K pathway and thus resulting in cellular death. Legend: AMPK, AMP-activated protein kinase; ERK1/2, Extracellular Signal-Regulated Kinases 1 and 2; KSR, Kinase Suppressor of Ras; LKB1, Liver kinase B1; P70S6K, 70kDa ribosomal protein S6 kinase; RHEB, Ras homolog enriched in brain; TSC1/2, Tuberous sclerosis protein 1/2.
AMPK inhibition partially abrogates CYP1A1siRNA-mediated apoptosis
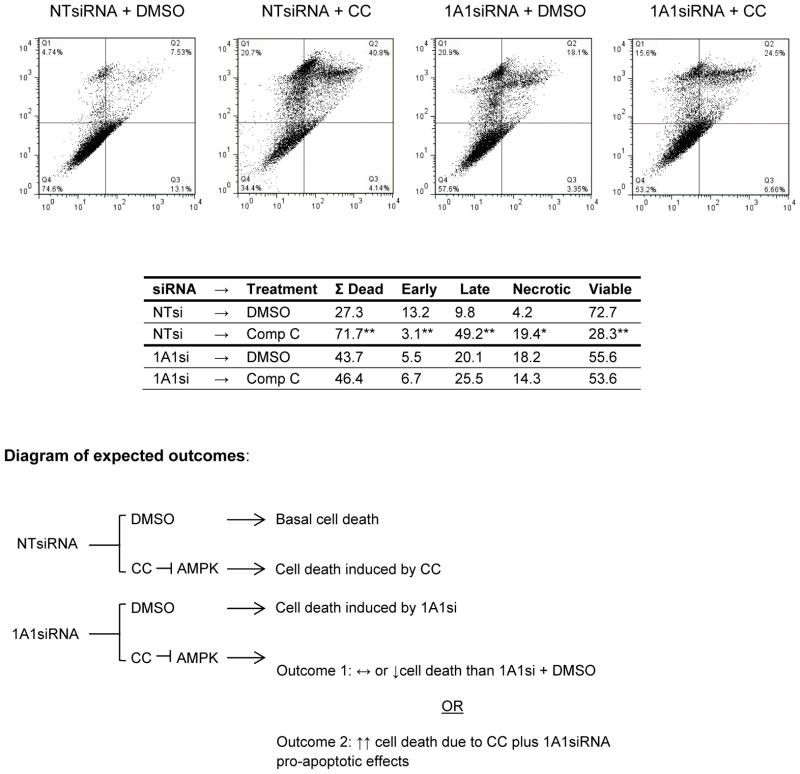
We hypothesized that if AMPK signaling is necessary for the biological effects of CYP1A1 knock down, then blocking AMPK activation should abrogate these effects. To test this hypothesis MCF7 cells were transfected for 24h with CYP1A1siRNA, followed by treatment for 24h with either vehicle (DMSO) or the AMPK inhibitor compound C (6-[4-(2-Piperidin-1-yl-ethoxy)-phenyl)]-3-pyridin-4-yl-pyyrazolo[1,5-a] pyrimidine; from now on abbreviated as CC). The reverse order of treatment (i.e. 24h treatment with DMSO or CC followed by NT/CYP1A1siRNA transfection) was also performed, leading to similar results. Following treatment, apoptosis was measured by flow cytometry and the percentage of overall cell death was calculated as follow: ΣDead= % early apoptotic + % late apoptotic + % necrotic populations. It should be noted that, because compound C induces cell death in MCF7 line (ref. (30) and confirmed in Fig. 5), the results are interpreted only within the context of treatment (i.e. DMSO or CC) as shown in the diagram on Fig. 5. Therefore, within the context of vehicle treatment CYP1A1siRNA increases cell death (Fig. 5; ΣDead (NTsi+DMSO) = 27% vs. ΣDead (1A1si+DMSO) = 44%; p =0.01). This pro-apoptotic effect of CYP1A1siRNA in the presence of vehicle is consistent with our results in Figure 3 and confirms that these effects are due to CYP1A1siRNA and not vehicle treatment.
Figure 5. AMPK inhibition partially abrogates CYP1A1siRNA-mediated apoptosis.
To support the hypothesis that the pro-apoptotic effects of CYP1A1siRNA require AMPK, MCF7 cells were transfected for 24hr and then treated with 10uM of the AMPK inhibitor compound C for 24hrs. Cells were collected, stained with propidium iodide and Annexin V-FITC, and analyzed by flow cytometry. A diagram of expected outcomes is included, Outcome 1 was observed in our experiments. (n=3, *p < 0.01, **p < 0.001, comparison between DMSO and compound C treatment)
Within the context of CC treatment two potential outcomes could be expected depending on whether AMPK is necessary for the pro-apoptotic effects of CYP1A1siRNA (Fig. 5 diagram): (a) if AMPK is necessary, then inhibition of AMPK would prevent CYP1A1siRNA-induced apoptosis and thus less cell death would be observed with “1A1si + CC” treatment compared to “NTsi + CC” treatment; (b) on the other hand if AMPK in not necessary, then inhibition of AMPK would not abrogate the pro-apoptotic effects of CYP1A1siRNA but would instead add to the pro-apoptotic effects of CYP1A1siRNA, thus resulting in an increase in cell death when treated with “1A1si + CC” compared to “NTsi + CC”. Our results agree with the first scenario, 72% total cell death is observed with “NTsi + CC” while 46% total cell death is observed in “1A1si + CC” (Fig. 5; p=0.003). This reduction of cell death observed for CYP1A1 knock down cells treated with the AMPK inhibitor (CC) indicates that AMPK is necessary for the effects of CYP1A1siRNA and agrees with our findings showing that CYP1A1siRNA induces AMPK signaling (Fig. 4). Together, these results suggest that AMPK phosphorylation may be repressed by CYP1A1 and reduction of CYP1A1 levels promotes AMPK phosphorylation. In this manner AMPK may be required for the effects of CYP1A1siRNA on cell death (see Figure 7).
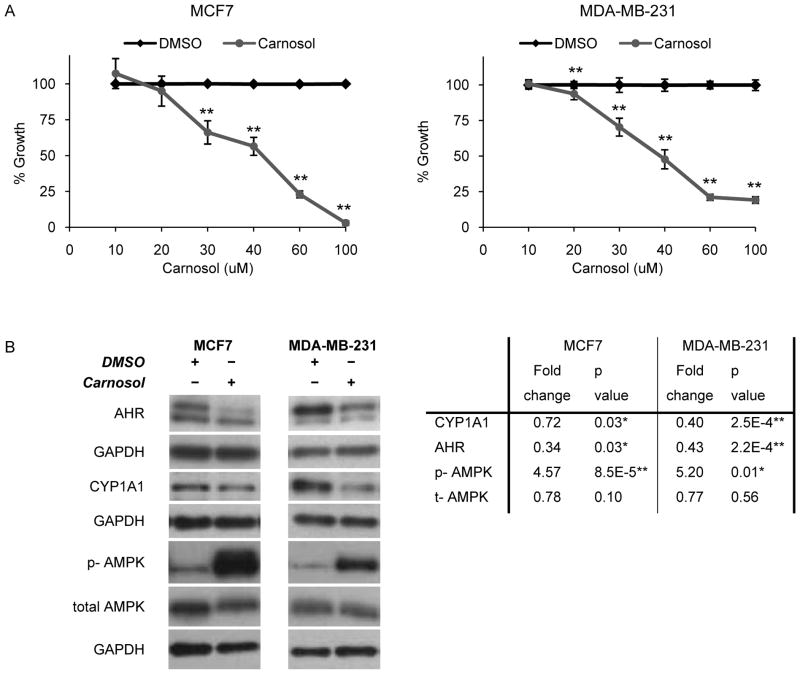
Carnosol impairs proliferation, in part, via reduction of CYP1A1 and activation of AMPK
To further test whether CYP1A1 signals, in part, through AMPK, we sought a pharmacologic approach to reduce CYP1A1 levels (31). Carnosol inhibits the aryl hydrocarbon receptor (AhR), a transcription factor that regulates the inducible and basal expression of CYP1 family members including CYP1A1 (31). Carnosol has been shown to reduce basal CYP1A1 expression in pre-malignant tongue and bronchial lines and in prostate cancer lines (31–33). Treatment with carnosol inhibits the proliferation of the MCF7 and MDA-MB-231 lines exhibiting IC50 values of ~40 μM for both lines (Fig 6A; p < 0.001). We performed time course experiments to investigate early effects (i.e. 2–12h) of carnosol treatment on CYP1A1 expression and determined that the optimal time for CYP1A1 reduction is 8h (Fig. 6B; p < 0.05). Carnosol treatment reduces AhR levels in both lines by over 50% (Fig. 6B; p < 0.05). In agreement with CYP1A1siRNA results (Fig. 4), treatment of the MCF7 and MDA-MB-231 lines with 40 μM carnosol for 8hrs results in the activation of AMPK (Fig. 6B; p < 0.01). This suggests that CYP1A1 reduction, whether by siRNA or carnosol, is associated with inhibition of proliferation mediated, in part, through activation of AMPK signaling.
Figure 6. The AHR inhibitor carnosol impairs proliferation, in part, via reduction of CYP1A1 and activation of AMPK.
An independent method to reduce CYP1A1 levels and evaluate downstream signaling was utilized. A, The IC50 of carnosol was determined by MTT assay. B, Cell extracts were collected after treatment with 40 μM carnosol for 8hrs the indicated proteins were evaluated by western blot. Fold changes are expressed relative to CYP1A1siRNA knock down (n=3, *p < 0.05, **p < 0.001).
Because carnosol inhibits both AhR and CYP1A1 levels, we tested whether the anti-proliferative effects of carnosol were mediated through AhR-dependent mechanisms. As previously shown, knock down of AhR does not reduce basal CYP1A1 levels (Supplementary Fig.S1A), suggesting that carnosol inhibits basal CYP1A1 through an AhR-independent mechanism. Therefore, to better understand these mechanisms, we tested whether AhR is required for the therapeutic effects of carnosol. If so, we would expect AhR knock down to shift the dose-response curve of carnosol. Treatment of AhR-knock down cells with carnosol does not result in a shift of the dose response curve (Supplementary Fig. S1C), further supporting that carnosol’s effects may be independent of AhR. Therefore, together these results suggest that carnosol’s anti-proliferative effects are primarily due to CYP1A1 reduction, AMPK activation, and potentially other yet unidentified mechanisms.
CYP1A1 targeting: expression vs. activity
To this point we have focused on the impact of CYP1A1 levels in the biology of breast cancer cells. Nonetheless, for therapeutic development purposes we should distinguish between the expression levels and the enzymatic activity of CYP1A1. To address this issue we tested whether inhibition and/or induction of CYP1A1 activity affects breast cancer cell proliferation.
First, the effects of inhibiting CYP1A1 activity on cell proliferation were determined. To achieve this we first evaluated whether CYP1A1siRNA affects the activity of CYP1A1. To test this, cells were transfected with CYP1A1siRNA for 48h and TCDD-induced CYP1A1 activity was measured by EROD assay. CYP1A1 knock down reduces CYP1A1 EROD activity by 38% (p = 0.04) in MCF7 line, but it is unaffected (p = 0.52) in MDA-MB-231 line (Supplementary Fig. S2A). These results led us to hypothesis that, while reduction of CYP1A1 levels is necessary for impaired proliferation (Fig. 1), this effect on proliferation may not be dependent on the inhibition of CYP1A1 activity. To further test this hypothesis, we measured the effects of inhibiting CYP1A1’s activity on cell proliferation. Cells were treated with 1 uM of alpha-naphthoflavone, an inhibitor of CYP1A1 activity that does not affect CYP1A1 levels (Supplementary Fig. S2 B and data not shown). Inhibition of CYP1A1 activity by alpha-naphthoflavone does not affect cell proliferation (Supplementary Fig. S2C), supporting the hypothesis that reduction CYP1A1 levels, but not its activity, is required for impairment of proliferation and survival (Fig. 1).
Secondly, the effects of inducing CYP1A1 activity on cell proliferation were determined. Cells were treated with the CYP1A1 inducer 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), which results in increased CYP1A1 levels and activity. Treatment with 5–20 nM TCDD induces CYP1A1 activity, but does not stimulate cell proliferation (10 nM shown in Supplementary Fig. S2D–E). It is noteworthy that the induction of CYP1A1 activity is greatest in the MCF7 line, when compared to MDA-MB-231, which is likely attributable to differences in AhR status in these lines (Supplementary Fig. S2D). These results suggest that breast cancer lines MCF7 and MDA-MB-231 have an optimal amount of CYP1A1 protein and further increasing its level or activity may not enhance the proliferative capability of these lines.
Together, our findings suggest that the development of therapeutic strategies to target CYP1A1 should consider the expression levels of the protein and not just its activity. Nonetheless, it remains to be determined whether CYP1A1’s enzymatic activity or a yet unidentified function(s) is mechanistically responsible for the proliferative and survival cell signaling identified in the present studies.
Discussion
CYP1A1 in breast cancer biology
Recent studies showing that CYP1A1 is expressed in breast tumors (7, 8) led us to investigate the functional roles of CYP1A1 in the proliferation, survival, and signal transduction of breast cancer cells. Although CYP1A1 has been extensively studied in context of extrahepatic drug metabolism, little is known about its roles in cancer progression and cancer cell signal transduction in the absence of xenobiotics. In this study we provide evidence that CYP1A1 silencing impairs proliferation and survival, in part, through activation of AMPK phosphorylation and reduction of AKT, ERK, and P70S6K signaling. These results mean that CYP1A1 is not only involved in the metabolism of xenobiotics, but has its own role in breast cancer progression.
The PI3K/AKT and the MEK/ERK pathways are critical for breast cancer progression (19–22). Knock down of CYP1A1 correlates with decreased phosphorylation of AKT (Ser473) and ERK1/2 (Thr202/Tyr204). CYP1A1 silencing also correlates with induction of AMPK tumor suppressor via phosphorylation of Thr172 in the catalytic subunit alpha. The AKT oncogene promotes proliferation via inhibition of the TSC1/TSC2 complex upstream of the mTOR/P70S6K pathway (34). In contrast, AMPK activates the TSC1/TSC2 complex thereby inhibiting protein synthesis and growth (35, 36). In this manner AKT and AMPK signaling converge on P70S6K to regulate cell proliferation (24, 37).
The effects of CYP1A1 knock down on cell proliferation correlate with cell cycle arrest and increased apoptosis. Our results indicate that cyclin D1 is suppressed by CYP1A1 knock down and correlates with a decrease in G1 to S cell cycle progression. In contrast, in the absence of TCDD induction, AhR knock down does not appear to significantly affect basal CYP1A1 expression (Supplementary Fig. S1A), cyclin D1 or cell cycle profile (38). AKT and ERK1/2 promote G1/S transition of the cell cycle by stabilizing cyclin D1, while AMPK activation inhibits this transition by decreasing cyclin D1 levels, (39–42). Additionally, AKT and ERK1/2 regulate apoptosis through the anti-apoptotic protein survivin (43, 44). Other feedback regulations between AKT, ERK1/2, and AMPK have also been described (26–28, 45). Therefore, based on these previous findings, the effects of CYP1A1 silencing on proliferation, cell cycle, and apoptosis are consistent with inhibition of AKT, ERK1/2, and P70S6K and activation of AMPK signaling. Moreover, our results showing that AMPK inhibition by compound C abrogates CYP1A1siRNA-mediated apoptosis further suggest that CYP1A1 signals through AMPK.
In light of previous findings and the evidence presented in this study we propose a model where CYP1A1 silencing inhibits AKT and ERK phosphorylation, thereby activating AMPK signaling (Fig. 7; steps 1–4). AMPK activation and concurrent loss of AKT signaling results in the inhibition of mTOR/P70S6K signaling (Fig. 7; steps 5–8) which consequently decreases synthesis of proliferative and pro-survival proteins such as cyclin D1 and survivin (Fig. 7; step 9). These findings differ from other cytochrome P450 enzymes, such as CYP3A4, which may act primarily through activation of STAT3 and regulation of the G2/M checkpoint (46). Our model, however, presents its limitations as it remains to be determined the CYP1A1 functions that affect these signaling pathways. The results presented suggest that CYP1A1 expression is critical for these biological functions and the roles of CYP1A1 activity in cancer cell growth may not be abrogated by inhibition of its measurable (EROD) enzymatic activity alone. Moreover, it is possible that CYP1A1 may be carrying functions distinct from its canonical metabolic functions. Novel hypotheses regarding non-enzymatic and enzymatic functions of CYP1A1 in cancer cell growth remain to be investigated.
Clinical impact
Inhibition of the PI3K/AKT and MEK/ERK pathways correlating with CYP1A1 silencing is important because cross talk between these pathways displays synergistic effects when combined inhibition is utilized for cancer therapeutics (23). The involvement of CYP1A1 in both pathways suggests that CYP1A1 may be a promising target for cancer therapeutics. Furthermore, because CYP1A1 silencing significantly inhibits proliferation and survival of ER+ and triple-negative breast cancer lines, the results presented in this study may have therapeutic implications for breast cancer independent of ER status. The effect of CYP1A1 knock down on cell death appears to be greater in ER+ MCF7 line than in triple-negative MDA-MB-231 line, whereas both lines appear to be strongly inhibited at the G1/S checkpoint. Whether strategies to inhibit CYP1A1 would be more effective in ER+ compared to triple-negative breast cancer or effective in both remains to be determined.
The widespread expression of CYP1A1 in breast cancer has been exploited as a strategy to activate pro-drug compounds to cytotoxic intratumoral metabolites. For example, drugs of the 2-(4-aminophenyl)benzothiazole class such as 5F 203 (Phortress) (47) and aminoflavone drugs (48) exhibit potent anti-tumor properties in xenograft models and have been moved forward to clinical trials (9, 12, 47, 49). The proposed mechanism of action is that these agents induce and are activated by CYP1A1 into electrophilic metabolites that bind to and damage DNA, thus resulting in tumor growth arrest. Of considerable interest, the benzothiazoles and aminoflavone appear to be active in ER+ but not triple negative breast cancer (10, 47). Our results suggest that an alternative approach to exploit CYP1A1 expression in breast cancer through reduction of basal levels may extend the range of CYP1A1 targeted approaches to triple negative breast cancer.
Consistent with observations in other cell types (31, 32, 50), our results indicate that carnosol treatment inhibits breast cancer cell proliferation. This inhibition of proliferation in breast cancer lines is associated with reduced CYP1A1 expression and activation of AMPK, which has not been previously described in breast cancer. While the activation of AMPK phosphorylation by carnosol may be part of a generalized stress response, these results provide new mechanistic information that carnosol is likely to affect bioenergetics in breast cancer in addition to exhibiting antioxidant properties. Our results suggest that the relevant target of carnosol in breast cancer may be reduction of CYP1A1 levels, resulting in AMPK phosphorylation, rather than modulation of AhR. The studies presented here suggest that reduction of CYP1A1 levels is a potential therapeutic strategy for breast cancer and that carnosol may be an approach to actualize this strategy.
In summary, we show that the basal level of CYP1A1, independent of measurable enzymatic (EROD) activity and AhR status, is important for breast cancer proliferation and survival. The identification of widespread expression of CYP1A1 in breast cancer suggests that its therapeutic potential could be exploited, either by induction followed by metabolism of pro-drugs to DNA damaging agents (9, 11, 47) or by a new approach of CYP1A1 reduction. Our study suggests that strategies that directly lower CYP1A1 levels using RNA silencing or carnosol may be a potential new approach for breast cancer therapeutics.
Supplementary Material
Acknowledgments
This study was supported by National Institute of Health grants F31CA1440842 and R01CA113570 and by the University of Minnesota OVPR Grant-in-Aid.
We would like to thank the expert advice of Drs. Chee-Onn Leong, Tracey D. Bradshaw, Douglas Yee, Ranjana Mitra, Zhijun Guo, and Monica Milani. We also appreciate Drs. James McCarthy, Carol Lange, Kaylee Schwertfeger for helpful discussions and serving on the thesis committee of MR. We thank Drs. Ian Blair and Narayan Avadhani for helpful discussions. We also thank Dr. Deepali Sachdev and Dr. Elizabeth Wattenberg for kindly providing the cell cycle and quantitative RT-PCR protocols, respectively. Thanks to Dr. Kaylee Schwertfeger for critical reading of the manuscript.
Footnotes
Conflict of Interest:
The authors confirm that there is no potential conflict of interest.
References
- 1.Spink DC, Eugster HP, Lincoln DW, 2nd, et al. 17 beta-estradiol hydroxylation catalyzed by human cytochrome P450 1A1: a comparison of the activities induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin in MCF-7 cells with those from heterologous expression of the cDNA. Archives of biochemistry and biophysics. 1992;293(2):342–8. doi: 10.1016/0003-9861(92)90404-k. [DOI] [PubMed] [Google Scholar]
- 2.Das M, Asokan P, Don PS, Krueger GG, Bickers DR, Mukhtar H. Carcinogen metabolism in human skin grafted onto athymic nude mice: a model system for the study of human skin carcinogenesis. Biochemical and biophysical research communications. 1986;138(1):33–9. doi: 10.1016/0006-291x(86)90242-1. [DOI] [PubMed] [Google Scholar]
- 3.van Cantfort J, Gielen JE. Ontogenetic variation in rat liver, lung and kidney monooxygenase induction by low doses of benzo(A)pyrene and cigarette-smoke condensate. British journal of cancer. 1981;44(6):902–10. doi: 10.1038/bjc.1981.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Androutsopoulos VP, Tsatsakis AM, Spandidos DA. Cytochrome P450 CYP1A1: wider roles in cancer progression and prevention. BMC cancer. 2009;9:187. doi: 10.1186/1471-2407-9-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sergentanis TN, Economopoulos KP. Four polymorphisms in cytochrome P450 1A1 (CYP1A1) gene and breast cancer risk: a meta-analysis. Breast cancer research and treatment. 2012;122(2):459–69. doi: 10.1007/s10549-009-0694-5. [DOI] [PubMed] [Google Scholar]
- 6.Singh V, Parmar D, Singh MP. Do single nucleotide polymorphisms in xenobiotic metabolizing genes determine breast cancer susceptibility and treatment outcomes? Cancer investigation. 2008;26(8):769–83. doi: 10.1080/07357900801953196. [DOI] [PubMed] [Google Scholar]
- 7.Murray GI, Patimalla S, Stewart KN, Miller ID, Heys SD. Profiling the expression of cytochrome P450 in breast cancer. Histopathology. 2010;57(2):202–11. doi: 10.1111/j.1365-2559.2010.03606.x. [DOI] [PubMed] [Google Scholar]
- 8.Vinothini G, Nagini S. Correlation of xenobiotic-metabolizing enzymes, oxidative stress and NFkappaB signaling with histological grade and menopausal status in patients with adenocarcinoma of the breast. Clin Chim Acta. 2010;411(5–6):368–74. doi: 10.1016/j.cca.2009.11.034. [DOI] [PubMed] [Google Scholar]
- 9.Chua MS, Kashiyama E, Bradshaw TD, et al. Role of Cyp1A1 in modulation of antitumor properties of the novel agent 2-(4-amino-3-methylphenyl)benzothiazole (DF 203, NSC 674495) in human breast cancer cells. Cancer research. 2000;60(18):5196–203. [PubMed] [Google Scholar]
- 10.Trapani V, Patel V, Leong CO, et al. DNA damage and cell cycle arrest induced by 2-(4-amino-3-methylphenyl)-5-fluorobenzothiazole (5F 203, NSC 703786) is attenuated in aryl hydrocarbon receptor deficient MCF-7 cells. British journal of cancer. 2003;88(4):599–605. doi: 10.1038/sj.bjc.6600722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradshaw TD, Stevens MF, Westwell AD. The discovery of the potent and selective antitumour agent 2-(4-amino-3-methylphenyl)benzothiazole (DF 203) and related compounds. Curr Med Chem. 2001;8(2):203–10. doi: 10.2174/0929867013373714. [DOI] [PubMed] [Google Scholar]
- 12.Nandekar PP, Sangamwar AT. Cytochrome P450 1A1-mediated anticancer drug discovery: in silico findings. Expert opinion on drug discovery. 2012;7(9):771–89. doi: 10.1517/17460441.2012.698260. [DOI] [PubMed] [Google Scholar]
- 13.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nature protocols. 2008;3(6):1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 14.Sinal CJ, Bend JR. Aryl hydrocarbon receptor-dependent induction of cyp1a1 by bilirubin in mouse hepatoma hepa 1c1c7 cells. Molecular pharmacology. 1997;52(4):590–9. doi: 10.1124/mol.52.4.590. [DOI] [PubMed] [Google Scholar]
- 15.Yu Z, Hu D, Li Y. Effects of zearalenone on mRNA expression and activity of cytochrome P450 1A1 and 1B1 in MCF-7 cells. Ecotoxicology and environmental safety. 2004;58(2):187–93. doi: 10.1016/j.ecoenv.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Lukas J, Bartkova J, Bartek J. Convergence of mitogenic signalling cascades from diverse classes of receptors at the cyclin D-cyclin-dependent kinase-pRb-controlled G1 checkpoint. Molecular and cellular biology. 1996;16(12):6917–25. doi: 10.1128/mcb.16.12.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka K, Iwamoto S, Gon G, Nohara T, Iwamoto M, Tanigawa N. Expression of survivin and its relationship to loss of apoptosis in breast carcinomas. Clin Cancer Res. 2000;6(1):127–34. [PubMed] [Google Scholar]
- 18.Yang L, Cao Z, Yan H, Wood WC. Coexistence of high levels of apoptotic signaling and inhibitor of apoptosis proteins in human tumor cells: implication for cancer specific therapy. Cancer research. 2003;63(20):6815–24. [PubMed] [Google Scholar]
- 19.Sun M, Paciga JE, Feldman RI, et al. Phosphatidylinositol-3-OH Kinase (PI3K)/AKT2, activated in breast cancer, regulates and is induced by estrogen receptor alpha (ERalpha) via interaction between ERalpha and PI3K. Cancer research. 2001;61(16):5985–91. [PubMed] [Google Scholar]
- 20.Sun M, Wang G, Paciga JE, et al. AKT1/PKBalpha kinase is frequently elevated in human cancers and its constitutive activation is required for oncogenic transformation in NIH3T3 cells. The American journal of pathology. 2001;159(2):431–7. doi: 10.1016/s0002-9440(10)61714-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sivaraman VS, Wang H, Nuovo GJ, Malbon CC. Hyperexpression of mitogen-activated protein kinase in human breast cancer. The Journal of clinical investigation. 1997;99(7):1478–83. doi: 10.1172/JCI119309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xing C, Imagawa W. Altered MAP kinase (ERK1,2) regulation in primary cultures of mammary tumor cells: elevated basal activity and sustained response to EGF. Carcinogenesis. 1999;20(7):1201–8. doi: 10.1093/carcin/20.7.1201. [DOI] [PubMed] [Google Scholar]
- 23.She QB, Halilovic E, Ye Q, et al. 4E-BP1 is a key effector of the oncogenic activation of the AKT and ERK signaling pathways that integrates their function in tumors. Cancer cell. 2010;18(1):39–51. doi: 10.1016/j.ccr.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fingar DC, Richardson CJ, Tee AR, Cheatham L, Tsou C, Blenis J. mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Molecular and cellular biology. 2004;24(1):200–16. doi: 10.1128/MCB.24.1.200-216.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo Z, Zang M, Guo W. AMPK as a metabolic tumor suppressor: control of metabolism and cell growth. Future oncology (London, England) 2010;6(3):457–70. doi: 10.2217/fon.09.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kovacic S, Soltys CL, Barr AJ, Shiojima I, Walsh K, Dyck JR. Akt activity negatively regulates phosphorylation of AMP-activated protein kinase in the heart. The Journal of biological chemistry. 2003;278(41):39422–7. doi: 10.1074/jbc.M305371200. [DOI] [PubMed] [Google Scholar]
- 27.Du J, Guan T, Zhang H, Xia Y, Liu F, Zhang Y. Inhibitory crosstalk between ERK and AMPK in the growth and proliferation of cardiac fibroblasts. Biochemical and biophysical research communications. 2008;368(2):402–7. doi: 10.1016/j.bbrc.2008.01.099. [DOI] [PubMed] [Google Scholar]
- 28.Kim MJ, Park IJ, Yun H, et al. AMP-activated protein kinase antagonizes pro-apoptotic extracellular signal-regulated kinase activation by inducing dual-specificity protein phosphatases in response to glucose deprivation in HCT116 carcinoma. The Journal of biological chemistry. 2010;285(19):14617–27. doi: 10.1074/jbc.M109.085456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hawley SA, Boudeau J, Reid JL, et al. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. Journal of biology. 2003;2(4):28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin J, Mullen TD, Hou Q, et al. AMPK inhibitor Compound C stimulates ceramide production and promotes Bax redistribution and apoptosis in MCF7 breast carcinoma cells. J Lipid Res. 2009;50(12):2389–97. doi: 10.1194/jlr.M900119-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohebati A, Guttenplan JB, Kochhar A, et al. Carnosol, a constituent of Zyflamend, inhibits aryl hydrocarbon receptor-mediated activation of CYP1A1 and CYP1B1 transcription and mutagenesis. Cancer Prev Res (Phila) 2012;5(4):593–602. doi: 10.1158/1940-6207.CAPR-12-0002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Johnson JJ, Syed DN, Heren CR, Suh Y, Adhami VM, Mukhtar H. Carnosol, a dietary diterpene, displays growth inhibitory effects in human prostate cancer PC3 cells leading to G2-phase cell cycle arrest and targets the 5′-AMP-activated protein kinase (AMPK) pathway. Pharm Res. 2008;25(9):2125–34. doi: 10.1007/s11095-008-9552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Offord EA, Mace K, Ruffieux C, Malnoe A, Pfeifer AM. Rosemary components inhibit benzo[a]pyrene-induced genotoxicity in human bronchial cells. Carcinogenesis. 1995;16(9):2057–62. doi: 10.1093/carcin/16.9.2057. [DOI] [PubMed] [Google Scholar]
- 34.Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Molecular cell. 2002;10(1):151–62. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 35.Corradetti MN, Inoki K, Bardeesy N, DePinho RA, Guan KL. Regulation of the TSC pathway by LKB1: evidence of a molecular link between tuberous sclerosis complex and Peutz-Jeghers syndrome. Genes & development. 2004;18(13):1533–8. doi: 10.1101/gad.1199104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115(5):577–90. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 37.Zhao P, Meng Q, Liu LZ, You YP, Liu N, Jiang BH. Regulation of survivin by PI3K/Akt/p70S6K1 pathway. Biochemical and biophysical research communications. 2010;395(2):219–24. doi: 10.1016/j.bbrc.2010.03.165. [DOI] [PubMed] [Google Scholar]
- 38.Abdelrahim M, Smith R, 3rd, Safe S. Aryl hydrocarbon receptor gene silencing with small inhibitory RNA differentially modulates Ah-responsiveness in MCF-7 and HepG2 cancer cells. Molecular pharmacology. 2003;63(6):1373–81. doi: 10.1124/mol.63.6.1373. [DOI] [PubMed] [Google Scholar]
- 39.Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes & development. 1998;12(22):3499–511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhuang Y, Miskimins WK. Cell cycle arrest in Metformin treated breast cancer cells involves activation of AMPK, downregulation of cyclin D1, and requires p27Kip1 or p21Cip1. J Mol Signal. 2008;3:18. doi: 10.1186/1750-2187-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lavoie JN, L’Allemain G, Brunet A, Muller R, Pouyssegur J. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. The Journal of biological chemistry. 1996;271(34):20608–16. doi: 10.1074/jbc.271.34.20608. [DOI] [PubMed] [Google Scholar]
- 42.Meloche S, Pouyssegur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene. 2007;26(22):3227–39. doi: 10.1038/sj.onc.1210414. [DOI] [PubMed] [Google Scholar]
- 43.Asanuma H, Torigoe T, Kamiguchi K, et al. Survivin expression is regulated by coexpression of human epidermal growth factor receptor 2 and epidermal growth factor receptor via phosphatidylinositol 3-kinase/AKT signaling pathway in breast cancer cells. Cancer research. 2005;65(23):11018–25. doi: 10.1158/0008-5472.CAN-05-0491. [DOI] [PubMed] [Google Scholar]
- 44.Fornaro M, Plescia J, Chheang S, et al. Fibronectin protects prostate cancer cells from tumor necrosis factor-alpha-induced apoptosis via the AKT/survivin pathway. The Journal of biological chemistry. 2003;278(50):50402–11. doi: 10.1074/jbc.M307627200. [DOI] [PubMed] [Google Scholar]
- 45.Tao R, Gong J, Luo X, et al. AMPK exerts dual regulatory effects on the PI3K pathway. J Mol Signal. 2010;5(1):1. doi: 10.1186/1750-2187-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitra R, Guo Z, Milani M, et al. CYP3A4 mediates growth of estrogen receptor-positive breast cancer cells in part by inducing nuclear translocation of phospho-Stat3 through biosynthesis of (+/−)-14,15-epoxyeicosatrienoic acid (EET) The Journal of biological chemistry. 2011;286(20):17543–59. doi: 10.1074/jbc.M110.198515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leong CO, Gaskell M, Martin EA, et al. Antitumour 2-(4-aminophenyl)benzothiazoles generate DNA adducts in sensitive tumour cells in vitro and in vivo. British journal of cancer. 2003;88(3):470–7. doi: 10.1038/sj.bjc.6600719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McLean L, Soto U, Agama K, et al. Aminoflavone induces oxidative DNA damage and reactive oxidative species-mediated apoptosis in breast cancer cells. International journal of cancer. 2008;122(7):1665–74. doi: 10.1002/ijc.23244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fichtner I, Monks A, Hose C, Stevens MF, Bradshaw TD. The experimental antitumor agents Phortress and doxorubicin are equiactive against human-derived breast carcinoma xenograft models. Breast cancer research and treatment. 2004;87(1):97–107. doi: 10.1023/B:BREA.0000041586.64371.88. [DOI] [PubMed] [Google Scholar]
- 50.Johnson JJ, Syed DN, Suh Y, et al. Disruption of androgen and estrogen receptor activity in prostate cancer by a novel dietary diterpene carnosol: implications for chemoprevention. Cancer Prev Res (Phila) 2010;3(9):1112–23. doi: 10.1158/1940-6207.CAPR-10-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.